Abstract
Adenosine receptors were studied on heart cells grown in cultures by the radioligand binding technique. We used the hydrophilic A1 adenosine receptor radioligand [3H]-8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX), to monitor the level of the receptors on intact cardiocytes. The binding showed high affinity (Kd = 0.13 nM) and the number of [3H]CPX binding sites (Bmax) was 23.1 fmol/dish (21 fmol/mg protein). The Ki of the agonists R-N6-(2-phenylisopropyl)-adenosine (R-PIA) and S-N6-(2-phenylisopropyl)-adenosine (S-PIA), and of the antagonists CPX and theophylline were 3.57, 49.0, 1.63 and 4880 nM, respectively. The number of adenosine receptors was very low during the first days in cultures (5 fmol/dish) and increased gradually until it reached a plateau on days 8–10. Treatment with norepinephrine or isoproterenol which accelerated the rate of contractions, induced up regulation of the receptors. Bmax increased 2–3-fold by application of norepinephrine for 4 days, while receptor affinity to the radioligand was unaffected. Lactate dehydrogenase (LDH) and creatine kinase (CK) activity increased only by 22 and 38%, respectively. Similarly, 3 days treatment with triiodothyronine (T3, 10−8 M), which also accelerated heart rate, increased the number of adenosine receptors by 56% without a significant change in the affinity of the receptors to [3H]CPX. Carbamylcholine (5 × 10−6 M), which reduced the rate of heart contractions, caused 26% down regulation while the affinity of the receptors remained unchanged. It is concluded that there is a linkage between the rate of cardiac contractions and the level of adenosine receptors. Thus, the level of adenosine receptors may respond to drug-induced chronic changes in cardiac contractile activity so as to restore conditions to normal (basal) contractions.
Keywords: CPX, heart-rate, norepinephrine, thyroid hormones
Adenosine modulates a variety of physiological functions in the heart. These actions are mediated by cell surface adenosine receptors, which can be classified into A1 and A2 subtypes [1], based upon their properties of activating (A2) or inhibiting (A1) adenylate cyclase activity, and according to the order of agonist potency [2,3].
In conditions of stress like hypoxia or ischemia, the concentration of adenosine in the extracellular fluid rises dramatically, mainly through the breakdown of ATP [4]. In these conditions adenosine has therapeutic and protective effects on the heart [5,6]. Adenosine causes negative chronotropic, dromotropic and ionotropic effects on the cardiac tissue via A1 receptors [7,8]. Adenosine also acts as a coronary vasodilator via A2 receptors [9]. These effects of adenosine occur also without prior catecholamine treatment [10]. Stimulation that enhances the cAMP content (catecholamines, forskolin, amrinone), makes the heart more sensitive to adenosine [11-14]. In addition, adenosine causes hyperpolarization by activation of K+ channels [15] via G proteins [16, 17]. These K+ channels are the same channels that are stimulated by acetylcholine in the cardiac tissue [18-20].
Chronic treatment with an adenosine agonist or antagonist is capable of regulating the density of adenosine A1 receptors in the cardiac tissue [21-23]. Since these treatments affect the rate of heart contractions, we addressed the question of whether modulation of cardiac contractility contributes to regulating the synthesis of A1 adenosine receptors. We describe here the binding properties of adenosine receptors in intact rat cardiocytes grown in culture using [3H]CPX (DPCPX)‡, which has been reported to be an A1 selective antagonist [24]. The radioligand binding technique has been used to study the possible mechanisms involved in up or down regulation of adenosine receptors as a result of treatment with agents affecting contractile activity. We show that NE or TH, which accelerate the heart rate of contractions, cause an elevation in the receptor level, whereas Carb, which attenuates the rate of contractions, reduces the level of adenosine receptors. Thus, our data indicates that contractile activity plays a major role in controlling the level of adenosine receptors in the heart.
