Abstract
Human immune system (HIS) mice, immunodeficient mice engrafted with human cells (with or without donor-matched tissue), offer a unique opportunity to study pathogens that cause disease predominantly or exclusively in humans. Several HIS mouse models have recently been used to study Ebola virus (EBOV) infection and disease. The results of these studies are encouraging and support further development and use of these models in Ebola research. HIS mice provide a small animal model to study EBOV isolates, investigate early viral interactions with human immune cells, screen vaccines and therapeutics that modulate the immune system, and investigate sequelae in survivors. Here we review existing models, discuss their use in pathogenesis studies and therapeutic screening, and highlight considerations for study design and analysis. Finally, we point out caveats to current models, and recommend future efforts for modeling EBOV infection in HIS mice.
Keywords: Ebola virus, hemorrhagic fever, humanized mice
Introduction
Ebola virus (EBOV; species Zaire ebolavirus; family Filoviridae) is the primary etiologic agent of Ebola virus disease (EVD), a zoonotic viral hemorrhagic fever. Current animal models of EVD include rodents (mice, hamsters, guinea pigs), ferrets, and several non-human primate (NHP) species. Wild-type (WT) EBOV causes lethal disease in ferrets and NHPs [1–3]. NHPs remain the gold standard model for studies of EVD. Ferrets are new and promising models, but are currently not well established, lack tools for immunological analyses and present additional challenges compared to other small animal models, including handling and housing requirements. Rodents exhibit no, or very mild clinical signs despite viral replication, and require viral serial adaptation to cause severe disease [4–6]. In rodents, disease from WT-EBOV infection is restricted to immunocompromised mice, such as STAT−/−, IFNAR−/−, A129, and SCID strains [7–9]. Despite the limitations, rodent models have been important in early investigations of viral pathogenesis and for preliminary therapeutic and vaccine screening studies, including for the development of viral-vectored EBOV vaccines that are currently in clinical evaluation [10,11].
The discrepancies between mouse and human immune systems [12] are of considerable concern, especially for diseases in which pathogenesis is, at least partially, immune-mediated. Advanced mouse models attempt to address these discrepancies. “Humanized mice” is a general designation for mice that express human genes, or contain human cells and/or tissues (e.g., transgenic mice or xenograft mouse models). Included in the term “humanized mice” are human immune system (HIS) mice, mice engrafted by a variety of approaches, including the use of human hematopoietic stem cells (HSC) or induced pluripotent stem cells (iPSC), resulting in reconstitution of human immune lymphoid and myeloid cells that are both present and functional to varying degrees [13,14]. HIS mice are increasingly being used to study human cancer and tumor biology, immunity, infections, autoimmunity, allergies, organ transplantation, vaccine development, and immune regulation [15].
EVD involves a complex combination of virus-mediated and immune-mediated alterations in organ and vascular function. The initial targets of filoviral infection and replication are immune cells, including macrophages and myeloid dendritic cells, which contribute to viral dissemination and immune dysregulation [16,17]. Using HIS mice to study EVD is promising for several reasons. The human immune cells in HIS mice provide an abundance of susceptible targets for filovirus replication in a rodent model that would otherwise efficiently control WT virus replication [18]. With this unique human immune system milieu, HIS mice are a platform to investigate early events in the infection of immune cells, to study the development of adaptive immune responses, and to test vaccines and immunomodulatory therapies.
EBOV infection in human immune system mice
All of the current HIS mouse models of EBOV infection are derived from the highly immunodeficient NOD-scid/IL2Rγ−/− (NSG) mouse background (Figure 1). The humanization process increases susceptibility of the mice to disease from EBOV infection. Unengrafted NSG mice do not develop disease until 3 to > 6 weeks post-infection [19,20][Spengler & Prescott, unpublished data], similar to SCID mice that are deficient in adaptive immune responses, lacking both B and T cells, but capable of type I IFN responses [4]. Although current HIS models are from the NSG background, they differ in engraftment approach, expression of human transgenes, and potential for human HLA/MHC compatibility, differences that result in complex variation in human and mouse immune system functionality. Early investigations of EBOV infection in humanized mice were challenging due to inherent limitations of the existing models. The NSG-huPBL model, engrafted with peripheral blood lymphocytes, is primarily reconstituted with T cells that have a mature phenotype, and is plagued with a rapid onset of graft-versus-host disease (GVHD), which limited studies to about a month post-engraftment. The NSG-huPBL model was used to investigate human lymphocyte apoptosis in mice inoculated with mouse-adapted EBOV (MA-EBOV) or WT-EBOV (Table 1). Increased levels of several human cytokines and significant lymphocyte apoptosis were reported in MA-EBOV compared to WT-EBOV infection, and only MA-EBOV infection resulted in a lethal disease [21], similar to other rodent models.
