Abstract
Because of its cardio-protective effects, a low Na, high K diet (LNaHK) is often warranted in conjunction with diuretics to treat hypertensive patients. However, it is necessary to understand the renal handling of such diets in order to choose the best diuretic. Wild type (WT) or Renal Outer Medullary K channel (ROMK) knockout mice (KO) were given a regular (CTRL), LNaHK, or high K diet (HK) for 4–7 days. On LNaHK, mice treated with either IP furosemide for 12 hrs, or given furosemide in drinking water for 7 days, exhibited decreased K clearance. We used free-flow micropuncture to measure the [K+] in the early distal tubule (EDT [K+]) before and after furosemide treatment. Furosemide increased the EDT [K+] in WT on CTRL but decreased that in WT on LNaHK. Furosemide did not affect the EDT [K+] of KO on LNaHK or WT on HK. Furosemide-sensitive Na+ excretion was significantly greater in mice on LNaHK than those on CTRL or HK. Patch clamp analysis of split-open TALs revealed that 70-pS ROMK exhibited a higher open probability (Po) but similar density in mice on LNaHK, compared with CTRL. No difference was found in the density or Po of the 30 pS K channels between two groups. These results indicate mice on LNaHK exhibited furosemide-sensitive net K+ secretion in the TAL that is dependent on increased NKCC2 activity and mediated by ROMK. We conclude that furosemide is a K-sparing diuretic by decreasing the TAL net K+ secretion in subjects on LNaHK.
Keywords: K secretion, thick ascending limb, furosemide, ROMK, NKCC2
Introduction
In contrast to the modern Western diet that has high Na and low K contents, the “ancient” diets, such as the Paleolithic and Mediterranean diets, comprise a preponderance of fruits and vegetables and are low in Na and high in K. The ancient diets are well known for their health benefits in cardiovascular diseases, diabetes, obesity, and multiple cancers1–5. Our understanding of such diets have, in large part, derived from studies of the South American Yanomami, an isolated culture known for consuming a high K alkaline diet with nearly zero Na6. Our lab has developed and studied the renal K handling of mice on a low Na high K, alkaline diet (LNaHK) as an exaggeration of the ancient diet consumed by the Yanomami7–9. Our understanding of the mechanism for handling LNaHK is crucial to choosing effective diuretics and preventing the drastic consequences of hypokalemia or hyperkalemia for patients on such diets10.
Potassium ions are freely filtered in the nephron and reabsorbed by the proximal tubule and the thick ascending limb of Henle’s loop (TAL). Potassium is secreted into the lumen in the aldosterone-sensitive distal nephron (ASDN) by the renal outer medullary K channel (ROMK) and the Ca-activated large conductance K channel (BK) 11,12. Potassium secretion is driven by the negative lumen potential generated by Na+ reabsorption through the epithelial Na channel (ENaC) 13,14. On a high K diet, the K+ concentration ([K+]) in the medullary interstitium can reach 39–53 mM by medullary K+ recycling15. The ex vivo microperfusion experiments by Stokes et. al. showed that high medullary interstitial [K+] may inhibit NaCl reabsorption via NKCC2 and increase distal flow and Na+ delivery to stimulate K+ secretion in the distal nephron16. This is a recognized cause for the diuretic effect of high K diets13,17. On the other hand, studies showed that NKCC2 activity is increased in mice on a low Na diet18,19. The dietary effect of both low Na and high K on the ion transport in the TAL remains uncertain.
Ex vivo microperfusion studies showed that K+ was reabsorbed in the isolated perfused medullary TAL but secreted in the cortical TAL20,21. However, net K+ secretion in the TAL has not been shown in vivo, and the physiological conditions and implications of net K+ secretion in the TAL remain unclear. In the current study, we found that after mice were adapted to LNaHK, furosemide, an inhibitor of NKCC2, enhanced urinary Na+ and decreased urinary K+ excretion. Given the high interstitial [K+] generated by medullary K+ recycling, we hypothesized that, in mice on LNaHK, NKCC2 is still active with net K+ secretion in the TAL which can be inhibited by furosemide.
Results
K+ secretion in the TAL with LNaHK
In traditional understanding, a high K diet inhibits NKCC2 in the TAL, causing its diuretic effect16. We first examined the effect of furosemide in mice on LNaHK. On both diets, wild-type mice (WT) receiving IP furosemide exhibited significantly higher urinary Na+ clearance and lower urine osmolality than those treated with vehicle (Figure 1A, Table 1). Thus, Na+ is still actively reabsorbed via NKCC2 in mice on LNaHK.
Figure 1. Effect of furosemide on urinary Na+ and K+ clearances in mice on CTRL and LNaHK.
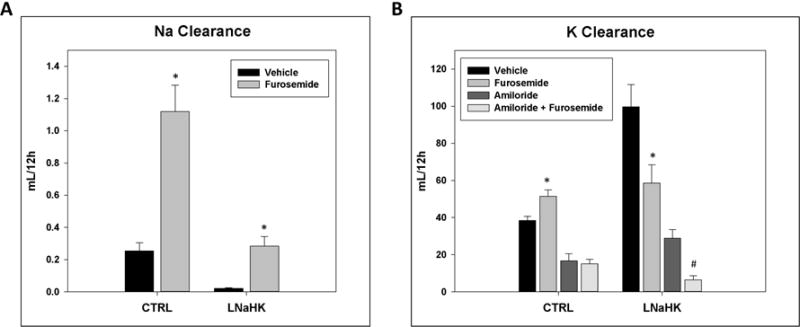
A. Twelve-hour urinary Na+ clearance of mice on CTRL or LNaHK treated with IP injections of vehicle or furosemide. B. Twelve-hour urinary K+ clearance of mice on CTRL or LNaHK treated with IP injections of vehicle, furosemide, amiloride, or amiloride + furosemide. N = 4 – 14 per group. *p < 0.05 vs vehicle; #p < 0.05 vs amiloride analyzed using two-way ANOVA with a post-hoc Tukey test.
Table 1.
