Abstract
The present study is premeditated to extenuate the role of Ficus virens extract and its bioactive compound on cigarette smoke, an important risk factor for CVD, induced oxidative stress and hyperlipidemia. Cigarette smoke (CS) exposure to rats results in significant loss of body weight and increases blood carbon monoxide saturation (carboxyhemoglobin), nicotine, plasma TC, TG, and LDL-C levels but reduced level of antiatherogenic HDL-C. Moreover, owing to substantial oxidative stress generated in rats due to cigarette smoke a significant increase in plasma and erythrocytes lipid peroxidation products were observed which was well correlated with increase in ex-vivo BDC (48%) and MDA (53%) level (p < 0.001). Simultaneous administration of FVBM extract at higher dose (100 mg/rat) and F18 (n-Octadecanyl-O-α-D-glucopyranosyl(6’→1’’)-O-α-D-glucopyranoside) compound to CS-exposed rats effectively blocked the increase in plasma lipid and lipoprotein levels (p < 0.001) which was due to the marked suppression in the hepatic HMG-CoA reductase activity (p < 0.001) and significantly inhibit the lipid peroxidation process thus preventing the membrane damage, LDL oxidation, and in turn subsequent atherosclerosis. Thus, the results clearly demonstrated the protective role of FVBM extract and F18 compound in risk factor induced cardiovascular disease.
Key Words: Cigarette smoke, Oxidative stress, Hyperlipidemia, Ficus virens, Bioactive compound
Introduction
Cardiovascular diseases (CVD) contribute around 17.3 million deaths globally (1). Cigarette smoking is one of the major risk factor for CVD and is the leading cause of preventable death and a major public health concern (1). Majority of compounds in cigarette smoke such as nicotine and carbon monoxide (CO) have been reported to further increase the risk of CVD in chronic smokers (2). Smoking can raise the cholesterol and free fatty acid concentrations in blood by increasing plasma total cholesterol (TC), triglycerides (TG), and low density lipoprotein-cholesterol (LDL-C) including Apo-B and decreasing the cholesterol and ApoA-1 level of high density lipoprotein (HDL) (3). Significant generation of free radicals and subsequent oxidative stress during smoking causes lipid peroxidation, LDL oxidation and decreased levels of antioxidants in the plasma of smokers (4) that ultimately results in CVD (3). Cigarette smokers encounter a sustained free radical load that can contribute to the oxidation of LDL in-vitro (5, 6), although data are conflicting (7). Moreover, erythrocytes from CS induced-hypercholesterolemia or oxidative stress may result in accelerated peroxidation reactions, cellular aberration, and alterations in lipid and protein structure (8). Thus, natural compounds with antioxidant properties contribute the protection of cell and tissue against deleterious effects caused by CS generated reactive oxygen species (ROS). Several natural antioxidants have been experimentally proved as protective agents against smoke induced-oxidative stress and hyperlipidemia (9-11). Ficus species due to their strong antioxidant and biological properties are known previously to diffuse the toxic free radical and can be used as a possible protective agent for treatment of oxidative stress related disorders (12-14). We previously described and documented that Ficus virens bark methanolic (FVBM) extract contained large amount of antioxidant with significant hypolipidemic property (15, 16), the work presented in this manuscript extenuate the protective role of FVBM extract and its principal bioactive compound, n-Octadecanyl-O-α-D-glucopyranosyl(6’→1’’)-O-α-D-glucopyranoside in CS-induced oxidative stress and hyperlipidemia.
Experimental
Chemicals
Bradford dye (Sigma Aldrich, India), hydrogen peroxide (H2O2), isopropeol, glacial acetic acid (Merck Pvt Ltd, India), hemoglobin assay kit, total cholesterol (TC) and triglycerides (TG) kits were procured from Span Diagnostics Ltd. (India). Rodent Chow (Ashirwad pellets), capston cigarette (Capston, India) and all other chemicals were procured from Himedia Laboratories, Mumbai, India. All other chemicals and solvents used in this study were of analytical grade.
Isolation of Bioactive compound
Bioactive compound; n-Octadecanyl-O-α-D-glucopyranosyl(6′→1″)-O-α-D-glucopyranoside (F18) from FVBM extract has been isolated according to Iqbal et al. (16).
Animals
Male Sprague-Dawley (SD) rats weighed around 100-150 gm were procured from Indian Institute of Toxicology Research Center, Lucknow. The study protocol was approved by Institutional Animal Ethics Committee (IAEC) (registration number: IU/Biotech/project/CPCSEA/13/11). The rats have been housed 5 per cage for one week in the animal house for acclimatization at a temperature of 21-22 ˚C with 12 h light and dark cycle. The rats were given standard diet and water ad libitum.
Dose preparation
Sequentially extracted FVBM extract, its bioactive fraction (F18) and reference drug atorvastatin were dissolved in 10% dimethyl sulfoxide (DMSO) at different concentrations and were homogenized with saline. The doses of the extracts were selected on the basis of previously published reports (16-18).
Diet/exposure to cigarette smoke
FVBM extract, its bioactive fraction (F18) and atorvastatin suspension was administrated through gastric intubation in two divided doses (morning and evening) of 0.5 mL each/rat/day. Rats in smoking control group received 0.5 mL of saline containing 10% DMSO (vehicle) twice daily while rats in normal control group received 0.5 mL of saline containing 10% DMSO twice daily. The rats were divided randomly and equally (5 rats in each group) in groups as illustrated in Table 1. Rats were exposed to cigarette smoke in morning by keeping two rats in bottomless metallic container (10 ×11 × 16 inch), having two holes of 3 and 1.5 cm diameter, one on the either side. A burning cigarette was introduced through one hole (3 cm) and the other hole (1.5 cm) was used for ventilation. Animals were exposed to CS for 30 minutes, daily for 4 weeks with interval of 10 min between each 10 min exposure, using 3 cigarettes/day/2 rats in each group (11).
Table 1.
Protocol for the treatment of cigarette smoke induce hyperlipidemia in rats.
| Group | Treatment |
|---|---|
| AT | Smoke-exposed + standard (Atorvastatin) (1 mg/rat/day) |
| N-C | Normal control |
| S-C | Cigarette smoking control + vehicle |
| FVT-1 | Smoke-exposed + plant extract (FVBM) (50 mg/rat/day) |
| FVT-2 | Smoke-exposed + plant extract (FVBM) (100 mg/rat/day) |
| CT | Smoke-exposed + bioactive compound (F18) (1 mg/rat/day) |
Table 3.
Impact of FVBM extract, F18 bioactive compound and atorvastatin on blood haemoglobin, carbon monoxide saturation and nicotine in cigarette smoke-exposed rats after 4 weeks of treatment
| Group * |
Haemoglobin
(g/dL) |
Carbon monoxide
saturation (SCO%) |
Nicotine
(µg/mL) |
|---|---|---|---|
| N-C | 14.86 0.764 | 3.26 0.089 | 0.998 0.012 |
| S-C | 11.60 0.515 (-21.9%)b |
5.78 0.095 (+77.3%)a |
2.16 0.033 (+116.4%) a |
| FVT-1 | 12.35 0.617 (+6.4%)c |
5.16 0.080 (-10.7%) a |
2.11 0.024 (-2.3%)d |
| FVT-2 | 14.62 0.721 (+26.1%) b |
4.07 0.091 (-29.6%) a |
1.48 0.042 (-31.5%) a |
| C-T | 14.74 0.732 (+27.1%) b |
4.76 0.078 (-17.6%) a |
1.59 0.035 (-26.4%) a |
| A-T | 13.99 0.677 (+20.6%) b |
3.97 0.043 (-31.3%) a |
1.98 0.022 (-8.3%)b |
Values are mean SD from blood of 5 rats in each group.