Figure 1.
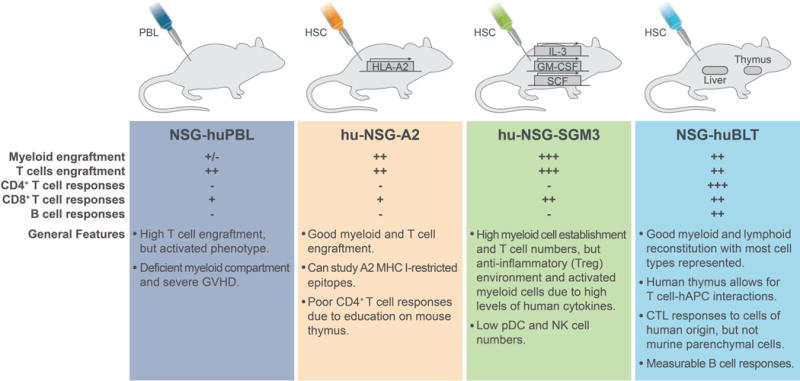
Characteristics of human immune system mouse models used in Ebola virus infection studies. All strains are derived from a NOD-scid/IL2Rγ−/− (NSG) background and are injected intravascularly with either human peripheral blood lymphocytes (PBL) or hematopoietic stem cells (HSC). Strains vary in human transgene expression, and in presence or absence of co-engrafted, donor-matched human liver and thymic tissue. Human cell engraftment and responses are indicated as (−) absent, (+) present, or (+/−) may only be present in low numbers and may not be functional. CTL: cytotoxic T lymphocyte; GVHD: graft versus host disease; NK: natural killer cells; pDC: plasmacytoid dendritic cells; Treg: regulatory T cells.
Table 1.
EBOV-infection in human immune system mouse models.
| Model | EBOV Strain | Route/Dose | Survival | Pathology/Immune response | Ref. |
|---|---|---|---|---|---|
| NSG-huPBL | MA-EBOV | IP, 1000 PFU | 0% | Human lymphocyte apoptosis | [21] |
| Mayinga | IP, 1000 PFU | 100% | Absence of human lymphocyte apoptosis | ||
| Hu-NSG-A2 | Mayinga | IP, 1000 FFU | 0% – 25% | Focal hemorrhage in liver, splenomegaly, steatosis; elevated hepatic enzymes | [19] |
| Hu-NSG-SGM3 | Makona (C07) | IP, 10 PFU | 100% | NR | [25] |
| IP, 1000 PFU | 50% | NR | |||
| IM, 10 PFU | 33% | NR | |||
| IM, 1000 PFU | 33% | Macrophage infiltration, lymphocytes in liver and spleen; increased T cell frequency in blood, T cell activation in the spleen | |||
| NSG-huBLT | Mayinga | IP, 100–100000 TCID50 | 0%* | Hepatic viral inclusion bodies and cellular necrosis; elevated hepatic enzymes; upregulation of human cytokines and chemokines | [20] |
| Makona (LBR/2014) | IP, 100 TCID50 | 50% | NR |
Excludes one survivor that was most likely a result of infection failure; unlike other survivors, there was no evidence of viral RNA in any tissues at time of euthanasia. MA, mouse adapted; NR, not reported.
