Abstract
Purpose
To compare cup to disc ratio (CDR) measurements from images taken with a portable, 45° non-mydriatic fundus camera to images from a traditional table-top mydriatic fundus camera.
Design
Prospective, cross sectional, comparative instrument validation study.
Methods
Setting
Clinic-based.
Study Population
422 eyes of 211 subjects were recruited from the Tilganga Institute of Ophthalmology (Kathmandu, Nepal). Two masked readers measured CDR and noted possible evidence of glaucoma (CDR ≥0.7 or the presence of a notch or disc hemorrhage) from fundus photographs taken with a non-mydriatic portable camera and a mydriatic standard camera. Each image was graded twice.
Main Outcome Measures
Effect of camera modality on CDR measurement, inter- and intra-observer agreement for each camera for the diagnosis of glaucoma.
Results
196 eyes (46.5%) were diagnosed with glaucoma by chart review. 41.2%–59.0% of eyes were remotely diagnosed with glaucoma over grader, repeat measurement, and camera modality. There was no significant difference in CDR measurement between cameras after adjusting for grader and measurement order (estimate=0.004, 95% confidence interval (CI), 0.003–0.011, p=0.24). There was moderate inter-observer reliability for the diagnosis of glaucoma (Pictor (κ=0.54, CI, 0.46–0.61); Topcon (κ=0.63, CI, 0.55–0.70) and moderate intra-observer agreement upon repeat grading (Pictor (κ=0.63 and 0.64, for Graders 1 and 2, respectively); Topcon (κ=0.72 and 0.80, for Graders 1 and 2, respectively).
Conclusions
A portable, non-mydriatic, fundus camera can facilitate remote evaluation of disc images on par with standard mydriatic fundus photography.
Keywords: telemedicine, glaucoma, screening, portable technologies, non-mydriatic imaging, diagnostic testing
Introduction
Glaucoma is the second leading cause of blindness globally.1–4 The number of individuals with glaucoma worldwide is projected to increase from 64 million in 2013 to 112 million in 2040.5 Approximately 50% of persons with glaucoma remain undiagnosed in the United States (US), with rates as high as 85% and 75% for African Americans and Hispanics, respectively, and there exist significant disparities in the prevalence of blindness due to glaucoma across ethnicities.1,6–14 These variations are thought to be in part due to minority groups traditionally having poor access to ophthalmic care.15–17 The rate of undiagnosed glaucoma is even higher in low-income countries. In Nepal, where there is a paucity of paved functioning roads, especially in mountainous areas, rates of undiagnosed glaucoma are estimated to be as high as 96%, with glaucoma remaining a leading cause of preventable blindness in the country.18,19
Glaucoma poses a serious personal and economic burden throughout the world. As glaucoma progresses from early to end-stage disease, the costs of care increase by as much as four fold in both Europe and the United States.20 Additionally, a disparity exists in the prevalence of blindness between high-income versus low- and middle-income countries. Not only is the prevalence of blindness higher in low- and middle-income countries, but those with visual impairment also have higher mortality rates.21–25 With populations in developing regions increasing more rapidly than in developed regions, the economic burden posed by glaucoma worldwide will increase.26
Glaucoma is a growing population health crisis and needs improved screening methods. The United States Preventive Services Task Force (USPSTF) does not currently recommend glaucoma screening for the general population due to “inadequate evidence on the accuracy of screening;” therefore, further research into how to best screen for glaucoma is crucial.27–30 A secondary challenge for glaucoma screening programs is cost. Screening technologies would ideally use relatively inexpensive portable imaging devices enabling screening in remote and underserved communities.7 Portable, non-mydriatic, lower-cost fundus cameras have recently become commercially available. If these image capturing systems could be used to provide accurate screening for specific, high-risk groups, screening could extend to remote, resource-limited communities.29,31
This study aims to compare cup to disc ratio (CDR) measurement from images taken with a portable, 45° non-mydriatic fundus camera (Pictor, Volk, Mentor, OH) to images taken with a traditional table-top mydriatic fundus camera in a Nepali sample at the Tilganga Eye Centre, Kathmandu, Nepal. Findings from this study will add to the literature on how to build cost-conscious, accurate glaucoma screening programs.
Methods
We conducted a single site (Tilganga Institute of Ophthalmology, Kathmandu, Nepal), cross sectional, clinic-based, comparative instrument validation study. All adult participants agreed to the study by written informed consent and those under the age of 18 provided assent with written informed consent provided by a parent or legal guardian. The Institutional Ethics Committee at the Tilganga Institute of Ophthalmology approved this study. The study followed the ICH-GCP guidelines and fulfilled the tenets of the Declaration of Helsinki.
