Abstract
Background
Eligibility criteria and screening procedures are designed to optimize the scientific yield and maximize the safety of clinical trials. However, they may also heighten trial complexity, hinder enrollment, decrease generalizability, and increase costs. We analyzed the types and number of eligibility criteria and screening procedures among thoracic oncology clinical trials sponsored or endorsed by the Eastern Cooperative Oncology Group (ECOG).
Methods
We identified trials and obtained protocols from the ECOG website. Eligibility criteria were grouped and categorized as comorbidity (classified by organ system), administrative requirements, prior treatment, and measurable disease requirement. Associations between trial characteristics and eligibility criteria were analyzed using Kruskal-Wallis and Wilcoxon tests.
Results
A total of 74 lung cancer trials activated 1986–2016 were identified. The total number of eligibility criteria was associated with trial principal therapy (median 9 for surgical, 18 for radiation, 20 for medical; P=0.02), trial primary endpoint (median 20 for OS, 28 for PFS, 17 for other; P=0.001), number of therapies (P=0.05), and year of activation (median 16 for 1986–1995, 19 for 1996–2005, 27 for 2006–2016; P<0.001). The increase in trial eligibility requirements over time was limited to medical therapy trials. Over time, there was also an increase in blood test screening procedures (P=0.05), but not for imaging, cardiac assessment, or pulmonary function screening procedures.
Conclusions
The number of eligibility criteria and screening procedures in medical therapy lung cancer clinical trials continues to rise. Continued efforts to simplify protocol eligibility and procedures are warranted to promote trial adherence, enrollment, completion, and generalizability.
Keywords: Accrual, Clinical research, Enrollment, Exclusion criteria, Inclusion criteria, Lung cancer
Introduction
Despite longstanding efforts to improve cancer clinical trial accrual, completion rates, and generalizability, fewer than two percent of adults with cancer in the United States participate in clinical trials.1,2 This low rate of enrollment reflects a wide range of factors, including patient trust in the healthcare system and understanding of study protocols; physician attitudes toward patients and communication skills; and experience of clinical research staff.2–10 Limited trial availability also hinders enrollment at many centers.11,12 Yet, even at sites heavily invested in clinical research that maintain diverse clinical trial portfolios and attract motivated populations, only a small minority of patients are enrolled in research studies.13
For decades, it has been recognized that clinical trial eligibility criteria present a critical barrier to study accrual.14–17 Indeed, the validity of and justification for many exclusion factors have been questioned.18 Analyses in the 1990s demonstrated that, over the preceding 25 years, despite calls to simplify and provide rationale for study eligibility, inclusion and exclusion criteria had become increasingly numerous and stringent.19 Since that time, national organizations including the Institute of Medicine, the National Cancer Institute, and the American Society of Clinical Oncology have joined the call to streamline cancer clinical trial processes to promote participation.20,21
To evaluate contemporary trends in cancer clinical trial inclusiveness and complexity, we quantified and categorized eligibility criteria in lung cancer clinical trials sponsored or endorsed by the Eastern Cooperative Oncology Group (ECOG) thoracic committee from 1986 through 2016. For each available protocol, we quantified and categorized inclusion and exclusion criteria and determined associations with trial characteristics.
Methods
Clinical trial selection and characterization
This study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. Clinical trial protocols were obtained from the ECOG thoracic committee website (http://www.ecog.org/), which was most recently accessed on January 30, 2017. When full study protocols were not available online, documents were requested from the ECOG coordinating center. For each clinical trial, we recorded year of activation, target patient accrual, trial phase (1/pilot, 2, 3), stage (early, locally advanced, advanced) and histology (non-small cell lung cancer [NSCLC], small cell lung cancer [SCLC], non-squamous NSCLC [NS-NSCLC]) of the lung cancer under study, primary endpoint (overall survival [OS], progression-free survival [PFS], response rate [RR], and other), and principal treatment modality (surgery, radiation, medical). If a single trial had more than one phase (e.g., phase 2/3), we assigned the higher of the two stages. If a single trial featured multiple treatment modalities, we assigned principal treatment to the modality most relevant to the primary research question. We recorded whether or not submission of archival tumor tissue was mandated. For each trial, we also calculated the total number of therapeutic interventions under investigation. For this calculation, each medical therapy was considered a separate intervention, as was each type of radiation (eg, thoracic versus cranial) and surgical therapy.