Significantly different from N-C at p < 0.01.
Significantly different from S-C at p < 0.001
Significantly different from S-C at p < 0.01.
Significantly different from S-C at p < 0.01.
Non-significantly different from S-C at p > 0.05.
Table 5.
Antioxidant impact of FVBM, F18 bioactive compound and atorvastatin on plasma total antioxidants, CD, LOOH and MDA contents in cigarette smoke-exposed rats after 4 weeks of treatment
| Group * | CD | LOOH | MDA | Total antioxidants |
|---|---|---|---|---|
| N-C | 2.86 ± 0.141 | 1.02 ± 0.022 | 1.24 ± 0.015 | 85.47 ± 3.46 |
| S-C | 7.52 ± 0.325 (+162.9%)a |
2.88 ± 0.104 (+182.4%) a |
2.68 ± 0.125 (+116.1%) a |
44.21 ± 2.04 (-48.27%) a |
| FVT-1 | 5.28 ± 0.216 (-29.8%) a |
1.96 ± 0.083 (-31.9%) a |
1.95 ± 0.098 (-27.2%)b |
56.42 ± 2.79 (+27.6%) a |
| FVT-2 | 3.21 ± 0.110 (-57.3%) a |
1.29 ± 0.034 (-55.2%) a |
1.46 ± 0.062 (-45.5%) a |
81.53 ± 3.16 (+84.4%) a |
| C-T | 2.91 ± 0.108 (-61.3%) a |
1.08 ± 0.028 (-62.5%) a |
1.28 ± 0.042 (-52.2%) a |
79.18 ± 3.73 (+79.1%) a |
| A-T | 5.74 ± 0.209 (-23.7%)b |
2.28 ± 0.026 (-20.8%) a |
2.14 ± 0.044 (-20.1%) c |
51.79 ± 2.92 (+17.1%) a |
N-C: normal control, S-C: smoke-exposed control, FVT-1: fed 50 mg FVBM extract/rat/day, FVT-2: fed 100 mg FVBM extract/rat/day, C-T: fed 1 mg F18 bioactive compound/rat/day and A-T: given 1 mg atorvastatin/rat/day for 4 weeks.
CD: Conjugated diene, LOOH: Lipid hydroperoxide, MDA: Malondialdehyde.
Values are mean (µmole/dL) ± SD from plasma of 5 rats in each group.
Significantly different from N-C at p < 0.001.
Significantly different from S-C at p < 0.001.
Significantly different from S-C at p < 0.01.
Significantly different from S-C at p < 0.05.
Collection of blood, plasma and packed erythrocytes
At the end of the experiment, all the rats were anaesthetized and blood was collected in heparinized tubes by cardiac puncture. Plasma was collected from blood by centrifugation at 2,500 rpm for 30 min, aliquoted and stored at either 4 or −20 ˚C for future use.
Packed erythrocytes hemolysate was prepared as described by Lakshmi and Rajagopal (19). The packed erythrocytes obtained after the separation of plasma and buffy coat, were washed thrice with normal saline and a portion of washed erythrocytes was lysed in hypotonic (10 mM) sodium phosphate buffer, pH 7.4. A portion of the washed packed erythrocytes was stored at 4 ˚C for further use.
Collection of organ and preparation of homogenate
After the experiment, liver from the rats was promptly excised and chilled in ice-cold saline. After washing with saline, it was blotted and weighed. One g of wet tissues were cut into pieces and homogenized with 9 mL of chilled 0.1 M sodium phosphate buffer, pH 7.4 (containing 1.17% KCl) in a waring blender. The homogenate was centrifuged at 1,000 rpm for 10 min at 4 ˚C and finally was aliquoted and stored at −20 ˚C.
Determination of hemoglobin (Hb) in blood
Hemoglobin level was estimated by cyanmethemoglobin method of Drabkin and Austin [20] according to the procedure described in the instruction sheet enclosed with the reagent kit supplied by Span diagnostic. The percent blood hemoglobin was determined by measuring the absorbance of cyanomethemoglobin at 540 nm in eppendorf spectrophotometer using a hemoglobin standard.
Determination of nicotine content in blood
Nicotine content in blood samples was determined by the method of Varley et al. (21). Nicotine standard was treated in the similar manner and used in the calculation of nicotine present in the blood samples.
Determination of carbon monoxide saturation in a mixture containing hemoglobin and carboxy hemoglobin
Carbon monoxide saturation (SCO) in blood samples was determined by the method of Varley et al. (21). The carbon monoxide saturation was calculated from the equation.
SCO% = (2.44 D538/ D578-2.68) 100
The constants 2.44 and 2.68 have been calculated from a series of 30 measurements of D538/ D578 to 0% COHb (100% Hb) and 100% COHb. The isobestic point was established at λ = 578 ± 0.5 nm (n = 100).
Isolation of Plasma LDL and HDL
The precipitation method described by Wieland and Seidel (22) was used for the isolation of plasma LDL and the method of Patsch et al. (23) was used for the isolation of HDL.
Measurement of ex-vivo and in-vitro Cu++-mediated susceptibility of LDL to oxidation
The ex-vivo and in-vitro Cu++-mediated susceptibility of isolated LDL to oxidation was assessed by determining the lag phase of conjugated diene (CD) formation using the method of Esterbauer et al. (24, 25). Conjugated diene was calculated by using an extinction coefficient of 2.52×104 M-1 cm-1 and expressed as nmole malondialdehyde (MDA) equivalent per mg LDL protein. The MDA content in LDL was assayed by the method of Niehaus et al. (26). The MDA concentration of the samples was calculated by using an extinction coefficient (1.56×105 M-1cm-1).
Measurement of plasma “total antioxidant power” (FRAP)
The method of Benzie and Strain (27) was used for measuring the ferric reducing ability of plasma (FRAP assay) which estimates the “total antioxidant power”, with minor modification.
Measurement of MDA release from intact erythrocytes
The procedure of Cynamon et al. (28) was employed for the determination of MDA release from erythrocytes.
Determination of MDA content in erythrocytes
The determination of MDA in erythrocytes was carried out by adopting standardized protocol of Stocks and Dormandy (29).
Determination of plasma triglycerides and very low density lipoprotein-cholesterol
Plasma TG was determined by using enzymatic kit (Merck, India) based on glycerol-3-phosphate oxidase peroxides (GPO-POD) method (30). The very low density lipoprotein-cholesterol (VLDL-C) in plasma was calculated by dividing plasma TG values (mg/dL) by a factor of 5 as described by Friedewald et al. (31).
Determination of total cholesterol in plasma and lipoprotein
Plasma TC, LDL-C and HDL-C were determined by using cholesterol enzymatic kit (Merck, India) based on cholesterol oxidase phenol aminophenazone (CHOD-PAP) method and the results were expressed as mg/dL.