Participants
Participants were individuals ≥13 years of age living near Kathmandu, Nepal who attended the Tilganga Institute of Ophthalmology. Two groups of participants were evaluated: (1) control subjects with healthy eyes and visual pathways except for corrected refractive errors or diabetes with or without retinopathy and (2) patients with clinically confirmed glaucoma. Participants were excluded if they had a best-corrected visual acuity of 20/60 or worse to ensure clear ocular media, an uncorrected refractive error of greater than 4 diopters sphere and/or 3 diopters cylinder to exclude myopic discs. We excluded those with high myopia to exclude myopic discs and excluded those with high hyperopia or astigmatism as that may have impacted their ability to complete the tablet-based visual field test in our companion study. Participants were also excluded if they had other known ocular, neurologic or systemic conditions that may affect visual field sensitivity, or were taking medications that were known to affect visual field sensitivity. Healthy control participants had eyes with intraocular pressure (IOP) of <21 mm Hg and no disease of the posterior pole. Glaucoma patients had eyes with evidence of optic nerve abnormalities alongside characteristic visual field changes (Humphrey Field Analyzer 24-2 SITA Standard). Diabetic patients had a diagnosis that was based on hemoglobin A1C levels. All subjects underwent a comprehensive ophthalmic examination for glaucoma and diabetic retinopathy, which included a best corrected visual acuity, measurement of IOP, biomicroscopy of the anterior segment, gonioscopy, and a dilated fundus examination by a fellowship trained glaucoma specialist (ST). Non-mydriatic optic disc photographs and visual field testing with the Humphrey Visual Field Analyzer (SITA Standard testing) and an iPad based app (Visual Fields Easy, see companion paper) were obtained prior to dilation and mydriatic optic disc photographs were obtained after dilation. The fellowship trained glaucoma specialist designated whether each eye had glaucoma based on his examination and ancillary testing.
Photography protocol
Patients satisfying the inclusion criteria underwent non-mydriatic fundus photography using the Pictor camera (Volk Optical, Mentor, Ohio) followed by mydriatic imaging (Topcon TRC 50 DX, Oakland, New Jersey) on the same day. All imaging was performed in a darkened room. The Pictor is a handheld, non-mydriatic 45° digital fundus camera with a five mega-pixel image sensor that weighs 400g (0.88 pounds). The Pictor camera has autofocus capability, a built-in LED light source, is Wi-Fi enabled, and produces an image resolution of 2560x 1920 pixels which was compressed to 1280 × 960 pixels post transmission. In the United States, the camera costs approximately US $8,000 and approximately US $4,000 when purchased in India. The Topcon camera table top system has an attached Canon SLR camera that produced an image resolution of 1078 × 960 pixels post transmission. The Topcon system weighs approximately 35kg (77 pounds) and costs approximately US $25,000. All photographs for both cameras were stored as Joint Photographic Experts Group (JPEG) files after removing all patient identifiers and assigning a randomly generated unique number linked both to the eye and participant.
A single ophthalmic assistant previously unfamiliar with the portable camera was trained to take all Pictor photographs in this study. The assistant took photographs of the posterior pole on a training sample of patients prior to taking images for the study. A glaucoma specialist (ST) reviewed these images until it was determined that the assistant produced adequate quality images to begin photographing patients for the study, which occurred after he photographed twenty patients. Once photography for the study began, the assistant took 2–3 photos of the posterior pole with each camera and selected the best for study inclusion.
Remote interpretation of the fundus photographs
Two glaucoma specialists, masked to the patients and their diagnoses, graded the Pictor and Topcon photographs for evidence of glaucoma (IP, PN). The glaucoma specialists measured CDR to the nearest 0.05 interval. Specialists also recorded if a notch or disc hemorrhage was present. A presumptive epidemiologic diagnosis of glaucoma was defined as presence of at least one of the following: a vertical CDR ≥0.7, a notch, or disc hemorrhage.32
The two glaucoma specialists received de-identified images of the posterior pole in batches containing approximately 400 randomly chosen images taken from both of the photographic modalities: 1) non-mydriatic Pictor and 2) mydriatic Topcon. Eyes from the same patient were not presented concurrently and the two specialists were masked to photographic modality. To evaluate intra-observer reliability, the readers re-graded all the images after one month.
The computer screen used to view the images at each institution (IP, Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA and PANC, Kellogg Eye Center, Ann Arbor, Michigan, USA) was at least 17 diagonal inches per NHS guidelines.33 Standard luminance and contrast settings set by Windows (Microsoft, Washington, US) were used.33
Outcome measures
The primary outcome was CDR as measured from the non-mydriatic portable camera images and the mydriatic tabletop camera images. The graders’ inter- and intra-observer reliability for glaucoma diagnosis were assessed for each camera.
Statistical analysis
Descriptive statistics of the sample were summarized with means and standard deviations (SD) for continuous variables and with frequencies and percentages for categorical variables.
