Abstract
Background
In a recent randomized placebo-controlled trial, consolidation treatment with brentuximab vedotin (BV) decreased the risk of Hodgkin lymphoma (HL) progression after autologous stem-cell transplantation (ASCT). However, the impact of BV consolidation on overall survival, quality of life, and health care costs are unclear.
Methods
We constructed a Markov decision-analytic model to measure the costs and clinical outcomes for BV consolidation therapy compared to active surveillance in a 33-year-old patient cohort at risk for HL relapse following ASCT. Life-time costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were calculated for each post-ASCT strategy.
Results
After quality-of-life adjustments and standard discounting, upfront BV consolidation was associated with an improvement of 1.07 quality-adjusted-life years (QALYs) compared to active surveillance with BV as salvage. However, the strategy of BV consolidation led to significantly higher healthcare costs ($378,832 versus $219,761), causing the ICER for BV consolidation compared with active surveillance to be $148,664/QALY. If indication-specific pricing were implemented, our model estimates BV price reductions of 18% to 38% for the consolidative setting would translate to ICERs of $100,000/QALY and $50,000/QALY, respectively. Findings were consistent on one-way and probabilistic sensitivity analyses.
Conclusions
BV as consolidation at current US pricing is unlikely to be cost-effective at a willingness to pay threshold of $100,000/QALY. However, indication-specific price reductions for the consolidative setting could reduce ICERs to widely acceptable values.
Keywords: brentuximab vedotin, brentuximab vedotin consolidation, Hodgkin lymphoma, consolidation therapy, cost-effectiveness analysis, markov model, autologous stem cell transplantation
Introduction
Although the majority of individuals with Hodgkin lymphoma (HL) experience durable remissions following modern first-line chemotherapy, upwards of 20–30% are refractory to initial treatment or experience disease relapse after initial remission.1–4 For relapsed or primary refractory HL, the standard of care is high dose chemotherapy followed by autologous stem cell transplantation (ASCT). Despite this intensive treatment, approximately 50% will relapse and succumb to HL following ASCT.5–10 Primary refractory disease, progression within 12 months of an initial response to frontline therapy, and extranodal involvement prior to salvage therapy portend the greatest risk of post-ASCT relapse.8, 11, 12 For these individuals at greatest risk for HL progression, additional therapeutic strategies following transplantation may offer clinical benefit.
The anti-CD30 antibody drug conjugate, brentuximab vedotin (BV), was first shown to be highly efficacious in individuals with relapsed HL following ASCT or in those not deemed to be candidates for intensive therapy.13, 14 More recently, the double blind, placebo-controlled phase 3 AETHERA trial evaluated BV as early consolidation after ASCT in individuals with HL and unfavorable risk factors.15 The study found significant improvement in progression free survival (PFS) for BV-treated patients compared to the placebo group (42.9 months vs. 24.1 months).15 At a median observation time of 30 months, no significant difference in overall survival was detected. However, the PFS improvement led the FDA to add post-ASCT consolidation to BV’s label and converted BV’s accelerated approval status to traditional approval.16
Adding preemptive BV to the post-ASCT setting comes at a time of increased scrutiny on drug prices, with many calling for interventions to better align drug prices with clinical utility.17–19 Fortunately, robust clinical trial and quality of life data were captured when testing BV consolidation, affording the opportunity to appraise consolidative BV using value-based assessment. In this study, we use a Markov decision-analytic model to compare the cost-effectiveness of BV as consolidation therapy compared to active surveillance with BV as salvage following ASCT.
