Abstract
Introduction
RTOG-0937 is a randomized phase-II trial evaluating 1-year OS with PCI or PCI plus consolidative radiation therapy (cRT) to intra-thoracic disease and extracranial metastases for ED-SCLC.
Methods
Patients with 1–4 extracranial metastases were eligible after CR or PR to chemotherapy. Randomization was to PCI or PCI+cRT to the thorax and metastases. Original stratification included PR vs CR after chemotherapy and 1 vs 2–4 metastases; age < 65 vs ≥ 65 was added after an observed imbalance. PCI was 25GY/10 fractions. cRT was 45GY/15 fractions. To detect an OS improvement from 30% to 45% with a 34% hazard reduction (HR=0·66) under a 0.1 type-1 error (1-sided) and 80% power, 154 patients were required.
Results
Ninety-seven patients were randomized between March, 2010 and February, 2015. Eleven patients were ineligible (nine PCI, two PCI+cRT), leaving 42 randomized to PCI and 44 to PCI+cRT. At planned interim analysis the study crossed the futility boundary for OS and was closed prior to meeting accrual target. Median follow-up was 9 months. One-year OS was not different between the groups: 60.1% [95% CI: 41.2–74.7%] for PCI and 50.8% [95% CI:34.0–65.3%] for PCI+cRT (p=0.21). Three and 12-month rates of progression were 53.3% and 79.6% for PCI, and 14.5% and 75% for PCI+cRT. Time to progression favored PCI+cRT, HR=0.53 (95% CI: 0.32–0.87, p=0.01). One-patient in each arm had Grade-4 therapy related toxicity and one had Grade-5 therapy related pneumonitis with PCI+cRT.
Conclusions
OS exceeded predictions for both arms. Consolidative RT delayed progression but did not improve 1-year OS.
Keywords: Small cell lung cancer, extensive disease, thoracic radiation therapy, PCI, oligometastases
Introduction
SCLC comprises approximately 13% of lung cancers diagnosed in the United States. The majority of patients with SCLC have metastatic disease.1 Primary therapy for ED-SCLC is 4–6 cycles of platinum-based chemotherapy followed by PCI in select patients. Although response rates to chemotherapy are high at 60–70%, time-to-progression and median survival are modest (4–6 months and < 10 months, respectively).1,2 Response and improvement in survival is limited for second-line chemotherapy, particularly if progression is within 30–90 days.3,4 PCI has been shown to decrease brain metastases and improve 1-year OS in patients that respond to chemotherapy.5 The use of consolidative thoracic radiation for ED-SCLC remains controversial even though it has been shown to improve survival and locoregional control in both prospective randomized trials6,7 and retrospective reviews.8,9
RTOG-0937 was designed to address a select patient population with oligometastitc small cell lung cancer (1–4 distant metastases) with consolidative RT to the locoregional thoracic disease and active sites of metastases after chemotherapy. The underlying hypothesis was that delaying progression of disease with radiation therapy will delay progression and improve overall survival in this favorable patient population.
We conducted a randomized phase-II trial designed to evaluate PCI alone or PCI and consolidative RT (PCI+cRT) to intrathoracic disease and limited extracranial metastases in patients with oligometastatic small cell lung cancer.
Materials and Methods
Patient selection
NRG Oncology/RTOG 0937 is a two-arm randomized phase-II study designed to assess whether the use of cRT in addition to PCI (PCI+cRT) would improve OS compared to PCI alone for patients with ED-SCLC. Protocol approval was received from the Institutional Review Board at each study site. Informed consent was obtained from each patient prior to participation. Patients were randomly assigned to PCI or PCI+cRT to the intrathoracic disease and residual metastases. Eligible patients had pathologically proven SCLC without brain metastases and with 1–4 extracranial metastases at diagnosis based on CT scan of the chest/abdomen and bone scan or PET/CT. Brain imaging was not required prior to chemotherapy in the absence of symptoms but was required prior to randomization. Eligible patients had PR or CR to 4–6 cycles of platinum based chemotherapy in a minimum of one site of disease and no evidence of progression at any site. Restaging was required within eight weeks of study entry. Imaging included CT of the chest/abdomen or PET/CT, bone scan or PET, and MRI of the brain (or CT if MRI was contraindicated). Other eligibility criteria included Zubrod PS 0–2, serum ALT and AST within 2.5×ULN and bilirubin <1.5×ULN within one week prior to study entry for patients with liver metastases, serum creatinine < 1.5×ULN for patients with renal or peri-renal metastases, absolute neutrophil count ≥ 1,000 cells/mm3, platelets ≥ 75,000 cells/mm3 and hemoglobin ≥ 8 g/dl.