CDR measurements were summarized with descriptive statistics by grader and photographic modality for first and second measurements. In addition, the CDR measurements between cameras for the same eye were displayed with scatterplots to show agreement and deviations from it. Linear mixed regression modeling was used to evaluate for fixed effects of grader, measurement order (first versus second), and camera modality on CDR measurement and estimates with 95% confidence intervals (CI) are reported. This model accounted for the correlation between repeated CDR measures on an eye, as well as the correlation between eyes of a subject. The absolute difference between first and second measurement of CDR was evaluated for deviations of 0.05 or larger. Repeated measures logistic regression with generalized estimating equations (GEE)13 was used to model the probability that the absolute difference between CDR measures was 0.05 or larger. This model also accounted for the dependency in the data. Fixed effects of grader and camera were investigated and odds ratios (OR) with 95% CI are reported.
Inter- and intra-observer agreement for the diagnosis of glaucoma for each camera was investigated with kappa statistics. In addition, kappa statistics for agreement of glaucoma diagnosis between imaging modalities but within grader were calculated. Kappa statistics were interpreted as follows: 0–0.2, slight agreement; 0.2–0.4, fair agreement; 0.4–0.6, moderate agreement; 0.6–0.8, substantial agreement; >0.81, high level of agreement.34 Tests for equal kappa statistics between cameras were also performed.
P-values <0.05 were considered statistically significant. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
A total of 211 participants completed study procedures, providing data on 422 eyes. Subjects had a mean age of 45.2 ± 15.4 years and 38.2% were female. In the study sample, 196 eyes (46.5%) were diagnosed with glaucoma and 226 eyes (53.5%) were diagnosed as not having glaucoma by the glaucoma specialist.
Glaucoma detection by photographs
A total of 41.2%–59.0% of eyes were diagnosed with glaucoma stratified over all levels of grader, repeat measurement, and camera, based on the study criteria (at least one of: CDR ≥0.7, or the presence of a notch, or disc hemorrhage). CDR measurement was ≥0.7 in 39.6%–55.8% of eyes photographed with the Topcon camera and 41.0%–58.6% of eyes photographed with the Pictor camera, over all levels of grader and repeat measurement. The presence of a notch was noted in 20.4%–25.4% of eyes photographed with the Topcon camera and 16.6%–23.3% of eyes photographed with the Pictor camera, across grader and repeat grading. Hemorrhage was noted less frequently, ranging from 0.2%–1.9% of eyes photographed by Topcon and 0.2%–1.4% of eyes photographed by Pictor, across graders and repeat grading. Grader 1 had a discrepancy in glaucoma diagnosis by 18.7% of eyes when comparing first measurements from Topcon images to Pictor images (20.4% on repeat grade). Grader 2 has a discrepancy of 27.6% and 22.0% for first and second grades, respectively.
Variation in measuring the CDR
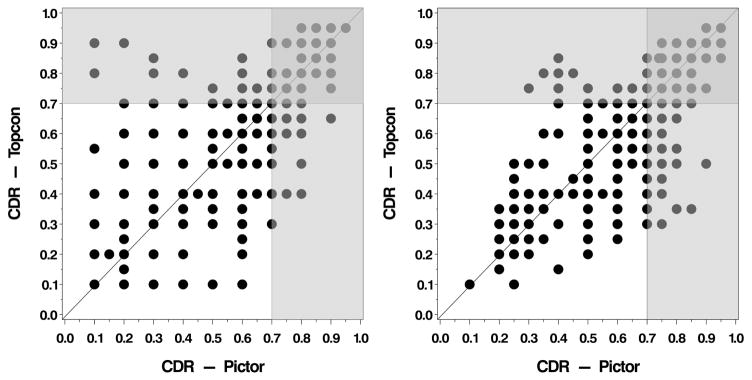
Descriptive statistics of CDR measurement by grader, photographic modality, and repeat measure are displayed in Table 1. Average CDR measures showed a range from 0.56–0.65 across all strata (grader, photographic modality, repeat measure). A multivariable linear mixed regression model found no statistically significant difference in CDR measurement between cameras (Estimate = 0.004, CI −0.003–0.011, p=0.24). However, a statistically significant effect for grader on CDR measurement was observed, such that Grader 1 measured CDR on average 0.07 lower than Grader 2 (estimate = −0.074, CI −0.081–0.067, p<0.0001). In addition, a significant effect of measurement order suggested that first measurements of CDR were on average 0.007 lower than second measurements (estimate = −0.007, CI −0.014 to −0.0000045, p=0.0498). Figure 1 displays initial CDR measurements obtained from Topcon versus Pictor images, stratified by grader. A positive linear trend is observed for both graders measuring CDR between image modalities, such that larger CDR measures from Pictor images are associated with larger measurements on Topcon images. However, deviations in CDR measures between cameras are apparent. Of particular importance are those images where CDR is measured above the threshold for a diagnosis of glaucoma (≥0.7) by one camera but not the other (light gray shaded areas, Figure 1). Specifically, Grader 1 had a discrepancy in glaucoma diagnosis by CDR alone in 18.0% of eyes when comparing measurements from Topcon images to Pictor images. Grader 2 had a discrepancy of 25.4% of eyes.
