Abstract
Nutrients that regulate methylation processes may modify susceptibility to the effects of air pollutants. Data from the National Birth Defects Prevention Study (United States, 1997–2006) were used to estimate associations between maternal exposure to nitrogen dioxide (NO2), dietary intake of methyl nutrients, and the odds of congenital heart defects in offspring. NO2 concentrations, a marker of traffic-related air pollution, averaged across postconception weeks 2–8, were assigned to 6,160 nondiabetic mothers of cases and controls using inverse distance-squared weighting of air monitors within 50 km of maternal residences. Intakes of choline, folate, methionine, and vitamins B6 and B12 were assessed using a food frequency questionnaire. Hierarchical regression models, which accounted for similarities across defects, were constructed, and relative excess risks due to interaction were calculated. Relative to women with the lowest NO2 exposure and high methionine intake, women with the highest NO2 exposure and lowest methionine intake had the greatest odds of offspring with a perimembranous ventricular septal defect (odds ratio = 3.23, 95% confidence interval: 1.74, 6.01; relative excess risk due to interaction = 2.15, 95% confidence interval: 0.39, 3.92). Considerable departure from additivity was not observed for other defects. These results provide modest evidence of interaction between nutrition and NO2 exposure during pregnancy.
Keywords: air pollution, birth defects, cardiovascular malformation, methionine, methyl donor, prenatal nutrition, ventricular septal defect
Research on the relationship between exposure to traffic-related air pollution (TRAP) during pregnancy and congenital heart defects (CHDs) in offspring has been inconsistent (1–10). Two recent meta-analyses observed significant associations between nitrogen dioxide (NO2) levels, a marker of TRAP, and coarctation of the aorta (COA) (11, 12). Subsequent studies have reported associations between NO2 exposure and COA as well as pulmonary valve stenosis (9) and an association between nitrogen oxides and ventricular septal defects (10). One of the potential biological pathways through which maternal TRAP exposure could affect cardiogenesis is by the induction of epigenetic changes, including alterations in DNA methylation (13, 14).
Epigenetic factors may be important in the regulation of gene expression during the complex process of cardiogenesis (15). Epidemiologic studies report associations between measures of maternal and fetal DNA hypomethylation and several CHD phenotypes (16–19). Exposure to TRAP such as particulate matter can contribute to lower levels of DNA methylation in adults (20, 21) and to decreased methylation in the placenta of pregnant women exposed in early pregnancy (22). Because TRAP exposure is associated with decreased DNA methylation and maternal DNA methylation status is associated with CHDs, it is plausible that mechanisms related to altered DNA methylation may underlie hypothesized associations between NO2 and CHDs.
Dietary nutrients such as choline, methionine, folate/folic acid, and vitamins B6 and B12 act as methyl donors and are necessary to initiate and regulate DNA methylation processes. These methyl nutrients are important for normal fetal development (23). Periconceptional maternal folate/folic-acid consumption reduces the risk of neural tube defects (24) and may also reduce the risk of CHDs (25). The mechanisms underlying these lower risks are not completely understood, but experimental research suggests that deficiencies in methyl nutrients can lead to changes in DNA methylation status (26).
Studies demonstrate that other aspects of the maternal diet may modify associations between air pollution, adverse birth outcomes, and neurodevelopment (27, 28). Additionally, research has found that intake of dietary methyl nutrients can modify the association between air pollutant exposure and cardiac outcomes in older adult populations (29). This raises the possibility that low dietary intake of methyl nutrients may modify maternal/fetal susceptibility to air pollutants, such as NO2 (13).
The goal of this analysis was to build upon a previous analysis of the National Birth Defects Prevention Study (NBDPS) (9) and estimate the joint association of exposure to NO2 and dietary intake of methyl nutrients with the odds of CHDs.
METHODS
The NBDPS is a population-based case-control study of over 30 structural defects. Cases were live births, stillbirths greater than 20 weeks’ gestation, and elective terminations with major structural birth defects, recruited from birth defects monitoring programs of 9 sites within the United States (Arkansas, California, Iowa, Massachusetts, Metropolitan Atlanta, New York, North Carolina, Texas, and Utah). Cases with recognized single-gene disorders or chromosomal abnormalities were not included in the study. Controls were live births without major birth defects that were randomly selected from birth certificates or hospital records, depending upon the site. Cases and controls were not matched. CHD cases were classified by the presence (or absence) of extracardiac defects and by the number of CHDs present within the infant/fetus, according to standardized NBDPS criteria (30). More detailed descriptions of the NBDPS design have been published previously (9, 31).
CHD phenotype homogeneity is an important component in understanding the heterogeneity in risk-factor profiles for different types of cardiovascular malformations. Therefore, cases were restricted to those with a single CHD and no extracardiac defects. As part of NBDPS protocol, CHD phenotypes were also classified into broader defect groupings that shared anatomic and developmental characteristics (30). The CHD phenotypes examined in this study, listed by their defect groupings, were the following: septals (atrial septal defect, perimembranous ventricular septal defect (VSDpm)), left-ventricular outflow-tract obstructions (COA, hypoplastic left heart syndrome, aortic stenosis), right-ventricular outflow-tract obstructions (pulmonary valve stenosis, pulmonary/tricuspid atresia), conotruncal defects (dextro-transposition of the great arteries, tetralogy of Fallot, other conotruncal defects (common truncus, interrupted aortic arch of type B and not otherwise specified, double outlet right ventricle, and conoventricular septal defects)), atrioventricular septal defect, and total anomalous pulmonary venous return.
Controls and cases had an estimated date of delivery from October 1, 1997, when the study began, through December 31, 2006. Mothers had a recorded residential history during pregnancy, lived within 50 km of a NO2 stationary air monitor, and responded to the interview questions regarding dietary intake before pregnancy. Women with pregestational diabetes were excluded from this analysis due to the strong association between maternal diabetes and CHDs in offspring (32).