Methods
Patients and Intervention
Our baseline sample was constructed to mirror the population enrolled in the AETHERA trial.15 The age of our patient cohort was 33 years and all individuals had at least one risk factor for progression after ASCT: primary refractory HL, relapsed HL with an initial remission duration of less than 12 months, or extranodal involvement at the start of pre-transplantation salvage chemotherapy. Similar to the clinical trial, individuals entered our model following ASCT and received either consolidation with BV (1.8 mg/kg every 3 weeks for maximum of 16 cycles) or active surveillance with BV at time of HL progression (placebo arm).15
Model Construction
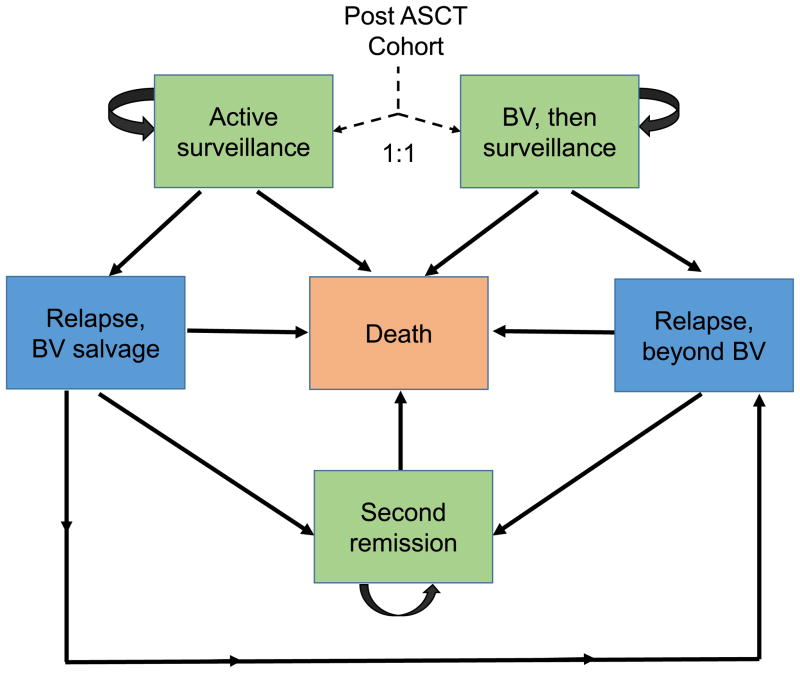
We developed a Markov decision-analytic model to compare the costs and clinical outcomes associated with post-ASCT BV consolidation compared to active surveillance. In the baseline model (Figure 1), individuals enter the model following ASCT and receive either BV consolidation or active surveillance. Four additional health transition states are used to capture the post-ASCT period until death: HL relapse treated with BV, HL relapse post BV treatment, long-term durable second remission, and death. Individuals who relapsed on active surveillance first received salvage with BV. Patients who had HL progression following BV consolidation or BV salvage entered the relapse-post BV health state where they received salvage chemotherapy, immunotherapy (nivolumab), and/or allogeneic stem cell transplantation.
Figure 1. Markov model of post-ASCT brentuximab vedotin consolidation versus active surveillance with use of brentuximab vedotin rescue at time of HL progression.
ASCT indicates autologous stem cell transplantation; BV, brentuximab vedotin.
We used 3-month transition-state cycles and a lifetime horizon to estimate direct healthcare costs and utilities experienced as individuals transitioned from ASCT until death. Costs and utilities were assigned to each health state, and transition probabilities were derived from published studies. Cost-benefit was conducted from a societal perspective within the United States, and all future costs and benefits were discounted at a standard rate of 3% annually.20 The primary outputs of the model were used to calculate the incremental cost in US dollars for an additional QALY gained comparing BV consolidation to active surveillance (incremental cost-effectiveness ratio, ICER). We assumed a willingness to pay threshold of $100,000/QALY gained. The resulting model was constructed using TreeAge Pro software (TreeAge Software, Williamstown, MA). Additional statistical analyses were performed using STATA 13 (StataCorp, College Station, TX) and R (R Core Team, www.R-project.org).
Transition Probabilities
Base-case estimates and ranges for health-state transition probabilities are listed in Table 1. When available, results from randomized controlled trials were used to inform clinical probabilities. We estimated rates of HL progression following ASCT out to 5 years using standard extrapolation methods.15, 21–23 Here, we first reconstructed individual patient-level PFS data from AETHERA’s Kaplan Meier curves and at-risk tables published at a median observation time of 30 months.15 The individual-level data were then fit with standard parametric models (Gompertz, exponential, log-logistic, Weilbull).21, 22, 24, 25 Both BV consolidation and placebo arms were best fit with Gompertz functions and the predicted 5-year PFS was 54% for BV consolidation compared to 43% for placebo (Figure 1 in Supplemental Material). All patients in our model remained at risk for HL progression up to 5 years post-ASCT. After the five years, individuals experienced age-adjusted mortality from other causes based on US Life Tables. Finally, we incorporated a number of recently published studies to derive transition probabilities following HL relapse. This included recently published long term follow-up data for BV monotherapy in the salvage setting and separate reports supporting the use of nivolumab after BV failure.26–28
Table 1.