Table 1.
Descriptive statistics of cup-disc ratio measurements
| First Measurement | Second Measurement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Camera, Graderd | N | Mean | SDa | Minb | Maxc | Median | N | Mean | SD | Min | Max | Median |
| Topcon | ||||||||||||
| Grader 1 | 422 | 0.56 | 0.22 | 0.10 | 0.95 | 0.60 | 422 | 0.57 | 0.21 | 0.10 | 0.96 | 0.60 |
| Grader 2 | 420 | 0.62 | 0.18 | 0.10 | 0.95 | 0.65 | 421 | 0.64 | 0.18 | 0.10 | 0.99 | 0.70 |
| Pictor | ||||||||||||
| Grader 1 | 422 | 0.56 | 0.22 | 0.10 | 0.95 | 0.60 | 422 | 0.56 | 0.22 | 0.00 | 0.95 | 0.60 |
| Grader 2 | 412 | 0.64 | 0.17 | 0.10 | 0.95 | 0.70 | 415 | 0.65 | 0.17 | 0.20 | 0.95 | 0.70 |
SD = Standard Deviation
Min = Minimum
Max = Maximum
Figure 1.
Initial cup to disc ratio (CDR) measurements obtained from the Topcon versus the Pictor images of the posterior pole, stratified by grader (Left. Grader 1; Right. Grader 2). The light gray shaded area indicates where the CDR is measured above the threshold for a diagnosis of glaucoma (≥0.7) by one camera but not the other.
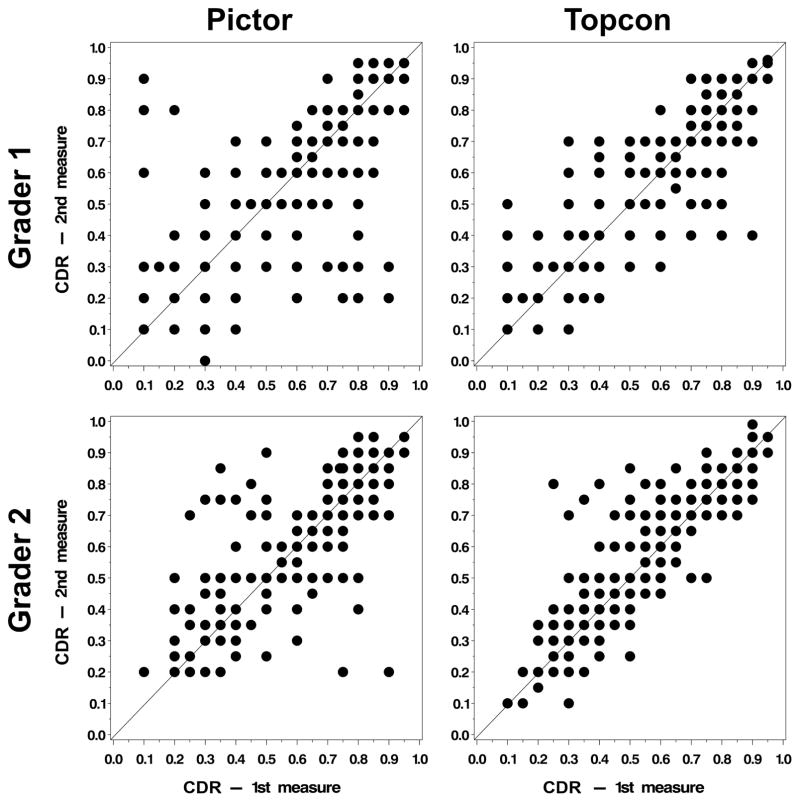
Descriptive statistics of the absolute difference between first and second measurement of CDR for each grader are displayed in Table 2. Scatterplots of first measure by second measure are displayed in Figure 2. The average absolute difference between first and second measurement of CDR ranged from 0.06–0.09, depending on grader and camera. The absolute difference between first and second measure of CDR was 0.05 or greater in 58.5% of eyes for Grader 1 with Topcon images, 61.9% of eyes for Grader 1 with Pictor images, 67.4% of eyes for Grader 2 with Topcon images, and 60.7% of eyes for Grader 2 with Pictor images. Repeated measures logistic regression showed no statistically significant effect for camera (OR for Pictor versus Topcon=0.93, CI 0.76–1.14, p-value=0.50) or grader (OR for Grader 1 versus Grader 2=0.85, CI 0.70–1.02, p-value=0.09) on the probability of the absolute difference between CDR measurements of 0.05 or greater.
