Abstract
Eukaryotic genomes are littered with sequences of diverse viral origins, termed endogenous viral elements (EVEs). Here we used examples primarily drawn from mammalian endogenous retroviruses to document how the influx of EVEs has provided a source of prefabricated coding and regulatory sequences that were formerly utilized for viral infection and replication, but have been occasionally repurposed for cellular function. While EVE co-option has benefited a variety of host biological functions, there appears to be a disproportionate contribution to immunity and antiviral defense. The mammalian embryo and placenta offer opportunistic routes of viral transmission to the next host generation and as such they represent hotbeds for EVE cooption. Based on these observations, we propose that EVE cooption is initially driven as a mean to mitigate conflicts between host and viruses, which in turn acts as a stepping-stone toward the evolution of cellular innovations serving host physiology and development.
Introduction
Endogenous viral elements (EVE) are sequences of viral origin that have integrated into the host germline genome and, as a result, become vertically inherited in the host population. Viral endogenization is pervasive across all branches of cellular life resulting in the accumulation of EVEs of diverse origins and varying ages within the genomes of infected species [1–4]. As such, EVEs represent a fossil record of past viral infections that can be harnessed to trace the deep origins of viruses and decipher their intricate co-evolution with their hosts [1–12]. As a source of genetic material added to the host genome, EVEs provide a rich compendium of sequences previously serving viral replication that natural selection can act upon at the level of the host organism to foster the emergence of novel cellular function. Here we review a variety of molecular processes, cellular mechanisms, and biological pathways that appear to have repeatedly benefited from such viral co-option events. We place emphasis on recently described examples involving mammalian EVEs, but certainly the phenomenon of EVE cooption is not restricted to mammals [13–15]. While it is now clear that virtually any major type of virus can be endogenized, most coopted mammalian EVEs derive from endogenous retroviruses (ERVs) [4,16]. This bias reflects in part the fact that ERVs are the most common EVEs in mammals, where they account for ~5–15% of nuclear genome content [2,17,18].
EVE as restriction factors: fighting fire with fire
Antiviral function is a recurrent theme of EVE cooption. When expressed, EVE products can in principle interfere with any step of viral infection, thereby acting as restriction factors. The most direct mechanisms of restriction are those involving direct interactions between EVE-derived peptides with viral or cellular proteins that control virus replication (Figure 1). In multiple vertebrates, ERV-encoded envelope (Env) proteins protect host cells from viral entry by competing with exogenous Env for cell surface receptors, a phenomenon analogous to superinfection resistance [19] (Figure 1A and Figure 2). To date, no human ERV (HERV) Env have been reported to restrict modern exogenous retroviruses. However, a recent ‘paleovirological’ study revealed that a primate-specific env derived from a copy of the HERV-T gammaretrovirus family is capable of restricting an experimentally reconstituted HERV-T Env-mediated infection [20]. These data suggest that the acquisition of this endogenous HERV-T Env gene, which has evolved under functional constraint in the human lineage, may have led to the extinction of the cognate retrovirus infecting our ancestors [20,21]. It cannot be excluded, however, that this HERV-T Env locus has been evolutionary preserved to serve another cellular function distinct from viral restriction [20].
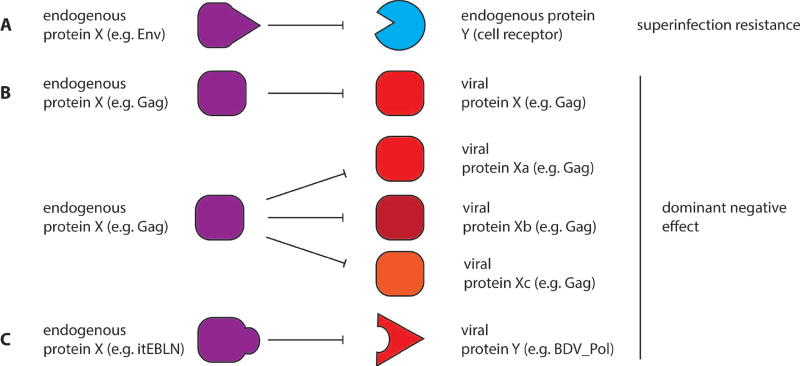
Figure 1. Direct interference of EVE proteins with exogenous viral replication.
Coopted EVE proteins can compete with virus replication by binding cellular proteins otherwise bound by exogenous virus (A). Physical interactions between coopted EVE proteins and homologous (B) or non-homologous (C) proteins encoded by exogenous viruses can result in dominant-negative effects on virus replication.
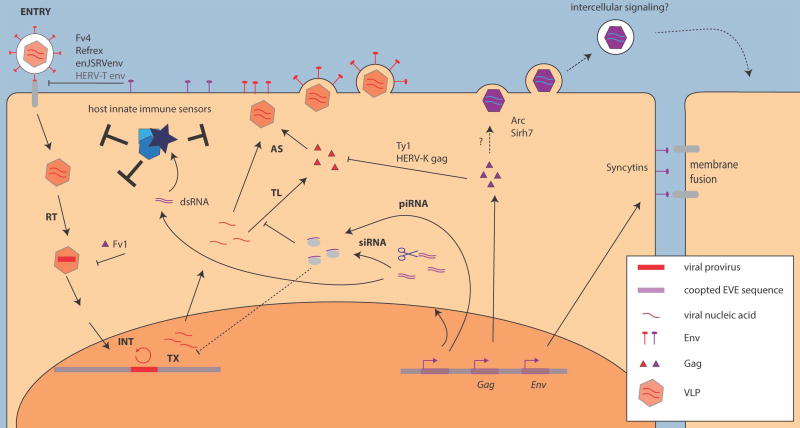
Figure 2. Mechanisms of EVE co-option for antiviral immunity and cell physiology.
A prototypical retroviral life cycle (shown in red) proceeds through cell entry (ENTRY), reverse transcription (RT), chromosomal integration (INT), proviral transcription (TX), translation (TL) and particle assembly (AS). EVE-encoded proteins and RNAs (shown in purple) can interfere with many steps of virus replication. EVE-encoded proteins may block virus entry (Env), provirus release (Gag), virus genome replication, and capsid assembly (Gag). Small RNAs (piRNAs, siRNAs) derived from EVE loci may also repress virus expression transcriptionally or post-transcriptionally. EVEs can also mediate cell fusion (Env) and may be involved in intercellular signaling (Gag). Viral proteins and nucleic acids can be recognized by host innate immune sensors (shown in blue) resulting in stimulation of the innate immune response.