Baseline Clinical Parameters
| Result/Transition | Estimate | Range | References |
|---|---|---|---|
| Cohort age at start (years) | 33 | 18–60 | 15 |
| PFS following BV consolidation within 5 years of ASCT | Gompertz Function λ = 0.0263 γ = −0.0385 |
- | 15, 21–23 |
| PFS on active surveillance within 5 years of ASCT | Gompertz Function λ = 0.0891 γ = −0.106 |
- | 15, 21–23 |
| Probability of remission after BV salvage | 0.09 | 0.05 to 0.13 | 13, 26, 44 |
| Number of BV doses in salvage setting | 10 | 8–12 | 13 |
| Probability of receiving alloSCT | 0.3 | 0.26 to 0.35 | 13, 44–47 |
| Probability of receiving salvage chemotherapy | 0.50 | 0.3–0.8 | Expert opinion |
| Probability of receiving nivolumab | 0.70 | 0.6–1 | Expert opinion |
| Number of nivolumab doses in salvage setting | 16 | 12 – 37 | 27, 28 |
| Probability of durable remission after alloSCT | 0.54 | 0.32 to 0.59 | 45–47 |
| Average survival with refractory disease post-ASCT (years) | 2.5 | 1 to 3.5 | 58–60 |
| Background mortality rate | CDC Life Table | - | |
| Discount rate | 0.03 | 0.015 to 0.06 | 20 |
Abbreviations: PFS indicates progression free survival; BV, brentuximab vedotin; ASCT, autologous stem cell transplantation; and alloSCT, allogeneic stem cell transplantation.
Costs
Baseline direct medical costs were derived from the 2016 Medicare fee schedule and medical literature (Table 2). Costs were converted into 2016 US dollars using the Medical Care component of the Consumer Price Index. Consistent with the Medicare pricing policy, we used 106% of the manufacturer’s 2016 average sales price for drug costs.17, 29 Our drug cost calculations assume 70 kg patients, but account for drug wastage by rounding up to the next full single-use vial size available for each dose administered.30, 31 For example, BV is only available in 50 mg single-use vials. Therefore, three vials per dose were used for our 70 kg patients (1.8 mg/kg/dose) and BV costs were based on the full 150 mg charged per dose. Costs for allogeneic transplantation and traditional salvage chemotherapy were based on values used in previous studies.32–35 The cost of monitoring included office visits and routine lab tests. End of life health care costs were estimated from published data on the cost of care in the last year of life for cancer patients compared to the general Medicare population.36, 37
Table 2.
Costs
| Service | CPT Code | Baseline ($ USD) | Range ($ USD) | References |
|---|---|---|---|---|
| Routine follow-up clinic visit | 116 | 100 to 250 | ||
| History and physical examination | 99213 | 79 | ||
| Complete blood count with differential | 85025 | 12 | ||
| Comprehensive metabolic panel | 80053 | 16 | ||
| Lactate dehydrogenase | 83615 | 9 | ||
|
| ||||
| Chemotherapy IV, up to 1 hr administration | 96413 | 149 | 75 to 175 | |
|
| ||||
| Lymphoma-related end-of-life | 53,265 | 37,792 to 64,786 | 36 | |
|
| ||||
| Non-lymphoma end-of-life | 42,543 | 32,393 to 53,988 | 37 | |
|
| ||||
| Brentuximab vedotin | J9042 | 19,393/dosea | - | |
|
| ||||
| Nivolumab | J9299 | 5,653/doseb | - | |
|
| ||||
| Conventional dose salvage chemotherapy | 17,199 | 15,000 to 45,000 | 32, 34 | |
|
| ||||
| Allogeneic stem cell transplantation | 255,408 | 106,915 to 582,094 | 33, 35 | |
70 kg patient, 1.8 mg/kg, requiring three 50 mg vials per dose, $129.287/mg for 106% of average sales price
70 kg patient, 3 mg/kg, requiring one 100 mg vials and three 40 mg vial per dose, $25.694/mg for 106% of average sales price
Abbreviations: CPT indicates current procedural terminology
Utilities
Baseline clinical utilities for various health states were based on values used in previous studies and published literature (Table 3). In particular, results from AETHERA’s companion quality of life study informed our clinical utilities in the post-ASCT setting.38 Individuals enrolled in AETHERA completed EQ-5D questionnaires every 3 months for the first two years on study, with published results displaying scores by treatment arm (BV consolidation vs. placebo) and disease status (ongoing remission vs. progressive disease).38 We used digitizer software to directly capture the mean EQ-5D score reported by participants of the AETHERA trial (GetData Graph Digitizer version 2.24, http://www.getdata-graph-digitizer.com).38 EQ-5D scores at 3 month intervals by treatment arm informed our model utilities through the first two years, while pooled mean scores at two years were used for our long-term remission (0.89) and progression (0.67) states. Further, we considered a range of clinical utilities based on prior literature during sensitivity analysis.39
Table 3.