Treatment
All patients were to receive 25Gy PCI at 2.5Gy/fraction. In Arm 2, thoracic radiation therapy to primary and involved regional nodes was required for all patients unless they had had palliative radiation therapy to the primary at diagnosis. Radiation was delivered to post-chemotherapy volumes including the site of the primary and involved nodal regions at diagnosis. Metastases were treated if they did not have a CR to chemotherapy. The recommended radiation dose to all extracranial sites was 45Gy delivered in 15 daily fractions of 3Gy. 30–40Gy was acceptable if dose reduction was necessary to meet normal tissue dose constraints. It was recommended that PCI be started concurrently with cRT although sequential therapy was allowed at the discretion of the treating physician.
Patients were evaluated after therapy at two weeks, one, two, six, nine, and 12 months, every six months for 2–3 years, then annually. CT of the chest/abdomen or PET/CT and brain imaging were required at each visit starting at two months.
Statistical Analysis
The study was designed to detect a 33.7% relative reduction in the risk of death (HR, 0.663) in the PCI+cRT as compared with PCI alone, at the significance level of 0.1 (1-sided) with 80% power. Based on the above, a total of 112 events and a sample size of 146 were required. Adjusting for an ineligibility or insufficient follow-up rate of up to 5%, the final targeted accrual was 154. Two interim analyses and a final analysis were planned for early stopping for efficacy and futility when 37 and 75 deaths from both arms were observed. The efficacy testing was based on the power family of test 10 with ∆=0, and the futility testing boundary was based on Rule C (at a nominal significance level of 0.005) as proposed by Freidlin and Korn.11 NRG Oncology/RTOG Data Monitoring Committee (DMC) oversaw the interim monitoring. Patients were equally randomized into the two arms, based on Zelen’s allocation scheme and stratified by response to treatment (CR vs. PR), number of metastases (1 vs. 2–4), and age (<65 vs. ≥65, added on July 15, 2014). Age was added as a stratification factor at the recommendation of the DMC when an unbalanced distribution between treatment arms was observed without knowledge of efficacy information.
All outcome times were calculated from the date of randomization to the date of event or, if no event, the date of last follow-up. For OS, the event is death due to any cause. The Kaplan-Meier method12 was used to estimate OS rates. For time to progression, the event was the first occurrence of recurrence/progression of pre-existing disease (regardless of prior response or treatment with RT) or development of new metastases. Death without failure was considered as a competing risk. Actual rates of time to progression were estimated using the cumulative incidence method13 by taking into account competing risks. The log-rank test14 was used to compare OS event rates between treatments. The Cox regression model15 was used to compare the treatment differences. For time to progression, competing risk events (death without progression) were censored at the time of competing event per the cause-specific competing risks analysis method.16 Adverse events were evaluated using the NCI CTCAE v4.0.
Results
The study opened to accrual March 18, 2010 and closed February 27, 2015. A planned interim analysis indicated that the study crossed the futility boundary for the primary endpoint and was closed prior to meeting the accrual target of 154 patients. The final analysis was performed in August 2015 with a median follow-up of nine months, based on all data received at NRG Oncology Statistics and DMC through July 15, 2015. 97 patients were randomized from 57 participating sites. Eleven patients were ineligible and excluded from analysis, nine patients randomized to PCI and two to PCI+cRT. Reasons for ineligibility included failure to receive protocol therapy (two), inability to confirm eligibility (two), testing completed outside the required time frame (four), brain metastases (one), chemotherapy completed > eight weeks from study entry (one), and platelet count outside of required range (one). 86 patients, 42 randomized to PCI and 44 to PCI+cRT, were included in the analysis (Figure 1).
Figure 1.
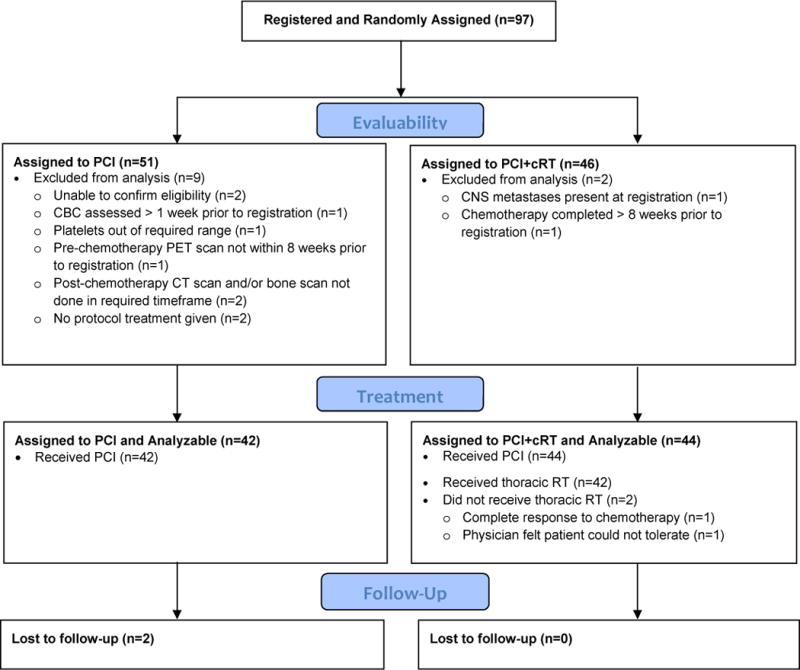
CONSORT Flow Diagram
Pretreatment characteristics were evenly distributed with the exception of age (Table 1). 54.5% of patients randomized PCI+cRT and 38.6% to PCI arm were ≥ 65 (p=0.03). CR to chemotherapy (prior to study entry) in the primary lung tumor was 15.9% and PR 68.1%. 36% of patients had one metastasis, and 36%, 15.1% and 12.8% had two, three and four metastases. The median time from diagnosis to start of radiation was 22 weeks for each group. Median time from end of chemotherapy to start of radiation was 5.9 weeks and 6.9 weeks for PCI and PCI+cRT.