Metabolic cage measurements for 12 hours, effects of diuretics.
| CTRL + vehicle (5) | CTRL + furosemide (8) | LNaHK + vehicle (12) | LNaHK + furosemide (7) | |
|---|---|---|---|---|
| Body weight (g) | 21.9 ± 0.3 | 22.0 ± 0.1 | 22.8 ± 0.3 | 19.8 ± 0.3# |
| Kidney weight (g) | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.28 ± 0.01 | 0.27 ± 0.01 |
| Food intake (g/day) | 0.2 ± 0.1 | 0.7 ± 0.3 | 0.6 ± 0.1 | 0.2 ± 0.1# |
| Water intake (mL/day) | 0.4 ± 0.1 | 1.3 ± 0.4 | 2.1 ± 0.3$ | 1.9 ± 0.6 |
| Urine volume (mL/day) | 1.3 ± 0.2 | 2.6 ± 0.5 | 2.1 ± 0.2 | 3.4 ± 0.6# |
| Urine osmolality (mOsm) | 2276 ± 332 | 1521 ± 308 | 1878 ± 186 | 786 ± 123# |
| UNaV (μmol/day) | 142 ± 63 | 315 ± 47* | 6.2 ± 0.6$ | 72.6 ± 15.7# |
| UKV (μmol/day) | 339 ± 17 | 390 ± 48 | 1132 ± 148$ | 609 ± 113# |
| Hematocrit (%) | 41.2 ± 1.2 | 48.2 ± 1.4* | 40.4 ± 1.0 | 42.4 ± 2.5 |
| Plasma osmolality (mOsm) | 296.7 ± 3.3 | 295.0 ± 2.7 | 288.2 ± 4.0 | 293.3 ± 6.1 |
| Plasma [Na+] (mM) | 155 ± 3 | 140 ± 3* | 151 ± 2 | 141 ± 1# |
| Plasma [K+] (mM) | 4.2 ± 0.2 | 4.1 ± 0.2 | 6.0 ± 0.3$ | 5.8 ± 0.2 |
| Urine pH | 6.4 ± 0.2 | 5.8 ± 0.1* | 6.9 ± 0.1$ | 6.0 ± 0.2# |
Parentheses indicate the number of animals. Data are shown as mean ± SEM.
p < 0.05 compared to CTRL + vehicle;
p < 0.05 compared to LNaHK + vehicle;
p < 0.05 compared to CTRL + vehicle analyzed by two-way ANOVA with a post-hoc Tukey test.
On a regular diet (CTRL), the mice treated with furosemide exhibited a higher urinary K+ clearance than the vehicle group. This is expected since furosemide is a K-wasting diuretic by stimulating K+ secretion in the distal nephron22,23. However, on LNaHK, furosemide-treated mice had a lower urinary K+ clearance than the vehicle group (Figure 1B). In order to exclude the complicating effects of K+ secretion in the distal nephron, we treated mice with furosemide + amiloride and amiloride alone, which inhibits the Na+ reabsorbing driving force required for both ROMK- and BK-mediated K+ secretion10,14. Compared to the amiloride-treated group, the furosemide + amiloride group had lower urinary K+ clearance on LNaHK, but not on CTRL (Figure 1B). These results indicate that the furosemide effect on K clearance is independent of the distal K secretion in mice on LNaHK.
To assess the chronic effect of furosemide, we kept WT on LNaHK for 7 days and then treated them with either furosemide water or control water for 7 days. The results are shown in figure 2 and table 2. For mice on control water, urinary K excretion (UKV) was unchanged after 7 days. For mice on furosemide water, however, UKV was reduced on the first day of furosemide treatment (Figure 2A). After 7 days, plasma [K+] was significantly higher and K+ clearance significantly lower in mice on furosemide water compared to control water (Figure 2B, C). These results indicate that furosemide is a K-sparing diuretic and can raise plasma [K+] in mice on LNaHK.
Figure 2. Chronic effect of furosemide on renal K+ handling in mice on LNaHK.
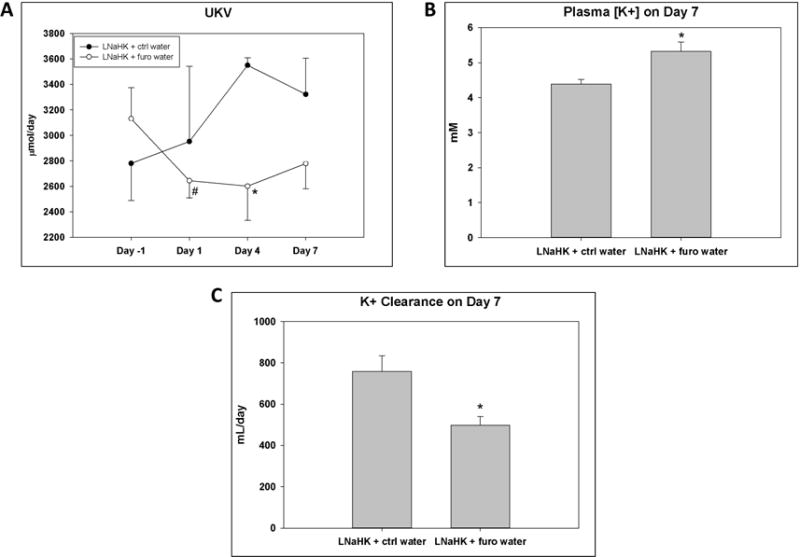
A. Urinary K+ excretion (UKV) on the day before treatment (day -1), day 1, day 4, and day 7 after treatment with control (ctrl) or furosemide (furo) water in WT on LNaHK. * p < 0.05 compared to LNaHK + ctrl water analyzed with two-way ANOVA with post-hoc Tukey test; # p < 0.05 compared to Day -1 analyzed with a paired t-test. B. Plasma [K+] after 7 days of treatment with ctrl or furo water in WT on LNaHK. C. Urinary K+ clearance after 7 days of treatment with ctrl or furo water in WT on LNaHK. * p < 0.05 compared to LNaHK + ctrl water analyzed with one-way ANOVA.
Table 2.
Metabolic cage measurements for chronic effect of furosemide.
| LNaHK + ctrl water (4) | LNaHK + furo water (4) | |
|---|---|---|
| Body weight (g) | 22.1 ± 0.8 | 20.9 ± 0.7 |
| Food intake (g/day) | 3.1 ± 0.2 | 3.4 ± 0.3 |
| Water intake (mL/day) | 8.4 ± 0.8 | 15.4 ± 1.6* |
| Urine volume (mL/day) | 4.0 ± 0.2 | 6.0 ± 0.2* |
| Urine osmolality (mOsm) | 1872 ± 105 | 1002 ± 77* |
| UNaV (μmol/day) | 35.7 ± 7.5 | 43.6 ± 2.8 |
| UKV (μmol/day) | 3323 ± 283 | 2620 ± 152 |
| Hematocrit (%) | 41.4 ± 1.2 | 42.3 ± 1.7 |
| Plasma osmolality (mOsm) | 295 ± 2.6 | 295 ± 2.6 |
| Plasma [Na+] (mM) | 139.5 ± 1.1 | 137.1 ± 1.3 |
| Plasma [K+] (mM) | 4.4 ± 0.1 | 5.3 ± 0.3* |
| K Clearance (mL/day) | 758 ± 76 | 498 ± 42* |
| Urine pH | 9.2 ± 0.1 | 8.6 ± 0.1* |
Parentheses indicate the number of animals. Data are shown as mean ± SEM.
p < 0.05 compared to LNaHK + ctrl water analyzed by one-way ANOVA.