Table 2.
Descriptive statistics of the absolute difference between repeated measurements of cup-disc ratio
| Absolute Difference | Absolute Difference ≥ 0.05 | |||||||
|---|---|---|---|---|---|---|---|---|
| Camera, Gradere | N | Mean | SDa | Minb | Maxc | Median | Freqd | Percent |
| Topcon | ||||||||
| Grader 1 | 422 | 0.07 | 0.08 | 0.00 | 0.50 | 0.05 | 247 | 58.5 |
| Grader 2 | 420 | 0.06 | 0.07 | 0.00 | 0.55 | 0.05 | 283 | 67.4 |
| Pictor | ||||||||
| Grader 1 | 422 | 0.09 | 0.12 | 0.00 | 0.80 | 0.05 | 261 | 61.9 |
| Grader 2 | 412 | 0.06 | 0.09 | 0.00 | 0.70 | 0.05 | 250 | 60.7 |
SD = Standard Deviation
Min = Minimum
Max = Maximum
Freq = Frequency
Figure 2.
Scatterplots of the first measure by the second measure of CDR are shown for each camera type and grader.
Reliability to detect glaucoma
Table 3 summarizes the inter- and intra-observer reliability for diagnosing glaucoma (at least one of: CDR ≥0.7, or the presence of a notch, or disc hemorrhage) by non-mydriatic Pictor images or mydriatic Topcon images. For initial grading, the inter-observer reliability between glaucoma specialists grading the images for a remote diagnosis of glaucoma showed kappa statistic values of κ=0.54 (CI, 0.46–0.61) for Pictor imaging and κ=0.63 (CI, 0.55–0.70) for Topcon imaging. The re-grading of images showed similar kappa values for inter-observer reliability. The intra-observer reliability for detecting glaucoma upon repeat grading of images showed kappa value of κ=0.63 (CI, 0.56–0.70) and 0.64 (CI, 0.56–0.71) for Pictor imaging, and κ=0.80 (CI, 0.74–0.86) and 0.72 (CI, 0.65–0.78) for Topcon imaging for graders 1 and 2, respectively. Inter-observer reliability of glaucoma diagnosis was not significantly different between Pictor and Topcon imaging (all p>0.05). Intra-observer reliability for Grader 1 showed a statistically significant difference between cameras such that Topcon graded images showed better agreement upon repeat grading than Pictor (p=0.0005). Grader 2 showed no significant difference between cameras with respect to intra-observer reliability.
Table 3.
Agreement for the diagnosis of glaucoma
| Grader 2 - 1st Dx | ||||
|---|---|---|---|---|
| Grader 1 - 1st Dxa | Normal | Glaucoma | Kappa | P-valueb |
|
| ||||
| Pictor | ||||
| Normal | 154 | 81 | 0.54 (0.46, 0.61) | 0.0915 |
| Glaucoma | 17 | 160 | ||
| TOPCON | ||||
| Normal | 192 | 55 | 0.63 (0.55, 0.70) | |
| Glaucoma | 23 | 150 | ||
| Grader 2 - 2nd Dx | ||||
| Grader 1 - 2nd Dx | Normal | Glaucoma | Kappa | |
|
| ||||
| Pictor | ||||
| Normal | 145 | 80 | 0.50 (0.42, 0.58) | 0.1717 |
| Glaucoma | 25 | 165 | ||
| TOPCON | ||||
| Normal | 159 | 70 | 0.58 (0.50, 0.65) | |
| Glaucoma | 20 | 172 | ||
| Grader 1 - 1st Dx | ||||
| Grader 1 - 2nd Dx | Normal | Glaucoma | Kappa | |
|
| ||||
| Pictor | ||||
| Normal | 198 | 32 | 0.63 (0.56, 0.70) | 0.0005 |
| Glaucoma | 45 | 147 | ||
| TOPCON | ||||
| Normal | 218 | 12 | 0.80 (0.74, 0.86) | |
| Glaucoma | 30 | 162 | ||
| Grader 2 - 1st Dx | ||||
| Grader 2 - 2nd Dx | Normal | Glaucoma | Kappa | |
|
| ||||
| Pictor | ||||
| Normal | 133 | 34 | 0.64 (0.56, 0.71) | 0.1348 |
| Glaucoma | 38 | 207 | ||
| TOPCON | ||||
| Normal | 167 | 12 | 0.72 (0.65, 0.78) | |
| Glaucoma | 48 | 193 | ||
Dx = Diagnosis
test for equality of kappa statistics for Pictor versus Topcon graded images
Agreement of glaucoma diagnosis between imaging modalities was also investigated. Agreement of glaucoma diagnosis on first grading of images between Pictor and Topcon cameras showed kappa statistics of κ=0.62 for Grader 1 (CI, 0.54–0.69) and κ=0.45 for Grader 2 (CI, 0.36–0.53). Agreement of glaucoma diagnosis on second grading of images between Pictor and Topcon cameras showed kappa statistics of κ=0.59 for Grader 1 (CI, 0.51–0.67) and κ=0.55 for Grader 2 (CI, 0.47–0.63).