The first lethal humanized mouse model of EVD was reported in hu-NSG-A2, humanized mice [19] (Table 1, Figure 1). Hu-NSG-A2 have functional human HLA-A2-restricted T-cell responses [22,23], which is beneficial for investigating A2-restricted antigens involved in the immune response to EBOV infection [24]. In hu-NSG-A2 mice engrafted with HSC, EBOV was lethal in an engraftment level-dependent manner; lethality was uniform in mice with high peripheral engraftment (>40% human CD45+ of total peripheral blood leukocytes), but sub-lethal in mice with low peripheral engraftment (20–40% CD45+). In hu-NSG-A2 mice, EBOV infection was disseminated, and liver damage and hemorrhage were observed [19]. EBOV studies in hu-NSG-A2 concluded that the association between disease severity and engraftment levels might be a result of immunopathology rather than the availability of human immune target cells of infection to overcome restricted viral replication in traditional mouse models [19]. However, support for these conclusions would require further investigation of immunopathogenic processes in this model.
Subsequent studies emphasized that contributors to EVD in HIS models are complex and still unclear. Hu-NSG-SGM3 mice are derived from the triple transgenic NSG-SGM3 (NSGS) mice expressing human IL-3, GM-CSF, and SCF cytokines that support the stable engraftment of myeloid lineages and result in high levels of regulatory T cell (Treg) populations [25,26] (Table 1, Figure 1). Hu-NSG-SGM3 studies were the first to demonstrate susceptibility of humanized mice to EBOV infection after intramuscular (IM) inoculation. This is notable, as rodents only develop disease when adapted virus is administered intraperitoneally (IP), whereas peripheral inoculation routes result in vaccination and protection against subsequent lethal challenge [4]. In hu-NSG-SGM3, despite high myeloid and lymphoid reconstitution, infection with EBOV-Makona did not result in uniform lethality; lethality was ~50% in IP-inoculated mice (103 FFU) and ~70% in IM-inoculated mice (10 or 103 FFU). In NHPs, EVD is characterized in part by hepatocyte necrosis and apoptosis [27], a result of high levels of cytokine expression leading to immunopathogenesis [28]. Interestingly, in hu-NSG-SGM3 mice with severe or lethal disease hepatocytes did not appear to be responding to inflammatory cells, as the typical pathology seen in NHPs was not observed in the mice, despite the presence of higher levels of mononuclear cells. This may be due to a lack of epitope-restricted responses in hu-NSG-SGM3. Alternatively, this may be due to high levels of Tregs that may modulate an anti-inflammatory response to EBOV, as transfer of human CD34+ HSC into NSG-SGM3 also significantly increases the CD4+CD25+Foxp3+ Treg population. In these models, Tregs have been shown to suppress polyclonal T cell proliferation [26], and to prevent virus-induced liver fibrosis [29], potentially explaining the absence of severe hepatic pathology in this EBOV model.
NSG-huBLT (bone marrow/liver/thymus) mice are prepared by co-transplanting human fetal liver, thymus, and autologous HSC (Table 1, Figure 1). A subset of cellular responses (Th1, Th2, and CTL), and of antigen-specific antibody responses that generate consistent IgM, but variable IgG [30–32] are functional in NSG-huBLT. Advantages of the hu-NSG mice with donor-matched thymic and liver tissue, such as the NSG-huBLT, include multilineage hematopoiesis (myeloid and lymphoid), T cell maturation in autologous thymus, HLA restriction, and mucosal human cell reconstitution [33]. In this model, human antigen presenting cells can present antigen to T cells in an MHC I- and II-restricted manner. Disadvantages to the model include the development of a wasting disease and the requirement of donor-matched tissue for implantation. Studies of EBOV infection in NSG-huBLT highlighted several advantages to humanized mouse studies, including the ability to lower the challenge dose to reduce lethality, and were the first to demonstrate the ability to use EBOV strains other than the prototypic Mayinga isolate from the first EVD outbreak in 1976, to which all adapted rodent models have previously been restricted [20]. EBOV infection in NSG-huBLT mice resulted in disease and pathology consistent with that seen in hu-NSG-A2, suggesting that HLA restriction may contribute to altered pathogenesis in the model. Although disease in NSG-huBLT was consistent with the findings in hu-NSG-A2, lethality in NSG-huBLT did not appear to be associated with engraftment levels in peripheral blood; engraftment ranged from ~25% to >50% total human CD45+ in peripheral blood, and no association with outcome was observed.