Assay of HMG-CoA reductase activity in liver homogenate
HMG-CoA reductase enzyme activity in liver homogenate was estimated indirectly by method of Rao and Ramakrishnan (32).
Protein estimation
The protein concentration of plasma, LDL and liver homogenate was analysed by the method of Bradford (33), using bovine serum albumin as standard. Aliquots were first precipitated with 10% TCA followed by centrifugation at 1500 rpm for 10 min. The pellets containing protein were dissolved in 0.5 N NaOH and suitable aliquots were used for protein determination.
Data analysis
For all assays, samples were analyzed in triplicate and the results were expressed as mean ± SD and the results were evaluated using one-way analysis of variance (ANOVA) and two tailed Students t-test. Statistical significance were expressed as *p < 0.05, **p < 0.01 and ***P < 0.001.
Results
Average body weight of rats in each group before and after 4 weeks of treatment
As shown in Table 2, there was a significant decrease in body weight of smoke-exposed rats from 130.85 gm ± 5.56 in N-C to 80.56 gm ± 3.14 gm in S-C (p < 0.001) rats after 4 weeks of exposure to CS. Whereas, the average body weight of smoke-exposed rats treated with different doses of F. virens methanolic extract (FVBM-50 and FVBM-100), bioactive compound (F18), and atorvastatin was 90.28 ± 3.14, 115.57 ± 4.45, 125.28 ± 5.14, and 120.57 ± 5.45 (gm) (p < 0.001), respectively, which indicates a significant regain of average body weight when compared to S-C rats.
Table 2.
Average body weight of rats in each group before and after 4 weeks of FVBM extract, F18 bioactive compound and atorvastatin treatment
| Group * | Before treatment | After treatment |
|---|---|---|
| N-C | 125.14 ± 2.67 | 130.85 ± 5.63 |
| S-C | 135.85 ± 3.93 | 80.56 ± 3.14 a |
| FVT-1 | 125.28 ± 7.86 | 90.28 ± 3.14 c |
| FVT-2 | 133.46 ± 6.07 | 115.57 ± 4.45 a |
| C-T | 122.24 ± 7.86 | 125.28 ± 5.14 a |
| A-T | 126.78 ± 6.07 | 120.57 ± 5.45 a |
N-C: normal control, S-C: smoke-exposed control, FVT-1: fed 50 mg FVBM extract/rat/day, FVT-2: fed 100 mg FVBM extract/rat/day, C-T: fed 1 mg F18 bioactive compound/rat/day and A-T: given 1 mg atorvastatin/rat/day for 4 weeks.
Values are mean (grams) SD from 5 rats in each group.
Significantly different from N-C at p < 0.001.
Significantly different from S-C at p < 0.001.
Significantly different from S-C at p < 0.05.
Impact on hemoglobin (Hb), blood carbon monoxide saturation and blood nicotine in smoke-exposed rats treated for 4 weeks
The results presented in Table 3, indicated the Hb, blood carbon monoxide saturation (carboxyhemoglobin) and blood nicotine level in N-C, S-C, and plant extract bioactive compound treated rats. Hemoglobin level was significantly reduced by 22% (p < 0.01) in smoke-exposed (S-C) rats, when compared to N-C value. However, treatment with higher dose of plant extract (100 mg/rat), purified compound (F18) and atorvastatin showed a highly significant increase in Hb concentration of 26%, 27% and 21% (p < 0.01) respectively, when compared to S-C rats. Both FVBM extract and F18 bioactive compound administration to smoke-exposed rats restored the Hb levels close to normal value. Furthermore, in S-C rats blood carbon monoxide saturation and blood nicotine levels were increased from 3.26 (SCO%) and 0.998 µg/mL in N-C to 5.78 (77%) and 2.16 µg/mL (116%) (p < 0.001) respectively. After 4 weeks of treatment blood carbon monoxide saturation and blood nicotine levels showed reduction of 11% (p < 0.001) and 2% (p < 0.05) in FVT-1; 30% (p < 0.001) and 32% (p < 0.001) in FVT-2, 18% (p < 0.001) and 26% (p < 0.001) in C-T and 31% (p < 0.001) and 8% (p < 0.01) in A-T rats, respectively, in comparison to values in S-C rats.
Effect on plasma lipids and lipoprotein levels
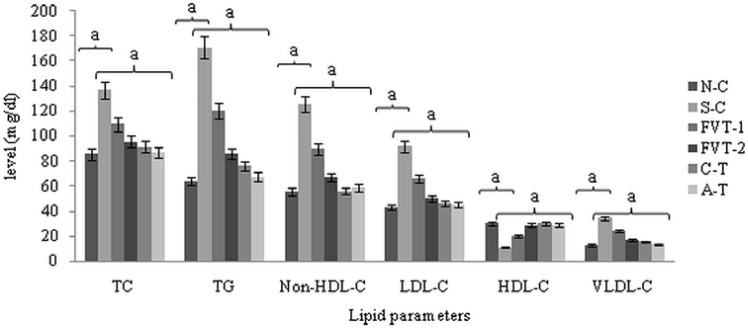
The results illustrated in Figure 1 showed that all the plasma lipids parameters, TC, TG, and non-HDL-C were significantly increased from 85.42, 64.05, and 55.53 mg/dL in N-C rats to 136.87, 171.05, and 125.93 mg/dL (p < 0.001) respectively in S-C rats. After 4 weeks of treatment with FVBM extract (50 and 100 mg/rat/day), levels of TC, TG and non-HDL-C were significantly decreased by 20%, 30%, 29% and 30%, 50%, 47% (p < 0.001) respectively, when compared to corresponding S-C values. Whereas, marked reduction of 33%, 55%, 56% and 36%, 61%, 54% (p < 0.001) was observed in TC, TG and non-HDL-C level of F18 and atorvastatin treated rats, when compared to corresponding values in S-C group.
Figure 1.
Effect of FVBM extract, F18 bioactive compound and atorvastatin on plasma triglycerides, total cholesterol, non-HDL-cholesterol, LDL-C, HDL-C and VLDL-C in cigarette smoke-exposed rats after 4 weeks of treatment.
†For the calculation of non-HDL-C, data is taken from Figure 1. *Values are mean (mg/dL) SD from plasma, LDL-C, HDL-C and VLDL-C of 5 rats in each group. Significantly different from N-C at ap < 0.001. Significantly different from S-C at ap < 0.001.
Moreover, plasma LDL-C and VLDL-C levels were significantly increased from 42.7 and 12.8 mg/dL in N-C to 91.7 mg/dL (115%) and 34.2 mg/dL (167%) (p < 0.001) respectively, in S-C rats. After 4 weeks of treatment with FVBM extract (at higher dose), both LDL-C and VLDL-C levels showed a significant reduction of 46% and 50%, (p < 0.001) respectively, whereas, FVT-1 group exhibited much less reduction. Furthermore, LDL-C and VLDL-C level in C-T group were significantly reduced by 50% and 55% (p < 0.001) respectively, in comparison to corresponding values in S-C rats
which was almost equivalent to the reduction observed in atorvastatin treated rats. Plasma HDL-C level were decreased from 30 mg/dL in N-C to 11 mg/dL (63%) (p < 0.001), in S-C values which was subsequently attenuated after the treatment with FVBM extract, bioactive compound and standard. Further, our result also depicted a significant decrease in HDL-C/LDL-C (5.8 fold) and HDL-C/TC (4.4 fold) ratio and a increase in TC/HDL-C (4.4 fold) and LDL-C/HDL-C (5.9 fold) ratios in CS-exposed hyperlipidemic rats (p < 0.001). The atorvastatin and bioactive compound treated rats exhibited marked increase of 4.1, 5.3 and 4.4, 5.8 fold (p < 0.001) in HDL-C/TC and HDL-C/LDL-C ratio (Table 4).