One possible explanation for the effect of furosemide in mice on LNaHK is that net K+ secretion in the TAL is inhibited. To test this hypothesis more directly, we used micropuncture techniques to measure the K+ concentration in the early distal tubule (EDT [K+]) before and after intravenous (IV) administration of vehicle or furosemide in mice on CTRL or LNaHK. As shown in Figure 3, vehicle administration did not affect the EDT [K+] in mice on either diet. Before furosemide treatment, the EDT [K+] was significantly higher in mice on LNaHK than CTRL. Furosemide infusion increased the EDT [K+] in mice on CTRL but decreased EDT [K+] in mice on LNaHK without affecting MAP or GFR during the collection period (Table 3). These results indicate furosemide-sensitive net K+ reabsorption in the TAL of mice on CTRL and net K+ secretion in mice on LNaHK.
Figure 3. Effect of furosemide on EDT [K+] in mice on CTRL or LNaHK.
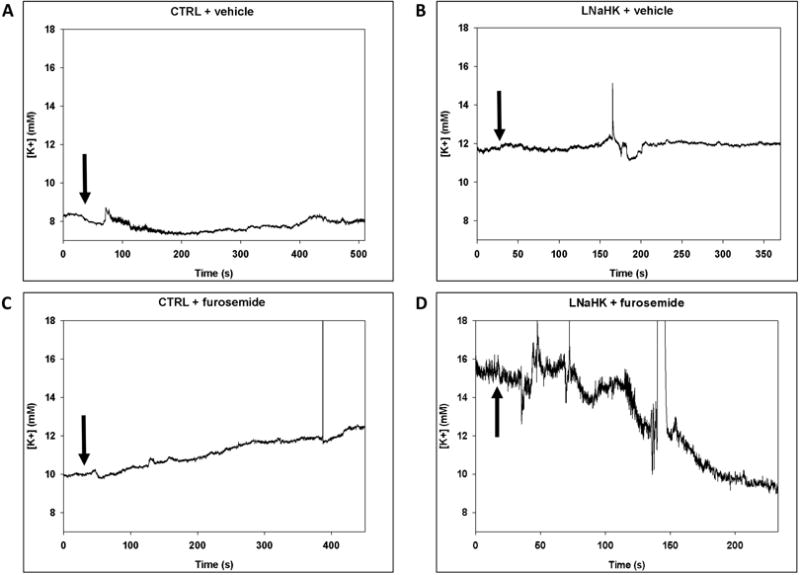
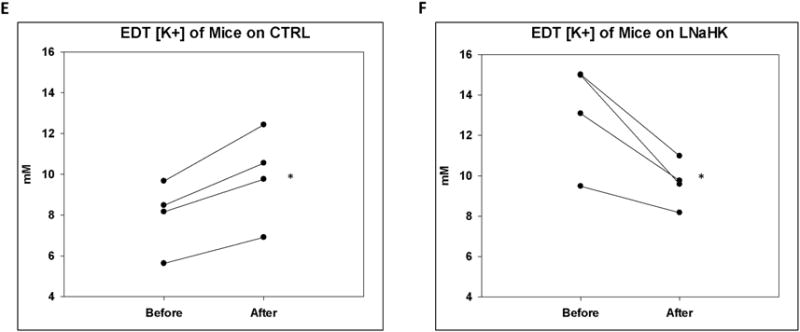
A-D. Representative recordings of K-selective microelectrodes in the lumen of EDT before and after IV injections of vehicle or furosemide in WT on CTRL or LNaHK. Arrows indicate the time of injections. E and F. EDT [K+] of mice on CTRL (E) or LNaHK (F) before and after IV furosemide injections. N = 4 per group. *p < 0.05 vs before furosemide analyzed using a paired t-test.
Table 3.
Hemodynamic measurements in micropuncture experiments.
| WT CTRL (4) | WT LNaHK (5) | WT HK (4) | ROMK KO LNaHK (4) | ||
|---|---|---|---|---|---|
| MAP (mmHg) | Before furo | 108.7 ± 6.6 | 102.7 ± 3.0 | 100.0 ± 8.6 | 96.4 ± 8.4 |
| After furo | 107.7 ± 7.4 | 103.4 ± 3.6 | 100.0 ± 10.0 | 96.0 ± 9.2 | |
| GFR (μL/min/g) | Before furo | 9.3 ± 1.7 | 9.3 ± 1.1 | 7.1 ± 0.6 | 4.8 ± 0.6* |
| After furo | 9.4 ± 0.5 | 8.4 ± 1.1 | 6.0 ± 1.1 | 5.5 ± 0.3* | |
Numbers in parentheses indicate the number of animals. Data are shown as mean ± SEM.
p < 0.05 compared to WT LNaHK analyzed by one-way ANOVA with a post-hoc Tukey test.
Normal Na, high K diet (HK)
The net K+ secretion in the TAL may be partly due to the high interstitial K+ concentration generated by medullary K+ recycling in mice on a high K diet16,24. We examined whether there was net K+ secretion in the TAL of mice on HK. As revealed by the results of micropuncture experiments in Figure 4, furosemide did not affect the EDT [K+] of WT on HK despite decreased urine osmolality as an indication of its effectiveness (Figure 4A and B). There was no significant difference in MAP and GFR before and after furosemide treatment (Table 3).
Figure 4. Effect of furosemide on EDT [K+] and urine osmolality of mice on HK.
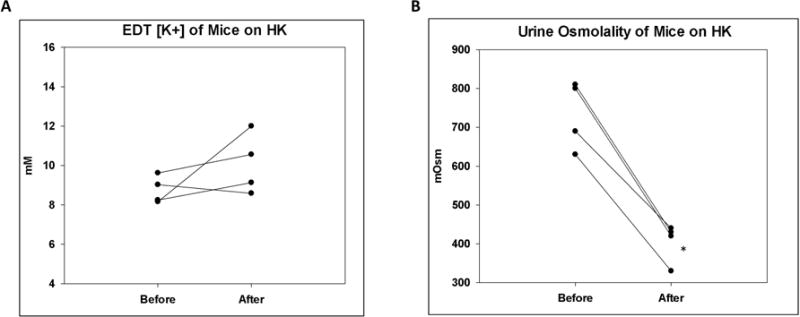
A. EDT [K+] of mice on HK before and after IV furosemide injection. B. Urine osmolality of mice on HK before and after furosemide. N = 4 per group. *p < 0.05 vs before furosemide analyzed using a paired t-test.