MATERIALS AND METHODS
Preparation of heart cells cultures
Rat hearts (l–2 days old) were removed under sterile conditions and washed three times in PBS to remove excess blood cells. The hearts were minced to small fragments and then gently agitated in a solution of proteolytic enzyme-RDB (Ness-Ziona, Israel) prepared from a fig tree extract. The RDB was diluted 1:50 in PBS, at 25° for a few cycles of 10 min each, as described previously [25-27]. The supernatant suspensions containing dissociated cells, to which medium containing 10% horse serum (Biolab, Jerusalem, Israel) was added, were centrifuged at 150 g for 5 min. After centrifugation, the supernatant phase was discarded and cells were resuspended in high glucose (5 mg/mL) DMEM (Gibco, Uxbridge, U.K.) supplemented with 10% heat-inactivated horse serum and 2% chick embryo extract. The suspension of cells was diluted to 1.2 × 106 cells/mL and 1.5 mL were placed in 35 mm collagen-gelatin-coated plastic culture dishes. Cultures were incubated in a humidified atmosphere of 10% CO2, 90% air at 37°. The incubated cells were washed well 24 hr after plating to remove unattached cells. Confluent monolayers, which exhibit spontaneous contractions, developed in culture within 2 days. The growth medium was replaced every 3–4 days.
Radioligand binding studies
Intact cells were incubated at room temperature (22–25°) for 60 min, with various concentrations of [3H]CPX. 1 μg/mL dipyridamole, 0.2 U/mL adenosine deaminase in PBS, pH 7.4. Incubation was stopped by quick rinsing the cells five times with cold (4–10°) PBS. The cells were solubilized with 0.2 mL 0.5 N NaOH and neutralized upon addition of 0.1 mL 2 N Tris–HCl, pH 3.7. After addition of 3 mL of scintillation liquid radioactivity was determined using a βcounter. Non-specific binding of [3H]CPX was defined as the amount of radioactivity remaining after incubation with theophylline (5 mM). Specific [3H]CPX binding was calculated as the total radioactivity bound minus the non-specific binding (less than 20%).
For calculation of the association rate constant, k1, specifically bound [3H]CPX (0.6 nM) was determined as a function of time as indicated in the graph (Fig. 1).
Fig. 1.
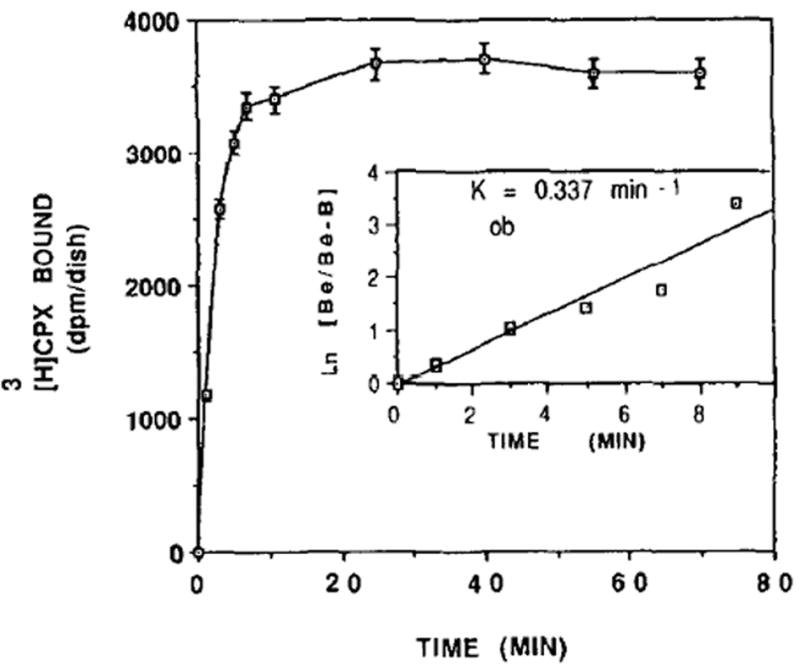
Time-course of [3H]CPX binding to heart cells. Six-day-old cardiocytes were incubated in the presence of 0.6nM [3H]CPX at 25°. Specific binding was determined as the [3H]CPX binding displaceable by 5 mM theophylline. Each value is the mean ± SE of triplicate determinations from a representative experiment of sister cultures. Inset: Pseudo first order kinetic plot of [3H]CPX binding. B = amount of [3H]CPX bound at each time. Be = amount of [3H]CPX bound at equilibrium.