Several ERV-derived Gag proteins are known to interfere with post-entry steps of the infection cycle of exogenous retroviruses. For example, the mouse Fv1 protein restricts murine leukemia virus (MLV) prior to chromosomal integration (Figure 2), by restricting capsid disassembly through direct binding to MLV capsid proteins [22,23]. As Gag proteins accumulate mutations, while remaining expressed, endogenous Gags may also interfere with their exogenous counterparts by exerting trans-dominant negative effects on virus particle assembly or release [24–26] (Figure 1B). This restriction mechanism has been documented for the sheep enJSRV [24] and a similar mechanism involving the production of truncated Gag isoforms is used by the yeast Ty1 long terminal repeat (LTR) retrotransposon, a retroviral-like element, as a form of copy number control [25,26] (Figure 2).
Such direct, conflicting interactions between EVE- and viral-encoded proteins are likely to drive rapid adaptive evolution of both viral and coopted endogenous genes. The resulting allelic diversification of EVE-derived genes may lead to the selection of alleles that expand the range of viruses restricted by this mechanism (Figure 1B). This scenario would explain why Fv1, which exhibits a strong signature of diversifying selection in mouse populations, presently restricts murine leukemia virus (MLV), despite being derived from an evolutionary distant lineage of retroviruses (ERV-L) [27,28]. Human-specific HERV-K Gag, which interferes with HIV-1 capsid assembly and release, may currently be serving such a restricting activity [29,30]. These observations indicate that co-option of ERV-derived proteins for viral defense is a common, dynamic, and ongoing evolutionary process.
It is also conceivable that EVE-derived proteins could interfere with exogenous viral replication by interacting with non-homologous viral proteins (Figure 1C). This model is supported by a recent study of endogenous bornavirus-like nucleoproteins (EBLN) encoded in the ground squirrel genome (itEBLN). Cell culture experiments showed that itEBLN expression conferred resistance to human Borna Disease Virus infection by inhibiting viral polymerase activity [31]. These observations may point to a more common theme of EVE cooption for viral defense that merits further investigation.
A recent study of the Mavirus virophage, a small DNA virus that parasitizes the machinery of the giant DNA virus Cafeteria roenbergensis virus (CroV) suggests a path through which EVE-mediated antiviral immunity may be established [32]. The authors show that Mavirus integrates within the genome of its marine host protozoan, but lays dormant until transcriptionally activated in response to CroV superinfection. Lysis of cells containing Mavirus particles inhibits CroV replication in neighboring cells thereby enhancing host survival while permitting Mavirus replication [32]. This study illustrates how mutualistic interactions between a virus capable of endogenization and its host may pave the way towards cooption.
Immune systems under EVE influence
There is growing evidence that the acquisition of EVEs can shape host immune systems in various ways. Notably EVE-derived noncoding sequences may act as cis-regulatory DNA enhancers of antiviral or pro-inflammatory genes (Figure 3A). The LTRs of mammalian ERVs frequently contain interferon-inducible enhancers that in some instances have been coopted to regulate adjacent host genes encoding critical innate immune factors [33,34]. A need for more efficient immune induction may have provided the selective pressure on ERV LTR sequences, which initially controlled proviral genes, to be maintained in the host population. Over the course of evolution, recombination between proviral LTRs, which results in the loss of internal ERV genes, would have eliminated the potential fitness cost of expressing ERV sequences while still providing the beneficial enhancer effects of the LTR.
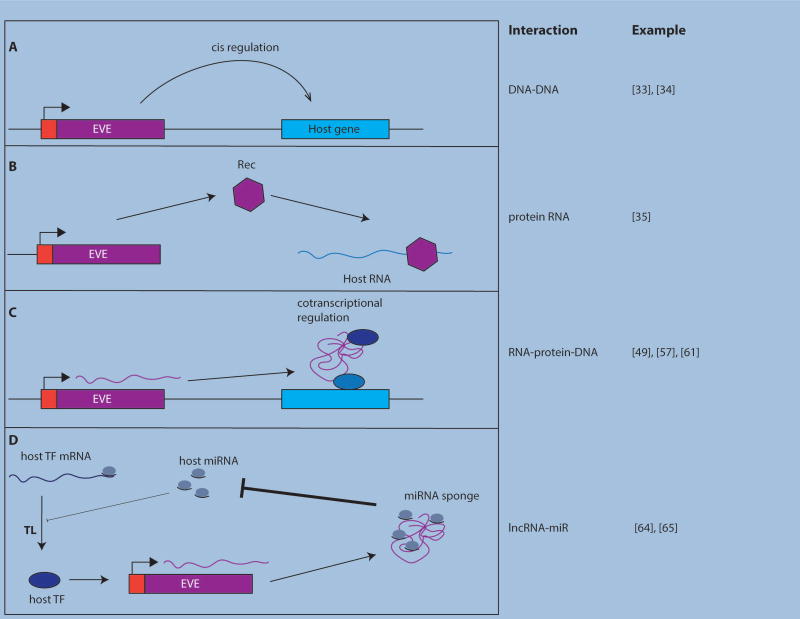
Figure 3. Coopted EVEs affect host gene expression by diverse mechanisms.
(A) EVE sequences may function as cis-regulatory DNA elements such as enhancers or promoters. (B) EVE-derived lncRNAs can also affect gene expression by acting as co-transcriptional regulators (C) or miRNA sponges (D). EVE-encoded proteins may also regulate gene expression. For instance, Rec and Gag proteins may bind to and modulate host mRNA stability, localization, or translation.