Model Utilities
| Utility | QALY | Range | References |
|---|---|---|---|
| Complete Remission | 0.89 | 0.75 to 0.95 | 38, 39 |
|
| |||
| Relapsed Disease | 0.67 | 0.50 to 0.79 | 33, 39 |
|
| |||
| BV Consolidation, in remission | |||
| Month 0–24 | 0.82 – 0.90 | 38 | |
| Beyond 24 months (durable remission) | 0.89 | 0.75 to 0.95 | 33, 38, 39 |
|
| |||
| Active surveillance, in remission | |||
| Month 0–24 | 0.87 – 0.92 | 38 | |
| Beyond 24 months (durable remission) | 0.89 | 0.75 to 0.95 | 33, 38, 39 |
Abbreviations: QALY indicates quality-adjusted life-year; BV, brentuximab vedotin.
Sensitivity Analysis
We performed a series of sensitivity analyses to account for uncertainty and evaluate the robustness of our model conclusions. In one-way sensitivity analyses, we varied the value of our model parameters one at a time to examine the effects on the ICER. During probabilistic sensitivity analysis, we performed 10,000 Monte Carlo simulations, each time randomly sampling from the distributions of model inputs. Clinical probabilities and health utilities were represented by beta distributions, whereas costs were represented by gamma distributions. Patient age also varied during probabilistic sensitivity analysis and mirrored participant age in the AETHERA trial.15
Results
Baseline Analysis
Results of the baseline cost effectiveness analysis are summarized in Table 4. Under the baseline model, compared to active surveillance, BV consolidation led to significantly higher healthcare costs ($378,832 vs $219,761) with an incremental cost of $159,071. The average life-years gained with BV consolidation compared to active surveillance was 2.1 years (mean [median] OS: 28.0 [29.6] vs 25.9 [24.1]). After applying quality-of-life adjustment and future discounting, BV as consolidation therapy was associated with an improvement of 1.07 QALYs compared to active surveillance. Therefore, the incremental cost-effectiveness ratio for BV consolidation compared to active surveillance was estimated at $148,664/QALY.
Table 4.
Baseline Cost-Effectiveness Analysis and Probabilistic Sensitivity Analysis
| Strategy | Baseline Model | PSA Model ICER 95% CI ($/QALY) |
||||
|---|---|---|---|---|---|---|
| Costs ($) | Incremental Costs ($) | Effectiveness (QALY) | Incremental Effectiveness | ICER ($/QALY) | ||
| Active surveillance | 219,761 | - | 13.50 | - | - | |
| BV consolidation | 378,832 | 159,071 | 14.57 | 1.07 | 148,664 | 97,134 to 282,171 |
Abbreviations: PSA indicates probabilistic sensitivity analyses; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; CI, confidence interval; BV, brentuximab vedotin.
Sensitivity Analyses
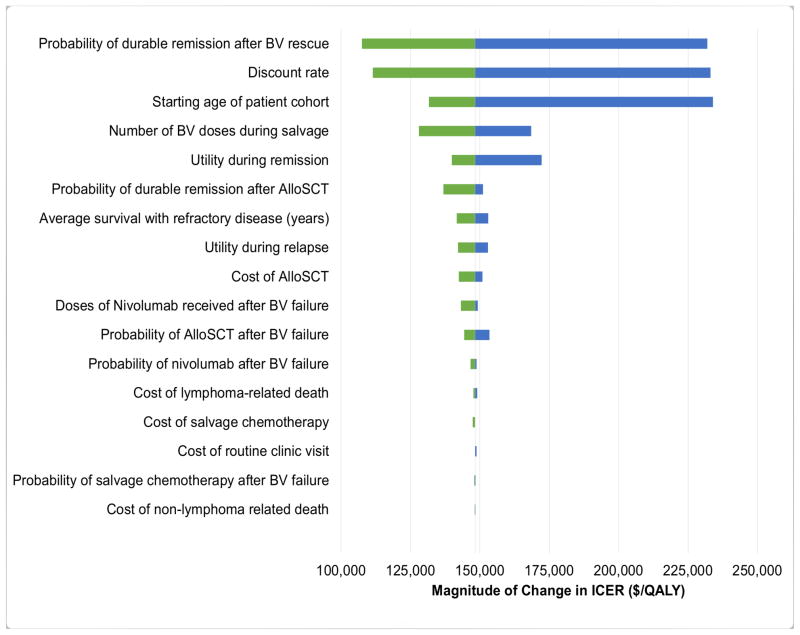
The results of one-way sensitivity analyses are presented in Figure 2. Model parameters with the greatest influence on the ICER were discount rate, probability of remission after BV rescue, and starting age of the patient cohort. For example, varying the probability of long-term durable remission after salvage BV between 5% and 12.7% caused the ICER to increase from $107,540 to $231,904. Less pronounced ICER changes were observed when varying non-drug costs and clinical utilities. Across broad ranges for each parameter, all ICERs in our one-way sensitivity analyses remained greater than $100,000 per QALY.