These results suggest that the low Na content of LNaHK is essential for net K+ secretion in the TAL. Studies have shown that a high K diet can reduce NKCC2 activity, probably by inhibiting Cl- exit from the TAL16. In agreement with this notion, compared to vehicle, the furosemide-treated mice exhibited higher urinary Na+ excretion (UNaV) than vehicle group in WT on CTRL, but not in WT on HK (Figure 5A). In contrast to WT on HK, the furosemide group exhibited higher UNaV than vehicle in mice on LNaHK (Figure 1A). Because of the difference in dietary Na content, we were unable to compare the furosemide-sensitive UNaV between mice on CTRL and LNaHK using metabolic cage experiments. We thus evaluated the furosemide-sensitive UNaV during micropuncture experiments, in which all mice received the same IV infusion for the same time period. The mice on LNaHK exhibited significantly greater furosemide-sensitive UNaV than those on CTRL or HK. There was no significant difference in furosemide-sensitive UNaV between mice on CTRL and HK (Figure 5B).
Figure 5. Effect of furosemide on urinary Na+ excretion in mice on CTRL or HK.
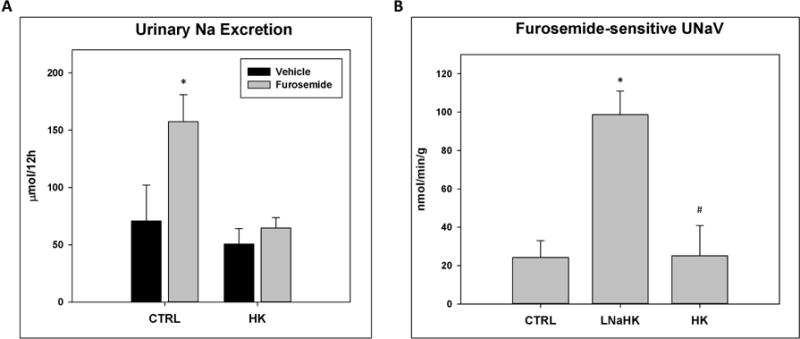
A. Twelve-hour UNaV of mice on CTRL or HK treated with IP injections of vehicle or furosemide. N = 8 per group. *p < 0.05 vs vehicle analyzed using two-way ANOVA with a post-hoc Tukey test. B. Furosemide sensitive UNaV normalized to body weight of mice on CTRL, LNaHK, or HK. N = 4 – 9 per group. *p < 0.001 vs CTRL; #p < 0.05 vs LNaHK analyzed by one-way ANOVA.
Role of ROMK in net K+ secretion
Both 30 pS and 70 pS inward rectifying K channels (Kir) have been reported to represent ROMK in the apical membrane of TAL25,26. To investigate which K channel mediates net K+ secretion, we performed single-channel patch clamp experiments on split-open TALs from WT and ROMK knockouts (KO) on CTRL or LNaHK. Whereas we found the 70 pS Kir as one or two channels in a patch, we often found the 30 pS Kir as multiple channels (2–5) in a patch. Figure 6A shows recordings of a single 30 pS Kir (top; −Vp = −60 mV) and multiple 30 pS Kir (middle and bottom; −Vp = −40 mV) in cell-attached patches from TALs of WT and KO. No difference was found in Po of the 30 pS Kir from mice on LNaHK compared with CTRL (Figure 6C). However, as shown by the recordings of Figure 6B and the summary plot of Figure 6C, the Po of the 70 pS Kir (−Vp = −40 mV) was greater in mice on LNaHK compared with CTRL.
Figure 6. Patch clamp recordings of apical K channels in the TAL of mice on CTRL and LNaHK.
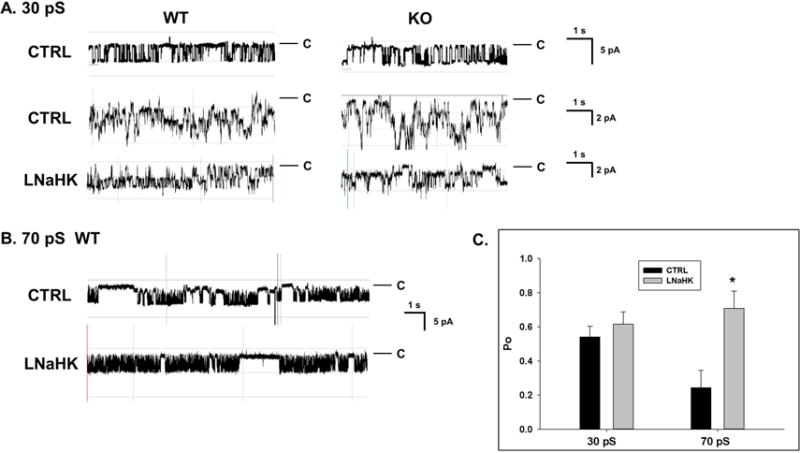
A. Recordings of single (top; CTRL, −Vp = −60 mV) and multiple (−Vp = −40 mV) 30 pS (inward currents) K channels from cell-attached patches of split-open TAL of WT and KO mice on CTRL or LNaHK. Pipette solution contained 140 mM KCl. Red lines denote closed states. B. Recordings of 70 pS Kir channels in apical membrane of WT mice. The recordings indicate 2 closed states (long and short) with an increased duration in long closed state in channels of mice on CTRL. C. Summary of Po at −Vp = −40 mV of the 30 pS and 70 pS Kir in the TAL from WT on CTRL and LNaHK. *p < 0.05 vs CTRL analyzed by one-way ANOVA.
Table 4 shows the number and conductance of apical K channels in the TALs from WT and KO on CTRL or LNaHK. The single channel conductance of the “30 pS” Kir varied with a range of 25 to 38 pS (inward currents) in cell-attached patches and was not significantly different among the four groups. Likewise, the single channel conductance of the 70 pS Kir and the BK channel was not different in WT on LNaHK compared with CTRL. We found the same density (channels per patch) of 30 pS Kir in all four groups. The densities of the 70-pS Kir and BK in WT were also not significantly different between the two diets. However, unlike the 30 pS Kir, we found no 70 pS Kir in the TAL from KO on CTRL nor LNaHK. Overall, these results show that only the 70 pS Kir is ROMK in the TAL and the 70 pS ROMK channel was more active in the apical membranes in mice on LNaHK, compared to CTRL.
Table 4.
Number and conductance of apical K channels in the TAL.
| Diet | N of mice | N of patches | 30 pS Kir | 70 pS Kir | BK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P# | C# | G (pS) | P# | C# | G (pS) | P# | C# | G (pS) | ||||
| WT | CTRL | 8 | 17 | 7 | 13 | 31.4 ± 1.4 | 7 | 11 | 70.7 ± 2.2 | 2 | 5 | 165.0 ± 5.0 |
| WT | LNaHK | 7 | 27 | 4 | 5 | 34.7 ± 0.5 | 5 | 6 | 69.8 ± 0.9 | 4 | 6 | 185.2 ± 24.3 |
| KO | CTRL | 5 | 20 | 6 | 12 | 35.5 ± 0.6 | 0* | 0 | 1 | 1 | 225.8 | |
| KO | LNaHK | 4 | 17 | 6 | 9 | 34.2 ± 1.7 | 0 | 0 | 0 | 0 | ||
“P#” denotes the number of patches that contain one or more respective channel. “C#” denotes the total number of respective channels. “G” denotes single channel conductance (mean ± SEM).
p < 0.05 compared to WT analyzed by a chi-squared test.