Table 1.
Demographics
| Patient or Tumor Characteristic | PCI (n=42) n (%) |
PCI and Consolidative RT (n=44) n (%) |
Total (n=86) n (%) |
|---|---|---|---|
| Age | |||
| Median (Range) | 60.5 (47 – 81) | 66 (35 – 86) | 63 (35 – 86) |
| < 65 | 30 (71.4%) | 20 (45.5%) | 50 (58.1%) |
| ≥ 65 | 12 (28.6%) | 24 (54.5%) | 36 (41.9%) |
| Gender | |||
| Male | 18 (42.9%) | 21 (47.7%) | 39 (45.3%) |
| Female | 24 (57.1%) | 23 (52.3%) | 47 (54.7%) |
| Zubrod Performance Status | |||
| 0 | 21 (50.0%) | 18 (40.9%) | 39 (45.3%) |
| 1 | 21 (50.0%) | 25 (56.8%) | 46 (53.5%) |
| 2 | 0 (0.0%) | 1 (2.3%) | 1 (1.2%) |
| Response to initial treatment (locoregional/metastases) |
|||
| CR/CR | 10 (23.8%) | 7 (15.9%) | 17 (19.8%) |
| CR/PR | 5 (11.9%) | 6 (13.6%) | 11 (12.8%) |
| PR/PR or Stable | 27 (64.3%) | 31 (70.5%) | 58 (67.4%) |
| Number of metastatic lesions | |||
| 1 | 17 (40.5%) | 14 (31.8%) | 31 (36.0%) |
| 2–4 | 25 (59.5%) | 30 (68.2%) | 55 (64.0%) |
| Lesion locations† | |||
| Head and neck | 4 (9.5%) | 2 (4.5%) | 6 (7.0%) |
| Gastrointestinal | 1 (2.4%) | 1 (2.3%) | 2 (2.3%) |
| Liver | 10 (23.8%) | 10 (22.7%) | 20 (23.3%) |
| Renal | 1 (2.4%) | 0 (0.0%) | 1 (1.2%) |
| Adrenal | 6 (14.3%) | 11 (25.0%) | 17 (19.8%) |
| Bone | 11 (26.2%) | 8 (18.2%) | 19 (22.1%) |
| Distant lymph nodes | 13 (31.0%) | 10 (22.7%) | 23 (26.7%) |
| Skin | 0 (0.0%) | 1 (2.3%) | 1 (1.2%) |
| Contralateral lung | 3 (7.1%) | 6 (13.6%) | 9 (10.5%) |
| Other location | 13 (31.0%) | 21 (47.7%) | 34 (39.5%) |
Patients may have more than one site; percentages will not add to 100.
Among the PCI+cRT patients, two (4.5%) did not have TRT. Treating physicians withheld radiation due to CR (1) and anticipated poor tolerance (1). Of the patients treated with cRT, 90.5% received thoracic radiation per protocol (30–45Gy). Two patients received less than 30Gy (22.5Gy and 24Gy) and two patients received > 45Gy (50 and 65Gy). 95.3% of all patients received PCI per protocol.
At the time of this analysis, 51 deaths, including 22 with PCI, and 29 with PCI+cRT, were reported. The one-year OS rates were 60.1% [95% CI: 41.2–74.7%] for PCI and 50.8% [95% CI: 34.0–65.3%] for PCI+cRT (Figure 2). Median Survival was 15.8 months for PCI and 13.8 months for PCI+cRT. The two-sided log-rank test p-value was 0.21, and the unadjusted HR is 1.44 (HR favors PCI, 95% CI: 0.82–2.53). The majority of patients died of disease progression (76.5%). Patients with CR to chemotherapy at all sites of disease appeared to have better survival outcome than those with PR (any site), with a HR of 2.1 (HR favors CR, 95% CI: 0.97, 4.56) and p=0.06. Due to the limited sample of patients with CR (all sites), PCI (10) and PCI+cRT (7), it was not possible to determine if PCI+cRT had a differential treatment effect over PCI alone between CR and PR patients.
Figure 2.