Figure 2. One-way sensitivity analysis.
Brentuximab vedotin consolidation versus active surveillance. BV indicates brentuximab vedotin; Allo, allogenic stem cell transplantation; ASCT, autologous stem cell transplantation; ICER, incremental cost-effectiveness ratios; QALY, quality-adjusted life-years.
In addition to standard one-way sensitivity analysis, we considered a scenario where indication-specific pricing was implemented. Here, BV utilized in the salvage setting remained at current pricing (approximately $6,464 per 50 mg vial), but BV for consolidation was discounted. Our model estimates 18% to 38% price reductions for BV in the consolidative setting would translate into ICERs of $100,000/QALY and $50,000/QALY, respectively (Figure 2 in Supplemental Material).
Summary of probabilistic sensitivity analysis is shown in Table 4. No iteration during our 10,000 sample simulation had an ICER below a threshold of $50,000 per QALY. Only 3% of iterations had an ICER below $100,000. In total, 95% of iterations during our Monte Carlo simulation produced ICERs for BV compared to active surveillance between $97,993 to $286,624 per QALY.
Discussion
Although first-line chemotherapy is highly effective for the majority of individuals with HL, outcomes in those with refractory or relapsed HL is poor.5–10, 40 As an anti-CD30 antibody-drug conjugate, BV has clear efficacy as a single-agent in the relapsed HL setting, including the potential for long-term durable remissions in a small subset of individuals.13, 14 With efficacy clearly established in relapsed and refractory HL, investigations testing BV in the first-line setting are underway.41, 42 In addition, the recently published AETHERA trial measured the efficacy of preemptive use of BV as consolidation therapy 4–6 weeks after ASCT for patients at high risk of HL relapse.15 In this trial, consolidation with BV was associated with significant improvements in PFS when compared to placebo.15
While consolidation with BV offers improvement in PFS, its impact on survival, quality of life, and health care costs was uncertain. Our analysis incorporated data from the AETHERA trial alongside clinical utilities and healthcare costs to calculate the cost-effectiveness of preemptive BV consolidation versus active surveillance with BV as salvage. The incremental clinical benefit of BV consolidation was associated with significant health care expenditures, producing an ICER of $148,664/QALY. ICERs remained greater than $100,000/QALY in one-way sensitivity analyses and conclusions were robust during probabilistic sensitivity analysis.
Decision-analytic models are subject to inherent limitations related to the data available to populate the model, however, our study has a number of notable strengths. First, our model was based on results from a large randomized placebo-controlled, double-blinded trial directly comparing BV consolidation to placebo (active surveillance in our model).15 Second, clinical utility estimates for BV consolidation and the post-ASCT surveillance were available from AETHERA’s companion study which measured quality of life during the clinical trial.15, 38 Our model also incorporated contemporary data to reflect recent advances in the treatment and outcomes of individuals with refractory or relapsed HL following ASCT.26–28 Lastly, cost-effectiveness analyses evaluating new drugs for hematologic malignancies infrequently consider drug wastage in cost calculations.31 Since the economic impact of drug wastage can be substantial,29 we chose to calculate costs for novel drugs based on the number of single-use vials used rather than the actual dose administered.