EVE-encoded proteins may also regulate the expression of innate immune factors (Figure 2). For instance, the HERV-K-encoded Rec protein is expressed in preimplantation embryos where it apparently modulates the translation of many cellular mRNAs (Figure 3B), which may have wide-ranging effects on embryonic function, including antiviral defenses [35]. Consistent with this idea, Rec overexpression in embryonic carcinoma cells confers resistance to H1N1 influenza virus infection [35]. Together these observations suggest that the expression of Rec during early development may prime embryonic cells for a rapid response to viral infection. In addition to their regulatory effects on immune gene expression, EVE-encoded proteins may also modulate host immunity more directly through processes linked to their viral origins. For instance, ERV-derived Env peptides can be recognized as antigens that effectively shape T cell repertoires and the humoral response [36,37]. In extreme cases, some endogenous Env can even behave as ‘superantigens’ eliciting non-specific T cell activation [38]. Yet other Env proteins can exert immunosuppressive effects that dampen the immune response [37,39]. While these various immune-modulatory properties have been investigated primarily in the context of ERV overexpression in certain disease states, it is tempting to speculate that some of these activities have coevolved with and become integral components of the host immune response. In all the cases described above, ancestral properties of ERV-encoded proteins appear to have been preserved to varying degrees for the benefit host immunity.
Other potentially protective effects of EVEs include the production of noncoding RNAs that act as adjuvants in antiviral systems (see Figure 2). For example, some EBLNs in rodents and primates appear to have inserted into piwi-interacting RNA (piRNA) genomic clusters and as a result produce piRNA-like RNAs in the testis [40]. Similarly, chickens also exhibit testis-specific piRNA expression, which appear to mostly map to young ALV derived ERV insertions, some of which are known to produce infectious viral particles [41]. It has been proposed that these small RNAs offer some protection to the host by silencing exogenous viral mRNAs [16,40,41]. It has also been reported that elevated levels of ERV-derived RNAs leads to the accumulation of cytosolic nucleic acids, including double-stranded RNAs and complementary DNAs, which are recognized by nucleic acid sensors that direct cells to mount an antiviral and inflammatory response [42–44]. These studies highlight how EVE-derived noncoding RNAs can directly or indirectly enhance antiviral immunity.
ERV choreography in early embryonic development
The early embryo represents a logical battleground for selfish genetic elements, including viruses, as it opens vulnerable routes for vertical and horizontal transmission [45]. In line with this paradigm, many genomics studies have revealed a complex interplay between ERV expression and early embryonic development [46–50]. For example, totipotent 2-cell (2C) mouse embryos are characterized by massive transcriptional activation of MERV-L loci [46,48]. Notably, a trio of recent studies showed that MERV-L activation is driven by the host transcription factor mouse Dux [51–53]. Past the 2C stage, mouse ESCs exhibit markedly reduced MERV-L transcription along with a subsequent peak in ERVK and MaLR expression [54] driven by binding of pluripotency-associated TFs like Nanog and Oct4 [54]. This choreography of ERV expression is likely to reflect regulatory pathways hijacked by different ERVs to take advantage of developmental niches that favor their own transcription and propagation [45]. But it raised the possibility that a subset of these elements has been coopted into the regulatory network orchestrating early mouse development. Consistent with this hypothesis, transient siRNA-mediated depletion of a subset of ERVK- and MaLR-derived long noncoding RNAs (lncRNA) highly expressed in mouse ESCs leads to reduced expression of cellular pluripotency markers, suggesting that these lncRNAs exert some form of control over the maintenance of a pluripotent state [54]. Similarly, a recent biochemical study showed that a lncRNA derived from a MERV-L locus, called LincGET, is required for in vitro embryonic development to proceed beyond the 2C stage [49]. Biochemical experiments and reporter assays suggest that LincGET functions as a scaffold for the recruitment of TFs and splicing factors (Figure 3C), some of which are known to be important for embryonic development [49,55].
A strikingly convergent pattern is emerging in human embryonic development involving primate-specific ERVs. Deep RNA sequencing has revealed that the expression of individual HERV families is precisely regulated during early embryonic development [35,51,56]. Notably, DUX4, a human homolog of mouse Dux, appears to be a crucial regulator of HERV-L LTR transcription in 4-cell-stage embryos [51,53]. Hundreds of ape-specific HERV-H elements are also transcriptionally activated by pluripotency TFs in human ESCs [57–60]. Knockdown experiments indicate that HERV-H transcript levels positively correlate with the expression of pluripotency factors and the ‘stemness’ of certain embryonic cell subpopulations [57,61,62]. Recent studies of the HERV-H-derived lncRNA lnc-RoR [63,64] and of another lncRNA called HPAT5 [65] derived from a distinct HERV family revealed that both lncRNAs, despite their distinct evolutionary origins, act as miRNA sponges (Figure 3D) to dampen miRNA-mediated translation repression of Nanog and other TFs. These results establish a mechanistic framework to understand how the levels of HERV-derived lncRNAs modulate the pluripotency of ESCs.
The data summarized above suggest that the finely tuned, stage-specific transcriptional activities of human and mouse ERVs may have been co-opted to orchestrate early embryonic development through cis- and trans-regulatory mechanisms. However, more work is needed to test whether these regulatory activities have become truly indispensable for proper embryonic development or are merely relics of selfish manipulations that facilitated ERV propagation.
The placenta as a hotspot of EVE cooption
At the interface between maternal and fetal tissues, the placenta must mediate nutrient exchange between mother and fetus, protect the fetus from infection by maternally carried pathogens, while avoiding stimulation of the maternal immune system. The trophoblast layer of the placenta exhibits globally elevated EVE expression, which is potentiated by a seemingly general hypomethylation of repetitive DNA [66–68]. In addition, the LTRs of several ERV families exhibit placenta-specific enhancer activity [69,70] (Figure 3A). Together these properties open the door for the cooption of certain LTRs to drive novel adaptive pattern of host gene expression. A recently described example is a primate-specific HERVP71A-LTR that functions as an enhancer for HLA-G expression in human extravillous trophoblasts, which confers maternal immune tolerance to the developing placenta by inhibiting natural killer cell-mediated cytotoxicity [70,71].