In experiments studying the dissociation of [3H]-CPX binding, cells were incubated at 25° with 0.6 nM [3H]CPX to equilibrium (60 min). At time zero, the binding mixture was replaced by PBS (1 mL) containing theophylline (5 mM). The experiment was terminated as in the ligand binding method. koff was calculated from the slope of the plot.
For the competition experiments, cardiomyocytes were incubated in a mixture containing the competing drug at various concentrations and 0.6 nM [3H]CPX for 60 min at room temperature.
Protein content, creatine kinase and lactate dehydrogenase activity
Protein determination was performed following washing of the cells with PBS (×2) according to Bradford [28] method, using bovine serum albumin as a standard.
The cardiomyocytes were washed with cold PBS (×2) and the cells were homogenized in the same buffer. CK and LDH activities were determined using CK and LDH-L kits (Sigma, St Louis, MO, U.S.A.) and the product of the enzyme was measured spectrometrically at a wavelength of 340 nm as described previously [27].
Beating rate determination
Number of beats/min were counted under a phase contrast microscope equipped with video motion detector system. Five dishes were monitored at random through a 25× objective.
Materials
Carb and Iso were dissolved in PBS. T3 was dissolved in 0.1 N NaOH and applied to the heart culture dishes to final concentration of 10 nM. Norepinephrine hydrochloride was dissolved in 0.1 mM of ascorbic acid. Dipyridamole was dissolved in 10% ethanol and applied to the cardiocytes to final concentration of 1 μM. Drugs and chemical used were from the Sigma Chemical Co. [3H]CPX (sp. act. 108.3 Ci/mmol) was purchased from Amersham International (Little Chalfont, Bucks, U.K.). R-PIA, S-PIA and CPX were obtained from Research Biochemicals International (Natick, MA, U.S.A.).
RESULTS
Characterization of adenosine receptors
Kinetics of [3H]CPX binding
The time-course of specific [3H]CPX binding to intact cardiomyocytes grown in culture is illustrated in Fig. 1. The binding reached equilibrium within 7 min of incubation and was maintained for an additional 70 min. The rate constant for the pseudo first order association reaction (kob) was calculated to be 0.337/min (Fig. 1, inset).
The dissociation of [3H]CPX from the receptor binding sites was reversible and temperature dependent. At 25°, 50% of the ligand was dissociated from the receptors within 8 min (Fig. 2). Equilibrium was achieved after 20 min of dissociation. The dissociation rate constant (koff) was calculated to be 0.099/min (Fig. 2, inset).
Fig. 2.