While our study has a number of strengths, limitations exist. First, our model accounts for treatment discontinuation due to toxicity, but it does not address dose reductions that can occur during treatment with BV.13, 15 Peripheral neuropathy is the most frequent cause of BV dose reduction and is more likely to occur in later cycles related to cumulative drug exposure.43 Since we calculated drug costs by the number of vials used per dose, our model is less sensitive to minor dose modifications. However, even when we performed a sensitivity analysis reducing the number of vials used in 20% of all BV administrations to 2 (from 3 vials), the ICER for BV consolidation remained greater than $100,000/QALY (data not shown).
Secondly, despite having randomized clinical trial information for much of our Markov model, uncertainly currently exists concerning post-ASCT relapse due to recent clinical advancements with relatively short term follow up.27, 28 In our model, patients who relapsed after BV (BV consolidation or BV salvage) received the same HL-directed treatment. In these heavily pre-treated patients, our model included the use of salvage chemotherapy, PD-1 blockade by nivolumab, and/or allogeneic stem cell transplantation.1 We grouped these treatments into a single health state by using published clinical data on therapy for HL patients relapsing after having received both ASCT and BV. The probability of receiving an allogeneic transplantation and the likelihood of a durable remission after allogeneic transplantation were extracted from available observational studies.13, 44–47
Likewise, the estimated cost of salvage therapy beyond BV was summed from the estimated percentage of heavily pre-treated patients who receive salvage chemotherapy, nivolumab, and/or allogeneic transplantation and the respective costs of the three treatment modalities. When available, we incorporated published literature to estimate the cost and utilities of relapse beyond BV and performed extensive sensitivity analysis. For example, we varied the number of nivolumab cycles from 12 to 37 based on the published report of nivolumab for HL after BV failure.27 Despite considering a wide range of nivolumab cycles during sensitivity analysis, calculated ICERs remained stable between $143,168 and $149,229/QALY. Even when we performed a sensitivity analysis attributing a cost of $1,000,000 per QALY gained during the post BV relapse state, our ICER for BV consolidation remained greater than $100,000/QALY.
Since our model is most influenced by parameters informing health transition states prior to BV-refractory disease, our model conclusions are robust despite the uncertainties surrounding recent treatment advances for HL beyond ASCT and BV. However, it remains possible that consolidation with BV would be cost-effective for a subgroup of patients with very high risk for relapse where consolidation with BV appeared to offer particular benefit.48 Furthermore, our AETHERA-derived model applies to individuals treated with chemotherapy in the pre-transplant setting and may not translate into future Hodgkin lymphoma settings where brentuximab and/or immunotherapy are used in earlier lines of therapy. Lastly, our cost-effectiveness analysis only considers direct health care expenditures, and future economic benefits related to improved survival in our relatively young patient population could be considerable.
Overall, our study suggests the use of BV as consolidation therapy at current US pricing is unlikely to be cost-effective at a willingness to pay threshold of $100,000/QALY. Although there continues to be deliberation about defining acceptable cost-effectiveness thresholds,49 recent data suggest rising costs of new anti-cancer drugs are only modestly associated with clinical benefits.50 With the cost of cancer treatments increasing ten-fold since 2000,51, 52 our finding of an unfavorable ICER is not unique among anti-cancer therapies.53, 54 In fact, a recently published systemic review found many treatments for hematologic cancers that were previously identified as having acceptable ICERs were no longer cost-effective when considering present-day US drug prices.55
Cancer treatment prices are increasingly recognized as unsustainable and a number of potential solutions are under consideration.18, 19 While cost-effectiveness analyses can inform value-based pricing, the quality of such analyses depends on the availability of robust comparative data. Our cost-effectiveness analysis utilized data from a randomized double-blinded clinical trial with a clinically relevant control arm and companion quality of life study. Unfortunately, this level of data is all too uncommon in today’s clinical trial environment, as industry sponsored trials frequently utilize control arms that do not represent current treatment standards.56, 57 This makes direct comparisons difficult in many oncology settings and increases uncertainty for cost-effectiveness analyses.
In the setting of HL after ASCT – approximately 50% of individuals will remain free from HL recurrence without additional therapy.5–10 Even when selecting patients at high risk using clinical parameters,15 a considerable portion remain in remission post-ASCT and are unlikely to benefit from upfront consolidation with BV. In patients with confirmed HL relapse post-ASCT, an overwhelming majority of patients will derive clinical benefit from BV therapy. Under indication-specific pricing, BV in the consolidation setting would be priced lower than BV used for post-ASCT salvage. Ultimately, our model found price reductions for BV in the consolidative setting of 18–38% produced more reasonable ICERs of $100,000/QALY and $50,000/QALY, respectively.