NO2 was selected as the exposure of interest because it is often used as a marker of TRAP (33), and prior research showed the most consistent associations with CHDs and NO2 (11). Exposure to NO2 was assigned using inverse distance-squared weighting (34). Daily maximum hourly concentrations of NO2 from up to 4 stationary air monitors within 50 km of the maternal residence were weighted by the inverse of the squared distance to the monitor and were then averaged to obtain a single daily estimate. If a monitor had more than 50% missing data, it was excluded, and the next closest monitor within 50 km was used in the exposure calculation. Exposure for women who lived within 5 km of at least 1 monitor (18.8%) was calculated by averaging over monitors only within the 5-km buffer, under the assumption that monitors within this short distance were more representative. If a woman had more than 1 residential address, exposures were assigned using the monitors closest to the residence that corresponded to the relevant day of pregnancy. Weighted daily estimates were averaged across weeks 2–8 of pregnancy to construct a single measure of exposure within the critical window of cardiac development (35). Exposure was categorized using the distribution of NO2 concentration among the controls into the following categories: less than the 10th percentile (referent), 10th percentile to the median, median to the 90th percentile, and greater than or equal to the 90th percentile. These categories captured the departure from linearity observed in initial analyses (9).
Dietary intake of methyl nutrients was assessed using a 58-item version of the Willett food frequency questionnaire with additional questions on fortified foods such as cereals and certain beverages (36). Women were asked about their dietary intake in the year before pregnancy and that was used as a proxy for dietary intake at the time of conception through the time cardiac structures develop (weeks 3–8 after conception) (35). Asking about diet in the year prior to pregnancy can avoid the potential bias that could occur if characteristics of the pregnancy (e.g., morning sickness) caused changes in a woman's diet, as well as capturing dietary intake at the very early stages of pregnancy, including the time prior to becoming aware of the pregnancy. Intake of the following nutrients was dichotomized at the 25th centile among controls to indicate relatively low intake: choline, methionine, dietary folate equivalents, vitamin B6, and vitamin B12. Dietary folate equivalents are a calculated combination of naturally occurring folate in foods and folic-acid supplementation of foods, such as grains (37).
Potential confounders were identified through review of the literature and directed acyclic graph analysis (38). The following variables obtained from the maternal interview were included in the final adjustment set: maternal age, race/ethnicity, educational attainment, household income, any maternal cigarette smoking in the first 3 months of pregnancy, any alcohol consumption during the first 3 months of pregnancy, and use of folic acid–containing supplements in the month prior to conception. Final models adjusted for the center-specific ratio of septal defects to total CHDs, because identification of septal defects is more vulnerable to differences in case ascertainment methods than other CHDs (39).
Two-stage hierarchical regression models were constructed to obtain adjusted odds ratios and 95% confidence intervals for the joint association of NO2 and dietary intake of methyl nutrients with CHD occurrence (40, 41). Separate models were created for each methyl nutrient. Hierarchical regression allows for the incorporation of prior knowledge about similarities between CHDs and adjusts coefficients from conventional regression models using information shared across those estimated associations. Estimates resulting from the second-stage model may be less sensitive to sampling error and model misspecification than those from a single-stage logistic regression model (41).
The first stage was a multinomial logistic regression model, adjusting for the confounders listed previously and containing 7 indicator variables corresponding to the combinations between the 4 levels of NO2 exposure and 2 levels of nutrient intake. The second stage model regressed the coefficients from the first-stage model on an 84 × 21 matrix composed of an intercept term and indicator variables for the level of NO2 exposure, high/low nutrient intake, type of defect, and the broader defect grouping. Additional details on the hierarchical regression model are provided in Web Appendix 1 (available at https://academic.oup.com/aje). We fixed the second-stage model variance at 0.5, corresponding to a prior belief with 95% certainty that the residual odds ratio will fall within a 16-fold span. This relatively larger variance was used due to the crude nature of information contained in the second-stage design matrix. We assessed how changes in model specification would influence our results by setting the value of the second-stage model variance to 0.25 (a 7-fold span in the residual odds ratio (OR)) and then setting it to a value of 0.83 (26-fold span).
Interactions on the additive scale between NO2 exposure and low intake of methyl nutrients were formally assessed using the relative excess risk due to interaction (RERI). Corresponding 95% confidence intervals were calculated based on Wald-type statistics using approximate variance estimators (42, 43). The RERIs compared women who had the highest levels of NO2 exposure and low dietary intake of each nutrient with women who had the lowest level of NO2 exposure and high dietary intake of each nutrient. Previous research suggests that deficiencies in one methyl nutrient can be compensated for by intake of the other methyl nutrients (44). A summary variable, constructed to indicate whether a woman had dietary intake less than the 25th centile for at least 1 of the 5 nutrients assessed, was used in a multinomial logistic regression model to assess the association between CHDs and having low intake of at least 1 nutrient in the context of higher intake of the other nutrients. To examine whether other maternal diet factors confounded our results, maternal obesity and total energy intake (in kilocalories) were assessed as potential confounders in subsequent models. All analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina) (45). The NBDPS was approved by the institutional review boards of all participating centers, and all participants provided informed consent prior to participation.
RESULTS
Of the nondiabetic NBDPS eligible cases and controls (4,844 cases and 7,056 controls), over 90% provided at least 1 geocoded residential address, and 63% of those participants (2,727 cases and 4,069 controls) lived within 50 km of at least 1 NO2 monitor. These women had complete data on diet during pregnancy. Women who had missing data on at least 1 confounder were excluded from the final models (9%; 223 cases and 413 controls). The demographic characteristics of the final analytical population (n = 2,504 cases, 3,656 controls) are similar to the full NBDPS population, although participants from the Arkansas and Iowa study sites were less likely to live near an NO2 monitor than were participants from other sites. A majority of the analytical population was white, had at least a high school education, and did not use tobacco or alcohol during early pregnancy, but the percentages varied by CHD phenotype (Table 1). For most case groups and controls, approximately one-third of women reported taking folic-acid supplements in the month prior to conception. Cases had similar or higher percentages of women with lower intakes of methyl nutrients than controls (Table 2). Spearman correlation coefficients between continuous nutrient intakes ranged from moderate (dietary folate equivalents and methionine: 0.48) to very strong (methionine and choline: 0.91).
Table 1.