Table 4.
Effect of FVBM Extract, F18 bioactive compound and atorvastatin on the ratios of plasma HDL-C/TC, HDL-C/LDL-C, TC/HDL-C and LDL-C/HDL-C in cigarette smoke-exposed rats after 4 weeks of treatment
| Group * /Ratio † | HDL-C/TC | HDL-C/LDL-C | TC/HDL-C | LDL-C/ HDL-C |
| N-C | 0.35 ± .015 | 0.70 ± 0.031 | 2.85 ± 0.105 | 1.43 ± 0.057 |
| S-C | 0.08 ± 0.003 (-4.4 f)a |
0.12 ± 0.006 (-5.8 f) a |
12.51 ± 0.46 (+4.4 f) a |
8.38 ± 0.35 (+5.9 f)a |
| FVT-1 | 0.18 ± 0.008 (+2.3 f) a |
0.30 ± 0.014 (+2.1 f) a |
5.55 ± 0.24 (-2.3 f) a |
3.33 ± 0.186 (-2.5 f) a |
| FVT-2 | 0.30 ± 0.013 (+3.8 f) a | 0.58 ± 0.026 (+4.8 f) a |
3.33 ± 0.135 (-3.8 f) a |
1.73 ± 0.079 (-4.8 f) a |
| C-T | 0.33 ± 0.016 (+4.4 f) a |
0.65 ± 0.029 (+5.8 f) a |
3.04 ± 0.091 (-4.4 f) a |
1.53 ± 0.061 (-5.9 f) a |
| A-T | 0.33 ± 0.015 (+4.1 f) a |
0.64 ± 0.028 (+5.3 f) a |
3.03 ± 0.129 (-4.1 f) a |
1.56 ± 0.064 (-5.4 f) a |
N-C: normal control, S-C: smoke-exposed control, FVT-1: fed 50 mg FVBM extract/rat/day, FVT-2: fed 100 mg FVBM extract/rat/day, C-T: fed 1 mg F18 bioactive compound/rat/day and A-T: given 1 mg atorvastatin/rat/day for 4 weeks.
For the calculation of ratios, data is taken from Figure 1.
Values are mean (ratio) SD from plasma of 5 rats in each group.
Significantly different from N-C at p < 0.001.
Significantly different from S-C at p < 0.001.
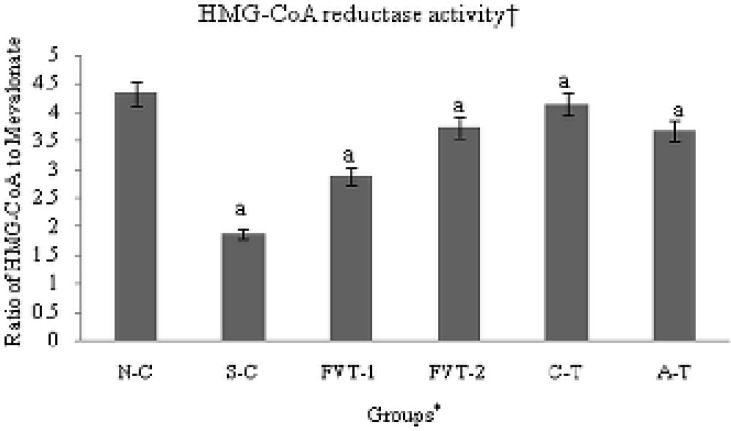
Regulation of enzymatic activity of hepatic HMG-CoA reductase
The result also exhibited a significant increase of 2.31 fold (p < 0.001) in hepatic HMG-CoA reductase activity; the rate limiting enzyme in the biosynthetic pathway of cholesterol when compared to N-C value (Figure 2). Among all the treated groups FVT-2 and C-T exhibited marked decline of 1.99 and 2.21 fold (p < 0.001) in HMG-CoA reductase activity, respectively (Figure 2), which was better or equivalent to the decline observed in atorvastatin treated rats.
Figure 2.
In-vivo regulation of hepatic HMG-CoA reductase activity in cigarette smoke-exposed rats treated with FVBM extract, F18 bioactive compound and atorvastatin for 4 weeks of treatment.
†Expressed as ratio of HMG-CoA to Mevalonate; lower the ratio higher the enzyme activity. *Values are mean SD from liver homogenate of 5 rats in each group. Significantly different from N-C at ap < 0.001. Significantly different from S-C at ap < 0.001.
Impact on plasma total antioxidants and lipid peroxidation products
Data in Table 5 demonstrate the antioxidant efficacy of FVBM extract, F18 bioactive compound and atorvastatin on ex-vivo plasma concentrations of total antioxidants, CD, LOOH, and MDA in CS-exposed rats. Data illustrated that cigarette smoke causes substantial decrease in plasma total antioxidants level and was reduced by 48% (p < 0.001) while CD, LOOH, and MDA levels were increased by 163%, 182%, and 116% (p < 0.001) respectively. Administration of FVBM extract (50 and 100 mg/rat/day), F18 and atorvastatin to smoke-exposed rats significantly increased the total antioxidants levels by 28%, 84%, 79% and 17% (p < 0.001), respectively, when compared to S-C value. On the other hand, CD, LOOH and MDA levels were significantly decreased by 30% (p < 0.001), 32% (p < 0.001) and 27% (p < 0.01) in FVT-1; 57%, 55% and 45% (p < 0.001) in FVT-2; 61%, 63% and 52% (p < 0.001) in C-T and 24% (p < 0.01), 21% (p < 0.001) and 20% (p < 0.05) in A-T rats, when compared to corresponding values in smoke-exposed rats.
Effect on membrane lipid peroxidation in erythrocytes
As seen in Table 6 , erythrocytes from smoke-exposed rats (S-C) group showed a greater susceptibility to hydrogen peroxide-induced lipid peroxidation than those from N-C group. The MDA level was substantially increased by 125% (p < 0.001) in S-C rats, when compared to N-C value. Formation of MDA was markedly decreased by 23%, 46%, 54%, and 25% (p < 0.001) after the administration of FVBM extract, F18 and atorvastatin, respectively, when compared to the corresponding values in S-C. Similarly, H2O2-mediated release of MDA erythrocytes was increased from 22.45 in N-C to 46.6 nmol/gHb (108%) (p < 0.001) in S-C rats. A highly significant decrease of 27%, 36%, 50% and 21% (p < 0.001) in MDA release was seen in smoke-exposed rats treated with FVBM extract, F18, and atorvastatin, respectively, when compared to corresponding values in S-C rats.
Table 6.