What do human immune system mice offer to the field of EBOV research?
There are many fundamental differences between mouse and human immune systems, including differences in blood cell populations; in MHC class expression by endothelial cells; in genomic inflammatory responses; and in innate immune molecules resulting in the functional loss or complete absence of homologous receptors [34]. These differences, coupled with the necessary use of rodent-adapted virus, present fundamental challenges for the use of mice in EBOV research. HIS mice offer several advances in rodent modeling of EBOV infection, including the study of (1) clinical isolates of EBOV; (2) early interactions of EBOV with human immune cells; (3) therapeutic interventions that modulate the human immune response to EBOV; and (4) EVD survivors.
The most valuable aspect of existing humanized mouse models of EVD is the ability to investigate early viral interactions with host cells and the resultant human immune responses. Early targets of EBOV infection include monocytes, macrophages, and dendritic cells, and EBOV’s ability to modulate the activation and maturation of these initial target cells correlates with its pathogenic potential [35,36]. Previously, studies of human immune cell responses were limited to ex vivo and in vitro experimental approaches. In humanized mice, the immune response to peripheral inoculation in resident immune cell populations can be investigated in an in vivo context, and humanized mice offer significant benefits for evaluating interventions that modulate these responses [37,38]. HIS models are particularly useful for studies of immunomodulatory therapies, including monoclonal antibodies that target specific immune pathways (e.g., anti-type II IFN).
NHPs develop acute, uniformly lethal disease and recapitulate many of the clinical signs and abnormalities noted in severe human disease. However, there are still several differences in the disease observed in NHPs versus humans, and studies of protracted or mild disease and of survivors can be challenging in these and other highly sensitive models of EVD. The 2013–2016 EVD outbreak in West Africa generated reports of ocular deficits, hearing loss, difficulty sleeping, arthralgias, and the potential for viral persistence in immunologically protected sites in survivors [39–41], strongly supporting the need for additional animal models in which EBOV infection is not uniformly lethal to study disease sequelae. There are currently no NHP models for mild and protracted Ebola disease or to study disease sequelae in survivors. The establishment of those models would take huge effort and expense, as high numbers of animals are needed to study such scenarios. In HIS mice, EBOV infection causes disease, but subsets of animals survive, permitting the study of protracted disease and investigation of sequelae (e.g., examining humanized mice for histological evidence of damage to the auditory system and CNS). While studies of survivors are an important and promising application of HIS mice, extrapolating observations to human disease will require further evaluation of the models based on current knowledge from EVD survivors to determine characteristics that mirror human disease and those that are dissimilar. Although some aspects may differ between HIS mice and human disease, we can still learn from these models. For example, virus appears to persist longer in surviving HIS mice than in humans. This may simply reflect poor humoral responses in current HIS mouse models, supporting the association between antibody levels and viral clearance in EVD patients [42].
Conclusions and future considerations
Characterization of HIS mouse models for the study of EVD is currently in its infancy. Many questions remain, including, and most importantly, what events lead to EVD manifestations, and what factors in the humanized milieu are unique in these processes. For example, in contrast to human cases, in which inflammation is a result of cytokine expression in response to viral infection, NSG-huBLT mice have a high residual mouse myeloid background, which could account for much of the inflammation seen in these animals. Thus, while NSG-huBLT demonstrated gross human cell-specific immunological alterations consistent with human disease, the mechanism leading to this result may not mirror the process in humans. As contributors to disease in these mice are better understood, the current complexity in interpreting findings in HIS mice and translating them to human disease will also significantly improve.
The studies described above represent significant breakthroughs in mouse modeling of EVD, but each presents its own caveats. Conclusions drawn from HIS models are highly dependent on knowledge of the particular model independently of challenge agent; the quantity of human and mouse immune cells (% and absolute), and the functional ability of reconstituted immune cell populations must be known. Without this information it is difficult to draw conclusions, such as those regarding the association of engraftment levels in HIS mice. Importantly, to date, engraftment comparisons have only been based on relative human and mouse contributions to cell populations in the blood, which may not reflect the potential impact of mouse cells in the leukocyte population. Further studies should examine this impact by assessing association of infection outcome with absolute cell counts of both species, which will vary by model.