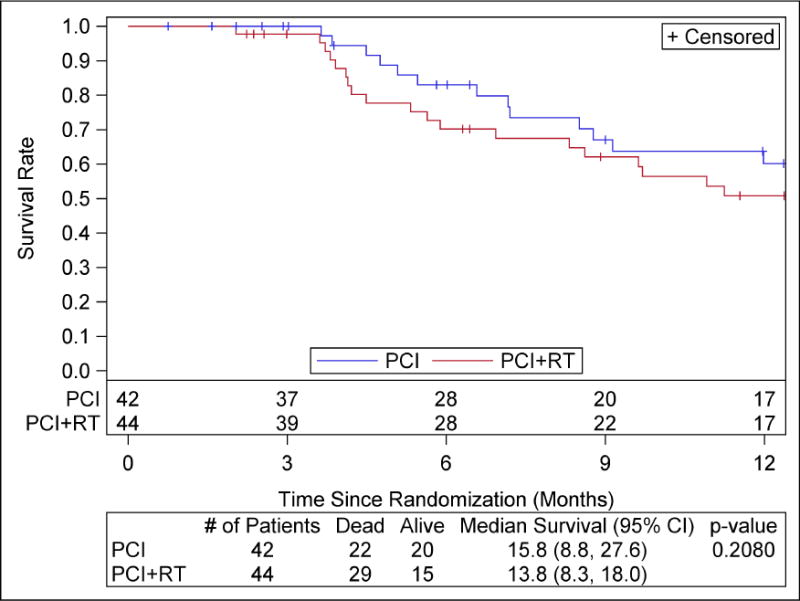
Overall Survival
There were 32 patients with progression in the PCI arm, and 31 in the PCI+cRT arm. The three-month and one-year rates of any progression were 53.3% [95% CI: 36.3–67.7] and 79.6% [95% CI: 60.6–90.2] for PCI, and 14.5% [95% CI: 5.8–27.0] and 75.0% [95% CI: 57.9–86.0] for PCI+cRT. The difference in the distribution of time to progression is statistically significant (log-rank test p-value 0.01) with a hazard ratio 0.53 (HR favors PCI+cRT, 95% CI: 0.32, 0.87) (Figure 3). There were only two and four deaths without progression with PCI and PCI+cRT, respectively. Patients with CR (all sites) had a longer time to progression than those with PR (any site), HR of 2.08 (95% CI: 1.04, 4.17) and p=0.04. Median Progression Free Survival was 2.9 months [95% CI: 2.4, 3.7] for PCI and 4.9 months [95%CI: 3.4, 6.0] for PCI+cRT, p-value 0.0148.
Figure 3.
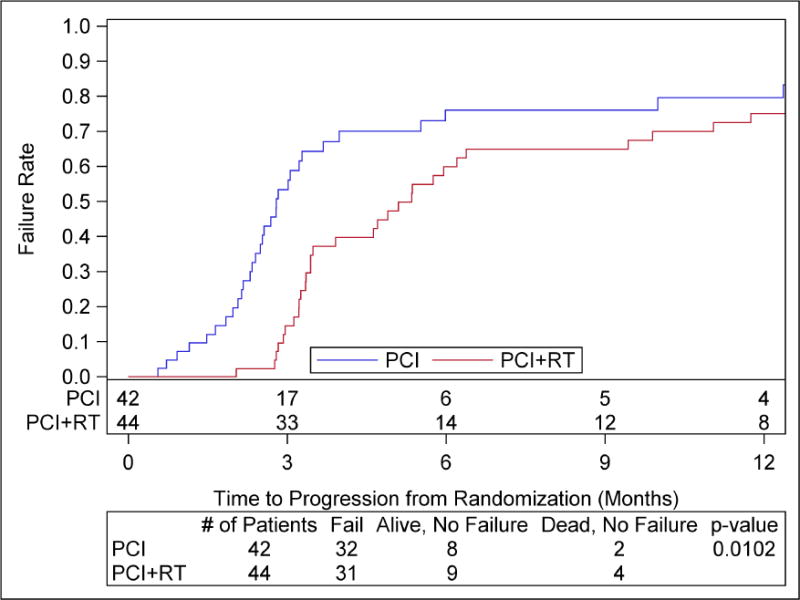
Time to progression
First site of failure was in the sites present at diagnosis in 25 (78.1%) patients with PCI and 13(41.9%) with PCI+cRT (Table 2). First site of failure at sites of locoregional disease was reported in 20 (62.5%) and 8(25.8%) of patients with PCI and PCI+cRT. New sites of disease (not present at diagnosis) were reported in 10 (31.3%) of the 32 patients with PCI and 19 (61.3%) of the 31 patients with PCI+cRT arm who progressed. First site of failure occurred outside of radiation fields in 17 (54.8%) patients with PCI+cRT. In patients treated with PCI+cRT there were ten (32.3%) first failures in the consolidative radiation fields (other than brain), and four (12.9%) first failures in the brain only. One-year cumulative incidence of brain metastases was 17% [95% CI: 6.6–40.2] and 18.5% [95% CI: 8.5–37.6] with PCI and PCI+cRT. Brain was a component of first failure in six (19.4%) of the 31 patients with failures with PCI+cRT and in none of the 32 patients with PCI only.