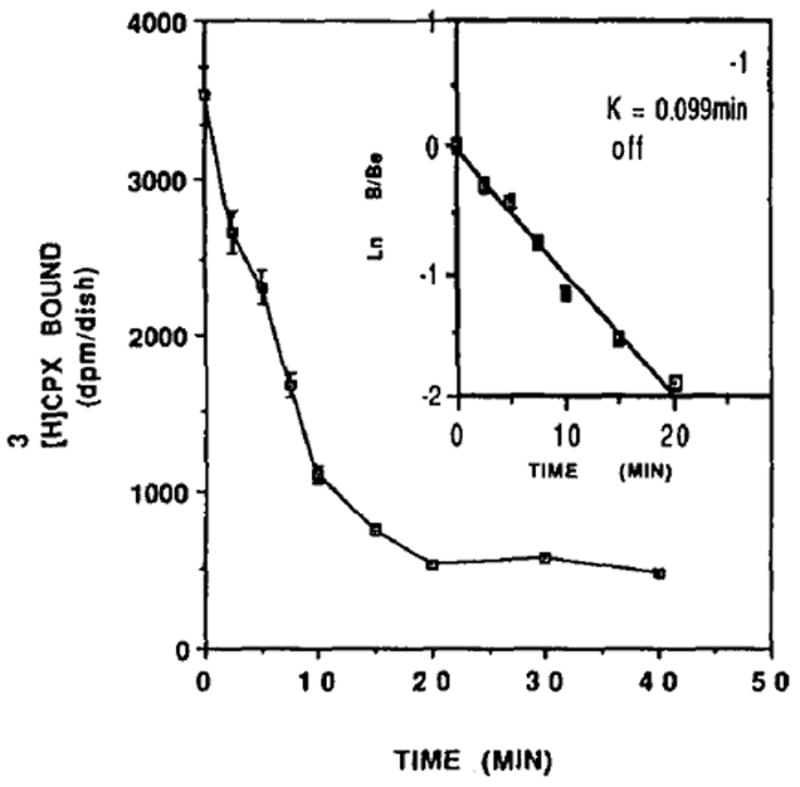
Dissociation curve of [3H]CPX binding from heart cells. Reverse kinetics of dissociation of [3H]CPX from cardiocytes (6 days old). Specific bindings at 25° were determined at subsequent time intervals. After 60 min of incubation the binding mixture (0.6 nM [3H]CPX in PBS) was removed and PBS containing 5 mM of theophylline was added. Each value is the mean ± SE of triplicate determinations from a representative experiment. Inset: First order kinetic plot of dissociation of [3H]CPX bound at each time after dilution of the binding mixture at 25°. Be = amount of [3H]CPX bound at time 0. B = amount of [3H]CPX bound at the indicated time. The association constant, koff = 0.099/min.
The association constant (k1) was calculated from the equation k1 = kob − koff/([3H]CPX) according to Cheng and Prusoff [29], and was found to be 0.397/min/nM. Thus, the equilibrium dissociation constant determined kinetically from the ratio koff/k1 was estimated to be 0.25 nM.
Saturability of [3H]CPX binding
To define the saturability of the ligand, intact cardiocytes (6 days old) were incubated at room temperature with various concentrations of [3H]CPX. For the non-specific binding the cardiocytes were incubated also with 5 mM theophylline. Figure 3 shows the relationship between the radioligand concentrations and the number of specific binding sites in the cultured cardiomyocytes. Maximal saturation of the antagonist occurred at a concentration of 0.2nM. Scatchard analysis [30] indicates that the maximum number of binding sites is 23.1 fmol/dish (21 fmol/mg protein) and the Kd for [3H]CPX is 0.13 nM (Fig. 3, inset), which is in adequate agreement with the kd value calculated from the kinetic experiments. The data best fits a straight line which indicates that there is one binding site on the receptor.