Other caveats involving immune function are illustrated in hu-NSG-SGM3 mice, in which selection of T cells on a murine thymic scaffold may result in rather modest adaptive immune responses [43]. Even in models with human thymic education, such as the NSG-huBLT, some T cells still develop on the mouse thymus, leading to a mix of mouse and human MHC-compatible cells. Furthermore, most models lack human cytokines critical for hematopoiesis and/or have insufficient interactions between cells of the murine stroma and human hematopoietic cells. This less than optimal lymphoid architecture can also affect adaptive immune responses. Alternatively, as in hu-NSG-SGM3 mice, cell populations under the influence of overexpressed transgenic human cytokines can lead to increased baseline inflammatory responses or an artificial cellular maturing environment, which may limit the cells’ ability to respond appropriately to infection.
Another factor in HIS models of EVD is the function of the humoral immune response. Currently, evaluation of the humoral immune response in EBOV-infected HIS mice is lacking. HIS mice can generate humoral responses upon immunization or viral infection [44], but IgM production has been the predominant response observed, with only low frequency production of specific IgG. Poor humoral responses can result from the inability to efficiently recognize antigens presented by human APCs and B cells in HIS mice, where the murine MHC positively selects human T cells during the intrathymic development process [45]. However, T-cell function does not appear to be the only factor important in B-cell maturation and class switching. In the BLT model, with enhanced T-cell engraftment and human MHC-restricted T-cell function, IgM remains the predominant antibody response [46]. Despite limitations in some models, vaccine studies are currently being performed using HIS mice [47,48]. Furthermore, recent reports demonstrating virus-specific and neutralization-capable IgM and IgG responses supports the use of huBLT models in vaccines studies [49,50]. Development of HIS mouse models with advanced humoral immune function is a focus in the field. As the availability of these models expands, future efforts should concentrate on characterizing them for EBOV vaccine studies.
Advances in HIS models also aim to ameliorate reconstitution of specific cell populations. EBOV targets myeloid cells, which historically were poorly reconstituted in HIS mice. Newer models, such as hu-NSG-SGM3 mice, demonstrate improved development of human myeloid-lineage cells. Monocyte, DC, granulocyte, and mast cell reconstitution can be selectively enhanced with expression of human transgenes, including IL-3, IL-4, and IL-15 with Flt-3 ligand (Flt3L) or macrophage CSF; or by replacing murine genes with human homologs [51–53]. In addition, other humanized mouse models offer unique opportunities to study EBOV. Humanized liver models, mice in which the mouse liver is replaced by xenografts of human primary hepatocytes (some at > 80% replacement) [52,54], have been successfully applied in studies of other hepatotropic viruses [55]. Efforts to combine technical advances in HIS mice with tissue engraftment may offer novel opportunities to further model EBOV pathogenesis.
EVD studies in HIS mice to date have emphasized the need for particular considerations in study design and data interpretation. Donor variability may affect the results depending on the model. A subset of NSG-huBLT studies that investigated this effect, although small in number, supported the presence of donor-associated outcome. However, this affect was not apparent in larger hu-NSG-SGM3 studies. The relative contribution of donor variability in HIS models may reflect differences in model characteristics, namely the presence or absence of HLA-restriction, but remains to be further investigated. Route of inoculation can also play a critical role in outcome, and IP is not always the preferred route, as is described in HIS mouse models of Plasmodium falciparum infection [56]. This was indicated in the EBOV studies in hu-NSG-SGM3, as increased lethality and reproducibility was seen with IM inoculation versus IP inoculation [25]. Finally, HIS mice are a dynamic milieu of human cell populations; the time post-engraftment that animals are inoculated can lead to different outcomes due to temporal variation in the relative presence of immune cell populations in both tissue and blood. Considerations in data analysis and reporting of age post-engraftment, engraftment levels, and donors’ HLA types when applicable, are essential to improve our knowledge of how and when these variables affect study outcome, and to further understand the EVD process in these models.