The frequent transcriptional activity of EVEs in the placenta may also facilitate the cooption of some of their gene products to foster the remarkable anatomical diversification of this organ. A classic example is provided by the syncytins, which are endogenous retroviral Env genes highly expressed in the placenta that have been coopted in diverse mammals [72,73]. Syncytins typically preserve the fusogenic activity of the ancestral Env, and genetic studies of mouse syncytins have established that this activity is essential for the formation of the bi-layered syncytiotrophoblast characteristic of the murid placenta [72,74,75] (Figure 2). Interestingly, multiple syncytins have been independently acquired from various ERVs in several mammalian lineages, suggesting Env co-option as a recurrent force driving the evolution of placentation [72,75]. Interestingly, the fusogenic properties of syncytins also appear to have been harnessed to support sex-specific muscle development because knockout of syncytin B in mouse results in reduced myoblast fusion and muscle mass in males [76] (Figure 2). These data illustrate how the biochemical properties of viral envelopes have been recycled multiple times during evolution to serve mammalian development.
Gag proteins from ancient LTR retrotransposons have also been repurposed for placenta biology in both marsupial and eutherian mammals [77]. Mouse knockout studies indicate that at least three ancient Gag genes derived from distinct retrotransposon families, Peg10, Peg11, and Sirh7, are required for successful completion of pregnancy [78–82]. Though biochemical studies of these Gag-derived proteins are sparse, current evidence suggests that they have distinct, non-redundant cellular functions [83–87]. This is not unexpected because retroviral and retrotransposon Gag proteins exert a variety of biochemical functions, including complex nucleic acid-, protein-, and lipid-binding activities [88–90]. It is therefore possible that the sole common factor driving co-option of these ancient Gags in placenta may have been placenta-specific expression of these genes. Interestingly, two of these genes (Peg10, Peg11) are only expressed from the paternal allele, yet reside in different regions of the genome – suggesting a predisposition for genomic imprinting and/or that their cooption was driven by parental conflict [91,92].
EVE coopted for brain function
Whereas most coopted EVEs tend to be derived from younger elements, several ancient retrotransposon-derived Gag proteins appear to have contributed to the evolution of the mammalian brain [93–95]. In particular, Arc has emerged as a significant player in memory formation and brain development [96,97]. Molecular studies indicate Arc regulates glutamate receptor turnover, a process key to the regulation of synaptic plasticity [94,98]. Additionally, Arc plays a role in synapse pruning during brain development [97]. Far less is known about Sirh11, another Gag-derived gene that is strongly conserved across eutherians and highly expressed in the brain [95,99]. Knockout of Sirh11 in mice has revealed behavioral alterations that may be explained by reduced extracellular noradrenaline levels in the prefrontal cortex [95]. Thus, like Arc, Sirh11 appears to play a role in neuronal signal transmission. While it is unclear what property these Gag-derived proteins share, it is likely that ancestral activities typical of Gag proteins, such as membrane binding or capsid assembly, may have been repurposed for cellular processes serving brain function.
Outlook
The viral life cycle is intimately intertwined with cell physiology because virus replication is inherently dependent on the cell’s machinery and function. Consequently, viruses have established complex interactions with host cellular factors, often involving direct physical interactions. The endogenization of viral sequences offers an opportunity for these activities to be deployed in a different cellular context, which may occasionally benefit host fitness leading to their fixation and cooption. Indeed, mechanistic studies of coopted EVEs have revealed that their functional activities are often directly descended from their ancestral viral sequences. For instance, the physical binding of cellular factors by coopted EVE-encoded proteins, such as Env [72,76] and Gag [25,26] can frequently be traced to ancestral protein interaction domains preexisting in the viral proteins. Likewise, coopted EVE-encoded regulatory sequences are typically derived from ancestral TF binding sites that were presumably used formerly by the virus to promote expression of their own genes [33,69,70,100]. This model does not preclude that some host-EVE adaptive interfaces evolve de novo through sequence modification and fortuitous interactions. The pairing of EVE-derived lncRNA with a host-encoded miRNA might represent such a fortuitous interaction that could have provided an initial selective advantage to the host, and possibly also to the virus, as a mechanism to dampen viral expression. Regardless of their origins, any emerging host-EVE interaction that mitigates the conflict between cell and virus is predicted to promote the fixation, retention, and diversification of an EVE [32]. In turn, this cascade might facilitate the emergence of novel adaptive contributions from the coopted EVE sequence. Such a steppingstone model might explain why some transitions from viral to cellular functions (e.g. Syncytins, LTRs, Fv1 [28,33,72,101]) have occurred repeatedly during evolution to establish seemingly redundant or convergent organismal function.
Highlights.
Endogenous viral elements (EVEs) account for a substantial fraction (~10%) of mammalian genomes
EVEs represent an abundant source of coding and noncoding sequences
EVEs of diverse origin and age have been coopted for cellular function
Host immunity and development have disproportionately benefited from EVE co-option
EVEs that relieve host-virus conflict may be a steppingstone toward cooption for other organismal processes, including host development
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feschotte C, Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat. Rev. Genet. 2012;13:283–296. doi: 10.1038/nrg3199. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WE. Endogenous Retroviruses in the Genomics Era. Annu. Rev. Virol. 2015;2:135–159. doi: 10.1146/annurev-virology-100114-054945. [DOI] [PubMed] [Google Scholar]