Eligibility quantification and categorization
Clinical trial eligibility criteria were initially documented by one investigator (S.G.) and subsequently reviewed by another investigator (D.E.G.). All instances of disagreement were discussed and resolved. Eligibility criteria were quantified on two levels: individual criteria and criteria groupings. For instance, white blood cell count, hemoglobin, and platelet count were considered individual eligibility criteria, all falling within the single grouping of hematologic parameters. To select category groupings and terms, we reviewed the relevant literature and adapted previously published systems that seemed most relevant to the disease type in question.19,22 We used a category of “unstable” conditions to broadly include a variety of excluded excluded states. Among others, these included recent or anticipated invasive procedures, substantial weight loss, and reference to non-specific conditions thought to interfere with study conduct or interpretation. Our analyses were performed using criteria groupings rather than individual criteria.
Statistical Analysis
Associations between trial characteristics and eligibility criteria were analyzed by nonparametric statistical methods, such as Wilcoxon two-sample test and Kruskal-Wallis test. All reported p-values were two-sided. A p-value less than 0.05 was considered as statistical significance. All statistical calculations were performed by SAS 9.4 for Windows (SAS Institute Inc., Cary, NC).
Results
We identified a total of 74 lung cancer clinical trials sponsored or endorsed by the ECOG Thoracic Committee for which full study protocols were available. Trial characteristics are listed in Table 1. Full protocol titles are listed in Supplementary Table 1.
Table 1.
Characteristics of trials included in the analysis
| Characteristic | Number (%) |
|---|---|
|
| |
| Total trials | 74 |
|
| |
| Phase of study | |
| 1 | 4 (5) |
| 2 | 40 (54) |
| 3 | 25 (34) |
| Other | 5 (7) |
|
| |
| Primary endpoint | |
| Overall survival | 27 (36) |
| Progression-free survival | 10 (14) |
| Other | 37 (50) |
|
| |
| Primary treatment modality | |
| Surgery | 8 (11) |
| Radiation | 9 (12) |
| Medical therapy | 54 (73) |
| Other | 3 (4) |
|
| |
| Year of study activation | |
| 1986–1995 | 27 (36) |
| 1996–2005 | 31 (42) |
| 2006–2016 | 16 (22) |
|
| |
| Archival tumor tissue required | 5 (7) |
|
| |
| Number of therapies administered | |
| 0–1 | 26 (35) |
| 2–3 | 32 (43) |
| 4–7 | 16 (22) |
Trends in total number of eligibility criteria over time, presented as both individual criteria and criteria categories, are shown in Figure 1. Temporal trends in each criteria category are shown in Figure 2. The increase in eligibility criteria was statistically significant for both time intervals: 1986–1995 vs 1996–2005: P=0.05; 1996–2005 vs 2006–2016: P<0.001. The following categories had statistically significant (P<0.05) increases in eligibility criteria: cardiac, concurrent medications, gastrointestinal, hematologic, hepatic, inflammatory, neurologic, renal, and prior cancer therapy. There was no significant change in the number of eligibility criteria in the pulmonary and endocrine categories.
Figure 1.
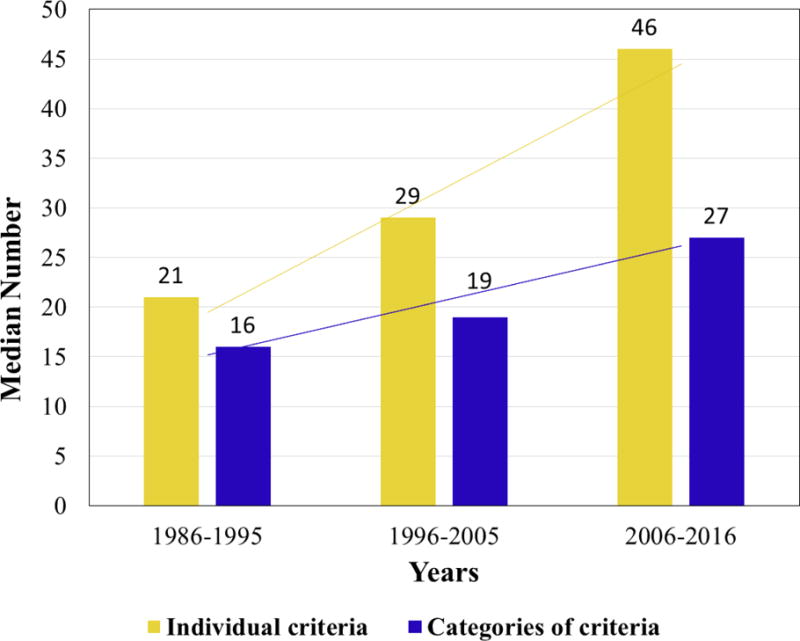
Median number of eligibility criteria over time
Figure 2.