Discussion
This study demonstrated that relatively lightweight and inexpensive, battery-operated, portable non-mydriatic fundus photography facilitated remote grading of cup to disc ratio (CDR) on par with fundus photography taken with a standard table-top camera that required dilation. After adjusting for grader and whether it was a first or second grading session, we found that there was no significant difference in CDR measurement between cameras. CDR measurements were not only similar between a portable fundus camera and a standard table-top camera, but also CDR measurements were similar despite the portable camera being non-mydriatic.
In the Philadelphia Glaucoma Detection and Treatment Project, they noted moderate intra-observer and inter-observer agreement when measuring cup to disc ratio between portable monoscopic non-mydriatic disc photos and ophthalmologist examination.35 For our study, there was moderate between-grader agreement for the remote diagnosis of glaucoma with images obtained from both the Pictor and the Topcon based on the ability to identify a CDR ≥0.7, a notch or a disc hemorrhage. On first and second readings, there was no significant difference in agreement between graders for images from the Pictor versus the Topcon. However, with both graders, there was a trend towards improved intra-observer reliability with the Topcon. One grader had significantly greater reliability in identifying glaucomatous optic nerve damage using the Topcon compared to the Pictor, with substantial (κ=0.80) intra-observer reliability with the Topcon images compared to only moderate (κ=0.63) intra-observer reliability with the Pictor images (p=0.0005). Furthermore, the graders had moderate agreement (κ=0.45–0.62) in their remote diagnosis of glaucoma between the Topcon and Pictor images. Though there may be some gain in reproducibility of image grading with standard dilated fundus photographs, the difference between the two modalities was not clinically significant as there was no significant difference between CDR measurements between the two modalities.
Given our findings that both cameras performed similarly for CDR measurement, the Pictor appears to be a reasonable tool to use in remote screening settings. The Pictor has several advantages with regards to its use in these settings that make it unique among the newly emerging market for portable fundus cameras.36–39 These include being lightweight, relatively inexpensive (compared to a table top camera), having a flexible black silicone eyecup that blocks ambient illumination maximizing physiological mydriasis in settings without darkened rooms, and providing a 45° image of the posterior p ole that can be used to screen for both glaucoma and other treatable diseases in patients with good vision, such as diabetic retinopathy. When screening for glaucoma, this might be combined with supra-threshold perimetry on devices such as a tablet (Johnson Chris, et al. IOVS 2015;56:ARVO E-Abstract 3179) as described in the companion paper. It also had the relative advantage of not requiring dilation, which is both relatively incapacitating for a patient and time consuming.
Using fundus photography alone is not likely a reasonable strategy for a screening program. Six studies40–45 have reported low sensitivities (50–67%) for detecting glaucoma with monoscopic disc photography alone.46 When no ancillary visual field testing was used, even a mydriatic fundus examination by an ophthalmologist had 59% sensitivity and 73% specificity in detecting glaucoma in one study.29 Kumar and colleagues demonstrated that combining demographic information (age >45 and family history of glaucoma), monoscopic photographs (CDR >0.5), and frequency doubling perimetry testing (abnormal results) increased the sensitivity to detect disease from 67% to 84% compared to disc photos alone.41 Layering information by combining structural and functional screening tools should greatly improve remote glaucoma screening.
This study had several limitations. First, participants were recruited from a tertiary care eye hospital. Our participants had greater access to care and perhaps better overall health than individuals in more remote locations who would be the target for these interventions. Furthermore, we excluded subjects without clear ocular media and with high myopia and astigmatism. We will need to assess whether the non-mydriatic camera is a reasonable tool in this population that is more difficult to image in the future. Additionally, though the training was relatively quick, a single ophthalmic assistant took all of the images. If a health care worker in a remote setting did not have substantial daily practice taking photographs, the quality of the images may be diminished.
The study has distinct strengths. Masking glaucoma specialists to image modality, patient identification, and randomly ordering eyes before grading helped eliminated grader bias. The variability of our results was further reduced by employing a single photographer to conduct all study imaging.
The opportunity to advance ophthalmic care in both remote and underserved populations can be met with new, low-cost portable technologies. The ability to image the optic nerve for glaucoma via a no-dilation method has broad implications for glaucoma screening programs. Portable equipment, such as the portable non-mydriatic fundus camera used in this study, could greatly enhance outreach efforts aimed at eliminating treatable blindness in remote and underserved populations. Future research should combine these images and ancillary testing to determine if layering information for remote graders can improve sensitivity and specificity levels to meet screening standards for community-based implementation of a remote glaucoma screening program.