- 3.Metegnier G, Becking T, Chebbi MA, Giraud I, Moumen B, Schaack S, Cordaux R, Gilbert C. Comparative paleovirological analysis of crustaceans identifies multiple widespread viral groups [Internet] Mob. DNA. 2015;6 doi: 10.1186/s13100-015-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiewsakun P, Katzourakis A. Endogenous viruses: Connecting recent and ancient viral evolution. Virology. 2015;479–480:26–37. doi: 10.1016/j.virol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert C, Feschotte C. Genomic fossils calibrate the long-term evolution of hepadnaviruses. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewiadomska AM, Gifford RJ. The Extraordinary Evolutionary History of the Reticuloendotheliosis Viruses. PLOS Biol. 2013;11:e1001642. doi: 10.1371/journal.pbio.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aswad A, Katzourakis A. Convergent capture of retroviral superantigens by mammalian herpesviruses. Nat. Commun. 2015;6:8299. doi: 10.1038/ncomms9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc G, Gallot-Lavallée L, Maumus F. Provirophages in the Bigelowiella genome bear testimony to past encounters with giant viruses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E5318–E5326. doi: 10.1073/pnas.1506469112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward A, Cornwallis CK, Jern P. Pan-vertebrate comparative genomics unmasks retrovirus macroevolution. Proc. Natl. Acad. Sci. 2015;112:464–469. doi: 10.1073/pnas.1414980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han G-Z, Worobey M. A Primitive Endogenous Lentivirus in a Colugo: Insights into the Early Evolution of Lentiviruses. Mol. Biol. Evol. 2015;32:211–215. doi: 10.1093/molbev/msu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diehl WE, Patel N, Halm K, Johnson WE. Tracking interspecies transmission and long-term evolution of an ancient retrovirus using the genomes of modern mammals. eLife. 2016;5:e12704. doi: 10.7554/eLife.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiewsakun P, Katzourakis A. Marine origin of retroviruses in the early Palaeozoic Era. Nat. Commun. 2017;8:13954. doi: 10.1038/ncomms13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik HS, Henikoff S. Positive Selection of Iris, a Retroviral Envelope–Derived Host Gene in Drosophila melanogaster [Internet] PLoS Genet. 2005;1 doi: 10.1371/journal.pgen.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinzelle L, Carradec Q, Paillard E, Bronchain OJ, Pollet N. Characterization of a Xenopus tropicalis endogenous retrovirus with developmental and stress-dependent expression. J. Virol. 2011;85:2167–2179. doi: 10.1128/JVI.01979-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henzy JE, Gifford RJ, Kenaley CP, Johnson WE. An Intact Retroviral Gene Conserved in Spiny-Rayed Fishes for over 100 My [Internet] Mol. Biol. Evol. 2016 doi: 10.1093/molbev/msw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda T, Tomonaga K. Endogenous non-retroviral RNA virus elements evidence a novel type of antiviral immunity [Internet] Mob. Genet. Elem. 2016;6 doi: 10.1080/2159256X.2016.1165785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewannieux M, Heidmann T. Endogenous retroviruses: acquisition, amplification and taming of genome invaders. Curr. Opin. Virol. 2013;3:646–656. doi: 10.1016/j.coviro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo X, Rho M, Feschotte C. Genome-Wide Characterization of Endogenous Retroviruses in the Bat Myotis lucifugus Reveals Recent and Diverse Infections. J. Virol. 2013;87:8493–8501. doi: 10.1128/JVI.00892-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfavon-Borja R, Feschotte C. Fighting Fire with Fire: Endogenous Retrovirus Envelopes as Restriction Factors. J. Virol. 2015;89:4047–4050. doi: 10.1128/JVI.03653-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Blanco-Melo D, Gifford RJ, Bieniasz PD. Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors. eLife. 2017;6:e22519. doi: 10.7554/eLife.22519. The authors demonstrate that a primate specific copy of HERV-T env can restrict infection mediated by an experimentally reconstituted HERV-T env. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parseval N, de Lazar V, Casella J-F, Benit L, Heidmann T. Survey of Human Genes of Retroviral Origin: Identification and Transcriptome of the Genes with Coding Capacity for Complete Envelope Proteins. J. Virol. 2003;77:10414–10422. doi: 10.1128/JVI.77.19.10414-10422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilditch L, Matadeen R, Goldstone DC, Rosenthal PB, Taylor IA, Stoye JP. Ordered assembly of murine leukemia virus capsid protein on lipid nanotubes directs specific binding by the restriction factor, Fv1. Proc. Natl. Acad. Sci. 2011;108:5771–5776. doi: 10.1073/pnas.1100118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstone DC, Walker PA, Calder LJ, Coombs PJ, Kirkpatrick J, Ball NJ, Hilditch L, Yap MW, Rosenthal PB, Stoye JP, et al. Structural studies of postentry restriction factors reveal antiparallel dimers that enable avid binding to the HIV-1 capsid lattice. Proc. Natl. Acad. Sci. 2014;111:9609–9614. doi: 10.1073/pnas.1402448111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mura M, Murcia P, Caporale M, Spencer TE, Nagashima K, Rein A, Palmarini M. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11117–11122. doi: 10.1073/pnas.0402877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha A, Mitchell JA, Nishida Y, Hildreth JE, Ariberre JA, Gilbert WV, Garfinkel DJ. A trans- Dominant Form of Gag Restricts Ty1 Retrotransposition and Mediates Copy Number Control. J. Virol. 2015;89:3922–3938. doi: 10.1128/JVI.03060-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida Y, Pachulska-Wieczorek K, Błaszczyk L, Saha A, Gumna J, Garfinkel DJ, Purzycka KJ. Ty1 retrovirus-like element Gag contains overlapping restriction factor and nucleic acid chaperone functions. Nucleic Acids Res. 2015;43:7414–7431. doi: 10.1093/nar/gkv695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan Y, Buckler-White A, Wollenberg K, Kozak CA. Origin, antiviral function and evidence for positive selection of the gammaretrovirus restriction gene Fv1 in the genus Mus. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3259–3263. doi: 10.1073/pnas.0900181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap MW, Colbeck E, Ellis SA, Stoye JP. Evolution of the Retroviral Restriction Gene Fv1 : Inhibition of Non-MLV Retroviruses. PLOS Pathog. 