Value-based and indication-specific pricing has the potential to align drug prices with their underlying clinical utility and continue to reward highly effective cancer therapy. While an academic exercise at present, future cost-effectiveness analysis similar to ours could assist with transparent and more equitable drug pricing in the US and abroad. This will only be possible with broad buy-in from health care stakeholders and a renewed focus on conducting clinical trials with relevant control arms and robust real-world comparative-effectiveness research.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the Lowe Endowment Fund, National Heart, Lung and Blood Institute of the National Institutes of Health (T35HL007649), and CTSA Grant KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not represent the official view of NIH.
Footnotes
Author Contributions: L.H. and S.F.H. contributed to the analysis and interpretation of data and wrote the manuscript. L.H. and S.F.H. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.M.Z., X.M., R.W., S.D.G, A.J.D. contributed to interpretation of data and helped write the manuscript.
Conflicts of interest disclosures: A.M.Z. has consulted and received honoraria payments from Celgene, Ariad, Incyte and Pfizer. X.M. consulted for Celgene and Incyte. S.F.H. has received honoraria from Pharmacyclics and consulted for Celgene and Janssen. S.D.G. has consulted for and receives research funding from Celgene and Boehringer Ingelheim. S.D.G. has also consulted for Kyowa, Pfizer, and Seattle Genetics. The remaining authors declare no competing financial interests.
References
- 1.Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:434–442. doi: 10.1002/ajh.24272. [DOI] [PubMed] [Google Scholar]
- 2.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 3.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 4.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol. 2008;19:1312–1319. doi: 10.1093/annonc/mdn052. [DOI] [PubMed] [Google Scholar]
- 8.Hahn T, McCarthy PL, Carreras J, et al. Simplified validated prognostic model for progression-free survival after autologous transplantation for hodgkin lymphoma. Biol Blood Marrow Transplant. 2013;19:1740–1744. doi: 10.1016/j.bbmt.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Societe Francaise de Greffe de Moelle. Bone Marrow Transplant. 1997;20:21–26. doi: 10.1038/sj.bmt.1700838. [DOI] [PubMed] [Google Scholar]
- 10.Smith SD, Moskowitz CH, Dean R, et al. Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: results from two transplant centres. Br J Haematol. 2011;153:358–363. doi: 10.1111/j.1365-2141.2011.08616.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24:2430–2434. doi: 10.1093/annonc/mdt206. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 13.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 16.Seattle Genetics Announces FDA Regular Approval of ADCETRIS (Brentuximab Vedotin) for Classical Hodgkin Lymphoma Patients at High Risk of Relapse or Progression as Post-Autologous Hematopoietic Stem Cell Transplantation Consolidation. [accessed May 12, 2017];BusinessWire [online article] 2015 Available from URL: http://www.businesswire.com/news/home/20150817006214/en/Seattle-Genetics-Announces-
- 17.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 18.Bach PB. Indication-specific pricing for cancer drugs. Jama. 2014;312:1629–1630. doi: 10.1001/jama.2014.13235. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Rajkumar SV. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc. 2015;90:500–504. doi: 10.1016/j.mayocp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. Jama. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 21.Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32:101–108. doi: 10.1007/s40273-013-0123-9. [DOI] [PubMed] [Google Scholar]
- 22.Latimer NR. Survival Analysis For Economic Evaluations Alongside Clinical Trials - Extrapolation with Patient-Level Data. London: National Institute for Health and Care Excellence (NICE); 2013. [accessed May 12, 2017]. NICE Decision Support Unit Technical Support Documents. Available from URL: http://www.nicedsu.org.uk/NICE%20DSU%20TSD%20Survival%20analysis.updated%20March%202013.v2.pdf. [PubMed] [Google Scholar]
- 23.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6(2):461–464. [Google Scholar]
- 25.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 26.Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach PB, Conti RM, Muller RJ, Schnorr GC, Saltz LB. Overspending driven by oversized single dose vials of cancer drugs. Bmj. 2016;352:i788. doi: 10.1136/bmj.i788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornberger J, Chien R, Friedmann M, et al. Cost-effectiveness of rituximab as maintenance therapy in patients with follicular non-Hodgkin lymphoma after responding to first-line rituximab plus chemotherapy. Leuk Lymphoma. 2012;53:2371–2377. doi: 10.3109/10428194.2012.694429. [DOI] [PubMed] [Google Scholar]