Consistent with the patch clamp results, immunofluorescence staining showed similar labeling of ROMK in the apical membrane of TAL for WT on both diets (Figure 7A–F). The antibody specificity was validated by ROMK KO as a negative control (Figure 7G–I).
Figure 7. ROMK localization in the TAL of mice on CTRL and LNaHK.
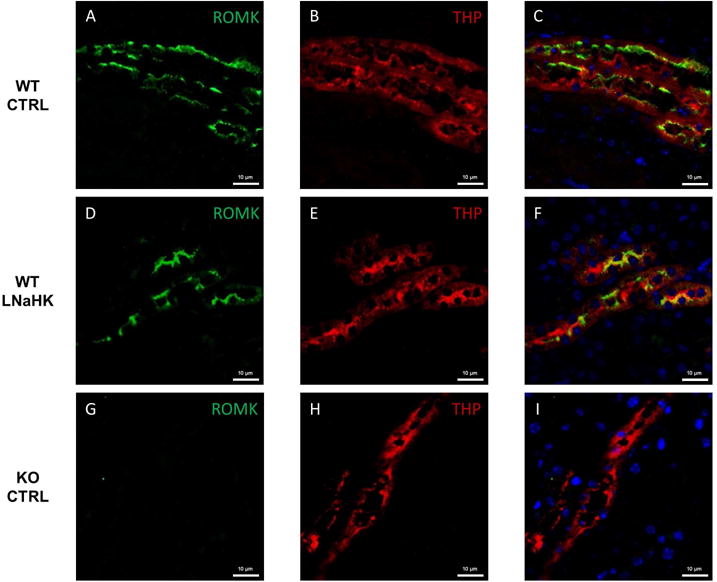
A-C. Immunostaining of ROMK (green), Tamm-Horsfall Protein (THP; marker of TAL; red), and both in the outer medulla of WT on CTRL. D-F. Immunostaining of ROMK (green), THP (red), and both in the outer medulla of WT on LNaHK. G-I. Immunostaining of ROMK (green), THP (red), and both in the outer medulla of KO on CTRL. All images were taken at 400× magnification. All scale bars are 10 μm.
To further determine the role of ROMK in the TAL net K+ secretion in vivo, we used micropuncture to measure the EDT [K+] before and after IV furosemide in ROMK WT and KO on LNaHK. As shown in Figure 8A, WT on LNaHK exhibited furosemide-sensitive net K+ secretion in the TAL. However, as shown in Figure 8B, KO on LNaHK did not exhibit furosemide-sensitive net K+ secretion. These results indicate that ROMK is required for the net K+ secretion in the TAL of mice on LNaHK.
Figure 8. Effect of furosemide on EDT [K+] in ROMK WT and KO on LNaHK.
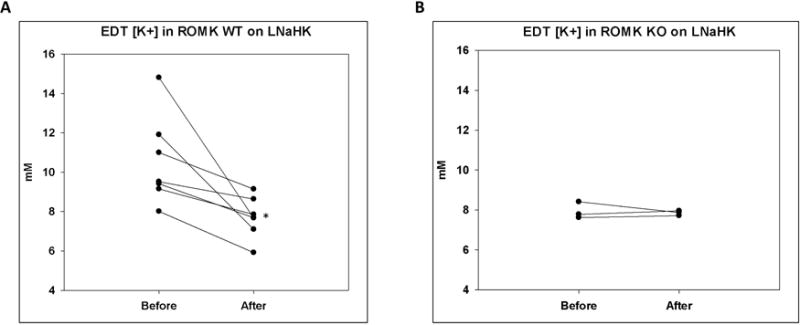
A and B. EDT [K+] of ROMK WT (A) and KO (B) on LNaHK before and after IV furosemide injection. N = 7 for WT; N = 3 for KO. *p < 0.05 vs before furosemide, analyzed by a paired t-test.
Discussion
The TAL reabsorbs about 25% of filtered K+ via the apical NKCC2 and basolateral K-selective channels. However, much of the K+ transported by the NKCC2 is recycled back to the lumen through the apical ROMK in order to maintain a continual supply of K+ for NaCl reabsorption27,28. In the ex-vivo microperfusion experiments with isolated hamster TAL, Tsuruoka et. al. found net K+ reabsorption in the medullary TAL but net K+ secretion in the cortical TAL20. Bailly et. al. also found a net K+ secretion in isolated perfused mouse cortical TALs21. However, two other groups failed to observe net K+ secretion with isolated perfused TALs29,30. The discrepancies are probably due to differences in experimental conditions. Nonetheless, net K+ secretion in the TAL has never been described in vivo, and its physiological conditions and implications remain unclear. The present study demonstrates in vivo net K+ secretion in the TAL of mice that are adapted to LNaHK, but not to a high K diet with normal Na. The 70 pS ROMK channel is the likely avenue for K+ secretion in mice on LNaHK.
Net K+ secretion in TAL with LNaHK
Furosemide is a widely used diuretic for the treatment of hypertension, edema, congestive heart failure, hepatic failure, and chronic kidney disease31,32. Empiric potassium is a recommended supplement for furosemide due to its K-wasting effect33. Furosemide inhibits NKCC2 in the TAL and increases distal Na+ delivery and flow, thus promoting distal K secretion in ASDN23. Consistent with this understanding, in mice on CTRL, furosemide increased renal K+ clearance. In mice on LNaHK, however, furosemide showed the opposite effect of reducing renal K+ clearance. The fact that furosemide reduced K+ clearance both with and without amiloride strongly suggests furosemide-sensitive net K+ secretion in the TAL. Notably, after 12 hours of IP furosemide injection, renal K+ clearance was decreased, but plasma [K+] was unaffected. This may be due to extrarenal K handlings including transcellular shift into muscle cells and excretion in the colon34. Chronic treatment with furosemide for 7 days decreased K+ clearance and elevated plasma [K+], demonstrating that furosemide changes from a K-wasting to a K-sparing diuretic when the mice are kept on LNaHK.
Additionally, in mice on LNaHK, elevated aldosterone along with other factors upregulate ENaC, ROMK, and BK expressions and activities to promote K secretion in the ASDN8,13,14,35. As shown in Table 2, furosemide still increased urine flow and decreased urine osmolality but did not increase UNaV after 7 days. This indicates that the increased distal Na+ delivery by furosemide is completely reabsorbed by ENaC, provided that Na-Cl-cotransporter (NCC) in distal convoluted tubule (DCT) is completely inhibited on LNaHK10. Along with increased distal flow, furosemide should further stimulate K+ secretion through ROMK and BK in ASDN. However, the overall effect of furosemide in mice on LNaHK is a decrease in UKV, suggesting that the inhibition of TAL net K+ secretion must exceed the stimulation of distal K+ secretion.