Humanized mice are highly promising for research of EVD and other hemorrhagic fever viruses, especially in light of continued advancement in HIS models. An ideal HIS mouse model would reconstitute all human immune cell populations in relative frequencies observed in humans, and be engineered to maintain natural cellular function and recapitulate both innate and adaptive immune responses. However, the complex dynamic between reconstituted cell populations and murine parenchymal cells will always be present, and should be characterized to aid in interpretation of experimental infection data. For future studies, the selection of which HIS mouse model to use will be pivotal and highly dependent on the questions being addressed. Appropriate model selection will rely on continued efforts to characterize and determine which factors, immune and non-immune, contribute to EVD susceptibility in these models, and how this compares to the human disease process.
Highlights.
Human immune system mice are useful tools for Ebola virus research.
Human lymphoid and myeloid immune cells are reconstituted in human immune system mice.
Wild-type Ebola virus causes disease in humanized mice.
Disease outcome varies with model, dose, route, donor, and time post-engraftment.
Knowledge of human immune cell levels and function in each model is key in interpreting studies.
Acknowledgments
The authors thank Ryan Kissinger and Austin Athman of Visual & Medical Arts, Research Technologies Branch, NIAID, NIH, for graphic design assistance, and Tatyana Klimova for assistance in editing the manuscript.
Financial support. This work was supported in part from CDC emerging infectious disease research core funds and the Intramural Research Program, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
References
- 1.Cross R, Mire CE, Borisevich V, Geisbert JB, Fenton K, Geisbert T. The Domestic Ferret (Mustela putorius furo) as a Lethal Infection Model for Three Different Species of Ebolavirus. J Infect Dis. 2016 doi: 10.1093/infdis/jiw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozak R, He S, Kroeker A, et al. Ferrets infected with Bundibugyo virus or Ebola virus recapitulate important aspects of human filoviral disease. J Virol. 2016 Aug; doi: 10.1128/JVI.01033-16. JVI.01033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisbert TW, Strong JE, Feldmann H. Considerations in the Use of Nonhuman Primate Models of Ebola Virus and Marburg Virus Infection. J Infect Dis. 2015:1–7. doi: 10.1093/infdis/jiv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1998;178:651–61. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 5.Connolly BM, Steele KE, Davis KJ, et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999;179(Suppl):S203–17. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 6.Wong G, He S, Wei H, et al. Development and characterization of a guinea pig-adapted Sudan virus. J Virol. 2015;90(1):392–9. doi: 10.1128/JVI.02331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond J, Bradfute S, Bray M. Filovirus infection of STAT-1 knockout mice. J Infect Dis. 2011;204(SUPPL. 3):986–990. doi: 10.1093/infdis/jir335. [DOI] [PubMed] [Google Scholar]
- 8.Smither SJ, Eastaugh L, Ngugi S, et al. Ebola Virus Makona Shows Reduced Lethality in an Immune-deficient Mouse Model. J Infect Dis. 2016:jiw145. doi: 10.1093/infdis/jiw145. [DOI] [PubMed] [Google Scholar]
- 9.Bray M. The role of the type I interferon response in the resistance of mice to filovirus infection. J Gen Virol Microbiology Society. 2001;82(Pt 6):1365–73. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 10.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78(10):5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SM, Stroher U, Fernando L, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;(S2):S404–12. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 12.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 14.Coughlan AM, Freeley SJ, Robson MG. Humanised mice have functional human neutrophils. J Immunol Methods. 2012;385(1–2):96–104. doi: 10.1016/j.jim.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Nat Rev Immunol. 11. Vol. 12. Nature Publishing Group; 2012. Humanized mice for immune system investigation: progress, promise and challenges; pp. 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnittler HJ, Feldmann H. Molecular pathogenesis of filovirus infections: role of macrophages and endothelial cells. Curr Top Microbiol Immunol. 1999;235:175–204. doi: 10.1007/978-3-642-59949-1_10. [DOI] [PubMed] [Google Scholar]