Basal MDA contents and its H2O2-induced MDA release in intact erythrocytes of cigarette smoke-exposed rats after 4 weeks of FVBM extract, F18 bioactive compound and atorvastatin treatment
| Group * |
MDA
content (nmole/g Hb) |
MDA release(percent) |
|---|---|---|
| N-C | 5.67 ± 0.24 | 22.45 ± 0.92 |
| S-C | 12.74 ± 0.64 (+124.7%)a |
46.6 ± 2.13 (+107.6%) a |
| FVT-1 | 9.78 ± 0.46 (-23.2%) a |
34.26 ± 1.92 (-26.5%) a |
| FVT-2 | 6.82 ± 0.31 (-46.4%) a |
29.87 ± 1.12 (-35.9%) a |
| C-T | 5.82 ± 0.23 (-54.3%) a |
23.68 ± 1.02 (-49.2%) a |
| A-T | 9.54 ± 0.32 (-25.1%) a |
36.81 ± 1.24 (-21.0%) a |
N-C: normal control, S-C: smoke-exposed control, FVT-1: fed 50 mg FVBM extract/rat/day, FVT-2: fed 100 mg FVBM extract/rat/day, C-T: fed 1 mg F18 bioactive compound/rat/day and A-T: given 1 mg atorvastatin/rat/day for 4 weeks.
CD: Conjugated diene, LOOH: Lipid hydroperoxide, MDA: Malondialdehyde.
Values are mean (µmole/dL) ± SD from packed erythrocytes of 5 rats in each group.
Significantly different from N-C at p < 0.001.
Significantly different from S-C at p < 0.001.
Significantly different from S-C at p < 0.01.
Significantly different from S-C at p < 0.05.
Antioxidant effect on basal and maximal level of CD formation and MDA content in LDL
As depicted in Table 7, the ex-vivo basal CD level of LDL in smoke-exposed rats was increased by 48%, in comparison to the corresponding N-C values. Administration of FVBM-50, FVBM-100, F18 and atorvastatin to these smoke-exposed stressed rats partially blocked their in-vivo LDL oxidation and reduced their basal CD levels by 11%, 27%, 29% and 8%, respectively, in comparison to the corresponding S-C values. The maximal CD value of LDL in N-C was substantially increased by 430% in comparison to corresponding basal CD value in N-C. When compared to corresponding maximal CD value in N-C rats, LDL associated CD formation of S-C rats was increased by 43%.
Table 7.
Ex-vivo and Cu++-catalyzed in-vitro oxidation of LDL, from plasma of cigarette smoke-exposed rats after 4 weeks of FVBM extract, F18 bioactive compound and atorvastatin treatment
| Group |
LDL oxidation
+
|
||||
|---|---|---|---|---|---|
| Conjugated diene formation * | MDA content # | ||||
| Basal | Maximal | Lag Phase ** | Basal | Maximal ¥ | |
| N-C | 178.49 | 945.86 (+430%) |
88 | 5.43±0.212 | 15.26 |
| S-C | 264.36 (+48.3%)† |
1348.56 (+42.6%)€ |
68 (-22.7%)¶ |
8.64±0.364a (+53.2%)† |
23.65 (+54.9%)€ |
| FVT-1 | 234.72 (-11.4%)†† |
1264.35 (-6.2%)α |
75 (+10.3%)§ |
7.43±0.202 b (-14.0%)†† |
20.26 (-14.3%) # |
| FVT-2 | 192.41 (-27.3%)†† |
1048.95 (-22.2%) α |
85 (+25.0%)§ |
6.24±0.244 a (-27.8%)†† |
18.45 (-21.9%) # |
| C-T | 187.32 (-29.2%)†† |
989.35 (-26.6%) α |
87 (+27.9%)§ |
5.87±0.231 a (-32.1%)†† |
16.6 (-29.8%) # |
| A-T | 244.54 (-7.5%)†† |
1294.28 (-4.0%) α |
73 (+7.4%)§ |
7.56±0.292 c (-12.5%)†† |
20.43 (-13.6%) # |
N-C: normal control, S-C: smoke-exposed control, FVT-1: fed 50 mg FVBM extract/rat/day; FVT-2: fed 100 mg FVBM extract/rat/day, C-T, fed 1 mg F18 bioactive compound/rat/day and A-T: given 1 mg atorvastatin/rat/day for 4 weeks.
MDA: Malondialdehyde.
Significantly different from N-C at
The CD values are expressed as nmole MDA equivalents/mg protein. Basal conjugated diene represent the in-vivo status of oxidized LDL.
The lag phase is defined as the interval between the intercept of the tangent of the slope of the curve with the time expressed in minutes.
The maximum in-vitro oxidation of LDL was achieved after 12 h of incubation with CuSo4 in each group,
Percent increase with respect to basal value in N-C,
Percent decrease with respect to basal value in S-C,
Percent decrease with respect to lag phase value in N-C,
Percent increase with respect to lag phase value in S-C,
Percent increase with respect to maximal value in N-C,
Percent decrease with respect to maximal value in S-C, Significantly different from N-C at
P < 0.001.
Values are obtained from LDL subpopulation, isolated from plasma of 5 rats in each group.
p < 0.01, significantly different from S-C at
p < 0.05.
Being a potent antioxidant, FVBM-100 and F18 significantly blocked the maximal CD concentration and reduced them by 22% and 27%, respectively, in comparison to corresponding maximal values in S-C rats. As expected, the lag phase time of LDL oxidation was reduced from 88 min in N-C to 68 min in S-C. Treatment of smoke-exposed rats with FVBM-50, FVBM-100, F18 and ATR, restored the lag phase time of LDL oxidation to 75 min, 85 min, 87 min, and 73 min, respectively. Similar to ex-vivo basal and in-vitro Cu++ catalyzed maximal CD values, the ex-vivo basal MDA content in LDL was significantly increased by 53% (p < 0.01) in S-C rats, when compared to corresponding values in N-C rats. FVBM-50, FVBM-100, F18 and atorvastatin treatment to smoke-exposed rats significantly blocked the ex-vivo increase in LDL MDA formation in S-C rats and reduced their levels by 14% (p < 0.01), 28% (p < 0.001), 32% (p < 0.001) and 13% (p < 0.05) respectively. An almost similar pattern was observed in maximal MDA content of LDL.
Discussion
Cigarette smoking (CS) is the foremost cause of morbidity and mortality worldwide (34, 35) and is considered to be the preventable risk factor for CVD (3). The current manuscript basically illustrates the use of FVBM extract and F18 compound in prevention and protection of CS-induced oxidative stress, hypercholesterolemia, and subsequent atherosclerosis. As shown in Table 2, there was significant decrease in body weight of smoke-exposed rats that may be due to reduced food intake or gastrointestinal irritation. Other reports also stated that smoker›s weight on average is about 4 kg less than non-smokers, mainly because of reduced food intake (36). Smoke-exposed rats treated with different doses of Ficus virens methanolic extract (FVBM-50 & FVBM-100), bioactive compound (F18) and atorvastatin indicates a significant regain of average body weight when compared to S-C rats.