Acknowledgments
Funding: This work was supported by the National Eye Institute, Bethesda, MD (K23 Mentored Clinical Scientist Award K23EY023596 (MAW), K23EY025320 (PANC)); and Research to Prevent Blindness (PANC). The funding sources had no role in the design, conduct, or interpretation of this study.
Footnotes
This work was presented, in part, as a paper presentation at the Annual Meeting of the Association for Research in Vision and Ophthalmology, May 2, 2016.
Financial Disclosures: Dr. Newman-Casey is a consultant for Blue Health Intelligence and Dr. Woodward is on the advisory board for Intelligent Retinal Imaging Systems. Dr. Robin The Pictor Camera was purchased by the University of Michigan and was loaned to the Tilganga Eye Institute. Dr. Robin is a consultant for Aerie pharmaceuticals, Biolight Pharmaceuticals and is a medical monitor for Macuclear.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascolini D, Mariotti S. Gobal estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 3.Kass M, Heuer D, Higginbotham E, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 4.Leske M, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Tham Y, Li X, Wong T, Quigley H, Aung T, Cheng C. Global prevalence of glaucoma and projetions of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Quigley H, West S, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119(12):1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 7.Varma R, Ying-Lai M, Francis B, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111(8):1429–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Sommer A, Tielsch J, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325(20):1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, Zhao D, Gualar E, Ko F, Boland M, Friedman D. Prevalence of glaucoma in the United States: the 2005–2008 National Health and Nutrition Examination Survey. Investig Opthalmology Vis Sci. 2016;57(4):2905–2913. doi: 10.1167/iovs.15-18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz B, West S, Rubin G, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118(6):819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 11.Dana M, Tielsch J, Enger C, Joyce E, Santoli J, Taylor H. Visual impairment in a rural Appalachian community. J Am Med Assoc. 1990;264(18):2400–2405. [PubMed] [Google Scholar]
- 12.Klein R, Klein B, Linton K, De Mets D. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 13.Shaikh Y, Yu F, Coleman A. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158(6):1121–1129. doi: 10.1016/j.ajo.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Waisbourd M, Prusan N, Johnson D, et al. The Philadelphia Glaucoma Detection and Treatment Project: detection rates and initial management. Ophthalmology. 2016;123(8):1667–1674. doi: 10.1016/j.ophtha.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson M, Eezzuduemhoi D. Ophthalmologic disorders in minority populations. Med Clin North Am. 2005;89:795–804. doi: 10.1016/j.mcna.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Tielsch J, Sommer A, Katz J, Quigley H, Ezrine S. Socioeconomic status and visual impairment among urban Americans. Arch Ophthalmol. 1991;109(5):637–641. doi: 10.1001/archopht.1991.01080050051027. [DOI] [PubMed] [Google Scholar]
- 17.Pleet A, Sulewski M, Salowe R, et al. Risk factors associated with progression to blindness from primary open-angle glaucoma in an African-American population. Ophthalmic Epidemiol. 2016;23(4):248–256. doi: 10.1080/09286586.2016.1193207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thapa S, Paudyal I, Khanal S, et al. A population-based survey of the prevalence and types of glaucoma in Nepal: the Bhaktapur Glaucoma Study. Ophthalmology. 2012;119(4):759–764. doi: 10.1016/j.ophtha.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Pokhrel R, Pant C, Mishra T, et al. In: Epidemiology of Blindness in Nepal, 2012. Sapkota Y, editor. Kathmandu: National Research and Monitoring Department of Nepal Netra Jyoti Sangh; 2012. [Google Scholar]
- 20.Lee P, Walt J, Doyle J, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124(1):12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Dandona R, Dandona L. Socioeconomic status and blindness. Br J Ophthalmol. 2001;85(12):1484–1488. doi: 10.1136/bjo.85.12.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prost A, Vaugelade J. La surmortalite des aveugles en zone de savane ouest-africaine. Bull World Health Organ. 1981;59(5):773–776. [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor H, Katala S, Munoz B, Turner V. Increase in mortality associated with blindness in rural Africa. Bull World Health Organ. 1991;69(3):335–338. [PMC free article] [PubMed] [Google Scholar]