- 17.Ryabchikova EI, Kolesnikova LV, Netesov SV. Animal pathology of filoviral infections. Curr Top Microbiol Immunol. 1999;235:145–73. doi: 10.1007/978-3-642-59949-1_9. [DOI] [PubMed] [Google Scholar]
- 18.Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J Gen Virol. 2001;82:1365–73. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 19 **.Lüdtke A, Oestereich L, Ruibal P, et al. Ebola virus disease in mice transplanted with human hematopoietic stem cells. J Virol. 2015;89(8):4700–4. doi: 10.1128/JVI.03546-14. Authors describe Ebola virus infection in Hu-NSG-A2 mice and introduce the importance of considering immune milieu in models by investigating effect of engraftement level on outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20 **.Bird BH, Spengler JR, Chakrabarti AK, et al. Humanized mouse model of Ebola virus disease mimics immune responses in human disease. J Infect Dis. 2016;213:703–11. doi: 10.1093/infdis/jiv538. This report describes Ebola virus infection in NSG-huBLT mice. Analyzing tissue RNA and plasma, authors characterize a cytokine reponse in Ebola virus-infected NSG-huBLT mice that mirrors human disease. They also present data on dose and donor variation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradfute SB, Warfield KL, Bray M. Mouse models for filovirus infections. Viruses. 2012;4(9):1477–508. doi: 10.3390/v4091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu I-M, Holdaway HA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J Virol. 2009;83(23):12101–7. doi: 10.1128/JVI.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal S, Pearson T, Friberg H, et al. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rγnull mice. PLoS One. 2009;4(10):e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundar K, Boesen A, Coico R. Computational prediction and identification of HLA-A2.1-specific Ebola virus CTL epitopes. Virology. 2007;360(2):257–263. doi: 10.1016/j.virol.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 25 **.Spengler JR, Lavender KJ, Martellaro C, et al. Ebola Virus Replication and Disease Without Immunopathology in Mice Expressing Transgenes to Support Human Myeloid and Lymphoid Cell Engraftment. J Infect Dis. 2016;214(suppl 3):S308–S318. doi: 10.1093/infdis/jiw248. Authors describe Ebola virus infection in hu-NSG-SGM3 mice. Intraperitoneal and intramuscular inoculation are compared at both high and low doses of virus. A lack of characteristic immunopathology in target organs is observed, despite human immune cell infiltration. These data further support the need to consider model differences when interpreting data, namely relative levels of human immune cell reconstitution and their function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2R{gamma}null humanized mice. Blood. 2011;117(11):3076–86. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett RS, Huzella LM, Jahrling PB, Bollinger L, Jr GGO, Hensley LE. Nonhuman Primate Models of Ebola Virus Disease. Curr Top Microbiol Immunol. 2017 Jun 23; doi: 10.1007/82_2017_20. [DOI] [PubMed] [Google Scholar]
- 28 *.Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol Nature Publishing Group. 2017;17(3):195–207. doi: 10.1038/nri.2016.138. This review provides a detailed summary of innate and adaptive immune responses to Ebola virus. [DOI] [PubMed] [Google Scholar]
- 29.Nunoya J-I, Washburn ML, Kovalev GI, Su L. Regulatory T cells prevent liver fibrosis during HIV type 1 infection in a humanized mouse model. J Infect Dis. 2014;209(7):1039–44. doi: 10.1093/infdis/jit548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 31.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8(1):65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajesh D, Zhou Y, Jankowska-Gan E, et al. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rγnull mice. Hum Immunol. 2010;71(6):551–559. doi: 10.1016/j.humimm.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013;25(3):403–409. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brehm MA, Bortell R, Verma M, Shultz LD, Greiner DL. Humanized Mice in Translational Immunology. Transl Immunol Mech Pharmacol Approaches. 2015:285–326. [Google Scholar]
- 35.Zaki SR, Shieh WJ, Greer PW, et al. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. J Infect Dis. 1999;179(Suppl):S36–S47. doi: 10.1086/514319. [DOI] [PubMed] [Google Scholar]