The gas phase and tar of cigarettes contain free radicals, responsible for oxidative stress, has been hypothesized to be involved in the pathogenesis of smoking-related atherosclerosis (6). Moreover, cigarette smoking generates many toxic and carcinogenic compounds harmful to the health and was an unlikely cause for atherosclerosis, such as nicotine, nitrogen oxides, carbon monoxide (CO), hydrogen cyanide and free radicals (37-40 and 2). Similar to these reports, our results presented in Table 3, indicates blood carbon monoxide saturation and blood nicotine levels in smoke exposed rats were significantly increased and after 4 weeks a significant decrease was observed, in comparison to values in S-C rats. Moreover, both FVBM extract and F18 bioactive compound administration to smoke-exposed rats restored the Hb levels close to normal value. These results specify a strong protective effect of FVBM extract and F18 compound, which may help in lowering the menace of myocardial infarction in smokers.
Numerous smoking consequences have been described as being atherogenic, like direct vascular actions, oxidative stress, thrombogenic factors and secondary dyslipidemia (4, 40 and 41). In this context, our results illustrated that, all the plasma lipids parameters, TC, TG, non-HDL-C, LDL-C and VLDL-C levels were significantly increased (Figure 1). This increase in cholesterol and TG level of CS-exposed rats is in consensus with previously published reports (3, 42 and 43). Whereas, HDL-C level was significantly decreased by 63.4% (p < 0.001) in S-C rats when compared to N-C value (Figure 1). The increase in plasma TC level in S-C rats is apparently due to increased cholesterol synthesis (42-44), through the induction of hepatic HMG-CoA reductase activity-the rate limiting enzyme in the biosynthetic pathway of cholesterol. Chen and Loo (7) earlier reported that the increase in the cholesterol content of TG rich VLDL might be due to CS-induced reduction of lipoprotein lipase, which is responsible for the removal of TG from VLDL particles. Thus, the increased level of VLDL-C in S-C rats is also responsible for high concentrations of atherogenic cholesterol rich circulating LDL. The observed high level of VLDL-C in CS-exposed rats may responsible for reduced level of antiatherogenic HDL-C because of decreased availability of phospholipid remnants needed for the configuration of HDL from VLDL and a concomitant decline in lecithin cholesterol: acyltransferase (LCAT) activity.
Administration of FVBM extract at higher dose (100 mg/rat) and F18 bioactive compound to CS-exposed rats effectively blocked the increase in plasma lipid and lipoprotein level and reversed them to a level close to their normal control values, which is almost comparable to amelioration exhibited by standard drug atorvastation in cigarette smoke-exposed rats. Moreover, the present study observed diminished level of HDL-C in cigarette smoke-exposed rats when compared to normal rats which were in agreement with earlier reports (44, 45). Simultaneous administration of FVBM extract and F18 compound to CS-exposed rats significantly increases the HDL-C level which might be due to its profound antioxidant activity which in turn offers better protection to LDL as well as HDL from oxidative stress through its associated antioxidant enzyme paraoxonase (PON) (46). Consistent with earlier reports (47, 48) that established lipoprotein ratios, LDL-C to HDL-C and HDL-C to TC, are good forecaster for the existence and severity of CAD, our results depicted a significant decrease in HDL-C/LDL-C and HDL-C/TC ratio and a increase in TC/HDL-C and LDL-C/HDL-C ratios in CS-exposed hyperlipidemic rats. The atorvastatin and bioactive compound treated rats exhibited marked increase in HDL-C/TC and HDL-C/LDL-C ratio. An opposite pattern was observed in TC/HDL-C and LDL-C/HDL-C ratios. The results indicated a significant restoration of these ratios close to the normal values, which in turn implify the normalization of lipoprotein associated cholesterol level in CS-exposed rats treated with FVBM extract, F18 and atorvastatin.
Enhanced plasma TC level in cigarette smoke-exposed rats is apparently due to significant amplification in hepatic HMG-CoA reductase activity-the rate limiting enzyme in the biosynthetic pathway of cholesterol (Figure 2). Moreover, the significant amelioration in plasma and lipoprotein lipid level exerted by these fractions was due to the marked suppression in the hepatic HMG-CoA reductase activity. Among all the treated groups FVT-2 and C-T exhibited marked decline in HMG-CoA reductase activity, respectively (Figure 2), which was better or equivalent to the decline observed in atorvastatin treated rats. These data suggest that FVBM extract/compound may exert their cholesterol lowering effect in CS-exposed hyperlipidemic rats via suppression of hepatic HMG-CoA reductase m-RNA expression which in turn cause inhibition of cholesterol synthesis (49). These data are in agreement with previous published reports that revealed hypolipidemic property of natural bioactive compound might be due to decrease in the efficiency of HMG-CoA reductase m-RNA translation and increasing the degradation of reductase protein post translationally (50-52). Our results represent an initial demonstration and exhibit strong rationale in support of the use of FVBM extract/bioactive compound, preferably F18, as a functional food in the prevention and treatment of tobacco induced dyslipidemia/hyperlipidemia and atherosclerosis.
It is well known that CS is associated with substantial increase in oxidative stress which is mainly due to increased lipid peroxidation or reduced antioxidants (3, 4). Throughout the course of CS-induced oxidative stress, oxygen derived free radicals like superoxide (O¨), hydrogen peroxide (H2O2), hydroxyl (•HO), peroxyl (ROO), alkoxy (RO) and nitric oxide (NO) are known to be generated in the cell, which can lead to oxidative modification of lipids and cause membrane damage, resulting in cell death (53, 4). It was previously found that serum MDA concentrations were higher in smokers and rats exposed to CS (54, 4). Similar to plasma, erythrocytes are also susceptible to damage by CS-induced oxidative stress because they are constantly exposed to both extracellular and intracellular sources of ROS and cause profound alteration in the structure and function of cell membrane that lead to cell death (8). Cigarette smoke is highly responsible for permanent inflammation and leads to imbalance in the profile of lipid peroxidation products (55), which can cause number of membrane changes including lipid peroxidation in CS-exposed erythrocytes of rats (8). The studies also showed that occupationally exposed human subjects indicate enhance lipid peroxidation and alter antioxidant systems in erythrocytes (56).
Consistent with these reports, our results demonstrated a significant increase in lipid peroxidation products in plasma and erythrocytes of subchronic cigarette smoke-exposed rats. The increase in plasma lipid peroxidation product is well correlated with a significant decline in plasma total antioxidant content which in turn is consistent with the prooxidant effect of CS in rats. These results are in consensus with previous finding (57), where an increase correlation between plasma MDA level and antioxidants has been reported in CS-exposed animals.
However, protective role of lipid lowering agent with potent antioxidant property, such as FVBM extract and F18 compound, on the formation of lipid peroxidation products in plasma and erythrocytes of CS-exposed rats has not been reported. Our results showed that supplementation of FVBM extract and F18 bioactive compound to CS-exposed rats caused a significant decrease in plasma and erythrocytes lipid peroxidation products with a concomitant and significant increase in plasma total antioxidants and restored their levels close to corresponding control values in N-C (Table 6). These results are in concordant with our previously published in-vitro data that demonstrated the profound antioxidant property of FVBM extract and F18 bioactive compound (14, 16). Here it is interesting to mention that F18 compound, at a dose of 1 mg showed significant amelioration in reducing CS-induced oxidative stress parameters which were almost comparable to the FVBM extract at higher dose. The above data indicate that FVBM extract at higher dose and F18 compound strongly inhibit the lipid peroxidation process initiated by free radicals, thus preventing the membrane damage.
