Abstract
This study investigated if pretreatment ethyl glucuronide (EtG) levels corresponding to light (100ng/mL), heavy (500ng/mL), and very heavy (1,000ng/mL) drinking predicted longest duration of alcohol abstinence (LDA) and proportion of EtG-negative urine tests in 40 outpatients receiving a 12-week EtG-based contingency management (CM) intervention for alcohol dependence. Only the 500ng/mL cutoff was associated with significant differences in LDA and proportion of EtG-negative samples during CM. Those with a pre-treatment EtG<500ng/mL attained a LDA 2.3 (alcohol) to 2.9 (drugs) weeks longer than pre-treatment heavy drinkers. Results suggest pre-treatment EtG cutoffs equivalent to heavy and very heavy drinking predict outcomes in CM.
Keywords: alcohol treatment, contingency management, ethyl glucuronide, serious mental illness, predicting treatment outcomes
Contingency management (CM) is an intervention in which reinforcers, such as vouchers or prizes, are provided when individuals demonstrate illicit drug abstinence, typically measured multiple times per week.1 While numerous studies have documented the efficacy of CM for illicit drugs and cigarette smoking, research investigating CM as a treatment for alcohol use disorders has been limited due to lack of a suitable alcohol biomarker to verify use for two or more days.2,3 However, ethyl glucuronide (EtG), a hepatic metabolite of alcohol detectable in urine, addresses this need.
In a recent study, the EtG alcohol biomarker was used to verify abstinence in a randomized controlled trial of CM for alcohol use disorders using a relatively low-cost EtG immunoassay.4 In this trial, 79 adults diagnosed with alcohol dependence and serious mental illness participated in 4-week pre-randomization observation period during which they submitted urine samples for EtG testing three times each week. They were randomized to receive 12-weeks of CM for EtG-negative urine samples (EtG<150ng/mL) and addiction treatment attendance, or non-contingent reinforcement in which they received reinforcement for submitting urine samples regardless of EtG results. Relative to controls, CM participants were three times more likely to submit an alcohol-negative urine sample during treatment and attained an average of 1.5 weeks of additional abstinence, as assessed by EtG.
While overall response to CM was positive, in previous studies pre-treatment drug-positive urine tests are associated with lower levels of abstinence during CM. 5,6,7 These studies used point-of-care urine drug tests, which provide limited information (positive/negative) with which to predict CM treatment response. In contrast, the present study used an EtG immunoassay, which provides continuous results from 0ng/mL to 2,000ng/mL. In our previous work we have found that EtG cutoffs of 100ng/mL, 500ng/mL, and 1,000ng/mL correspond to recent light, heavy, and very heavy drinking, respectively.8 Therefore, the EtG immunoassay allows us to determine which of these clinically relevant EtG cutoffs best predicts treatment outcomes.
In this secondary analysis, we investigated whether an EtG cutoff that was comparable to light, heavy or very heavy drinking best discriminated between those who did and did not respond to the CM intervention, as assessed by longest duration of abstinence (LDA) from alcohol and the odds of submitting an alcohol-negative EtG test during CM. Results could allow clinicians to use EtG tests, including new point-of-care tests, to identify individuals who are likely or unlikely to respond to CM.
Method
Participants
Participants were adults enrolled in state-certified outpatient addiction treatment at a multisite community mental health and addiction treatment agency, who met DSM-IV-TR criteria for alcohol dependence, and schizophrenia, schizoaffective disorder, bipolar I or II, or recurrent major depressive disorder as assessed by the MINI International Neuropsychiatric Interview. Seventy-nine individuals were randomized. This secondary data analysis included only those allocated to CM (n=40). Participants provided written informed consent and procedures were approved by the University of Washington’s Human Subjects Division.
Study design
Participants received treatment-as-usual including case management, medication management, and housing and vocational services throughout the study. Upon enrollment they participated in a 4-week pre-treatment observation period in which they received reinforcers contingent on providing urine samples three times per week regardless of EtG-results. Participants who attended at least one visit during week 4 and provided at least one EtG-positive urine sample were randomized.
Participants randomized to CM (n=40) submitted urine samples for EtG testing three times per week for 12 weeks. They engaged in the variable magnitude of reinforcement procedure each time they tested negative for EtG (<150ng/mL). This involved making “prize draws” from a bucket containing tokens representing different magnitudes of reinforcement.1 Prizes included toiletries, clothing, grocery and retail gift cards, small kitchen appliances, and electronics, (average total value earned by participants=$175.03, SD=$183.17). In addition, every week, participants received gift cards for attending all ($10) or at least one ($5) of their scheduled addiction treatment group sessions.