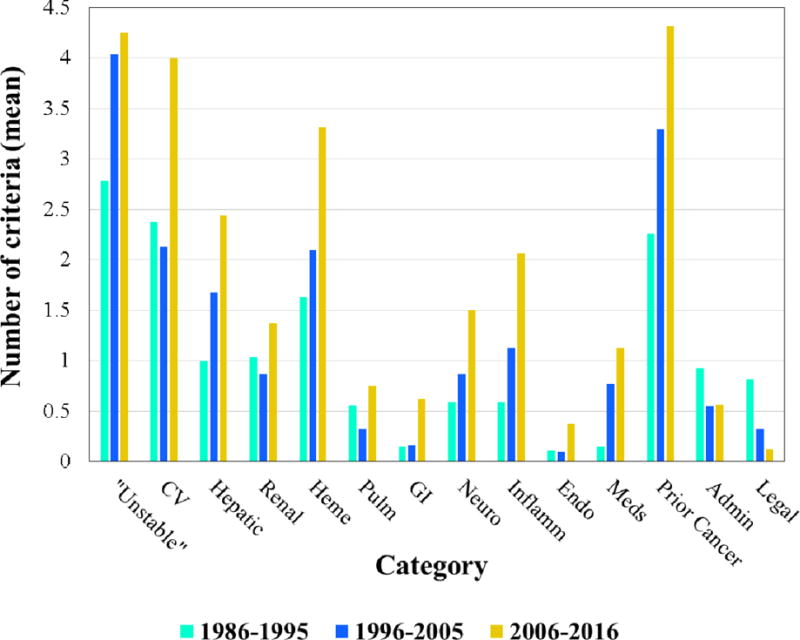
Mean number of eligibility criteria by category over time
Associations between trial characteristics and number of eligibility criteria are shown in Table 2. There was no association between trial phase and number of criteria (P=0.45). Similar to treatment modality, disease stage displayed a trend toward association with number eligibility criteria: median 12 for early stage, 21 for locally advanced stage, and 19 for advanced stage (P=0.08). There was also an association between maximum number of therapies administered and number of eligibility criteria (P=0.05). The increase in eligibility criteria over time was limited to medical therapy trials, for which the median number of eligibility criteria increased from 17 in 1986–1995 to 28 in 2006–2016 (P<0.001). There was no significant change in the number of eligibility criteria for surgery or radiation therapy trials (Table 3).
Table 2.
Association between trial characteristics and number of eligibility criteria*
| Characteristic | Median (IQR) | P value |
|---|---|---|
|
| ||
| Primary endpoint | 0.001 | |
| Overall survival | 20 (13–24) | |
| Progression-free survival | 28 (22–31) | |
| Other | 17 (12–23) | |
|
| ||
| Primary treatment modality | 0.02 | |
| Surgery | 9 (7–18) | |
| Radiation | 18 (18–21) | |
| Medical | 20 (17–25) | |
|
| ||
| Year of study activation | <0.001 | |
| 1986–1995 | 16 (10–20) | |
| 1996–2005 | 19 (13–23) | |
| 2006–2016 | 27 (24–31) | |
|
| ||
| Number of therapies administered | 0.05 | |
| 0–1 | 18 (9–22) | |
| 2–3 | 21 (15–24) | |
| 4–7 | 22 (17–28) | |
Eligibility criteria quantified by grouping
Table 3.