Table 2.
Failure Patterns
| PCI (n=32) n (%) |
PCI and Consolidative RT (n=31) n (%) |
|
|---|---|---|
| First failure at site of disease present at diagnosis | 25 (78.1%) | 13 (41.9%) |
| Locoregional disease as first failure | 20 (62.5%) | 8 (25.8%) |
| First failure brain | 0 (0.0%) | 6 (19.4%) |
| Failure at any time at new site | 10 (31.3%) | 19 (61.3%) |
Grade-3 or greater adverse events regardless of attribution to protocol therapy occurred in ten (23.8%) patients with PCI and 16 (36.4%) with PCI+cRT, p=0.24. Grade-3 or greater toxicity attributed to therapy was reported in four (9.5%) patients with PCI and 11 (25%) with PCI+cRT. Grade-3 toxicity with PCI+cRT included decreased blood counts, esophageal symptoms, tachycardia, fatigue and respiratory symptoms. Grade-4 dyspnea and tracheal stenosis was reported in one (2.4%) patient with PCI only and no Grade-5 toxicity attributed to therapy with PCI. Two (4.5%) patients had Grade-4 and one (2.3%) Grade-5 toxicity attributed to treatment with PCI+cRT. Grade-4 toxicity included hypoxia and respiratory failure during treatment, and decreased platelets at 12 days post therapy. Grade-5 pneumonitis attributed to therapy was reported in one patient with PCI+cRT. This patient received 42Gy to the chest with 37% of the lung receiving 20Gy or more, exceeding protocol normal tissue dose constraints (Table 3).
Table 3.
Grade 3+ toxicity related to therapy.
| Toxicity | PCI N=42 |
PCI+cRT N=44 |
|---|---|---|
| Anemia | 1 | 1 |
| Tachycardia | 0 | 1 |
| Retinal Detachment | 0 | 1 |
| Gastrointestinal* | 1 | 5 |
| Fatigue | 1 | 3 |
| Lung Infection | 0 | 2 |
| Metabolism# | 0 | 3 |
| Muscle Weakness | 0 | 1 |
| Nervous System | 1 | 1 |
| Respiratory (grade 3) | 0 | 2 |
| Respiratory (Grade 4) | 1 | 1 |
| Respiratory (Grade 5) | 0 | 1 |
| Hypotension | 0 | 2 |
| Decreased Platelets (grade 4) |
0 | 1 |
| Other** | 1 | 4 |
Diarrhea, dyspepsia, dysphagia, esophagitis, nausea
Hyperglycemia, anorexia, dehydration
Decreased lymphocytes, decreased white blood cells
Discussion
Thoracic radiation and PCI have been shown to decrease the incidence of locoregional failures and brain metastases and improve survival in both LD-SCLC17,18 and ED-SCLC.5–7 Although multimodality therapy for LD-SCLC is standard of care, it remains controversial for ED-SCLC. Patients with good response to chemotherapy and limited metastatic disease are the most likely patients with ED-SCLC to benefit from multimodality therapy. NRG Oncology RTOG 0937 was conducted to assess a potential survival advantage of consolidative extracranial irradiation in patients with oligometastatic ED-SCLC. Although a negative trial, there were several important findings: the first site of failure after chemotherapy is likely to be in sites of presenting disease; radiation therapy to these sites alters failure patterns; late radiation therapy without concurrent chemotherapy is not durable; and, oligometastatic ED-SCLC survival approaches that of LD SCLC. Ineffective radiation dose and schedule, advanced age and an imbalance in disease burden in the two groups all likely contributed to lack of survival advantage with consolidative radiation therapy in this trial.
Survival
Early randomized studies showed a survival advantage with thoracic radiation in addition to chemotherapy for LD-SCLC.19,20 These early LD-SCLC studies did not include the routine use of CTs and were conducted before the era of PET/CT and MRIs, therefore many patients likely had unrecognized ED-SCLC. The routine use of PET/CT has been shown to increase stage in 8–18% of patients.21,22 Thoracic radiation for ED-SCLC was supported by the Jeremic trial reported in 1999 which showed a survival advantage in patients with ED-SCLC treated with thoracic radiation. Despite compelling data, the routine use of thoracic radiation in ED-SCLC has not been regarded as standard of care. However, many clinicians have selectively used thoracic radiation and have published outcomes in several retrospective reviews published since the initiation of the current trial. These retrospective reviews and database analyses have shown improvement in survival with thoracic radiation (Table 4).6–9
Table 4.