Our micropuncture studies provided more direct evidence for net K+ secretion. Furosemide had strikingly opposite effects on EDT [K+] in mice on CTRL and LNaHK without altering MAP or GFR within the periods of measurements. Because of the high interstitial [K+], the paracellular pathway could mediate net K+ secretion. However, the EDT [K+], which were initially different in the two diets before treatment, reached similar concentrations a few minutes after furosemide injections. This result makes paracellular pathway unlikely. Furosemide would not block K+ secreted by the paracellular route. Moreover, for paracellular K+ secretion, the EDT [K+] after furosemide should still be greater in mice on LNaHK than on CTRL.
In vivo micropuncture of the mouse distal nephron has the limitation of the absence of flow measurements before and after systemic injections of furosemide. Intravenous injections of furosemide will diminish the medullary interstitial gradient and change the flow in the EDT. However, with the inhibition of NKCC2 by furosemide, we expect that the tubular fluid in EDT is similar to the fluid in late proximal tubule (LPT). For mice on LNaHK, the EDT [K+] before furosemide was higher than after furosemide, suggesting that the [K+] in the EDT was greater than [K+] in the LPT. Along with the opposite effect in mice on CTRL, we provide strong evidence for net K+ secretion in the TAL. Additionally, furosemide may inhibit carbonic anhydrase (CA)36, causing more K+ following Na+ delivery to the EDT, which would increase the EDT [K+]. Therefore, if furosemide inhibits CA, the actual amount of furosemide-sensitive K+ secretion in the TAL of LNaHK mice would be greater than that observed.
Role of NKCC2 in net K+ secretion
The diuretic effect of HK was thought to be due to inhibition of NKCC213,16. On LNaHK, however, NKCC2 activity is enhanced, indicating that the diuretic effect of LNaHK is not by the same mechanism as in HK. In fact, a recent study by Cornelius et. al. showed that in mice on LNaHK, the BK-αβ4-mediated K+ secretion in the distal nephron may contribute to the diuretic effect8.
Another important finding in our study is that the net K+ secretion in TAL seems to be unique for mice on LNaHK but not HK, probably because of upregulated NKCC2 activity. This is consistent with a previous ex vivo study showing that NKCC2 was inhibited by the high interstitial [K+] in mice on HK16. Without an active NKCC2, the Na-K-ATPase cannot supply enough intracellular K+ for secretion. This provides further evidence for transcellular K+ secretion. The exact mechanism for increased NKCC2 activity in mice on LNaHK needs investigation.
The low Na content of LNaHK may result in volume depletion leading to the activation of the renin-angiotensin-aldosterone system (RAAS) and elevated plasma vasopressin (AVP). Both AVP and angiotensin II (Ang II) are regulators of NKCC2. AVP increases NKCC2 expression via V2 receptor-mediated pathways37. However, studies showed that plasma and urinary AVP levels were either unchanged or decreased in animals fed a low Na diet38–40. The effect of LNaHK on plasma AVP is unclear. In our metabolic cage studies, plasma osmolality, the major stimulus of AVP release was not different between CTRL + vehicle and LNaHK + vehicle groups. Therefore, plasma AVP is unlikely to play a role in regulating NKCC2 in mice on LNaHK. Another possible candidate is Ang II. Studies showed that a low Na diet increased NKCC2 phosphorylation and caused a shift from the low-affinity NKCC2A to the high-affinity NKCC2B isoform, partly mediated by the actions of Ang II on AT1 receptors18,41. Ang II level is greatly increased in mice on LNaHK, but not on HK7,8,42. These findings suggest that elevated Ang II may be responsible for the upregulated NKCC2 activity in mice on LNaHK.
Role of ROMK in net K+ secretion
Other K channels, including large, Ca-activated K channels (BK), have been observed by our laboratory and others43 in the TAL. However, ROMK has been identified as the K channel involved in recycling the K+ that is reabsorbed by NKCC225–27. ROMK knockout mice (KO) exhibit type 2 Bartter’s with extreme natriuretic diuresis27. Consistent with the previous study26, our patch clamp experiments identified the 70 pS K channel as ROMK in the apical membrane of TAL. In contrast to the study by Lu et. al.25, we found the same density and Po of a 30 pS K channel in KO. It is possible that KO exhibited no furosemide-sensitive net K+ secretion in the TAL because of decreased NKCC2 activity. However, the increased activity of the ROMK channel, but not the 30 pS channel, in WT on LNaHK indicates that ROMK mediates the observed net K+ secretion.
Unlike ROMK in the ASDN, the mechanism for regulating ROMK in the TAL is not well understood. In the ASDN, ROMK activity is upregulated in response to high dietary K by increasing channel density rather than Po44. Aldosterone-dependent signaling pathways, including SGK1 and PKA, promote the forward trafficking of ROMK channels to the apical membrane of ASDN by phosphorylating ROMK13,45,46. Interestingly, our patch clamp experiments showed that TAL from mice on LNaHK exhibited increased Po rather than channel density of ROMK. The immunofluorescence staining also indicates that LNaHK did not affect ROMK trafficking. The recent study by Dong et. al. demonstrated that ROMK1 isoform, which is expressed in the CCD, but not TAL, was required for ROMK trafficking in response to high K intake47. This study suggests that the ROMK2 isoform in the TAL is regulated by different signaling pathways than the ROMK1 in the ASDN. Mice on LNaHK have highly elevated aldosterone and Ang II compared to CTRL7,8,42. Since TAL is absent of 11-β-hydroxysteroid dehydrogenase 2 (HSD11B2)48, aldosterone is unlikely to regulate ROMK in TAL. WNK1, WNK3, and WNK4 all regulate ROMK by affecting clathrin-dependent endocytosis in the ASDN49. Thus, the WNKs are unlikely to regulate the Po of ROMK in the TAL. The patch clamp study by Lu et. al. also showed that Ang II regulates the activity of the 70 pS ROMK in the TAL50. Thus, Ang II may play a role to increase ROMK activity in the TAL of mice on LNaHK.
Intracellular pH is another important regulator of ROMK activity. Like other Kir channels, the Po of ROMK is highly sensitive to intracellular pH51. The urine pH was higher in mice on LNaHK than on CTRL due to the alkaline dietary content. However, the dietary effect on the intracellular pH of TAL cells remains unclear.