2014;10:e1003968. doi: 10.1371/journal.ppat.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monde K, Terasawa H, Nakano Y, Soheilian F, Nagashima K, Maeda Y, Ono A. Molecular mechanisms by which HERV-K Gag interferes with HIV-1 Gag assembly and particle infectivity. Retrovirology. 2017;14:27. doi: 10.1186/s12977-017-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monde K, Contreras-Galindo R, Kaplan MH, Markovitz DM, Ono A. Human Endogenous Retrovirus K Gag Coassembles with HIV-1 Gag and Reduces the Release Efficiency and Infectivity of HIV-1. J. Virol. 2012;86:11194–11208. doi: 10.1128/JVI.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Fujino K, Horie M, Honda T, Merriman DK, Tomonaga K. Inhibition of Borna disease virus replication by an endogenous bornavirus-like element in the ground squirrel genome. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13175–13180. doi: 10.1073/pnas.1407046111. The authors demonstrate that BDV genome replication in the nucleus is restricted by a dominant negative effect of EVE derived itEBLN. This is the first study to show that an endogenized EBL protein can interfere with BDV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Fischer MG, Hackl T. Host genome integration and giant virus-induced reactivation of the virophage mavirus. Nature. 2016;540:288–291. doi: 10.1038/nature20593. The authors demonstrate that Mavirus provirophages are activated upon exogenous infection of their host by an unrelated giant virus and form viral particles that offer neighboring cells protection against CroV replication. [DOI] [PubMed] [Google Scholar]
- 33**.Chuong EB, Elde NC, Feschotte C. Regulatory evolution of innate immunity through cooption of endogenous retroviruses. Science. 2016;351:1083–1087. doi: 10.1126/science.aad5497. The authors demonstrate that diverse ERV LTR families possess STAT1 binding sites that can function as enhancers of innate immunity genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manghera M, Ferguson-Parry J, Lin R, Douville RN. NF-κB and IRF1 Induce Endogenous Retrovirus K Expression via Interferon-Stimulated Response Elements in Its 5′ Long Terminal Repeat. J. Virol. 2016;90:9338–9349. doi: 10.1128/JVI.01503-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Grow EJ, Flynn RA, Chavez SL, Bayless NL, Wossidlo M, Wesche DJ, Martin L, Ware CB, Blish CA, Chang HY, et al. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature. 2015;522:221–225. doi: 10.1038/nature14308. The authors demonstrate that HERV-K gene products, including the accessory protein Rec, are expressed and form virus-like particles in human preimplantation embryos. Furthermore, they show that Rec binds to and potentially modulate the expression a wide range of host mRNAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert PJR, Jiang S, Xie J, Li Q-J, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat. Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassiotis G, Stoye JP. Immune responses to endogenous retroelements: taking the bad with the good. Nat. Rev. Immunol. 2016;16:207–219. doi: 10.1038/nri.2016.27. [DOI] [PubMed] [Google Scholar]
- 38.Tai AK, Lin M, Chang F, Chen G, Hsiao F, Sutkowski N, Huber BT. Murine Vβ3+ and Vβ7+ T Cell Subsets Are Specific Targets for the HERV-K18 Env Superantigen. J. Immunol. 2006;177:3178–3184. doi: 10.4049/jimmunol.177.5.3178. [DOI] [PubMed] [Google Scholar]
- 39.Schlecht-Louf G, Renard M, Mangeney M, Letzelter C, Richaud A, Ducos B, Bouallaga I, Heidmann T. Retroviral infection in vivo requires an immune escape virulence factor encrypted in the envelope protein of oncoretroviruses. Proc. Natl. Acad. Sci. 2010;107:3782–3787. doi: 10.1073/pnas.0913122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parrish NF, Fujino K, Shiromoto Y, Iwasaki YW, Ha H, Xing J, Makino A, Kuramochi-Miyagawa S, Nakano T, Siomi H, et al. piRNAs derived from ancient viral processed pseudogenes as transgenerational sequence-specific immune memory in mammals. RNA. 2015;21:1691–1703. doi: 10.1261/rna.052092.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun YH, Xie LH, Zhuo X, Chen Q, Ghoneim D, Zhang B, Jagne J, Yang C, Li XZ. Domestic chickens activate a piRNA defense against avian leukosis virus. eLife. 2017;6:e24695. doi: 10.7554/eLife.24695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng M, Hu Z, Shi X, Li X, Zhan X, Li X-D, Wang J, Choi JH, Wang K, Purrington T, et al. MAVS, cGAS, and endogenous retroviruses in T-independent B cell responses. Science. 2014;346:1486–1492. doi: 10.1126/science.346.6216.1486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43*.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. See [44] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, Han H, Liang G, Jones PA, Pugh TJ, et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell. 2015;162:961–973. doi: 10.1016/j.cell.2015.07.056. This study and [43] demonstrate that loss of DNA methylation induces an interferon response through the transcriptional activation of ERVs. These studies demonstrate that, within a cancer context, ERV transcription can serve to stimulate an immune response through the activation of dsRNA sensors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haig D. Transposable elements: Self-seekers of the germline, team-players of the soma. BioEssays. 2016;38:1158–1166. doi: 10.1002/bies.201600125. [DOI] [PubMed] [Google Scholar]
- 46.Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A, Singer O, Trono D, Pfaff SL. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maksakova IA, Thompson PJ, Goyal P, Jones SJ, Singh PB, Karimi MM, Lorincz MC. Distinct roles of KAP1, HP1 and G9a/GLP in silencing of the two-cell-specific retrotransposon MERVL in mouse ES cells. Epigenetics Chromatin. 2013;6:15. doi: 10.1186/1756-8935-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiuchi T, Enriquez-Gasca R, Mizutani E, Bošković A, Ziegler-Birling C, Rodriguez-Terrones D, Wakayama T, Vaquerizas JM, Torres-Padilla M-E. Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat. Struct. Mol. Biol. 2015;22:662–671. doi: 10.1038/nsmb.3066. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Li X, Wang L, Li J, Zhao Y, Bou G, Li Y, Jiao G, Shen X, Wei R, et al. A novel long intergenic noncoding RNA indispensable for the cleavage of mouse two-cell embryos. EMBO Rep. 2016;17:1452–1470. doi: 10.15252/embr.201642051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi YJ, Lin C-P, Risso D, Chen S, Kim TA, Tan MH, Li JB, Wu Y, Chen C, Xuan Z, et al. Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells. Science. 2017;355:eaag1927. doi: 10.1126/science.aag1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendrickson PG, Doráis JA, Grow EJ, Whiddon JL, Lim J-W, Wike CL, Weaver BD, Pflueger C, Emery BR, Wilcox AL, et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017;49:925–934. doi: 10.1038/ng.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiddon JL, Langford AT, Wong C-J, Zhong JW, Tapscott SJ. Conservation and innovation in the DUX4-family gene network. Nat. Genet. 2017;49:935–940. doi: 10.1038/ng.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017;49:941–945. doi: 10.1038/ng.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fort A, Hashimoto K, Yamada D, Salimullah M, Keya CA, Saxena A, Bonetti A, Voineagu I, Bertin N, Kratz A, et al. Deep transcriptome profiling of mammalian stem cells supports a regulatory role for retrotransposons in pluripotency maintenance. Nat. Genet. 2014;46:558–566. doi: 10.1038/ng.2965. [DOI] [PubMed] [Google Scholar]
- 55.Zhou W, Chung YJ, Parrilla Castellar ER, Zheng Y, Chung H-J, Bandle R, Liu J, Tessarollo L, Batchelor E, Aplan PD, et al. Far Upstream Element Binding Protein Plays a Crucial Role in Embryonic Development, Hematopoiesis, and Stabilizing Myc Expression Levels. Am. J. Pathol. 2016;186:701–715. doi: 10.1016/j.ajpath.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Göke J, Lu X, Chan Y-S, Ng H-H, Ly L-H, Sachs F, Szczerbinska I. Dynamic Transcription of Distinct Classes of Endogenous Retroviral Elements Marks Specific Populations of Early Human Embryonic Cells. Cell Stem Cell. 2015;16:135–141. doi: 10.1016/j.stem.2015.01.005. See [59] [DOI] [PubMed] [Google Scholar]
- 57.Ng S-Y, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santoni FA, Guerra J, Luban J. HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency. Retrovirology. 2012;9:111. doi: 10.1186/1742-4690-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Wang J, Xie G, Singh M, Ghanbarian AT, Raskó T, Szvetnik A, Cai H, Besser D, Prigione A, Fuchs NV, et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–409. doi: 10.1038/nature13804. This study and [56] demonstrate that the transcriptional activity of ERV families can mark with remarkable precision certain stem cell populations during embryonic development. [DOI] [PubMed] [Google Scholar]
- 60.Izsvák Z, Wang J, Singh M, Mager DL, Hurst LD. Pluripotency and the endogenous retrovirus HERVH: Conflict or serendipity? BioEssays. 2016;38:109–117. doi: 10.1002/bies.201500096. [DOI] [PubMed] [Google Scholar]
- 61.Lu X, Sachs F, Ramsay L, Jacques P-É, Göke J, Bourque G, Ng H-H. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat. Struct. Mol. Biol. 2014;21:423–425. doi: 10.1038/nsmb.2799. [DOI] [PubMed] [Google Scholar]
- 62.Ohnuki M, Tanabe K, Sutou K, Teramoto I, Sawamura Y, Narita M, Nakamura M, Tokunaga Y, Nakamura M, Watanabe A, et al. Dynamic regulation of human endogenous retroviruses mediates factor-induced reprogramming and differentiation potential. Proc. Natl. Acad. Sci. 2014;111:12426–12431. doi: 10.1073/pnas.1413299111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loewer S, Cabili MN, Guttman M, Loh Y-H, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA Sponge lincRNA-RoR Regulates Oct4, Nanog, and Sox2 in Human Embryonic Stem Cell Self-Renewal. Dev. Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 65*.Durruthy-Durruthy J, Sebastiano V, Wossidlo M, Cepeda D, Cui J, Grow EJ, Davila J, Mall M, Wong WH, Wysocka J, et al. The primate-specific noncoding RNA HPAT5 regulates pluripotency during human preimplantation development and nuclear reprogramming. Nat. Genet. 2016;48:44–52. doi: 10.1038/ng.3449. The authors demonstrate that the ERV-derived lncRNA HPAT5 regulates pluripotency in naive human embryonic cells by acting as a miRNA sponge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman V, Forrester L, Sanford J, Hastie N, Rossant J. Cell lineage-specific undermethylation of mouse repetitive DNA. Nature. 1984;307:284–286. doi: 10.1038/307284a0. [DOI] [PubMed] [Google Scholar]
- 67.Sanford JP, Chapman VM, Rossant J. DNA methylation in extraembryonic lineages of mammals. Trends Genet. 1985;1:89–93. [Google Scholar]
- 68.Chuong EB. Retroviruses facilitate the rapid evolution of the mammalian placenta. BioEssays. 2013;35:853–861. doi: 10.1002/bies.201300059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chuong EB, Rumi MAK, Soares MJ, Baker JC. Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat. Genet. 2013;45:325–329. doi: 10.1038/ng.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70*.Ferreira LMR, Meissner TB, Mikkelsen TS, Mallard W, O’Donnell CW, Tilburgs T, Gomes HAB, Camahort R, Sherwood RI, Gifford DK, et al. A distant trophoblast-specific enhancer controls HLA-G expression at the maternal–fetal interface. Proc. Natl. Acad. Sci. 2016;113:5364–5369. doi: 10.1073/pnas.1602886113. The authors demonstrate that a primate-specific ERV1-family LTR functions as an enhancer to drive HLA-G expression in the placenta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The α1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: Is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc. Natl. Acad. Sci. 1997;94:5249–5254. doi: 10.1073/pnas.94.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T. Paleovirology of “syncytins”, retroviral env genes exapted for a role in placentation. Phil Trans R Soc B. 2013;368:20120507. doi: 10.1098/rstb.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornelis G, Vernochet C, Carradec Q, Souquere S, Mulot B, Catzeflis F, Nilsson MA, Menzies BR, Renfree MB, Pierron G, et al. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc. Natl. Acad. Sci. 2015;112:E487–E496. doi: 10.1073/pnas.1417000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupressoir A, Lavialle C, Heidmann T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta. 2012;33:663–671. doi: 10.1016/j.placenta.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Vernochet C, Redelsperger F, Harper F, Souquere S, Catzeflis F, Pierron G, Nevo E, Heidmann T, Dupressoir A. The Captured Retroviral Envelope syncytin-A and syncytin-B Genes Are Conserved in the Spalacidae Together with Hemotrichorial Placentation. Biol. Reprod. 2014;91:148. doi: 10.1095/biolreprod.114.124818. [DOI] [PubMed] [Google Scholar]