- 31.Lien K, Cheung MC, Chan KK. Adjusting for Drug Wastage in Economic Evaluations of New Therapies for Hematologic Malignancies: A Systematic Review. J Oncol Pract. 2016;12:e369–379. doi: 10.1200/JOP.2015.005876. [DOI] [PubMed] [Google Scholar]
- 32.Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24:4116–4122. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 33.Khera N, Emmert A, Storer BE, Sandmaier BM, Alyea EP, Lee SJ. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist. 2014;19:639–644. doi: 10.1634/theoncologist.2013-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huntington SF, Svoboda J, Doshi JA. Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol. 2015;33:1467–1474. doi: 10.1200/JCO.2014.58.5729. [DOI] [PubMed] [Google Scholar]
- 35.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DE, Lynn J, Louis TA, Shugarman LR. Medicare program expenditures associated with hospice use. Ann Intern Med. 2004;140:269–277. doi: 10.7326/0003-4819-140-4-200402170-00009. [DOI] [PubMed] [Google Scholar]
- 37.Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 2001;20:188–195. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey SD, Nademanee A, Masszi T, et al. Quality of life results from a phase 3 study of brentuximab vedotin consolidation following autologous haematopoietic stem cell transplant for persons with Hodgkin lymphoma. Br J Haematol. 2016;175(5):860–867. doi: 10.1111/bjh.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swinburn P, Shingler S, Acaster S, Lloyd A, Bonthapally V. Health utilities in relation to treatment response and adverse events in relapsed/refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2015;56:1839–1845. doi: 10.3109/10428194.2014.970542. [DOI] [PubMed] [Google Scholar]
- 40.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147:129–139. doi: 10.1111/j.1365-2141.2009.07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 42.America’s Health Insurane Plans. Ensuring quality through appropriate use of diagnostic imaging. [accessed May 12, 2017];Online publication. 2008 Available from URL: http://www.medsolutions.com/clinical_quality/facts/AHIP%202008%20Imaging%20Stats.pdf.
- 43.Seattle Genetics Adcetris (brentuximab vedotin) [accessed May 12, 2017];Package Insert. Available from URL: http://www.seattlegenetics.com/pdf/adcetris_USPI.pdf.
- 44.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen R, Palmer JM, Tsai NC, et al. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014;20:1864–1868. doi: 10.1016/j.bbmt.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146:158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderlini P, Saliba RM, Ledesma C, et al. Gemcitabine, Fludarabine, and Melphalan for Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation for Relapsed and Refractory Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2016;22:1333–1337. doi: 10.1016/j.bbmt.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moskowitz CH, Paszkiewicz-Kozik E, Nademanee A, et al. Analysis of primary-refractory Hodgkin lymphoma pts in a randomized, placebo-controlled study of brentuximab vedotin consolidation after autologous stem cell transplant [ICML abstract 120] Hematol Oncol. 2015;33(suppl 1):165. [Google Scholar]
- 49.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 50.Mailankody S, Prasad V. Five Years of Cancer Drug Approvals: Innovation, Efficacy, and Costs. JAMA Oncol. 2015;1:539–540. doi: 10.1001/jamaoncol.2015.0373. [DOI] [PubMed] [Google Scholar]
- 51.Kantarjian H, Zwelling L. Cancer drug prices and the free-market forces. Cancer. 2013;119:3903–3905. doi: 10.1002/cncr.28330. [DOI] [PubMed] [Google Scholar]
- 52.Light DW, Kantarjian H. Market spiral pricing of cancer drugs. Cancer. 2013;119:3900–3902. doi: 10.1002/cncr.28321. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33:1112–1118. doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein DA, Ahmad BB, Chen Q, et al. Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. J Clin Oncol. 2015;33:3727–3732. doi: 10.1200/JCO.2015.61.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chhatwal J, Mathisen M, Kantarjian H. Are high drug prices for hematologic malignancies justified? A critical analysis. Cancer. 2015;121:3372–3379. doi: 10.1002/cncr.29512. [DOI] [PubMed] [Google Scholar]
- 56.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533. doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- 59.Kaloyannidis P, Voutiadou G, Baltadakis I, et al. Outcomes of Hodgkin’s lymphoma patients with relapse or progression following autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:451–457. doi: 10.1016/j.bbmt.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 60.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program. 2008:326–333. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.