In summary, we have provided in vivo evidence of furosemide-sensitive net K+ secretion in the TAL of mice on LNaHK. Net K secretion is dependent on increased NKCC2 activity and may be mediated by ROMK. With net K secretion in the TAL, furosemide becomes a K-sparing diuretic and may lead to hyperkalemia. As summarized in Figure 9, furosemide inhibits the net K+ reabsorption in the TAL of mice on CTRL, increasing distal Na+ delivery and flow, thereby promoting K+ secretion through ROMK and BK channels in ASDN. In mice on LNaHK, upregulated ROMK and NKCC2 activity results in net K+ secretion in the TAL via ROMK channels. Elevated plasma aldosterone on LNaHK upregulates ENaC, ROMK, and BK in ASDN to promote distal K+ secretion. Furosemide not only inhibits the net K+ secretion in the TAL but also increases distal flow and Na+ delivery that further stimulate distal K+ secretion. The net result of these two opposing effects is a decrease in K+ excretion. Our finding is especially important when considering diuretic treatments for patients consuming a healthy low Na high K diet, which changes furosemide from a K-wasting to a K-sparing diuretic.
Figure 9. Summary figure of dietary effects on furosemide actions on urinary K excretion.
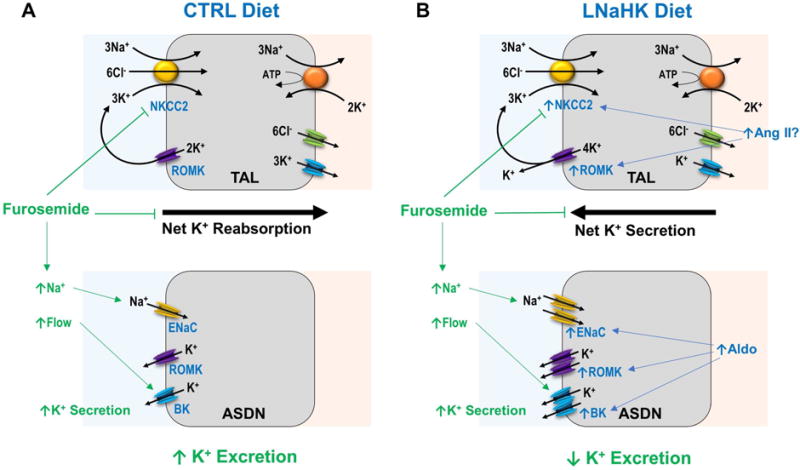
A. On CTRL diet, furosemide blocks NKCC2, inhibiting net K+ reabsorption in the TAL while increasing distal Na+ delivery and flow, which stimulates K+ secretion through ROMK and BK in ASDN. The net effect of furosemide is an increase in urinary K+ excretion. B. On LNaHK diet, up-regulated NKCC2 and ROMK, possibly by Ang II, results in net K+ secretion in the TAL. Elevated aldosterone (Aldo) from LNaHK upregulates ENaC, ROMK, and BK in the ASDN to promote distal K+ secretion. Furosemide blocks NKCC2, inhibiting net K+ secretion in the TAL. Although furosemide increases distal Na+ delivery and flow that stimulate K+ secretion in ASDN, the net result is a decrease in urinary K+ excretion. Transporters in the basolateral membrane and cell types of ASDN have been omitted for simplicity.
Methods
Animals
All animals were maintained in accordance with the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Wild-type mice on C57BL/6 background (Charles River Laboratories, Wilmington, MA), ROMK wild-type and knockout littermates on 129/SvJ background (generously provided by Dr. Tong Wang, Yale University School of Medicine) were housed in the UNMC animal facility and maintained on a 12-hour day-night cycle, with free access to water and food.
Metabolic Cage
Wild-type mice, at 10–12 weeks of age, were given either standard mouse chow (CTRL; 0.3% Na, 0.6% K), a high K diet (HK; 0.3% Na, 5% K with equimolar carbonate/citrate/Cl as anions; TD.07278; Envigo, Indianapolis, IN) or a low Na, high K diet (LNaHK; 0.01% Na, 5% K with equimolar carbonate/citrate/Cl as anions; TD.07278; Envigo, Indianapolis, IN) for 4–7 days. Before experiments, mice were placed in metabolic cages for 1 day for acclimation. For acute diuretic treatments, mice were given intraperitoneal injections of vehicle (100μL poly(ethylene glycol); PEG) or furosemide (15mg/kg) and placed in metabolic cages for 12 hours to collect urine and measure food and water consumption. For chronic furosemide treatments, mice were given either furosemide water (0.1 mg/mL, pH 8.4 with 0.7 mM K+) or control water (0.7 mM KHCO3, pH 8.4). Urine was collected in metabolic cages for 24 hours the day before treatment and on day 1, day 4, and day 7 after treatments. The mice were then anesthetized, sacrificed by exsanguination from the carotid artery, and blood and kidneys were collected. Urine and plasma osmolality were measured with an osmometer (Model 3250, Advanced Instruments, Norwood, MA). Urine and plasma Na+ and K+ concentrations were determined by a flame photometer (PFP7, Jenway, Burlington, NJ).
Micropuncture, patch clamp and immunofluorescence staining
Micropuncture, patch clamp, and immunofluorescence staining were performed as described previously52–56. Please see online supplementary materials.
Supplementary Material
Acknowledgments
This project was funded by National Institutes of Diabetes and Digestive and Kidney Diseases grants RO1 DK071014 (SCS), RO1 DK92474 (SCS), and F30 DK108456 (BW). We appreciate receiving the ROMK knockout mice from Drs. Tong Wang (Yale School of Medicine) and Gary Shull (University of Cincinnati College of Medicine).