Fig. 3.
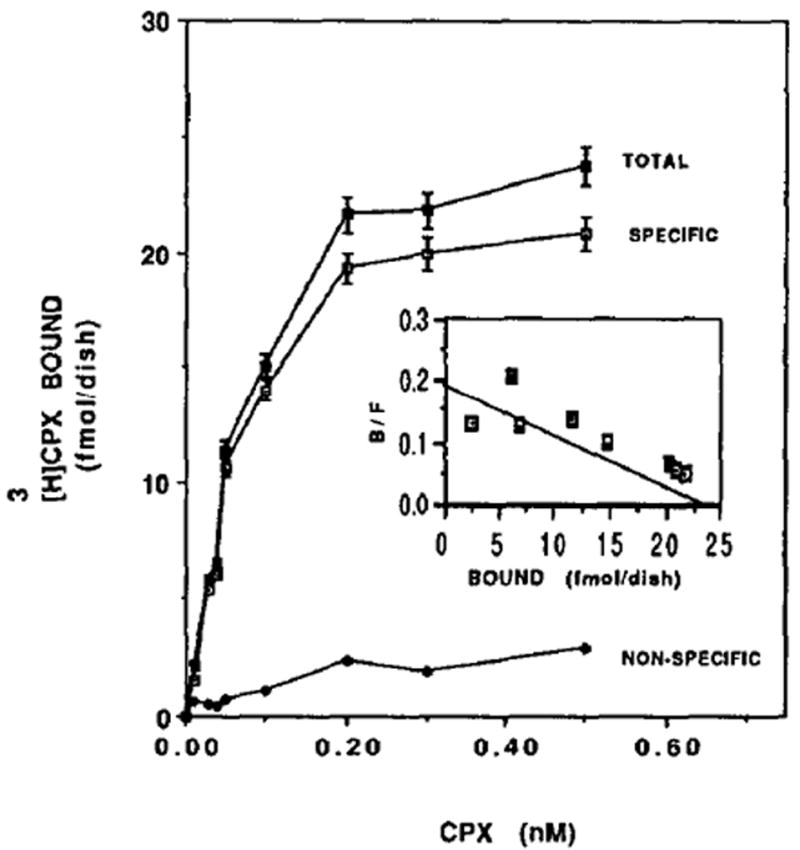
Specific binding of [3H]CPX to heart cells. Six-day-old cardiocytes were exposed to the indicated concentrations of the radioligand as described in Materials and Methods. Specific binding was determined as the [3H]CPX binding displaceable by 5 mM theophylline. Each value is the mean ± SE of triplicate determinations from a representative experiment of sister cultures. Inset: Scatchard plot of specific [3H]CPX binding of Fig. 3.The Kd for [3H]CPX was 0.13 ± 0.07 nM and the maximal binding capacity was 23.1 ± 2.9 nM (21 fmol/mg protein).
Competition of [3H]CPX binding
The agonists R-PIA and S-PIA, and the antagonists CPX and theophylline were examined in competition binding assays with [3H]CPX (Fig. 4). The inhibition constants (Ki) were calculated according to Bylund and Yamamura [31]. Figure 4 indicates that CPX is the most A1 potent antagonist (Ki = 1.6 nM). After CPX, the agonist R-PIA (Ki = 3.57) is more potent than the agonist S-PIA (Ki = 49.0 nM) and the antagonist theophylline (Ki = 4880 nM).
Fig. 4.
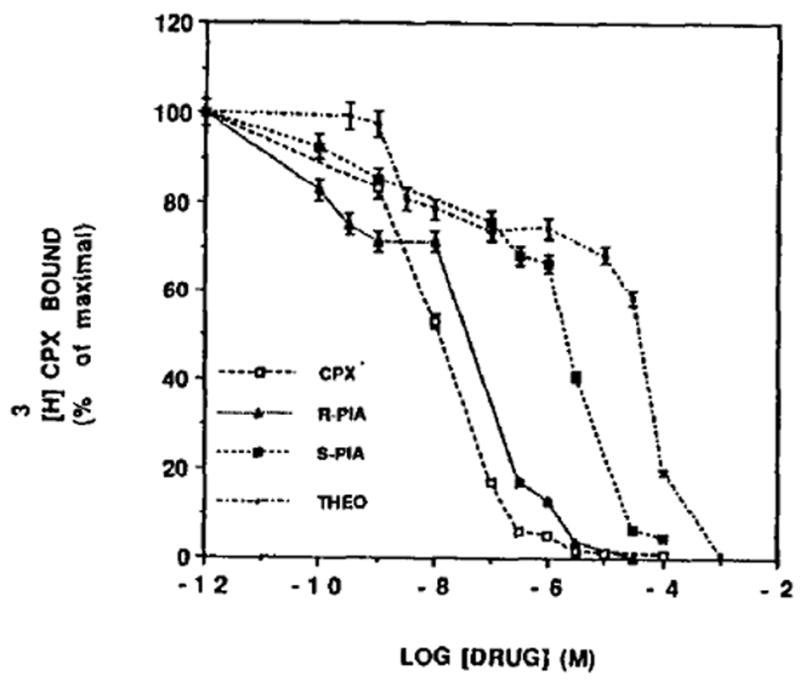
Inhibition of [3H]CPX binding to heart cells. Six-day-old cardiocytes were incubated in the presence of [3H]CPX (0.7 nM) and increasing concentration of different drugs. After 60min of incubation at room temperature, specific binding was estimated as described in Materials and Methods. The results are expressed as the percentage of [3H]CPX specifically bound. Data points represent means ± SE of triplicate determinations from a representative experiment. The Ki, values for CPX, R-PIA, S- PIA and theophylhne were 1.63, 3.57, 49 and 4880 nM, respectively.