- 36.Bosio CM, Aman MJ, Grogan C, et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188(11):1630–8. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 37.Leung C, Chijioke O, Gujer C, et al. Infectious diseases in humanized mice. Eur J Immunol. 2013;43(9):2246–2254. doi: 10.1002/eji.201343815. [DOI] [PubMed] [Google Scholar]
- 38.Vudattu NK, Waldron-Lynch F, Truman LA, et al. Humanized Mice as a Model for Aberrant Responses in Human T Cell Immunotherapy. J Immunol. 2014;193(2):587–596. doi: 10.4049/jimmunol.1302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15(8):905–912. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 40.Bausch DG. Sequelae after Ebola virus disease: even when it’s over it’s not over. Lancet Infect Dis. 2015;15(8):865–6. doi: 10.1016/S1473-3099(15)70165-9. [DOI] [PubMed] [Google Scholar]
- 41.Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med. 2015;372(25):2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spengler JR, McElroy AK, Harmon JR, Ströher U, Nichol ST, Spiropoulou CF. Relationship Between Ebola Virus Real-Time Quantitative Polymerase Chain Reaction-Based Threshold Cycle Value and Virus Isolation From Human Plasma. J Infect Dis. 2015;212(Suppl 2):1–4. doi: 10.1093/infdis/jiv187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nischang M, Gers-Huber G, Audigé A, Akkina R, Speck RF. Modelling HIV infection and therapies in humanised mice. Swiss Med Wkly. 2012;142:1–12. doi: 10.4414/smw.2012.13618. [DOI] [PubMed] [Google Scholar]
- 44.Garcia S, Freitas AA. Humanized mice: Current states and perspectives. Immunol Lett. 2012;146(1–2):1–7. doi: 10.1016/j.imlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Ito R, Shiina M, Saito Y, Tokuda Y, Kametani Y, Habu S. Antigen-specific antibody production of human B cells in NOG mice reconstituted with the human immune system. Curr Top Microbiol Immunol. 2008;324:95–107. doi: 10.1007/978-3-540-75647-7_6. [DOI] [PubMed] [Google Scholar]
- 46.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83(14):7305–21. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao Y, Lai R, Afkhami S, et al. A novel virus-vectored respiratory mucosal vaccine enhances anti-tuberculosis immunity in a humanized model system. J Infect Dis. 2017 doi: 10.1093/infdis/jix252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grover A, Troy A, Rowe J, et al. Humanized NOG mice as a model for tuberculosis vaccine induced immunity: A comparative analysis with the mouse and guinea pig models of tuberculosis. Immunology. 2017 May 14; doi: 10.1111/imm.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49 *.Crawford LB, Tempel R, Streblow DN, et al. Human Cytomegalovirus Induces Cellular and Humoral Virus-specific Immune Responses in Humanized BLT Mice. Sci Rep Springer US. 2017;7(1):937. doi: 10.1038/s41598-017-01051-5. Authors detect virus-specific IgM and IgG B-cell responses with the ability to neutralize virus. This supports the use of humanized BLT mice in vaccines studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50 **.Jangalwe S, Shultz LD, Mathew A, Brehm MA. Improved B cell development in humanized NOD-scid IL2Rγ null mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immunity, Inflamm Dis. 2016;4(4):427–440. doi: 10.1002/iid3.124. Authors detail reconstitution of a variety of human cell populations in NSG-huBLT and NSG-SGM3-huBLT. Higher levels of T regulatory cells and of mature naïve B cells in NSG-SGM3 BLT mice were found compared to NSG BLT mice. These and other data in this study are key to interpreting data generated in the respective models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106(51):21783–8. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9(3):208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rongvaux A, Willinger T, Takizawa H, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci. 2011;108(6):2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasegawa M, Kawai K, Mitsui T, et al. Biochem Biophys Res Commun. 3. Vol. 405. Elsevier Inc.; 2011. The reconstituted “humanized liver” in TK-NOG mice is mature and functional; pp. 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissig K-D, Wieland SF, Tran P, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest American Society for Clinical Investigation. 2010;120(3):924–30. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrer S. Humanised models of infection in the evaluation of anti-malarial drugs. Drug Discov Today Technol. 2013;10(3):351–357. doi: 10.1016/j.ddtec.2012.07.003. [DOI] [PubMed] [Google Scholar]