Several lines of research have established that lipoproteins play a pivotal role in atherogenesis (58, 59). Exposure of CS produces enhanced free radical that leads to the oxidative modification of lipoprotein (6). During oxidative modification native LDL is converted to Ox-LDL which is a key mediator of atherosclerosis and plasma concentrations has been found to be elevated in a range of cardiovascular diseases (60).
Previous studies showed that elevated level of Ox-LDL concentrations in plasma have been found in smoke-exposed rats (61). The ex-vivo BDC levels and MDA have been suggested to reflect the in-vivo oxidation of LDL (62). The result demonstrated that LDL in the CS-exposed dyslipidemic rats had an increased susceptibility to oxidation when compared to LDL of normal rats. Moreover, our result showed that the ex-vivo BDC and MDA levels of LDL in CS-exposed hyperlipidemic rats were significantly increased. This increase susceptibility may result from increased oxidative stress, decrease total antioxidant and increase LDL cholesterol content. To the best of our knowledge no one to date has demonstrated the potent protective effect of F. virens extract or the isolated bioactive compound on the ex-vivo and in-vitro Cu++-catalyzed oxisability of plasma LDL of CS-exposed dyslipideamic rats. Simultaneous supplementation of FVBM extract and bioactive compound to CS-exposed rats blocked the in-vivo and in-vitro oxidation of LDL. As expected, the lag phase time of LDL oxidation was reduced and restored significantly after treatment. Similarly, ex-vivo basal and Cu++ induced maximal formation of MDA in LDL was significantly decreased in CS-exposed rats treated with plant extract and the bioactive compound. The data summarises that FVBM extract at a lower dose was least effective as an antioxidant and was only able to partial restoration of the above lipid peroxidation parameters. The potent hypolipidemic property of FVBM extract (at higher dose) and the bioactive compound, as discussed above, is consistent with the excellent antioxidant property of these fractions.
The data summarises that FVBM extract at a lower dose was least effective as an antioxidant and was only able to partial restoration of the above lipid peroxidation parameters. The potent hypolipidemic property of FVBM extract (at higher dose) and the bioactive compound, as discussed above, is consistent with the excellent antioxidant property of these fractions.
Conclusions
In conclusion our combined results clearly demonstrated the protective role of FVBM extract and F18 compound in risk factor induced cardiovascular disease. Moreover, the test fractions/pure compound exhibited significant protection against CS-induced severe oxidative stress and hyperlipidemia, which indicates that F18/FVBM extract are highly promising natural antioxidant and also can be used as an antiperoxidative, hypolipidemic, and antiatherogenic agent. However, further large scale clinical trials in hyperlipidemic smokers with and without coronary heart disease are required to substantiate their antioxidative, hypolipidemic, and atheroprotective properties.
Acknowledgements
The author would like to appreciate Prof. S.W. Akhtar, vice chancellor, for providing state-of-the-art research laboratory and animal house for smooth succession of this work. The author would also like to thank Deanship of Scientific Research at Majmaah University for their support and contribution to this study.
References
- 1.WHO. World Health Organization (WHO) Cardiovascular diseases, Fact Sheet No. 317. 2015. Available from: URL: http://www.who.int/mediacentre/factsheets/fs317/en/,2015.
- 2.Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Effects of smoking on cardiovascular function: the role of nicotine and carbon monoxide. Health Sci. J. . 2014;8:274–90. [Google Scholar]
- 3.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol. . 2004;43:1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Mohod K, Ninghot A, Ansari AK, Garg N. Circulating lipid peroxide and antioxidant status in cigarette smokers: an oxidative damage phenomena. Int. J. Health Sci. Res. . 2014;4:59–65. [Google Scholar]
- 5.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. . 1985;64:111–26. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokode M, Nagano Y, Arai H, Ueyama K, Ueda Y, Kita T. Cigarette smoke and lipoprotein modification A possible interpretation for development of atherosclerosis. Ann. N.Y. Acad. Sci. . 1995;748:294–300. [PubMed] [Google Scholar]
- 7.Chen C, Loo G. Inhibition of lecithin: cholesterol acyltransferase activity in human blood plasma by cigarette smoke extract and reactive aldehyde. J. Biochem. Toxicol. . 1995;10:121–8. doi: 10.1002/jbt.2570100302. [DOI] [PubMed] [Google Scholar]
- 8.Achudume AC, Aina F. Oxidative stress and oxidative damage in male rat erythrocytes associated with prolonged exposure to smoke pollution. J. Environ. Prot. . 2012;3:414–9. [Google Scholar]
- 9.Ramesh T, Mahesh R, Begum HV. Effect of Sesbania grandiflora on lung antioxidant defense system in cigarette smoke exposed rats. Int. J. Biol. Chem. . 2007;1:141–8. [Google Scholar]
- 10.Ramesh T, Sureka C, Bhuvana S, Begum VH. Sesbania grandiflora diminishes oxidative stress and ameliorates antioxidant capacity in liver and kidney of rats exposed to cigarette smoke. J. Physiol. Pharmacol. . 2010;61:467–76. [PubMed] [Google Scholar]
- 11.Anbarasi K, Vani G, Balakrishna K, Shyamala Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. . 2006;78:1378–84. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Sirisha N, Sreenivasulu M, Sangeeta K, Chetty CM. Antioxidant properties of Ficus species – a review. Int. J. PharmTech. Res. . 2010;2:2174–82. [Google Scholar]
- 13.Shridhar NB. In-Vivo antioxidant evaluation of methanolic extract of Ficus virens leaves in CCl4 induced oxidative stress in rats. Indian J. Vet. Sci. . 2013;1:28. [Google Scholar]
- 14.Iqbal D, Khan MS, Khan A, Khan MS, Ahmad S, Srivastava AK, Bagga P. In-vitro screening for β-hydroxy-β-methylglutaryl-CoA reductase inhibitory and antioxidant activity of sequentially extracted fractions of Ficus Palmata Forsk. BioMed Res. Int. . 2014a;762620:1–10. doi: 10.1155/2014/762620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal D, Khan MS, Khan MS, Ahmad S, Srivastava AK. An In-Vitro and molecular informatics study to evaluate the antioxidative and β-hydroxy-β-methylglutaryl-CoA reductase inhibitory property of Ficusvirens ait. Phytother. Res. . 2014b;28:899–908. doi: 10.1002/ptr.5077. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal D, Khan MS, Khan MS, Ahmad S, Hussain MS, Ali M. Bioactivity guided fractionation and hypolipidemic property of a novel HMG-CoA reductase inhibitor from Ficusvirens ait. Lipids Health Dis. . 2015;14:1–15. doi: 10.1186/s12944-015-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayashree P, Shridhar NB, Vijaykumar M, Suhasini K, Jayakumar , Satyanarayana ML. Toxicological studies of Ficusvirens in wistar albino rats. Int. Res. J. Pharm. . 2012;3:84–7. [Google Scholar]
- 18.Hafeez A, Jain U, Sajwan P, Srivastava S, Thakur A. Evaluation of Carrageenan induced anti-inflammatory activity of ethanolic extract of bark of Ficusvirens Linn in swiss albino mice. J. Psychopharmacol. . 2013;2:39–43. [Google Scholar]
- 19.Lakshmi S, Rajagopal G. Reduced glutathione level and catalase activity in erythrocytes in patients with diabetes mellitus. Biomedicine . 1998;18:37–9. [Google Scholar]
- 20.Drabkin D, Austin J. Spectrophotometric studies: I Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. . 1932;98:719–33. [Google Scholar]
- 21.Varley H, Gowenlock AH. Practical Clinical Biochemistry. Vol. I; 5th (ed.), Chapter 19. London: Heinemann Medical Books; 1976. Bell M; pp. 557–8. [Google Scholar]