Change in eligibility criteria over time (median ± IQR)
| Year | P value | |||
|---|---|---|---|---|
| 1986–1995 | 1996–2005 | 2006–2016 | ||
| Surgery | 9 (7–23) | 8 (3–13) | N/A* | 0.50 |
| Radiation Therapy | 23 (21–24) | 18 (13–20) | 18 (11–27) | 0.35 |
| Medical Therapy | 17 (15–19) | 20 (17–24) | 28 (25–31) | <0.001 |
IQR, interquartile range
There were no trials with surgical therapy as the primary research question in the 2006–2016 period.
We also examined the number and mandated timing of study screening procedures. Over time, there was no significant change in the number of urine-based tests, pulmonary function parameters, and assessments of cardiac function. There was a significant increase in the number of required blood tests: median 11 in 1986–1995, 15 in 1996–2005, 19 in 2006–2016 (P=0.006). However, we observed a decrease in the number of required imaging tests: median 5 in 1986–1995, 2 in 1996–2005, 2 in 2006–2016 (P<0.001). Over time, the permitted timing interval for screening blood tests was median 14 days (1986–1995), 28 days (1996–2005), and 14 days (2006–2016) (P=0.09). We observed a decrease in the permitted timing interval for screening imaging tests as follows: median 42 days (1986–1995), 28 days (1996–2005), and 28 days (2006–2016) (P=0.04).
Discussion
For decades, it has been widely recognized that the number, complexity, and stringency of cancer clinical trial eligibility criteria limit enrollment.12,19,23 Complex and numerous screening procedures may hinder accrual as well. Given ongoing challenges in trial accrual and completion, there have been multiple calls to simplify eligibility considerations and study-related procedures.20,21,24 Nevertheless, our present analysis demonstrates an ongoing increase in the number of eligibility criteria over time in lung cancer clinical trials, as well as a clear increase in some screening requirements. Specifically, we observed a 50% growth in the number of eligibility criteria over the past 30 years. Within this time period, this increase accelerated in the most recent years, suggesting that this trend may continue unless dedicated interventions are undertaken.
Importantly, this increase in trial eligibility criteria appears to occur exclusively in medical therapy trials. Over time, surgery and radiation therapy trials have had stable numbers of eligibility criteria. Consistent with this observation, the majority of eligibility categories which sustained a significant increase over time (eg, hepatic, renal, hematologic, gastrointestinal, inflammatory, concurrent medications, and prior cancer therapy) are directly related to medical therapy. Categories without such increases (eg, pulmonary) could be considered more relevant to surgical and radiation therapy planning. Similarly, we observed a near doubling of required screening blood tests (most relevant to medical therapy) but no increase in the number of pulmonary function, cardiovascular, or disease imaging assessments. Notably, fewer than 10 percent of trials mandated submission of archival tumor tissue. This small proportion may reflect the broad time-frame of trials under study, the pragmatic nature of some trials, budgetary considerations, or other factors. Should this proportion increase in the future, other recent studies suggest that it will further complicate and limit enrollment.25,26
Why are medical therapy lung cancer trials becoming more stringent and complex, but not surgery and radiation therapy lung cancer trials? This observation likely reflects the nature of therapeutic advances. Over time, surgery and radiation therapy have become not only more effective, but also better tolerated. New techniques have not necessarily conveyed new toxicities, and principles of patient selection have not changed substantially. For medical therapies, however, advances have introduced new adverse events. Due to concerns for heightened toxicity, immunotherapy trials exclude patients with pre-existing autoimmune disease,27 a factor that is not addressed in trials of conventional chemotherapy or molecularly targeted therapy. Anti-angiogenic treatments, conveying risk of clotting and bleeding, require assessment of tumor- and comorbidity-related factors that could promote these events. New potential toxicities also impact study-related procedures. Among the 12 trials that require baseline urinalyses, eight of them feature bevacizumab, which requires regular monitoring for proteinuria. (One of the other four trials features ifosfamide, which requires regular monitoring for hemorrhagic cystitis.) Additionally, unlike conventional intravenous chemotherapy, oral targeted therapies require consideration of factors related to drug exposure (eg, gastrointestinal absorption, drug-drug interactions, hepatic function). As might be expected, we observed more eligibility criteria among trials involving more types of therapies. However, this effect appears to account for relatively little of the variation among studies, with a median of 18 eligibility criteria among those with the simplest treatment regimens compared to a median of 22 among those with the most complex therapies.