Development of adenosine receptors
[3H]CPX binding in cardiocytes was investigated during their development in culture. It was found that the number of A1 adenosine receptor binding sites increased with culture age, without reaching the plateau, whereas CK activity reached a plateau (0.70–0.75 U/dish) in 5-day-old cultures (Fig. 5).
Fig. 5.
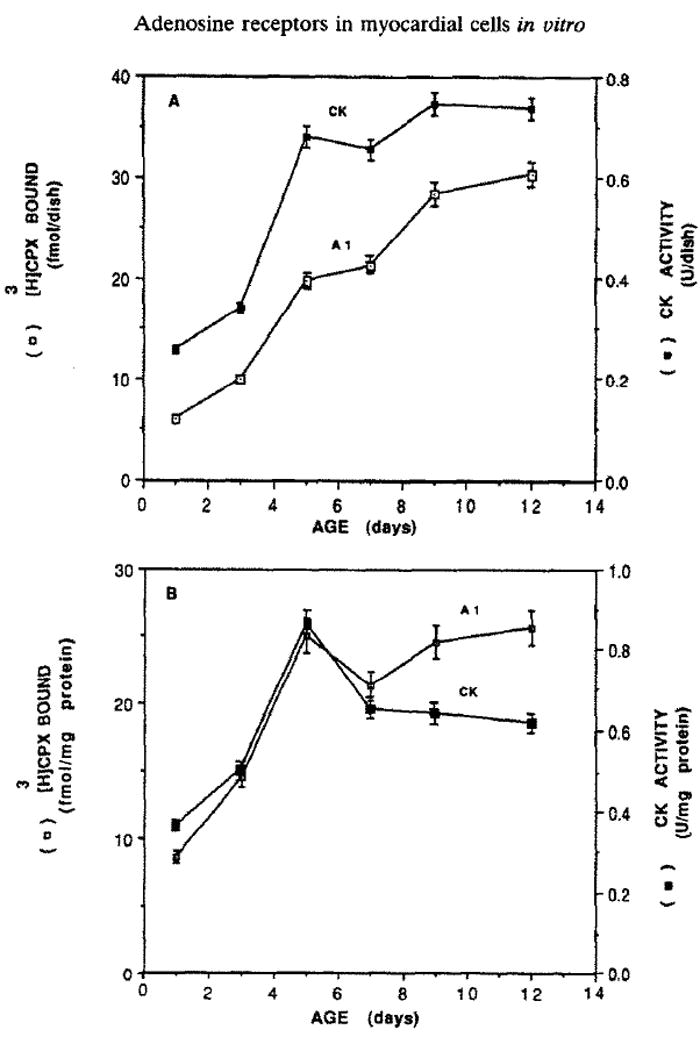
Age dependence of specific binding of [3H]CPX to heart cells in comparison to CK activity. The level of [3H]CPX binding and CK activity were determined at the indicated time on sister cultures according to Materials and Methods. The results are expressed (A) per dish, or (B) per protein (means ± SE of triplicate determinations from a representative experiment).