Prospective and retrospective trials evaluating consolidative radiation therapy for ED SCLC
| Study | Type | Patient Selection | Treatment | Med Survival | 1 year OS | 2yr OS | 5 yr OS | |
|---|---|---|---|---|---|---|---|---|
| RTOG 0937 | PhIIr (n=86) |
1–4 metastatic lesions RT after ChT response | ChT–>RT 45 Gy at 3Gy/fx | 13.8 mos | 50.8% | NA | NA | NS |
| ChT | 15.8 mos | 60.1% | ||||||
|
| ||||||||
| Jeremic 1999 | PhIIIr (n=109) |
All Patients with EDSLC with CR to DM with 3 cycles of ChT | ChT–>Cht/RT 54 Gy at 1.5Gy/fx |
17mos | 65% | 38% | 9.1% | p=0.041 |
| ChT | 11 mos | 46% | 28% | 3.7% | ||||
|
| ||||||||
| CREST 2015 | Phase IIIr (n=495) |
All patients with EDSCLC with ChT Response | ChT–>RT 30 Gy at 3Gy/fx |
11.8 mos | 33% | 13% | NA | p<0.0001 |
| ChT | 7.5 mos | 28% (NS) | 3% | |||||
|
| ||||||||
| Zhu 2011 | Single institution Retrospective (n-119) |
Variable | ChT/RT 40–60 Gy 1.8–2Gy/Fx |
17 mos | NA | 35% | 7.1% | p=0.014 |
| ChT | 19.3 mos | 17% | 5.1% | |||||
|
| ||||||||
| Ou 2009 | Regional Database (n=4782) |
Variable | ChT/RT | 8 mos | 27.8% | 9.3% | NA | p<0.0001 |
| ChT | 4 mos | 16.2% | 3.8% | |||||
The CREST trial, conducted in the Netherlands and the United Kingdon, was conducted during the same time as the current trial. It included 495 patients with ED-SCLC randomly assigned to PCI or PCI and thoracic radiation (30Gy/10 fractions) after response to chemotherapy. The number of metastases was not limited. OS at one year was the primary endpoint. One-year OS was not different between the two groups with 33% (95% CI 27–39) for thoracic radiation versus 28% (95% CI 22–34) for observation (hazard ratio [HR] 0.84, 95% CI 0.69–1.01; p=0.066). However, secondary analysis of two-year OS showed improvement with thoracic radiation, 13% (95% CI 9–19) versus 3% (95% CI 2–8; p=0.004). Toxicity was acceptable with 2% and 1.2% Grade-3 esophageal and pulmonary toxicity.6
Additional analysis of CREST to identify patients who may have benefitted most from thoracic radiation showed that thoracic radiation led to a significant difference in overall and progression-free survival in patients who had residual intrathoracic disease after chemotherapy. In these patients, the difference in OS was statistically significant (p=0.03; HR 0.81; 95% CI: 0.66–0.98; stratified). They concluded that thoracic radiation should be offered to patients with a good response or PR after chemotherapy, but not those with thorax CR.23 There were too few patients in 0937 to perform this analysis.
The trial published by Jeremic et al in 1999 supported thoracic radiation for EDSLC in patients with at least a PR to locoregional disease and a CR to extrathoracic sites with chemotherapy.7 Similar to 0937, the majority of patients had one to two sites of metastases at diagnosis, although this was not a requirement. Unlike NRG Oncology RTOG 0937, patients with good response to first 3 cycles of chemotherapy received high dose radiation therapy to the primary locoregional disease only with concurrent chemotherapy. Patients were treated initially with three cycles of cisplatin and etoposide. Those with a CR or PR locally and a CR at distant sites were treated with two cycles of carboplatin and etoposide +/− concurrent hyperfractionated RT to the thorax (54Gy/36 fractions over 18 days). Both groups received PCI. Median Survival (17 months versus 11 months, p=0.041), five-year survival (9.1% versus 3.7%, p=0.041), and median time to local recurrence (30 versus 22 months, p=0.062) were improved in the RT group. The investigators did not describe a difference between patients with and without residual disease in the chest after chemotherapy. Distant metastatic rate remained high in both groups. The pattern of failure relative to initial pattern of distant disease was not described.7
An important finding in NRG Oncology RTOG 0937 is that the median OS (15.8 months) and one-year OS (60.1% 95%CI: 41.2, 74.7) in the PCI arm in 0937 exceeded what was predicted with chemotherapy and PCI for ED-SCLC. For comparison, the median survival with chemotherapy and PCI was 15.8 months compared to 8 months in the CREST trial. Both of these trials calculated survival from randomization, which was after 4–6 cycles of chemotherapy and restaging. In 0937 the median time from diagnosis to start of radiation was greater than four months. The medians survival in the Jeremic trial with chemotherapy and PCI alone for patients with CR to distant sites was 11 months from randomization which was prior to starting chemotherapy. Additionally, the outcome of 0937 compares favorably to the intergroup trial for LD-SCLC with concurrent RT to 45Gy at 1.8Gy daily fractions or 1.5Gy twice daily fractions with medians survival from randomization prior to chemotherapy of 19 and 23 months respectively.24
The design of 0937 intentionally included a favorable patient population with oligometastases by rigorous staging at diagnosis and after chemotherapy. Perhaps a more appropriate treatment for this patient population with low volume systemic disease is early radiation concurrent with cycle three or four of chemotherapy in patients with a favorable response to cycles one and two of chemotherapy followed by PCI, similar to the Jeremic trial.