The increasing number of eligibility criteria that were broadly categorized as “unstable” medical conditions also merits consideration. Some instances reflect specific toxicity concerns, such as those related to antiangiogenic therapies; related examples include recent or planned surgical procedures, wounds, ulcers, and other conditions. Others attempt to encapsulate overall functional capacity, such as marked weight loss. Still others seek to target any situation that could hinder study conduct or interpretation. For example, E4508 excludes “any medical or psychiatric condition or addictive disorder, or laboratory abnormality that, in the opinion of the investigator, may increase the risks associated with study participation or study treatment or may interfere with the conduct of the study or interpretation of study results.” While such far-reaching clauses appear to cover numerous potential concerns, the lack of specificity could result in widely varying interpretation.
To simplify eligibility criteria and screening procedures for clinical trials while still preserving subject safety and scientific rigor, investigators, sponsors, and regulators must carefully consider the value of each requirement. For instance, the exclusion of patients with prior cancer from lung cancer trials is a common practice (>80% of protocols), results in the exclusion of more than 15% of potential patients, and does not appear to be justified.28–30 In recent years, trials have been more likely to limit this exclusion to a specific time-frame—most commonly within the past 5 years. However, because more than half of prior cancer diagnoses occur within this period, this practice still results in excluding a substantial proportion of patients.23
Organ function specifications also merit reconsideration. Protocols of molecularly targeted therapies and immunotherapy have incorporated new criteria reflecting unique toxicities of these drugs. However, most of these protocols continue to include exclusion criteria related to conventional chemotherapy, even if the therapies under study do not convey relevant toxicities. For example, EA5142 (“Adjuvant Nivolumab in Resected Lung Cancer [ANVIL]—A Randomized Phase III Study of Nivolumab After Surgical Resection and Adjuvant Chemotherapy in Non-Small Cell Lung Cancers”) has multiple eligibility criteria related to hematologic parameters (WBC ≥ 2,000/μL, neutrophils ≥ 1,000/μL, platelets ≥ 100×103/μL) even though nivolumab and other immune checkpoint inhibitors are not associated with hematologic toxicity. Similarly, E3503 (“A Pilot Study to Determine if Downstream Markers of EGFR Linked Signaling Pathways Predict Response to OSI-774 [Erlotinib] in the First-line Treatment of Patients with Advanced Non-Small Cell Lung Cancer”) requires adequate blood counts (neutrophils ≥ 1,500/mm3, platelets ≥ 100,000/m3) even though erlotinib and other epidermal growth factor receptor (EGFR) inhibitors do not cause myelosuppression. Over 80% of protocols in our analysis mandated adequate kidney function, commonly defined as a creatinine clearance ≥ 60 mL/min—a threshold that over 20% of lung cancer patients fall below at some point during their treatment history.31 Accordingly, such restrictions might be limited to trials of therapies cleared renally or conveying renal toxicity. These observations echo those from other fields, including a recent analysis of hematologic malignancy protocols which found that most eligibility criteria did not correlate with known, observed adverse events of study therapy.32
Similarly, the exclusion of medications that could exhibit drug-drug interactions with the study agent or prolong the QT interval should be limited to those medications conveying the highest potential risk. Otherwise, this practice is likely to exclude a large number of patients, as lists of CYP450 inducers, inhibitors, and substrates have grown to include more than 230 drugs33 and lists of QT-prolonging drugs now exceed 160 medications.34 Finally, treatment washout periods could also be re-visited. For instance, the majority of protocols in our sample mandate waiting times of 2–4 weeks after prior radiation therapy. While this delay may be appropriate following fractionated radiation therapy to a visceral site, it does not seem warranted after stereotactic radiation to an asymptomatic brain metastasis or palliative radiation therapy to a peripheral skeletal lesion, where the potential for overlapping toxicity with systemic therapy is limited. The administration of pre-study radiation therapy has been identified as a key factor limiting trial enrollment.35 Shortening post-radiation washout periods to clinically practical intervals that are unlikely to heighten toxicity may help enroll on protocol patients who currently receive standard therapy instead to expedite systemic treatment initiation.