Measures
Participants provided urine samples three times per week during pretreatment and CM. Urine samples were analyzed onsite using the Diagnostic Reagents Incorporated EtG-immunoassay with a Thermo Fisher Indiko analyzer (Fremont, CA). Samples were considered alcohol-negative if EtG<150ng/mL. Tests were conducted using EtG 100ng/mL, 500ng/mL, 1000ng/mL, 2000ng/mL, and Negative calibrators and 100ng/mL and 375ng/mL controls. The cutoff was set at 150ng/mL, 50ng/mL above the lower limit of detection for the EtG biomarker to allow for regular fluctuation in the Indiko instrumentation. Calibrations occurred weekly. Participants were asked to avoid non-beverage sources of ethanol (i.e., mouthwash and cough syrup).
Data analysis
The average pre-treatment EtG score of each participant was calculated based on 12 pre-treatment urine samples collected over 4-weeks. These mean scores were then recoded into three different variables based on the following cutoff levels: <100ng/mL (yes/no), <500ng/mL (yes/no), and <1,000ng/mL (yes/no). Longest duration of alcohol abstinence (highest number of consecutive EtG-negative urine tests; missing tests were considered EtG-positive and ended the period of abstinence) was compared across each of the three cutoff level variables using independent samples t-tests. We utilized generalized estimating equations (GEE) to investigate whether pretreatment period average EtG level predicted proportion of EtG-negative urine samples submitted during the CM phase. The main effect of each cutoff level variable was investigated in three separate analyses. For GEE analyses we present odds ratios, and 95% confidence intervals. The criterion for statistical significance was set at alpha p<0.05. Analyses were conducted using IBM SPSS version 23.
Results
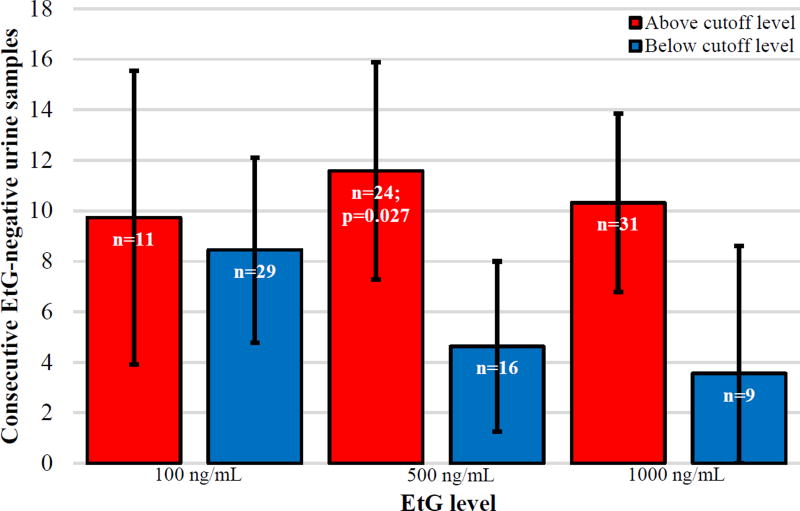
As Figure 1 demonstrates, those who attained an average pretreatment EtG level <100 ng/mL did not differ in LDA (n=11, M=9.73, 95%CI=3.91–15.55) relative to those who had an average pretreatment EtG>99 ng/mL (n=29, M=8.45, 95%CI=4.78–12.12), p=0.72. Those with an average pretreatment EtG level below EtG<500ng/mL had a significantly longer LDA (n=24, M=11.58, 95%CI=7.28–15.88), relative to those with a pretreatment EtG>499ng/mL (n=16, M=4.63, 95%CI=1.26–8), p<0.05. Individuals who averaged EtG<1,000ng/mL (n=31, M=10.32, 95%CI=6.79–13.85) did not attain a longer duration of abstinence that than those with EtG>999ng/mL (n=9, M=3.56, 95%CI=−1.49–8.61), p=0.071. For comparison, participants in the control condition attained an average LDA of 4.1 (95%CI=3.73–4.49).
Figure 1.