Maternal Characteristics of Cases and Controls, National Birth Defects Prevention Study, United States, 1997–2006
| Maternal Characteristics | Controls (n = 3,656), % | Cases, According to Congenital Heart Defect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD (n = 387), % | VSDpm (n = 437), % | AVSD (n = 50), % | Aortic Stenosis (n = 103), % | COA (n = 203), % | HLHS (n = 201), % | PVS (n = 305), % | Atresia (n = 82), % | TOF (n = 341), % | dTGA (n = 225), % | Other Conotruncal (n = 93), % | TAPVR (n = 77), % | ||
| Age, yearsa | 27.6 (6.0) | 27.9 (6.7) | 28.4 (6.3) | 28.6 (4.8) | 28.3 (5.3) | 28.8 (5.8) | 28.1 (5.6) | 28.3 (5.6) | 28.2 (6.2) | 29.1 (5.7) | 28.0 (6.3) | 27.8 (5.8) | 27.8 (6.9) |
| Race/ethnicity | |||||||||||||
| White, non-Latina | 57.5 | 56.8 | 52.2 | 76.0 | 83.5 | 68.0 | 63.2 | 66.2 | 61.0 | 58.1 | 66.7 | 54.8 | 62.3 |
| Black, non-Latina | 12.6 | 16.3 | 19.7 | 14.0 | 0.0 | 5.4 | 12.4 | 16.1 | 8.5 | 14.1 | 4.9 | 12.9 | 3.9 |
| Latina | 22.6 | 20.9 | 20.4 | 4.0 | 12.6 | 21.7 | 19.9 | 12.8 | 24.4 | 19.9 | 21.8 | 22.6 | 23.4 |
| Asian/Pacific Islander | 3.7 | 2.8 | 5.5 | 4.0 | 1.9 | 2.5 | 3.0 | 1.3 | 3.7 | 4.4 | 2.7 | 6.5 | 6.5 |
| Other | 3.5 | 3.1 | 2.3 | 2.0 | 1.9 | 2.5 | 1.5 | 3.6 | 2.4 | 3.5 | 4.0 | 3.2 | 3.9 |
| Educational attainment | |||||||||||||
| 0–11 years education | 14.7 | 17.6 | 14.4 | 8.0 | 4.9 | 13.3 | 14.4 | 9.8 | 11.0 | 12.6 | 14.2 | 21.5 | 11.7 |
| Completed high school/GED | 21.3 | 21.7 | 18.5 | 20.0 | 22.3 | 16.7 | 28.4 | 23.0 | 30.5 | 19.4 | 25.3 | 21.5 | 20.8 |
| Some college/trade school | 27.6 | 30.7 | 28.8 | 32.0 | 33.0 | 26.1 | 22.4 | 29.2 | 23.2 | 29.0 | 26.2 | 21.5 | 29.9 |
| Bachelor's degree or more | 36.4 | 30.0 | 38.2 | 40.0 | 39.8 | 43.8 | 34.8 | 38.0 | 35.4 | 39.0 | 34.2 | 35.5 | 37.7 |
| Household income, $ | |||||||||||||
| <10,000 | 17.1 | 19.4 | 14.9 | 12.0 | 10.7 | 9.9 | 13.4 | 14.8 | 18.3 | 16.1 | 17.3 | 15.1 | 19.5 |
| 10,000–50,000 | 43.3 | 43.9 | 45.5 | 44.0 | 41.7 | 44.3 | 45.3 | 40.7 | 40.2 | 37.2 | 41.8 | 45.2 | 42.9 |
| >50,000 | 39.6 | 36.7 | 39.6 | 44.0 | 47.6 | 45.8 | 41.3 | 44.6 | 41.5 | 46.6 | 40.9 | 39.8 | 37.7 |
| Alcohol consumptionb | |||||||||||||
| None | 61.6 | 63.8 | 64.1 | 58.0 | 67.0 | 67.5 | 66.2 | 64.3 | 57.3 | 56.9 | 63.1 | 62.4 | 67.5 |
| Yes, no binge drinking | 11.4 | 12.4 | 11.0 | 18.0 | 11.7 | 8.4 | 13.4 | 10.5 | 13.4 | 13.2 | 13.3 | 7.5 | 7.8 |
| Yes, and binge drinking | 27.0 | 23.8 | 24.9 | 24.0 | 21.4 | 24.1 | 20.4 | 25.2 | 29.3 | 29.9 | 23.6 | 30.1 | 24.7 |
| Tobacco useb | |||||||||||||
| Yes | 13.4 | 21.2 | 14.2 | 24.0 | 9.7 | 10.3 | 12.9 | 12.5 | 18.3 | 11.7 | 14.2 | 11.8 | 13.0 |
| Use of folic-acid supplementc | |||||||||||||
| Yes | 36.8 | 29.7 | 36.8 | 48.0 | 43.7 | 40.4 | 36.3 | 36.4 | 36.6 | 41.6 | 39.6 | 31.2 | 33.8 |
Abbreviations: ASD, atrial septal defect; AVSD, atrioventricular septal defect; COA, coarctation of the aorta; dTGA, dextro-transposition of the great arteries; GED, General Educational Development; HLHS, hypoplastic left heart syndrome; PVS, pulmonary valve stenosis; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; VSDpm, perimembranous ventricular septal defect.
a Expressed as mean (standard deviation).
b Time period of exposure was during the first 3 months of pregnancy.
c Time period of exposure was during the month prior to conception.
Table 2.