- 76*.Redelsperger F, Raddi N, Bacquin A, Vernochet C, Mariot V, Gache V, Blanchard-Gutton N, Charrin S, Tiret L, Dumonceaux J, et al. Genetic Evidence That Captured Retroviral Envelope syncytins Contribute to Myoblast Fusion and Muscle Sexual Dimorphism in Mice. PLOS Genet. 2016;12:e1006289. doi: 10.1371/journal.pgen.1006289. The authors demonstrate that mouse syncytins known to be crucial for placenta development are also important for male-specific muscle development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaneko-Ishino T. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals [Internet] Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sekita Y, Wagatsuma H, Nakamura K, Ono R, Kagami M, Wakisaka N, Hino T, Suzuki-Migishima R, Kohda T, Ogura A, et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat. Genet. 2008;40:243–248. doi: 10.1038/ng.2007.51. [DOI] [PubMed] [Google Scholar]
- 79.Ono R, Nakamura K, Inoue K, Naruse M, Usami T, Wakisaka-Saito N, Hino T, Suzuki-Migishima R, Ogonuki N, Miki H, et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2006;38:101–106. doi: 10.1038/ng1699. [DOI] [PubMed] [Google Scholar]
- 80*.Naruse M, Ono R, Irie M, Nakamura K, Furuse T, Hino T, Oda K, Kashimura M, Yamada I, Wakana S, et al. Sirh7/Ldoc1 knockout mice exhibit placental P4 overproduction and delayed parturition. Dev. Camb. Engl. 2014;141:4763–4771. doi: 10.1242/dev.114520. The authors demonstrate through mouse knockout experiments that the Gag-derived protein Sirh7 contributes to fetal development and parturition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ito M, Sferruzzi-Perri AN, Edwards CA, Adalsteinsson BT, Allen SE, Loo T-H, Kitazawa M, Kaneko-Ishino T, Ishino F, Stewart CL, et al. A trans-homologue interaction between reciprocally imprinted miR-127 and Rtl1 regulates placenta development. Dev. Camb. Engl. 2015;142:2425–2430. doi: 10.1242/dev.121996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koppes E, Himes KP, Chaillet JR. Partial Loss of Genomic Imprinting Reveals Important Roles for Kcnq1 and Peg10 Imprinted Domains in Placental Development [Internet] PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okabe H, Satoh S, Furukawa Y, Kato T, Hasegawa S, Nakajima Y, Yamaoka Y, Nakamura Y. Involvement of PEG10 in Human Hepatocellular Carcinogenesis through Interaction with SIAH1. Cancer Res. 2003;63:3043–3048. [PubMed] [Google Scholar]
- 84.Lux A, Beil C, Majety M, Barron S, Gallione CJ, Kuhn H-M, Berg JN, Kioschis P, Marchuk DA, Hafner M. Human Retroviral gag- and gag-pol-like Proteins Interact with the Transforming Growth Factor-β Receptor Activin Receptor-like Kinase 1. J. Biol. Chem. 2005;280:8482–8493. doi: 10.1074/jbc.M409197200. [DOI] [PubMed] [Google Scholar]
- 85.Inoue M, Takahashi K, Niide O, Shibata M, Fukuzawa M, Ra C. LDOC1, a novel MZF-1- interacting protein, induces apoptosis. FEBS Lett. 2005;579:604–608. doi: 10.1016/j.febslet.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 86.Chen H, Sun M, Liu J, Tong C, Meng T. Silencing of Paternally Expressed Gene 10 Inhibits Trophoblast Proliferation and Invasion [Internet] PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0144845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X, Xiao R, Tembo K, Hao L, Xiong M, Pan S, Yang X, Yuan W, Xiong J, Zhang Q. PEG10 promotes human breast cancer cell proliferation, migration and invasion. Int. J. Oncol. 2016;48:1933–1942. doi: 10.3892/ijo.2016.3406. [DOI] [PubMed] [Google Scholar]
- 88.Campillos M, Doerks T, Shah PK, Bork P. Computational characterization of multiple Gag-like human proteins. Trends Genet. 2006;22:585–589. doi: 10.1016/j.tig.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Matreyek KA, Engelman A. Viral and Cellular Requirements for the Nuclear Entry of Retroviral Preintegration Nucleoprotein Complexes. Viruses. 2013;5:2483–2511. doi: 10.3390/v5102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freed EO. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haig D. Retroviruses and the Placenta. Curr. Biol. 2012;22:R609–R613. doi: 10.1016/j.cub.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat. Rev. Genet. 2014;15:517–530. doi: 10.1038/nrg3766. [DOI] [PubMed] [Google Scholar]
- 93.Mortelmans K, Wang-Johanning F, Johanning GL. The role of human endogenous retroviruses in brain development and function. APMIS. 2016;124:105–115. doi: 10.1111/apm.12495. [DOI] [PubMed] [Google Scholar]
- 94**.Zhang W, Wu J, Ward MD, Yang S, Chuang Y-A, Xiao M, Li R, Leahy DJ, Worley PF. Structural Basis of Arc Binding to Synaptic Proteins: Implications for Cognitive Disease. Neuron. 2015;86:490–500. doi: 10.1016/j.neuron.2015.03.030. The authors present a crystal structure of Arc confirming its Gag retrotransposon origin and characterize its interactions with synaptic proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95*.Irie M, Yoshikawa M, Ono R, Iwafune H, Furuse T, Yamada I, Wakana S, Yamashita Y, Abe T, Ishino F, et al. Cognitive Function Related to the Sirh11/Zcchc16 Gene Acquired from an LTR Retrotransposon in Eutherians [Internet] PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005521. Through mouse knockout experiments, the authors demonstrate that the Gag-derived protein Sirh11 plays a role in cognitive function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 Is Essential for the Consolidation of Synaptic Plasticity and Memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 97.Mikuni T, Uesaka N, Okuno H, Hirai H, Deisseroth K, Bito H, Kano M. Arc/Arg3.1 Is a Postsynaptic Mediator of Activity-Dependent Synapse Elimination in the Developing Cerebellum. Neuron. 2013;78:1024–1035. doi: 10.1016/j.neuron.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irie M, Koga A, Kaneko-Ishino T, Ishino F. An LTR Retrotransposon-Derived Gene Displays Lineage-Specific Structural and Putative Species-Specific Functional Variations in Eutherians [Internet] Front. Chem. 2016;4 doi: 10.3389/fchem.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flemr M, Malik R, Franke V, Nejepinska J, Sedlacek R, Vlahovicek K, Svoboda P. A Retrotransposon-Driven Dicer Isoform Directs Endogenous Small Interfering RNA Production in Mouse Oocytes. Cell. 2013;155:807–816. doi: 10.1016/j.cell.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Esnault C, Cornelis G, Heidmann O, Heidmann T. Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV Syncytin, Captured for a Function in Placentation [Internet] PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]