Predicting Longest Duration of Abstinence from Alcohol
In GEE analyses, participants who attained a pretreatment EtG level <100ng/mL were 6.67 times more likely (95% CI=2.07–21.46) to submit EtG-negative urine samples in the treatment period, relative to those with EtG>99ng/mL. When the 500 ng/mL cutoff was examined, participants with pretreatment EtG<500ng/mL were 10.16 times more likely (95% CI=3.58–28.84) to submit EtG-negative urine samples, relative to those who had an average EtG>499ng/mL. Finally, those who attained a pretreatment EtG level <1000ng/mL were 12.33 times more likely (95% CI=2.78–54.67) to submit EtG-negative samples, compared to those with a pretreatment EtG>999ng/mL.
Discussion
Because pre-treatment drug-positive urine tests are a strong predictor of treatment outcome, particularly in CM, 5–7 we used EtG to identify a specific cutoff associated with CM treatment response. The 500ng/mL cutoff was the only cutoff associated with both LDA and proportion of EtG-negative samples submitted during CM. Participants who had a pretreatment mean EtG>499ng/mL attained 2.3 fewer weeks of alcohol abstinence than those with an EtG<500ng/mL, indicating that the CM intervention was ineffective for this subsample. The LDA for this group was equal to that of participants in the control condition.
The 100ng/mL and 1,000ng/mL cutoffs were also associated with the proportion of EtG samples submitted during CM. The strength of the association between mean pretreatment EtG levels and EtG-negative results during treatment increased as the pretreatment EtG cutoff increased (i.e. OR=6.61 at 100 ng/mL, OR=10.16 at 500ng/mL, OR=12.13 at 1,000ng/mL). Therefore, individuals with higher pretreatment EtG levels were less likely to submit EtG samples consistent with abstinence during CM.
Limitations include the relatively small sample size, restricting our ability to conduct more sophisticated analyses in which we could account for other variables that might predict CM outcomes. However, the effect of pre-treatment EtG levels on CM outcomes was large, and likely to remain even after accounting for confounds. Other limitations include recruitment from one site, the possibility that results may not generalize to adults without SMI, feasibility issues of the benchtop analyzer needed to conduct EtG tests, and the limited practicality of conducting multiple EtG tests prior to treatment (sensitivity analysis found the results of a single baseline EtG test were not associated with alcohol outcomes). Newly available point-of-care EtG immunoassays with a cutoff of 500ng/mL would allow clinicians to more feasibly identify CM responders vs. non-responders and implement CM.
EtG can be used to identify those who are likely to respond to an abstinence-based CM intervention for alcohol use disorders, with 500ng/mL predicting LDA and proportion of EtG-negative samples during CM. EtG has potential to personalize treatment for adults with alcohol use disorders. For those unlikely to respond to a typical CM intervention, modifications such as increasing the magnitude of reinforcement or requiring reductions in drinking before abstinence might improve outcomes. Alternatively, other interventions such as medication management may be needed to improve outcomes for CM non-responders. Future research should investigate whether pre-treatment EtG levels predict outcomes in other psychosocial and pharmacological interventions, and strategies for improving the efficacy of CM for heavy drinkers.
Acknowledgments
Financial acknowledgements: Funding for this study was provided by a grant from the National Institute on Alcohol Abuse and Alcoholism, (R01 AA020248, PI: McDonell).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction. 2014;109(9):1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118(2–3):391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68(2):250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- 4.McDonell M, Leickly E, McPherson S, et al. A randomized controlled trial of ethyl glucuronide-based contingency management for outpatients with co-occurring alcohol use disorders and serious mental illness. Am J Psychiat. 2017 doi: 10.1176/appi.ajp.2016.16050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelo FN, McDonell MG, Lewin MR, et al. Predictors of stimulant abuse treatment outcomes in severely mentally ill outpatients. Drug Alcohol Depend. 2013;131(1–2):162–165. doi: 10.1016/j.drugalcdep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson S, Barbosa-Leiker C, Daratha K, et al. Association of co-occurring serious mental illness with emergency hospitalization in people with chronic kidney disease. Am J Nephrol. 2014;39(3):260–267. doi: 10.1159/000360095. [DOI] [PubMed] [Google Scholar]
- 7.Stitzer ML, Petry N, Peirce J, et al. Effectiveness of abstinence-based incentives: interaction with intake stimulant test results. J Consult Clin Psychol. 2007;75(5):805–811. doi: 10.1037/0022-006X.75.5.805. [DOI] [PubMed] [Google Scholar]
- 8.McDonell MG, Skalisky J, Leickly E, et al. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug Alcohol Depend. 2015;157:184–187. doi: 10.1016/j.drugalcdep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]