Sources of Support: RO1 DK071014, RO1 DK092474, and F30 DK108456
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.Jonsson T, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol. 2014;25:20–26. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 3.Huo R et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.243. [DOI] [PubMed] [Google Scholar]
- 4.Lau KK et al. Mediterranean-Style Diet Is Associated With Reduced Blood Pressure Variability and Subsequent Stroke Risk in Patients With Coronary Artery Disease. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu195. [DOI] [PubMed] [Google Scholar]
- 5.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Cornelius RJ, Wang B, Wang-France J, Sansom SC. Maintaining K balance on the low Na, high K diet. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00330.2015. ajprenal 00330 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius RJ, et al. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One. 2015;10:e0115515. doi: 10.1371/journal.pone.0115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelius RJ, Wen D, Hatcher LI, Sansom SC. Bicarbonate promotes BK-alpha/beta4-mediated K excretion in the renal distal nephron. Am J Physiol Renal Physiol. 2012;303:F1563–F1571. doi: 10.1152/ajprenal.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen D, Cornelius RJ, Sansom SC. Interacting influence of diuretics and diet on BK channel-regulated K homeostasis. Curr Opin Pharmacol. 2014;15:28–32. doi: 10.1016/j.coph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluznick JL, Sansom SC. BK channels in the kidney: role in K(+) secretion and localization of molecular components. Am J Physiol Renal Physiol. 2006;291:F517–F529. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- 12.Sansom SC, Welling PA. Two channels for one job. Kidney Int. 2007;72:529–530. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 13.Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol. 2013;33:215–228. doi: 10.1016/j.semnephrol.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Wen D, et al. Relation between BK-alpha/beta4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int. 2014;86:139–145. doi: 10.1038/ki.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battilana CA, et al. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978;62:1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamison RL, Work J, Schafer JA. New pathways for potassium transport in the kidney. Am J Physiol. 1982;242:F297–312. doi: 10.1152/ajprenal.1982.242.4.F297. [DOI] [PubMed] [Google Scholar]
- 18.Schiessl IM, et al. Dietary salt intake modulates differential splicing of the Na-K-2Cl cotransporter NKCC2. Am J Physiol Renal Physiol. 2013;305:F1139–F1148. doi: 10.1152/ajprenal.00259.2013. [DOI] [PubMed] [Google Scholar]
- 19.Fraser SA, et al. AMPK couples plasma renin to cellular metabolism by phosphorylation of ACC1. Am J Physiol Renal Physiol. 2013;305:F679–690. doi: 10.1152/ajprenal.00407.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuruoka S, Koseki C, Muto S, Tabei K, Imai M. Axial heterogeneity of potassium transport across hamster thick ascending limb of Henle’s loop. Am J Physiol. 1994;267:F121–129. doi: 10.1152/ajprenal.1994.267.1.F121. [DOI] [PubMed] [Google Scholar]
- 21.Bailly C, Imbert-Teboul M, Roinel N, Amiel C. Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol. 1990;258:F1224–1231. doi: 10.1152/ajprenal.1990.258.5.F1224. [DOI] [PubMed] [Google Scholar]
- 22.Knochel JP. Diuretic-induced hypokalemia. Am J Med. 1984;77:18–27. doi: 10.1016/s0002-9343(84)80004-2. [DOI] [PubMed] [Google Scholar]
- 23.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int. 1985;28:477–489. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- 24.Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol. 1981;241:F395–F402. doi: 10.1152/ajprenal.1981.241.4.F395. [DOI] [PubMed] [Google Scholar]
- 25.Lu M, et al. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter’s) knockout mice. J Biol Chem. 2002;277:37881–37887. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu M, et al. ROMK is required for expression of the 70-pS K channel in the thick ascending limb. Am J Physiol Renal Physiol. 2004;286:F490–F495. doi: 10.1152/ajprenal.00305.2003. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz JN, et al. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter’s syndrome. J Biol Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- 28.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol. 2009;297:F849–F863. doi: 10.1152/ajprenal.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandon B, Siga E, Roinel N, de Rouffignac C. Ca2+, Mg2+ and K+ transport in the cortical and medullary thick ascending limb of the rat nephron: influence of transepithelial voltage. Pflugers Arch. 1993;424:558–560. doi: 10.1007/BF00374924. [DOI] [PubMed] [Google Scholar]
- 30.Di Stefano A, et al. Effects of glucagon on Na+, Cl−, K+, Mg2+ and Ca2+ transports in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch. 1989;414:640–646. doi: 10.1007/BF00582129. [DOI] [PubMed] [Google Scholar]
- 31.Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I) Clin Pharmacokinet. 1990;18:381–408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- 32.Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011;13:639–643. doi: 10.1111/j.1751-7176.2011.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160:2429–2436. doi: 10.1001/archinte.160.16.2429. [DOI] [PubMed] [Google Scholar]
- 34.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol. 1981;240:F257–268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 35.Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens. 2010;19:98–105. doi: 10.1097/MNH.0b013e328332bda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unwin RJ, Walter SJ, Giebisch G, Capasso G, Shirley DG. Localization of diuretic effects along the loop of Henle: an in vivo microperfusion study in rats. Clin Sci (Lond) 2000;98:481–488. [PubMed] [Google Scholar]
- 37.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol. 2015;309:F280–299. doi: 10.1152/ajprenal.00093.2015. [DOI] [PubMed] [Google Scholar]
- 38.Roos KP, et al. Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J Am Soc Nephrol. 2013;24:218–227. doi: 10.1681/ASN.2012050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cristia E, Amat C, Naftalin RJ, Moreto M. Role of vasopressin in rat distal colon function. J Physiol. 2007;578:413–424. doi: 10.1113/jphysiol.2006.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuguchi H, Schmid PG, Van Orden D, Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension. 1981;3:174–181. doi: 10.1161/01.hyp.3.2.174. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs G, Peti-Peterdi J, Rosivall L, Bell PD. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT(1) receptors. Am J Physiol Renal Physiol. 2002;282:F301–306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 42.van der Lubbe N, et al. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol. 2013;305:F1177–1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi JGW. Membrane stretch: a physiological stimulator of Ca2+-activated K+ channels in thick ascending limb. Am J Physiol. 1989;408:600–608. doi: 10.1152/ajprenal.1989.257.3.F347. [DOI] [PubMed] [Google Scholar]
- 44.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol. 1999;277:F805–812. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 45.Yoo D, et al. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem. 2003;278:23066–23075. doi: 10.1074/jbc.M212301200. [DOI] [PubMed] [Google Scholar]
- 46.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong K, et al. Romk1 Knockout Mice Do Not Produce Bartter Phenotype but Exhibit Impaired K Excretion. J Biol Chem. 2016;291:5259–5269. doi: 10.1074/jbc.M115.707877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bostanjoglo M, et al. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 49.Welling PA. Roles and Regulation of Renal K Channels. Annu Rev Physiol. 2016;78:415–435. doi: 10.1146/annurev-physiol-021115-105423. [DOI] [PubMed] [Google Scholar]
- 50.Lu M, et al. Effect of angiotensin II on the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1996;108:537–547. doi: 10.1085/jgp.108.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol. 1990;259:F494–502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 52.Wen D, et al. Increased Epithelial Sodium Channel Activity Contributes to Hypertension Caused by Na+-HCO3− Cotransporter Electrogenic 2 Deficiency. Hypertension. 2015;66:68–74. doi: 10.1161/HYPERTENSIONAHA.115.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallon V. Micropuncturing the nephron. Pflugers Arch. 2009;458:189–201. doi: 10.1007/s00424-008-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockand JD, et al. Arachidonic acid potentiates the feedback response of mesangial BKCa channels to angiotensin II. Am J Physiol. 1998;274:F658–664. doi: 10.1152/ajprenal.1998.274.4.F658. [DOI] [PubMed] [Google Scholar]
- 55.Foutz RM, Grimm PR, Sansom SC. Insulin increases the activity of mesangial BK channels through MAPK signaling. Am J Physiol Renal Physiol. 2008;294:F1465–F1472. doi: 10.1152/ajprenal.00012.2008. [DOI] [PubMed] [Google Scholar]
- 56.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. Journal of the American Society of Nephrology. 2010;21:634–645. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