There are a number of caveats to the interpretation of our findings. Importantly, an increase in the number of eligibility criteria does not necessarily imply a decrease in potential eligibility. In some instances, additional eligibility criteria may serve to define exclusion policies more precisely, thereby resulting in a net increase in eligibility. Nevertheless, the increase in eligibility criteria number does complicate the assessment of potential subjects and could therefore increase the risk of protocol deviations or violations, or delay initiation of therapy. We also recognize that our analysis includes only selected NCI-sponsored clinical trials, potentially limiting the generalizability of our findings. However, in an earlier analysis, we found that the specific practice of excluding patients with prior cancer was comparable between NCI- and industry-sponsored lung cancer clinical trials.23 While our results are not directly generalizable to clinical trials in other cancer types, they are likely representative. Increases in eligibility criteria have been reported in other settings, including breast, gastrointestinal, and gynecologic cancers.19,22 Small numbers of radiation therapy and surgery protocols may obscure significant changes in eligibility criteria, although the observed values suggests that this is unlikely. Finally, we do not attempt in the current analysis to determine the appropriateness of protocol eligibility criteria or screening procedures.
In conclusion, medical therapy lung cancer clinical trials are becoming more complex, with growing numbers of eligibility criteria and screening procedures. While this trend may hypothetically increase the scientific yield or safety of a protocol, it also potentially hinders accrual, decreases study completion rates, limits generalizability, and increases costs. The growth in medical therapy lung cancer trials appears to reflect the general practice of adding new criteria relevant to contemporary treatments such as immunotherapy and molecularly targeted therapies, without revisiting and removing criteria not pertinent to these interventions. With federal funding for cancer clinical trials decreasing and a substantial proportion of NCI-sponsored cancer clinical trials not completing accrual,36 ongoing efforts to simplify eligibility and procedures will be critical moving forward. Tailoring inclusion and exclusion criteria to match the intervention under study represents a key step in this process.
Supplementary Material
Acknowledgments
For their assistance obtaining clinical trial protocols, the authors thank BilliSue Sawyer, Laura Gagnon, and Elizabeth O’Conner from the Coordinating Center of the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair), which is supported in part by Public Health Service Grant CA23318 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. The authors also thank Helen Mayo, MLS, from the University of Texas Southwestern Medical Center Library for her assistance performing literature searches; and Ms. Dru Gray for assistance with manuscript preparation
Funding:
Funded in part by the UT Southwestern Medical Student Summer Research Program (to SG), a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG), and an NCI National Clinical Trials Network Lead Academic Site Award (5U10CA180870-02; to DEG). Biostatistical support was provided by the Biostatistics and Bioinformatics Shared Resource at the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-03. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number TL1TR001104 as well as CTSA NIH Grant UL1-RR024982.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentations:
This work was presented in abstract form at the International Association for the Study of Lung Cancer (IASLC) 2016 Chicago Multidisciplinary Symposium in Thoracic Oncology. September 22–24, 2016, Chicago, Illinois, and the 17th Annual IASLC Targeted Therapies for Lung Cancer Meeting (Santa Monica, CA, February 22–25, 2017).