Nitrogen Dioxide Exposure Distributions and Prevalence of Low Nutrient Intake Among Cases and Controls, National Birth Defects Prevention Study, United States, 1997–2006
| Percentiles of Exposure and Intake | Controls (n = 3,656), % | Cases, According to Congenital Heart Defect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD (n = 387), % | VSDpm (n = 437), % | AVSD (n = 50), % | Aortic Stenosis (n = 103), % | COA (n = 203), % | HLHS (n = 201), % | PVS (n = 305), % | Atresia (n = 82), % | TOF (n = 341), % | dTGA (n = 225), % | Other Conotruncal (n = 93), % | TAPVR (n = 77), % | ||
| NO2 and methionine | |||||||||||||
| <10th and ≥25th | 7.8 | 7.5 | 5.9 | 8.0 | 6.8 | 3.0 | 6.0 | 6.2 | 8.5 | 6.2 | 5.3 | 6.5 | 10.4 |
| <10th and <25th | 2.5 | 2.1 | 1.6 | 2.0 | 5.8 | 3.0 | 2.5 | 2.3 | 2.4 | 2.9 | 4.0 | 2.2 | 5.2 |
| 10th–50th and ≥25th | 30.0 | 33.3 | 29.7 | 30.0 | 27.2 | 22.2 | 28.9 | 25.9 | 32.9 | 31.7 | 28.0 | 28.0 | 28.6 |
| 10th–50th and <25th | 9.6 | 10.6 | 11.9 | 12.0 | 8.7 | 13.3 | 13.4 | 11.8 | 12.2 | 9.7 | 10.7 | 10.8 | 5.2 |
| 50th–90th and ≥25th | 30.0 | 27.1 | 28.6 | 26.0 | 32.0 | 34.5 | 31.3 | 29.2 | 23.2 | 27.0 | 32.4 | 26.9 | 28.6 |
| 50th–90th and <25th | 10.5 | 10.9 | 11.7 | 12.0 | 6.8 | 11.8 | 10.4 | 11.8 | 9.8 | 12.9 | 7.1 | 16.1 | 10.4 |
| ≥90th and ≥25th | 7.5 | 5.9 | 5.7 | 6.0 | 11.7 | 8.4 | 6.5 | 8.5 | 9.8 | 7.0 | 11.1 | 7.5 | 10.4 |
| ≥90th and <25th | 2.1 | 2.6 | 4.8 | 4.0 | 1.0 | 3.9 | 1.0 | 4.3 | 1.2 | 2.6 | 1.3 | 2.2 | 1.3 |
| NO2 and choline | |||||||||||||
| <10th and ≥25th | 7.3 | 7.2 | 5.3 | 6.0 | 6.8 | 3.0 | 7.0 | 6.2 | 8.5 | 6.2 | 4.4 | 7.5 | 11.7 |
| <10th and <25th | 2.9 | 2.3 | 2.3 | 4.0 | 5.8 | 3.0 | 1.5 | 2.3 | 2.4 | 2.9 | 4.9 | 1.1 | 3.9 |
| 10th–50th and ≥25th | 29.9 | 33.6 | 29.3 | 26.0 | 26.2 | 22.7 | 27.9 | 27.5 | 30.5 | 31.1 | 24.4 | 30.1 | 27.3 |
| 10th–50th and <25th | 9.7 | 10.3 | 12.4 | 16.0 | 9.7 | 12.8 | 14.4 | 10.2 | 14.6 | 10.3 | 14.2 | 8.6 | 6.5 |
| 50th–90th and ≥25th | 30.5 | 27.4 | 30.4 | 28.0 | 31.1 | 34.0 | 30.3 | 29.8 | 25.6 | 29.3 | 31.6 | 30.1 | 27.3 |
| 50th–90th and <25th | 10.0 | 10.6 | 9.8 | 10.0 | 7.8 | 12.3 | 11.4 | 11.1 | 7.3 | 10.6 | 8.0 | 12.9 | 11.7 |
| ≥90th and ≥25th | 7.4 | 6.5 | 6.2 | 8.0 | 10.7 | 7.9 | 6.0 | 9.8 | 9.8 | 7.3 | 10.7 | 6.5 | 10.4 |
| ≥90th and <25th | 2.2 | 2.1 | 4.3 | 2.0 | 1.9 | 4.4 | 1.5 | 3.0 | 1.2 | 2.3 | 1.8 | 3.2 | 1.3 |
| NO2 and dietary folate | |||||||||||||
| <10th and ≥25th | 7.5 | 8.0 | 6.2 | 4.0 | 7.8 | 3.0 | 8.0 | 6.9 | 8.5 | 5.9 | 4.9 | 7.5 | 9.1 |
| <10th and <25th | 2.7 | 1.6 | 1.4 | 6.0 | 4.9 | 3.0 | 0.5 | 1.6 | 2.4 | 3.2 | 4.4 | 1.1 | 6.5 |
| 10th–50th and ≥25th | 29.4 | 32.6 | 30.4 | 22.0 | 23.3 | 22.2 | 31.8 | 27.5 | 31.7 | 30.8 | 27.6 | 30.1 | 26.0 |
| 10th–50th and <25th | 10.2 | 11.4 | 11.2 | 20.0 | 12.6 | 13.3 | 10.4 | 10.2 | 13.4 | 10.6 | 11.1 | 8.6 | 7.8 |
| 50th–90th and ≥25th | 30.9 | 28.9 | 29.3 | 22.0 | 28.2 | 34.0 | 28.4 | 28.9 | 25.6 | 29.9 | 30.7 | 31.2 | 27.3 |
| 50th–90th and <25th | 9.5 | 9.0 | 11.0 | 16.0 | 10.7 | 12.3 | 13.4 | 12.1 | 7.3 | 10.0 | 8.9 | 11.8 | 11.7 |
| ≥90th and ≥25th | 7.2 | 6.2 | 7.6 | 8.0 | 8.7 | 9.4 | 6.5 | 8.5 | 8.5 | 7.9 | 9.3 | 4.3 | 7.8 |
| ≥90th and <25th | 2.4 | 2.3 | 3.0 | 2.0 | 3.9 | 3.0 | 1.0 | 4.3 | 2.4 | 1.8 | 3.1 | 5.4 | 3.9 |
| NO2 and vitamin B6 | |||||||||||||
| <10th and ≥25th | 7.5 | 8.0 | 6.9 | 8.0 | 8.7 | 3.0 | 7.0 | 6.6 | 8.5 | 6.5 | 6.2 | 6.5 | 11.7 |
| <10th and <25th | 2.7 | 1.6 | 0.7 | 2.0 | 3.9 | 3.0 | 1.5 | 2.0 | 2.4 | 2.6 | 3.1 | 2.2 | 3.9 |
| 10th–50th and ≥25th | 29.5 | 33.1 | 29.5 | 22.0 | 23.3 | 23.6 | 31.3 | 26.6 | 31.7 | 29.6 | 25.8 | 31.2 | 27.3 |
| 10th–50th and <25th | 10.1 | 10.9 | 12.1 | 20.0 | 12.6 | 11.8 | 10.9 | 11.1 | 13.4 | 11.7 | 12.9 | 7.5 | 6.5 |
| 50th–90th and ≥25th | 30.4 | 29.2 | 28.1 | 20.0 | 29.1 | 34.0 | 28.9 | 29.5 | 25.6 | 27.6 | 30.2 | 29.0 | 26.0 |
| 50th–90th and <25th | 10.1 | 8.8 | 12.1 | 18.0 | 9.7 | 12.3 | 12.9 | 11.5 | 7.3 | 12.3 | 9.3 | 14.0 | 13.0 |
| NO2 and vitamin B6 (continued) | |||||||||||||
| ≥90th and ≥25th | 7.3 | 5.7 | 7.3 | 6.0 | 9.7 | 7.9 | 6.0 | 9.5 | 9.8 | 7.6 | 10.7 | 4.3 | 9.1 |
| ≥90th and <25th | 2.4 | 2.8 | 3.2 | 4.0 | 2.9 | 4.4 | 1.5 | 3.3 | 1.2 | 2.1 | 1.8 | 5.4 | 2.6 |
| NO2 and vitamin B12 | |||||||||||||