- 22.Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J. Lipid Res. . 1983;24:904–9. [PubMed] [Google Scholar]
- 23.Patsch W, Brown SA, Morrisett JD, Gotto (Jr) AM, Patsch JR. A dual-precipitation method evaluated for measurement of cholesterol in high-density lipoprotein sub fractions HDL2 and HDL3 in human plasma. Clin. Chem. . 1989;35:265–70. [PubMed] [Google Scholar]
- 24.Esterbauer H, Gebicki J, Puhl H, Jugens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad. Biol. Med . 1992;13:341–90. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 25.Esterbauer H, Striegel G, Puhl H, Oberreither S, Rotheneder M, El Saadani M, Jurgens G. The role of vitamin E and carotenoids in preventing oxidation of low-density lipoproteins. Ann. N.Y. Acad. Sci. . 1989;570:254–67. doi: 10.1111/j.1749-6632.1989.tb14925.x. [DOI] [PubMed] [Google Scholar]
- 26.Niehaus WG, Samuelsson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biocem. . 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” The FRAP assay. Anal. Biochem. . 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28.Cynamon HA, Isenberg JN, Nguyen CH. Erythrocyte malondialdehyde release in-vitro: a functional measure of vitamin E status. Clin. Chim. Acta. . 1985;151:169–76. doi: 10.1016/0009-8981(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 29.Stocks J, Dormandy TL. The autooxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. . 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 30.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann. Clin. Biochem. . 1969;6:24–7. [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. . 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Rao V, Ramakrishnan A. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. . 1975;21:1523–5. [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. . 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Jha P, Jacob B, Gajalakshmi V, Gupta PC, Dhingra N, Kumar R, Sinha D, Dikshit R, Parida DK, Kamadod R, Boreham J, Peto R. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. . 2008;358:1137–47. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- 35.Alam DS, Jha P, Ramasundarahettige C, Streatfield PK, Niessen LW, Chowdhury MAH, Siddiquee AT, Ahmed S, Evans TG. Smoking-attributable mortality in Bangladesh: proportional mortality study. Bull. World Health Org. . 2013;91:75764. doi: 10.2471/BLT.13.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rang HP, Dale MM, Ritter JM, Gardner P. Pharmacology. 4th (ed.) New York: Churchill Livingstone; 2001. [Google Scholar]
- 37.Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, Schmid RW, Maurer G, Stefenelli T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J. Am. Coll. Cardiol. . 2002;39:251–56. doi: 10.1016/s0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- 38.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco Smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health . 2009;6:445–62. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochette L, Cottin Y, Zeller M, Vergely C. Carbon monoxide: mechanisms of action and potential clinical implication. Pharmacol. Ther. . 2013;137:133–52. doi: 10.1016/j.pharmthera.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Chung WS, Lin CL, Kao CH. Carbon monoxide poisoning and risk of deep vein thrombosis and pulmonary embolism: a nationwide retrospective cohort study. J. Epidemiol. Community Health. . 2015;69:557–62. doi: 10.1136/jech-2014-205047. [DOI] [PubMed] [Google Scholar]
- 41.Powell JT. Vascular damage from smoking: Disease mechanisms at the arterial wall. Vasc. Med. . 1998;3:21–8. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 42.Chitra S, Semmalar R, Shyamala devi CS. Effect of fish oil on cigarette smoking induced dyslipidemia in rats. Indian J. Pharmacol. . 2000;32:114–19. [Google Scholar]
- 43.Li X, Ip MSM, Mak JC, Liang YM. Alterations of cigarette smoke-induced lipid metabolism in rat heart and lung. Am. J. Respir. Crit. Care Med. . 2013;187:A4841. [Google Scholar]
- 44.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. Br. Med. J. . 1989;298:784–8. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vella JC, Linaje MJ, Perez-Iniguez B. Serum concentration of lipoprotein (a) and cholesterol in HDL subfractions in relation to cigarette consumption. Revista. De. La. Sociedad. Española. De. Quimica. Clin. . 1994;13:50–253. [Google Scholar]
- 46.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase, inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. . 1995;96:2882–91. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenai GR, Després JP. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch. Intern. Med. . 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 48.Millán J, Pintó X and Muñoz A. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk. Manag. . 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 49.Maurya SK, Srivastava AK. Glycyrrhizic acid attenuates the expression of HMG-CoA reductase mRNA in high fructose diet induced dyslipidemic hamsters. Prague. Med. Rep. . 2011;112:29–37. [PubMed] [Google Scholar]
- 50.Elson CE, Peffley DM, Hentosh P, Mo HB. Isoprenoid-Mediated inhibition of mevalonate synthesis: potential application to cancer. Proc. Soc. Exp. Biol. Med. . 1999;221:294–311. doi: 10.1046/j.1525-1373.1999.d01-87.x. [DOI] [PubMed] [Google Scholar]
- 51.Pakpour S. Towards application of bioactive natural products containing isoprenoids for the regulation of HMG-CoA reductase—a review. Am. J. Plant. Sci. . 2013;4:1116–26. [Google Scholar]
- 52.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. . 1993;268:11230–38. [PubMed] [Google Scholar]
- 53.Pryor WA, Stone K. Oxidants in cigarette smoke Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N.Y. Acad. Sci. . 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 54.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta . 2007;380:50–8. doi: 10.1016/j.cca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Vaart VDH, Postma DS, Timens W, Ten Hacken NHT. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax . 2004;59:713–21. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durak I, Serenay E, Bingöl NK, My BC, Murat K, Büyükkocak S, Öztürk HS. Effects of cigarette smoking with different tar content on erythrocyte oxidant/antioxidant status. Addict. Biol. . 2002;7:255–8. doi: 10.1080/135562102200120505. [DOI] [PubMed] [Google Scholar]
- 57.Thirumalai T, Therasa SV, Elumalai EK, David E. Effect in-vivo of cigarette smoke on lipid peroxidation and antioxidant status in male albino mice. J. Pharm. Sci. Res. . 2010;2:579–82. [Google Scholar]
- 58.Nordestgaard BG, Nielsen LB. Atherosclerosis and arterial influx of lipoproteins. Curr. Opin. Lipidol. . 1994;5:252–7. doi: 10.1097/00041433-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM, Ridker PM, Kastelein JJ. Association of LDL cholesterol, non–HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. . 2012;307:1302–9. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 60.Messner B, Bernhard D. Smoking and cardiovascular disease mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. . 2014;34:509–15. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 61.Ejaz SK. Oxidative stress-induced modifications of lipoproteins and antioxidant status in cigarette smoke exposed rats: protective actions of essential oil and methanol fractions from nigella sativa seeds. 2013. Available from: URL: http://shodhganga.inflibnet.ac.in/bitstream/ 10603/13194/9/ 09_chapter%201.pdf.
- 62.Planski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew RE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in-vivo. Proc. Natl. Acad. Sci. U.S.A. . 1989;86:1372–6. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]