Concurrent chemotherapy and radiation has been shown to be superior to sequential therapy.17 The importance of the early use of RT in LD-SCLC was demonstrated in the National Cancer Institute of Canada Clinical Trials Group in 1993.25 Patients were randomized to begin thoracic radiation on week 3 or 15 of chemotherapy. Patients without progressive disease received PCI following chemotherapy and radiation. Progression free survival (p=0.036) and OS (p=0.008) were superior with early thoracic radiation. Additionally patients with late radiation had a higher risk of brain metastases.25 A subsequent meta-analysis was undertaken by De Ruysscher et al to identify time factors for combined chemotherapy and radiotherapy that may influence OS.26 The time from first day of chemotherapy to last day of RT (Start of chemotherapy to End of Radiation (SER)) was the most important predictor of outcome. There was a significantly higher five-year survival rate in the shorter SER arms (relative risk [RR] = 0.62; 95% CI, 0.49 to 0.80; P =.0003), which was more than 20% when the SER was less than 30 days (upper bound of 95% CI, 90 days).20 For comparison the SER for 0937 was well over 150 days.
Failure Patterns
Brain failures are well described and reported, although extracranial failure patterns after chemotherapy have not been reported in detail. It is intuitive that the most likely site of failure after chemotherapy for ED-SCLC is in areas of measurable disease at diagnosis, particularly sites without at CR to chemotherapy. In the landmark trial showing an improvement in 1 year overall survival with PCI in patients with ED-SCLC published by Slotman et al, the chest was the most likely site of progression.5 Persistent intrathoracic disease was present in 75% of patients after chemotherapy and approximately 90% had intrathoracic progression in the first year. The CREST trial, evaluating thoracic radiation therapy following chemotherapy was a natural follow up to this trial.6 Unlike the CREST trial, NRG Oncology RTOG 0937 included consolidative RT to distant metastases in addition to thoracic radiation and is the first study to evaluate outcomes and report failure patterns in patients with oligometastatic ED-SCLC.
Patients on 0937 were more likely to fail in in sites of disease present at diagnosis. Fewer patients in the PCI+cRT arm had their locoregional disease as a component of first failure (25.8% vs 62.5%). Progression at presenting sites of disease was higher with PCI (78.1%) than PCI+cRT (41.9%). Similar to 0937, patients on the CREST trial had lower isolated intrathoracic progression of 19.8% with thoracic radiation compared 46% in the observation arm (p<0.0001).6 Likewise, local recurrence free survival in the Jeremic trial at five years was 20% with RT and 8.1% with chemotherapy only (p=0.062).7 Early concurrent chemotherapy and high dose radiation therapy in the Jeremic trial likely contributed favorable local recurrence free survival. An unexplained finding in the current trial was more new sites of disease and first-failures in the brain with PCI+cRT compared to PCI alone. There was not a clear explanation for this based on site, volume and number of metastases. Higher brain failures and new sites of disease suggests that there was an unaccounted for imbalance in systemic disease burden decreasing the likelihood of seeing a survival advantage with PCI+cRT.
Although consolidative radiation to locoregional disease and residual metastases after chemotherapy delayed progression at 3 months, this benefit was not durable. The 3-month rate of progression was 53.3% with PCI and only 14.5% with PCI+cRT, although by 1 year the progression rates were not different at 79.6% and 75%, respectively. A more aggressive course of radiation therapy would have likely resulted in a more durable response. PCI remains controversial in the treatment ED-SCLC despite a survival advantage at 1 year demonstrated by Slotman et al5 and advantages of PCI in early trials with subset analysis of patients with extensive disease.18 Adding to this controversy is a recent Japanese trial which did not show a survival benefit with PCI.27 PCI has consistently been shown to be effective for decreasing brain failures although it is not completely clear which patients will derive a survival benefit. Extent of disease at diagnosis, response to chemotherapy and immunotherapy are important considerations. Treatment of active extracranial disease is likely to also play an important role as active extracranial disease will continue to seed the brain after PCI.
Toxicity
NRG Oncology 0937 was closed early due to a futility analysis showing that it was unlikely there would be a survival advantage in the study arm. Unfortunately, unofficial communications and discussions have suggested that there was excess toxicity on this trial.