| <10th and ≥25th | 7.7 | 7.8 | 5.9 | 6.0 | 8.7 | 2.5 | 6.0 | 6.2 | 8.5 | 5.9 | 7.6 | 7.5 | 6.5 |
| <10th and <25th | 2.6 | 1.8 | 1.6 | 4.0 | 3.9 | 3.4 | 2.5 | 2.3 | 2.4 | 3.2 | 1.8 | 1.1 | 9.1 |
| 10th–50th and ≥25th | 29.4 | 33.3 | 28.8 | 26.0 | 27.2 | 23.6 | 28.9 | 27.5 | 34.1 | 28.2 | 27.1 | 31.2 | 24.7 |
| 10th–50th and <25th | 10.2 | 10.6 | 12.8 | 16.0 | 8.7 | 11.8 | 13.4 | 10.2 | 11.0 | 13.2 | 11.6 | 7.5 | 9.1 |
| 50th–90th and ≥25th | 30.5 | 27.1 | 29.1 | 28.0 | 27.2 | 32.5 | 27.4 | 27.2 | 26.8 | 28.7 | 30.2 | 30.1 | 20.8 |
| 50th–90th and <25th | 10.0 | 10.9 | 11.2 | 10.0 | 11.7 | 13.8 | 14.4 | 13.8 | 6.1 | 11.1 | 9.3 | 12.9 | 18.2 |
| ≥90th and ≥25th | 7.2 | 5.9 | 7.8 | 8.0 | 10.7 | 8.4 | 6.5 | 9.5 | 9.8 | 7.0 | 9.8 | 5.4 | 9.1 |
| ≥90th and <25th | 2.4 | 2.6 | 2.7 | 2.0 | 1.9 | 3.9 | 1.0 | 3.3 | 1.2 | 2.6 | 2.7 | 4.3 | 2.6 |
Abbreviations: ASD, atrial septal defect; AVSD, atrioventricular septal defect; COA, coarctation of the aorta; dTGA, dextro-transposition of the great arteries; HLHS, hypoplastic left heart syndrome; NO2, nitrogen dioxide; PVS, pulmonary valve stenosis; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; VSDpm, perimembranous ventricular septal defect.
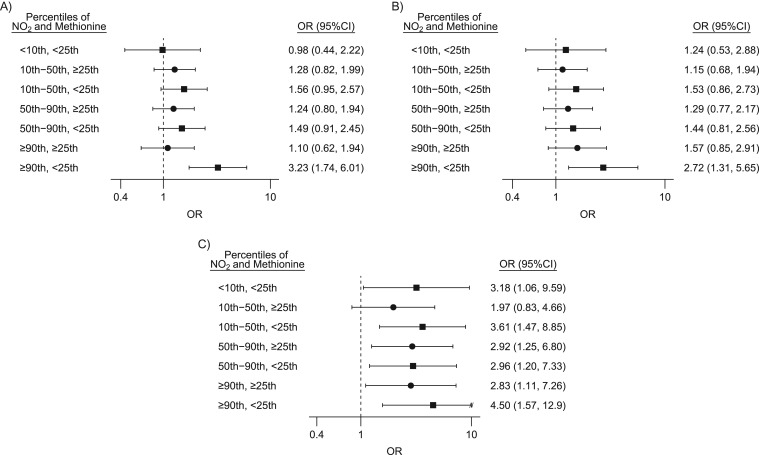
Three CHD phenotypes were found to be associated with greater exposure to NO2 and lower intake of methyl nutrients: VSDpm, PVS, and COA. Figure 1 shows the adjusted odds ratios and 95% confidence intervals from the 2-stage hierarchical regression to estimate the joint association of NO2 and methionine for these 3 phenotypes. Complete results for all CHDs and nutrients can be found in Web Figure 1 and Web Table 1 for corresponding numerical data. Crude odds ratios showed similar patterns, although slightly smaller in magnitude than fully adjusted estimates. Incorporating the second-stage model brought estimates closer to the null and slightly reduced the width of the confidence intervals but did not alter interpretations (data not shown). Women exposed to the highest levels of NO2 with low dietary intake of methionine had considerably elevated odds of offspring with VSDpm when compared with those who had low NO2 exposure and high methionine intake (OR = 3.23 95% confidence interval (CI): 1.74, 6.01; Figure 1A). Similar results were observed for choline (OR = 2.86, 95% CI: 1.50, 5.45). A pattern of elevated odds for PVS in offspring among women exposed to high NO2 and low methionine intake was also observed (Figure 1B). For analyses of both VSDpm and PVS, the largest odds ratios were observed when examining high NO2 and low methionine, with smaller effect estimates observed when examining other nutrients. Results for COA were different (Figure 1C); for all nutrients examined, women with all combinations of NO2 exposure and dietary intake consistently had greater odds of having offspring with COA when compared with women at the lowest level of NO2 exposure and high nutrient intake. Adjustment for total energy intake and maternal obesity did not considerably change estimates (i.e., less than a 10% change in estimate) and were not included in final models.
Figure 1.
Estimated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) between selected congenital heart defects and categories of nitrogen dioxide (NO2) exposure and dietary intake of methionine, National Birth Defects Prevention Study, United States, 1997–2006. For perimembranous ventricular septal defect (A), pulmonary valve stenosis (B), and coarctation of the aorta (C). Reference group for all comparisons is NO2 exposure less than the 10th centile and methionine intake at or greater than the 25th centile (high nutrient). The models adjusted for maternal race/ethnicity, age, education, household income, tobacco and alcohol use during pregnancy, use of folic-acid supplements 1 month prior to conception, and site-specific ratio of septal cases to total congenital heart defect cases. Filled circles correspond to ORs calculated for populations with methionine intake greater than the 25th centile, and filled squares correspond to ORs calculated for populations with methionine intake less than the 25th centile. “//” indicates truncation of the confidence interval.