References
- 1.Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65:2376–82. doi: 10.1002/1097-0142(19900515)65:10+<2376::aid-cncr2820651504>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 3.Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 4.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 5.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–76. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 6.Hietanen PS, Aro AR, Holli KA, et al. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncol. 2007;46:42–8. doi: 10.1080/02841860600849067. [DOI] [PubMed] [Google Scholar]
- 7.Avis NE, Smith KW, Link CL, et al. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006;24:1860–7. doi: 10.1200/JCO.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 8.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 9.Rasco DW, Xie Y, Yan J, et al. The impact of consenter characteristics and experience on patient interest in clinical research. Oncologist. 2009;14:468–75. doi: 10.1634/theoncologist.2008-0268. [DOI] [PubMed] [Google Scholar]
- 10.Gerber DE, Rasco DW, Skinner CS, et al. Consent timing and experience: modifiable factors that may influence interest in clinical research. J Oncol Pract. 2012;8:91–6. doi: 10.1200/JOP.2011.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux J, Goodwin PJ, Pritchard KI, et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol. 2008;26:4458–65. doi: 10.1200/JCO.2007.15.3726. [DOI] [PubMed] [Google Scholar]
- 13.Gotay CC. Accrual to cancer clinical trials: directions from the research literature. Soc Sci Med. 1991;33:569–77. doi: 10.1016/0277-9536(91)90214-w. [DOI] [PubMed] [Google Scholar]
- 14.McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials: a pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982;5:227–36. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- 15.Kotwall CA, Mahoney LJ, Myers RE, et al. Reasons for non-entry in randomized clinical trials for breast cancer: a single institutional study. J Surg Oncol. 1992;50:125–9. doi: 10.1002/jso.2930500215. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer. 1983;52:1014–6. doi: 10.1002/1097-0142(19830915)52:6<1014::aid-cncr2820520614>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–52. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–40. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 19.Fuks A, Weijer C, Freedman B, et al. A study in contrasts: eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program Pediatric Oncology Group. J Clin Epidemiol. 1998;51:69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Bernstein D, Hilsenbeck SG, et al. Modernizing Eligibility Criteria for Molecularly Driven Trials. J Clin Oncol. 2015;33:2815–20. doi: 10.1200/JCO.2015.62.1854. [DOI] [PubMed] [Google Scholar]
- 21.Kim ES, Atlas J, Ison G, et al. Transforming Clinical Trial Eligibility Criteria to Reflect Practical Clinical Application. Am Soc Clin Oncol Educ Book. 2016;35:83–90. doi: 10.1200/EDBK_155880. [DOI] [PubMed] [Google Scholar]
- 22.Srikanthan A, Vera-Badillo F, Ethier J, et al. Evolution in the eligibility criteria of randomized controlled trials for systemic cancer therapies. Cancer Treat Rev. 2016;43:67–73. doi: 10.1016/j.ctrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. 106:2014. doi: 10.1093/jnci/dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber DE, Pruitt SL, Halm EA. Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res. 2015;4:289–91. doi: 10.2217/cer.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia S, Saltarski JM, Yan J, et al. Time and Effort Required for Tissue Acquisition and Submission in Lung Cancer Clinical Trials. Clin Lung Cancer. 2017 doi: 10.1016/j.cllc.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim C, Sung M, Shepherd FA, et al. Patients with Advanced Non-Small Cell Lung Cancer: Are Research Biopsies a Barrier to Participation in Clinical Trials? J Thorac Oncol. 2016;11:79–84. doi: 10.1016/j.jtho.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Khan SA, Pruitt SL, Xuan L, et al. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol. 2016;2:1507–1508. doi: 10.1001/jamaoncol.2016.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laccetti AL, Pruitt SL, Xuan L, et al. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 107:2015. doi: 10.1093/jnci/djv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laccetti AL, Pruitt SL, Xuan L, et al. Prior cancer does not adversely affect survival in locally advanced lung cancer: A national SEER-medicare analysis. Lung Cancer. 2016;98:106–13. doi: 10.1016/j.lungcan.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruitt SL, Laccetti AL, Xuan L, et al. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer. 2017;116:717–725. doi: 10.1038/bjc.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutluk Cenik B, Sun H, Gerber DE. Impact of renal function on treatment options and outcomes in advanced non-small cell lung cancer. Lung Cancer. 2013;80:326–32. doi: 10.1016/j.lungcan.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Statler A, Radivoyevitch T, Siebenaller C, et al. The relationship between eligibility criteria and adverse events in randomized controlled trials of hematologic malignancies. Leukemia. 2017 doi: 10.1038/leu.2016.374. [DOI] [PubMed] [Google Scholar]
- 33.Indiand University School of Medicine DoM, Clinical Pharmacology: P450 Drug Interactions, Abbreviated #x0201C;Clinically Relevant” Table. 2017
- 34.Credible list of drugs that prolong QT and/or cause Torsades de Pointes (TDP), 2016
- 35.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to Enrollment in Non-small Cell Lung Cancer Therapeutic Clinical Trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 36.Medicine Io: A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