- 24.Karpa M, Mitchell P, Beath K, Rochtchina E, Cumming R, Wang J. Direct and indirect effects of visual impairment on mortality risk in older persons: The Blue Mountains Eye Study. Arch Ophthalmol. 2009;127(10):1347–1353. doi: 10.1001/archophthalmol.2009.240. [DOI] [PubMed] [Google Scholar]
- 25.Foong A, Fong C, Wong T, Saw S, Heng D, Foster P. Visual acuity and mortality in a Chinese population: The Tanjong Pagar Study. Ophthalmology. 2008;115(5):801–807. doi: 10.1016/j.ophtha.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 26.United Nations Department of Economic and Social Affairs Population Division. World Population Prospects: The 2015 Revision, Key Findings, and Advance Tables. New York: 2015. [Google Scholar]
- 27.Moyer V. Screening for glaucoma: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159(7):484–489. doi: 10.7326/0003-4819-159-6-201309170-00686. [DOI] [PubMed] [Google Scholar]
- 28.Burr J, Mowatt G, Hernandez R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess (Rockv) 2007;11(41):1–190. doi: 10.3310/hta11410. [DOI] [PubMed] [Google Scholar]
- 29.Fleming C, Whitlock E, Beil T, Smit B, Harris R. Screening for primary open-angle glaucoma in the primary care setting: an update for the US Preventive Service Task Force. Ann Fam Med. 2005;3(2):167–170. doi: 10.1370/afm.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ervin A-M, Boland M, Myrowitz E, et al. Screening for Glaucoma: Comparative Effectiveness. Rockville: Agency for Healthcare Research and Quality (AHRQ); 2012. [PubMed] [Google Scholar]
- 31.Prum B, Jr, Rosenberg L, Gedde S, et al. Primary Open-Ange Glaucoma Preferred Practice Pattern Guidelines. Elsevier Inc; 2015. [Google Scholar]
- 32.Foster P, Buhrmann R, Quigley H, Johnson G. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Facey K, Cummins E, Macpherson K, Morris A, Reay L, Slattery J. Organisation of Services for Diabetic Retinopathy Screening. Glasgow; 2002. [Google Scholar]
- 34.Viera A, Garrett J. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 35.Waisbourd M, Bond E, Sullivan T, et al. Evaluation of nonmydriatic hand-held optic disc photography grading in the Philadelphia Glaucoma Detection and Treatment Project. J Glaucoma. 2016;25(5):e520–e525. doi: 10.1097/IJG.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 36.Sharma A, Subramaniam S, Ramachandran K, Lakshmikanthan C, Krishna S, Sundaramoorthy S. Smartphone-based fundus camera device (MII Ret Cam) and technique with ability to image peripheral retina. Eur J Ophthalmol. 2016;26(2):142–144. doi: 10.5301/ejo.5000663. [DOI] [PubMed] [Google Scholar]
- 37.Ryan M, Rajalakshmi R, Prathiba V, et al. Comparison Among Methods of Retinopathy Assessment (CAMRA) Study: Smartphone, nonmydriatic, and mydriatic photography. Ophthalmology. 2015;122(10):2038–2043. doi: 10.1016/j.ophtha.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo A, Morescalchi F, Costagliola C, Delcassi L, Semeraro F. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J Ophthalmol. 2015;159(2):360–364. e361. doi: 10.1016/j.ajo.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Maamari R, Keenan J, Fletcher D, Margolis T. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol. 2014;98(4):438–441. doi: 10.1136/bjophthalmol-2013-303797. [DOI] [PubMed] [Google Scholar]
- 40.Chan H, Ong D, Kong Y, et al. Glaucomatous optic neuropathy evaluation (GONE) project: the effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am J Ophthalmol. 2014;157(5):936–944. doi: 10.1016/j.ajo.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, Giubilato A, Morgan W, et al. Glaucoma screening: analysis of conventional and telemedicine-friendly devices. Clin Experiment Ophthalmol. 2007;35(3):237–243. doi: 10.1111/j.1442-9071.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 42.Lichter P. Variability of expert observers in evaluating the optic disc. Trans Am Ophthalmol Soc. 1976;74:532–572. [PMC free article] [PubMed] [Google Scholar]
- 43.Maa A, Evans C, DeLaune W, Patel P, Lunch M. A novel tele-eye protocol for ocular disease detection and access to eye care services. Telemed J E-Health Off J Am Telemed Assoc. 2014;20(4):318–323. doi: 10.1089/tmj.2013.0185. [DOI] [PubMed] [Google Scholar]
- 44.Marcus D, Brooks S, Ulrich L, et al. Telemedicine diagnosis of eye disorders by direct ophthalmoscopy. A pilot study. Ophthalmology. 1998;105(10):1907–1914. doi: 10.1016/S0161-6420(98)91040-5. [DOI] [PubMed] [Google Scholar]
- 45.Yogesan K, Constable I, Barry C, et al. Evaluation of a portable fundus camera for use in the teleophthalmologic diagnosis of glaucoma. J Glaucoma. 1999;8(5):297–301. [PubMed] [Google Scholar]
- 46.Newman-Casey P, Verkade A, Oren G, Robin A. Gaps in glaucoma care: a systematic review of monoscopic disc photos to screen for glaucoma. Expert Rev Ophthalmol. 2014;9(6):467–474. doi: 10.1586/17469899.2014.967218. [DOI] [PMC free article] [PubMed] [Google Scholar]