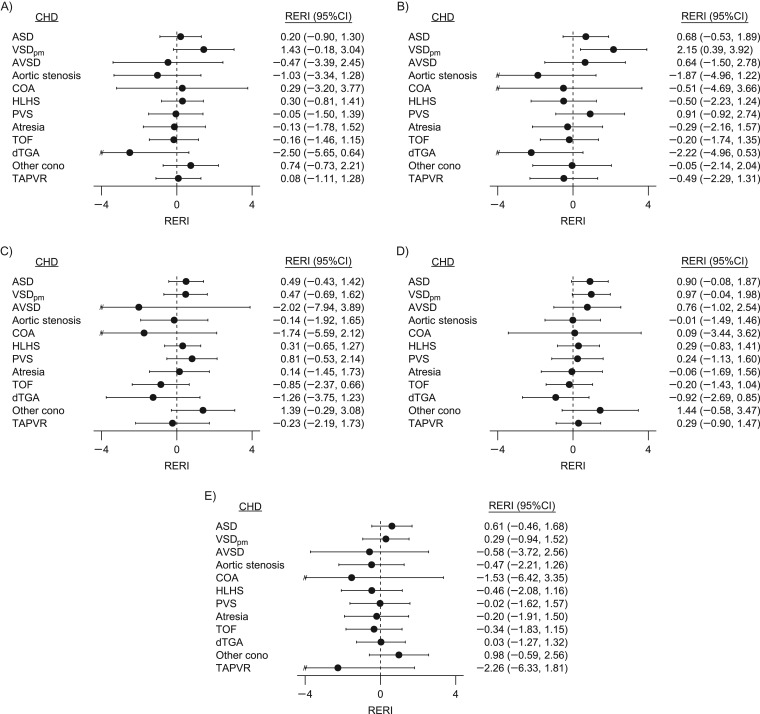
RERIs and corresponding 95% confidence intervals comparing the joint effect estimates of the highest level of NO2 exposure and low nutrient intake with low NO2 exposure and high nutrient intake for each CHD are plotted in Figure 2. The quantitative estimates, formatted into a heat map for identification of patterns, are available in Web Figure 2. Because many of the CHDs are not associated with greater exposure to NO2, the majority of results do not demonstrate evidence of departures from additivity. Greater than additive interaction was observed between NO2 exposure and methionine, choline, and vitamin B6 for VSDpm (Figure 2A, 2B, and 2D). Moderate evidence of greater than additive interaction was also observed for the category of other conotruncal defects. However, the RERI calculations were based on estimates with wide confidence intervals and do not clearly show elevated odds of “other conotruncals” among women exposed to greater levels of NO2 exposure and low dietary intake of dietary folate equivalents or vitamin B6 (Figure 2C and 2D).The largest negative RERIs were calculated for dextro-transposition of the great arteries, indicating the joint association was less than expected from summing individual associations. Large negative RERIs were also observed for COA, atrioventricular septal defects, and total anomalous pulmonary venous return, but the confidence intervals were wide and results were not consistent across nutrients.
Figure 2.
Relative excess risk due to interaction (RERI) between maternal nitrogen dioxide (NO2) exposure and methyl nutrient intake, National Birth Defects Prevention Study, United States, 1997–2006. For choline (A), methionine (B), dietary folate equivalents (C), vitamin B6 (D), and vitamin B12 (E). RERIs were calculated by comparing odds ratios of women with NO2 exposure greater than or equal to the 90th centile and nutrient intake less than the 25th centile, women with NO2 exposure greater than or equal to the 90th centile and nutrient intake greater than or equal to the 25th centile, and women with NO2 exposure less than the 10th centile and nutrient intake greater than or equal to the 25th centile (referent: women with NO2 exposure less than the 10th centile and nutrient intake greater than or equal to the 25th centile of nutrient intake). Confidence intervals (CIs) were calculated based on Wald-type statistics, as proposed by Hosmer and Lemeshow (42). ASD, atrial septal defect; AVSD, atrioventricular septal defect; CHD, congenital heart defect; COA, coarctation of the aorta; dTGA, dextro-transposition of the great arteries; HLHS, hypoplastic left heart syndrome; other cono, other conotruncal defects; PVS, pulmonary valve stenosis; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of Fallot; VSDpm, perimembranous ventricular septal defect. “//” indicates truncation of the confidence interval.
For analyses stratified by folic-acid supplement use, small sample size contributed to wide confidence intervals and unstable estimates (Web Table 2). Similar patterns to the main analysis were observed, including evidence of greater than additive interaction between methionine intake and NO2 exposure on the odds of VSDpm. The magnitude of these effect estimates was greater within the strata without folic-acid supplement use. Evidence of less than additive interaction was also observed between nutrient intake and NO2 exposure on the odds of dextro-transposition of the great arteries. Analysis of the summary measure of nutrient intake also provided evidence of greater than additive interaction for VSDpm and less than additive interaction for dextro-transposition of the great arteries, although effect estimates were smaller in magnitude than when examining the nutrients individually (Web Table 3). Our results were robust to varying the second-stage variance parameter (Web Table 4), with slight changes in magnitude and precision of individual estimates but no change in overall study conclusions.
DISCUSSION
The goal of this study was to investigate a biologically plausible hypothesis: that nutrients that help regulate methylation processes may affect maternal/fetal susceptibility to air pollutants. Support for this hypothesis was provided by results that showed greater than additive interaction of methionine, choline, and to a lesser extent vitamin B6 with NO2 exposure in increasing the odds of VSDpm. Analysis of dextro-transposition of the great arteries showed evidence of less than additive interaction. The majority of nutrient-pollutant-CHD combinations did not provide evidence of interaction, likely due to the lack of an overall association with greater NO2 exposure. In conjunction with the broader literature observing interactions between diet and TRAP in different populations (27, 28), these findings provide support for additional research investigating interactions between nutrient intake and environmental exposures during pregnancy.
The present study does not have measures of DNA methylation to directly investigate the role it plays, if any, within the observed associations between NO2, methyl nutrients, and VSDpm. Previous work exploring methylation patterns in cardiac tissue from fetuses with VSD (vs. controls) showed hypomethylation of 2 apoptosis-related genes (SIVA1 and MDM2) among only the VSD samples (46). However, there were also genes that were hypermethylated in case samples. Methionine and choline have common dietary sources, and it is possible that other nutrients within these dietary sources are confounding these associations. For example, fish also contain n-3 fatty acids, which could counteract the inflammation and oxidative stress caused by TRAP, such as NO2 (27).
Given the large number of comparisons made, in conjunction with the majority of analyses failing to provide evidence of departures from additive interaction, random variation cannot be ruled out as an explanation for the study findings. The analytical approach of hierarchical regression partially addresses multiple comparisons and improved estimation by accounting for similarities between the estimated associations (41). We also observed a greater magnitude of the association for the relationship between NO2 and low methionine within the stratum of women with no folic-acid supplement use in the month prior to conception. These preliminary results support further investigation of these relationships. However, there are potential sources of error and misclassification in exposure assessment for both NO2 and maternal intake of methyl nutrients that warrant consideration when interpreting the results of this study.