Toxicity in 0937 was as anticipated with the therapy delivered (Table 3). Grade 3+ toxicity was 23% for PCI and 36% for PCI+cRT, p=0.24. Grade-4 and 5 toxicity included hematologic and pulmonary toxicity. There was one death attributed to protocol therapy. This patient had radiation-induced pneumonitis. The radiation dose to normal lung exceeded dose constraints required by protocol. This emphasizes the importance of careful attention to minimizing normal tissue dose exposure to radiation. Toxicity in 0937 compares favorably to toxicity reported other trials evaluating thoracic RT for ED-SCLC and LD-SCLC. Jeremic et al reported 20% Grade-3 and 7% Grade-4 esophageal toxicity and 5% Grade-3 bronchopulmonary toxicity with radiation and similar to CREST.6,7 Future studies should encourage or require use RT techniques such as Intensity Modulated Radiation Therapy (IMRT) and Stereotactic Body Radiation Therapy (SBRT) to minimize dose to normal tissues including bone marrow to minimize hematologic toxicity and improve tolerance to systemic therapy.
Conclusion
Patients with ED-SCLC represent a diverse patient population with a wide range of anticipated outcomes. There is not one appropriate treatment regimen for all patients. This study was the first to prospectively evaluate the favorable population with oligometastatic SCLC. Consolidation radiation therapy delayed progression and altered failure patterns in this population. Toxicity was as anticipated with the therapy delivered and similar to published data. The lack of benefit seen with PCI+cRT in this very favorable cohort of patients with ED-SCLC may be due to lack of optimal timing, dose and fractionation of radiation therapy. This study has demonstrated that this favorable subpopulation of patients with ED-SCLC has OS similar to patients with LD-SCLC. Future trials for this population should focus on timing, dose, and fractionation of radiation therapy delivery.
Acknowledgments
Role of the funding source
The sponsors were not involved in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. The corresponding author (EMG) had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Funding: This project was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (IROC) from the National Cancer Institute (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Hu reports grants from National Cancer Institute (NCI), during the conduct of the study. Dr. Ramalingam reports serving on Advisory Boards for Amgen, Astra Zeneca, Abbvie, BMS, Lilly, Celgene, Genentech, and Novartis, outside the submitted work. Ms. Paulus reports grants from National Cancer Institute (NCI) during the conduct of the study.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Bunn PA, Langer C, et al. Randomized phase III trial comparing Irinotecan/Cisplatin with Etoposide/Cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz JL, McCoy F, Scullin P, Fennell DA. New advances in the second-line treatment of small cell lung cancer. Oncologist. 2009;14:986–94. doi: 10.1634/theoncologist.2009-0026. [DOI] [PubMed] [Google Scholar]
- 4.Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5 Suppl):e400S–19S. doi: 10.1378/chest.12-2363. [DOI] [PubMed] [Google Scholar]
- 5.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 6.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radio- therapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 7.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol. 1999;17:2092–99. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. 2011;117:5423–31. doi: 10.1002/cncr.26206. [DOI] [PubMed] [Google Scholar]
- 9.Ou SHI, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC). The importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol. 2009;4:37–43. doi: 10.1097/JTO.0b013e31819140fb. [DOI] [PubMed] [Google Scholar]
- 10.Pampallona S, Tsiatis AA. Group sequential designs for one-sided and two-sided hypothesis testing with provision for early stopping in favor of the null hypothesis. Journal of Statistical Planning and Inference. 1994;42:19–35. [Google Scholar]
- 11.Freidlin B, Korn EL. A comment on futility monitoring. Controlled clinical trials. 2002;23:355–66. doi: 10.1016/s0197-2456(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–81. [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–54. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemo Reports. 1966;50:163–70. [PubMed] [Google Scholar]
- 15.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34(series B):187–229. [Google Scholar]
- 16.Freidlin B, Korn EL. Testing treatment effects in the presence of competing risks. Statist Med. 2005;24:1703–12. doi: 10.1002/sim.2054. [DOI] [PubMed] [Google Scholar]
- 17.Takada M, Fukuoka M, Kawhara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–60. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 18.Aupérin A1, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 19.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell lung cancer? A meta-analysis. J Clin Oncol. 1992;10:890–95. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 20.Pignon JP, Arriagada R, Ihde D. Meta-analysis of small-cell lung cancer. NEJM. 1992;327:1618–24. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 21.Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: A prospective study. JCO. 2004;22:3248–54. doi: 10.1200/JCO.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 22.Niho S, Fujii H, Murakami K, et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET in LD-SCLC scan in apparent limited-disease small –cell lung cancer. Lung Cancer. 2007;57:328–33. doi: 10.1016/j.lungcan.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial (Authors’s reply) Lancet. 2015;385:1292–3. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 24.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 25.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer: The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–44. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 26.De Ruysscher D, Pijls-Johannesma M, Bentzen S, et al. Time Between the First Day of Chemotherapy and the Last Day of Chest Radiation Is the Most Important Predictor of Survival in Limited-Disease Small-Cell Lung Cancer. J Clin Oncol. 2006;24:1057–63. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 27.Seto T, et al. Prophylactic Cranial Irradiation (PCI) has a detrimental effect o the overall survival (OS) of patients with extensive disease small cell lung cancer (ED-SCLC): Results of a Japanese randomized phase III trial. JCO ASCO Am Meet Abstr. 2014;32:7503. [Google Scholar]