Use of central-site monitoring data within a 50-km radius for an exposure surrogate in lieu of personal exposure data is likely to lead to exposure error. Land covering a 50-km radius can vary substantially with respect to land use (e.g., urban vs. rural) and source mixtures. Differences in land use, climate, and other factors across the different study sites have the potential to influence the representativeness of the central site monitors over the 50-km radius. Time spent away from the residential location was also not captured. Exposure error is expected to be nondifferential with respect to case/control status, potentially attenuating the reported estimates towards the null. Additionally, the use of daily maxima, having complete residential history during pregnancy, and categorization of the NO2 concentrations used for exposure surrogates may have also reduced the probability and/or magnitude of exposure error.
It is difficult to disentangle the associations between NO2 and health outcomes from associations with other air pollutants through an epidemiologic study design. NO2 concentrations have been correlated with concentrations of other TRAP pollutants, such as particulate matter and carbon monoxide (47–50). Hence, it is possible that the measured NO2 concentration at a central site monitor is serving as a surrogate for the TRAP mixture rather than acting as an individual agent.
There are well-established limitations of dietary assessment through food frequency questionnaires (51). The food frequency questionnaire upon which the NBDPS is based has been validated previously in other populations (36). However, there is still the possibility of measurement error from using a food frequency questionnaire to assign relative intake of the methyl nutrients. Additionally, the dietary assessment focused on the year prior to pregnancy as a proxy for diet in the very early stages of conception and pregnancy. Previous research suggests that general dietary patterns in women of childbearing age are relatively stable prior to pregnancy and in early pregnancy (52), but differences could exist for specific foods that affect methyl nutrient intake. The inability to adjust for supplements and/or herbal products containing methyl nutrients but not folic acid could contribute to residual confounding. Additionally, this work does not account for genetic differences in individual ability to metabolize methyl nutrients. There could be genetically defined subsets of the population with varying levels of susceptibility to the interaction between air pollutant exposure and intake of methyl nutrients.
The NBDPS has a systematic classification scheme for CHDs (30), ensuring relatively homogeneous case groups and reducing the potential for outcome misclassification. Only women living within 50 km of an air monitor were included in the analysis. The lack of a strong association between demographics that contribute to residential location and CHDs in offspring minimizes the risk of selection bias due to excluding populations living more than 50 km from a monitor. However, our results may not be generalizable to women living in rural areas, farther from an air monitor.
In summary, this analysis of the NBDPS provides modest evidence of interaction between NO2 exposure and dietary intake of methyl nutrients, particularly methionine and choline, and VSDpm in offspring. Future research should address the methodological limitations of this initial study, including using more detailed dietary assessments and/or biomarkers of methyl nutrients as well as accounting for time spent at other locations when determining ambient air pollution exposure. Investigating the potential interactions between maternal nutrition and environmental exposures during pregnancy increases our understanding of the complex causes of CHDs and can potentially lead to interventions aimed at prevention. There is also a need for research that directly explores the hypothesis that disruptions to DNA methylation underlie the association between air pollutant exposure and CHDs.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, New York (Jeanette A. Stingone); National Center for Environmental Assessment, Office of Research and Development, US Environmental Protection Agency, Research Triangle Park, North Carolina (Thomas J. Luben, Jennifer Richmond-Bryant); Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, California (Suzan L. Carmichael, Gary M. Shaw); Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, North Carolina (Arthur S. Aylsworth); Department of Genetics, University of North Carolina School of Medicine, Chapel Hill, North Carolina (Arthur S. Aylsworth); Division of Medical Genetics, Department of Pediatrics, University of Utah, Salt Lake City, Utah (Lorenzo D. Botto); Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi (Adolfo Correa); Division of Medical Genetics, Department of Pediatrics, University of Mississippi Medical Center, Jackson, Mississippi (Adolfo Correa); Division of Congenital and Developmental Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia (Suzanne M. Gilboa); Birth Defects Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas (Peter H. Langlois); Arkansas Center for Birth Defects Research and Prevention, Department of Pediatrics, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, Arkansas (Wendy N. Nembhard); and Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, (Andrew F. Olshan).
This study was supported in part through cooperative agreements from the Centers for Disease Control and Prevention with the centers participating in the National Birth Defects Prevention Study (including cooperative agreement 5U01DD001036) and by the National Institute of Environmental Health Sciences (grant T32ES007018). Additionally, J.A.S. was supported by the Elizabeth Mascia Scholarship from the Mount Sinai Children's Environmental Health Center.
We thank all of the participating study centers, including the California Department of Public Health, Maternal Child and Adolescent Health Division, for providing data on study subjects for the National Birth Defects Prevention Study.
This work was presented as an oral presentation at the 47th Annual Meeting of the Society for Epidemiologic Research, June 24–27, 2014, Seattle, Washington.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the California Department of Public Health, the Massachusetts Department of Public Health, the Centers for Disease Control and Prevention, or the Environmental Protection Agency.
Conflict of interest: none declared.
REFERENCES
- 1. Ritz B, Yu F, Fruin S, et al. . Ambient air pollution and risk of birth defects in southern California. Am J Epidemiol. 2002;155(1):17–25. [DOI] [PubMed] [Google Scholar]
- 2. Gilboa SM, Mendola P, Olshan AF, et al. . Relation between ambient air quality and selected birth defects, Seven County Study, Texas, 1997–2000. Am J Epidemiol. 2005;162(3):238–252. [DOI] [PubMed] [Google Scholar]
- 3. Strickland MJ, Klein M, Correa A, et al. . Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol. 2009;169(8):1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolk H, Armstrong B, Lachowycz K, et al. . Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occup Environ Med. 2010;67(4):223–227. [DOI] [PubMed] [Google Scholar]
- 5. Dadvand P, Rankin J, Rushton S, et al. . Ambient air pollution and congenital heart disease: a register-based study. Environ Res. 2011;111(3):435–441. [DOI] [PubMed] [Google Scholar]
- 6. Agay-Shay K, Friger M, Linn S, et al. . Air pollution and congenital heart defects. Environ Res. 2013;124:28–34. [DOI] [PubMed] [Google Scholar]
- 7. Padula AM, Tager IB, Carmichael SL, et al. . The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. 2013;177(10):1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schembari A, Nieuwenhuijsen MJ, Salvador J, et al. . Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. 2014;122(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stingone JA, Luben TJ, Daniels JL, et al. . Maternal exposure to criteria air pollutants and congenital heart defects in offspring: results from the National Birth Defects Prevention Study. Environ Health Perspect. 2014;122(8):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farhi A, Boyko V, Almagor J, et al. . The possible association between exposure to air pollution and the risk for congenital malformations. Environ Res. 2014;135:173–180. [DOI] [PubMed] [Google Scholar]
- 11. Vrijheid M, Martinez D, Manzanares S, et al. . Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;119(5):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen EK, Zmirou-Navier D, Padilla C, et al. . Effects of air pollution on the risk of congenital anomalies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(8):7642–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nawrot TS, Adcock I. The detrimental health effects of traffic-related air pollution: a role for DNA methylation. Am J Respir Crit Care Med. 2009;179(7):523–524. [DOI] [PubMed] [Google Scholar]
- 14. Serra-Juhé C, Cuscó I, Homs A, et al. . DNA methylation abnormalities in congenital heart disease. Epigenetics. 2015;10(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nuhrenberg T, Gilsbach R, Preissl S, et al. . Epigenetics in cardiac development, function, and disease. Cell Tissue Res. 2014;356(3):585–600. [DOI] [PubMed] [Google Scholar]
- 16. Chowdhury S, Cleves MA, MacLeod SL, et al. . Maternal DNA hypomethylation and congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2011;91(2):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheng W, Wang H, Ma X, et al. . LINE-1 methylation status and its association with tetralogy of Fallot in infants. BMC Med Genomics. 2012;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu C, Yu ZB, Chen XH, et al. . DNA hypermethylation of the NOX5 gene in fetal ventricular septal defect. Exp Ther Med. 2011;2(5):1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheng W, Qian Y, Wang H, et al. . DNA methylation status of NKX2-5, GATA4 and HAND1 in patients with tetralogy of Fallot. BMC Med Genomics. 2013;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baccarelli A, Wright RO, Bollati V, et al. . Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Prins S, Koppen G, Jacobs G, et al. . Influence of ambient air pollution on global DNA methylation in healthy adults: a seasonal follow-up. Environ Int. 2013;59:418–424. [DOI] [PubMed] [Google Scholar]
- 22. Janssen BG, Godderis L, Pieters N, et al. . Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009;89(2):673S–677S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blom HJ, Shaw GM, den Heijer M, et al. . Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7(9):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng Y, Wang S, Chen R, et al. . Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies. Sci Rep. 2015;5:8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chango A, Pogribny IP. Considering maternal dietary modulators for epigenetic regulation and programming of the fetal epigenome. Nutrients. 2015;7(4):2748–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jedrychowski W, Perera F, Mrozek-Budzyn D, et al. . Higher fish consumption in pregnancy may confer protection against the harmful effect of prenatal exposure to fine particulate matter. Ann Nutr Metab. 2010;56(2):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guxens M, Aguilera I, Ballester F, et al. . Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baccarelli A, Cassano PA, Litonjua A, et al. . Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117(14):1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Botto LD, Lin AE, Riehle-Colarusso T, et al. . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. [DOI] [PubMed] [Google Scholar]
- 31. Reefhuis J, Gilboa SM, Anderka M, et al. . The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103(8):656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correa A, Gilboa SM, Besser LM, et al. . Diabetes mellitus and birth defects. Am J Obstet Gynecol 2008;199(3):237.e1–237.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brunekreef B. Health effects of air pollution observed in cohort studies in Europe. J Expo Sci Environ Epidemiol. 2007;17:S61–S65. [DOI] [PubMed] [Google Scholar]
- 34. de Mesnard L. Pollution models and inverse distance weighting: some critical remarks. Comput Geosci. 2013;52:459–469. [Google Scholar]
- 35. Gittenberger-de Groot AC, Bartelings MM, Deruiter MC, et al. . Basics of cardiac development for the understanding of congenital heart malformations. Pediatr Res. 2005;57(2):169–176. [DOI] [PubMed] [Google Scholar]
- 36. Willett WC, Reynolds RD, Cottrell-Hoehner S, et al. . Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 37. Carmichael SL, Gonzalez-Feliciano AG, Ma C, et al. . Estimated dietary phytoestrogen intake and major food sources among women during the year before pregnancy. Nutr J. 2011;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 39. Martin GR, Perry LW, Ferencz C. Increased prevalence of ventricular septal defect: epidemic or improved diagnosis. Pediatrics. 1989;83(2):200–203. [PubMed] [Google Scholar]
- 40. Liu X, Jorgenson E, Witte JS. Hierarchical modeling in association studies of multiple phenotypes. BMC Genet. 2005;6(suppl 1):S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenland S. A semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat Med. 1992;11(2):219–230. [DOI] [PubMed] [Google Scholar]
- 42. Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. [DOI] [PubMed] [Google Scholar]
- 43. Lundberg M, Fredlund P, Hallqvist J, et al. . A SAS program calculating three measures of interaction with confidence intervals. Epidemiology. 1996;7(6):655–656. [PubMed] [Google Scholar]
- 44. Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132(8 suppl):2333S–2335S. [DOI] [PubMed] [Google Scholar]
- 45. SAS Institute SAS/STAT 9.3 User's Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 46. Zhu C, Yu ZB, Chen XH, et al. . Screening for differential methylation status in fetal myocardial tissue samples with ventricular septal defects by promoter methylation microarrays. Mol Med Rep. 2011;4(1):137–143. [DOI] [PubMed] [Google Scholar]
- 47. Sarnat JA, Schwartz J, Catalano PJ, et al. . Gaseous pollutants in particulate matter epidemiology: confounders or surrogates. Environ Health Perspect. 2001;109(10):1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim D, Sass-Kortsak A, Purdham JT, et al. . Associations between personal exposures and fixed-site ambient measurements of fine particulate matter, nitrogen dioxide, and carbon monoxide in Toronto, Canada. J Expo Sci Environ Epidemiol. 2005;16(2):172–183. [DOI] [PubMed] [Google Scholar]
- 49. Andersen ZJ, Wahlin P, Raaschou-Nielsen O, et al. . Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol. 2007;17(7):625–636. [DOI] [PubMed] [Google Scholar]
- 50. Levy I, Mihele C, Lu G, et al. . Elucidating multipollutant exposure across a complex metropolitan area by systematic deployment of a mobile laboratory. Atmos Chem Phys. 2014;14(14):7173–7193. [Google Scholar]
- 51. Willett W. Nutritional Epidemiology. 3rd ed Oxford, United Kingdom: Oxford University Press; 2012. [Google Scholar]
- 52. Crozier SR, Robinson SM, Godfrey KM, et al. . Women's dietary patterns change little from before to during pregnancy. J Nutr. 2009;139(10):1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.