Regulation of the receptor level
Effect of catecholamines on [3H]CPX binding
Since NE and Iso stimulate the contraction of cardiomyocytes, we investigated whether this increase in the rate of heart contractions is associated with a change in the level of adenosine receptors. Thus, treatment with NE for 4 days caused an increase of 270% of [3H]CPX binding (Fig. 6). Scatchard analysis (Fig. 6, inset) shows that the affinity of the receptor for its ligand remained almost unchanged. There was no significant difference in protein content between control and NE-treated cells (1.08 ± 0.14 and 1.18 ± 0.18 mg/dish, respectively). However, in NE treated cells LDH and CK activity increased by 22 and 34%, respectively. The rate of heart contractions was doubled, whereas cell number was decreased by 17% (Table 1). The effect of catecholamines was time (Fig. 7) and dose dependent (Fig. 8). Figure 7 shows an increase of 30, 105 and 171% in [3H]CPX binding following 2, 3 and 4 days of NE treatment, respectively, and an increase of 41% after 2 days of Iso treatments. Figure 8 shows a dose–response of [3H]CPX binding following ISO or NE treatments. Increases of 20, 28 and 41% were achieved by 10−7, 10−6, 10−5 M of Iso treatments, respectively (Fig. 8). [3H]CPX binding was elevated by 36, 57 and 77% in 10, 20 and 100 μM, respectively, following 60 hr of NE treatment (Fig. 8).
Fig. 6.
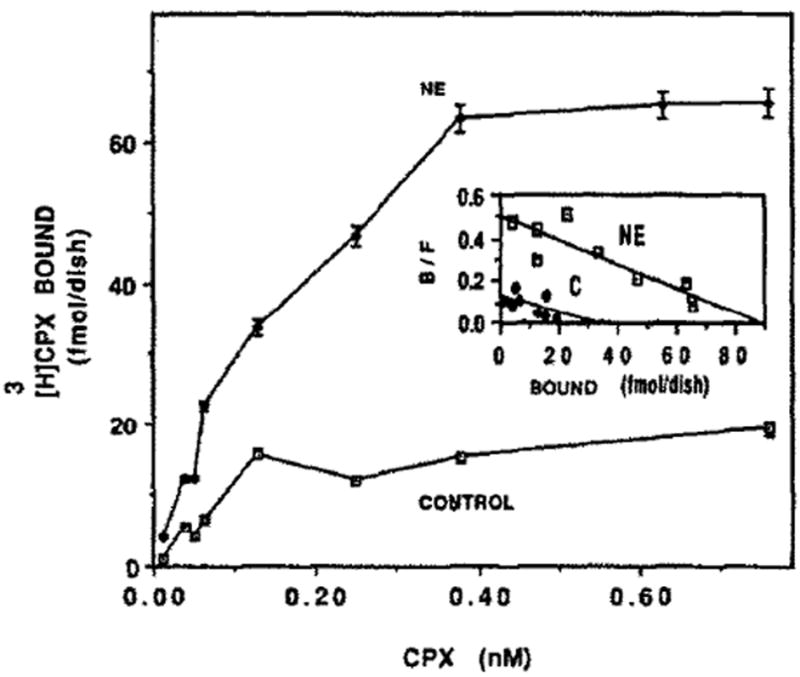
Effect of NE on [3H]CPX binding. Four-day-old cardiocytes were treated with 20 μm NE. Specific binding of [3H]CPX was determined following 4 days. Inset: Scatchard plot of specific [3H]CPX binding of Fig. 6. kd = 0.18 and 0.17nM, and Bmax = 31.4 and 88.2 fmol/dish in control and NE-treated cells, respectively.
Table 1.
The effect of NE on cardiac cells
| Control | NE | |
|---|---|---|
| CK (U/dish) | 0.75 ± 0.02 | 1.00 ± 0.04 |
| LDH (U/dish) | 2.02 ± 0.12 | 2.46 ± 0.01 |
| Protein (mg/dish) | 1.08 ± 0.14 | 1.18 ± 0.18 |
| Cell number (×104) | 91 ± 9 | 76 ± 9 |
| Rate of contractions | 82 ± 20 | 174 ± 16 |
| Adenosine receptors (fmol/dish) | 21.45 ± 2.22 | 58.03 ± 4.01 |
Four-day-old cardiomyocytes were treated with 20 μM NE for 4 days. Then, the levels of specific [3H]CPX binding were determined. Sister cultures were homogenized for measurement of proteins and enzyme activities. Rate of contractions and cell number were performed on separate group of cells. The results are expressed as means ± SE of at least triplicate determinations from a representative experiment.
Fig. 7.
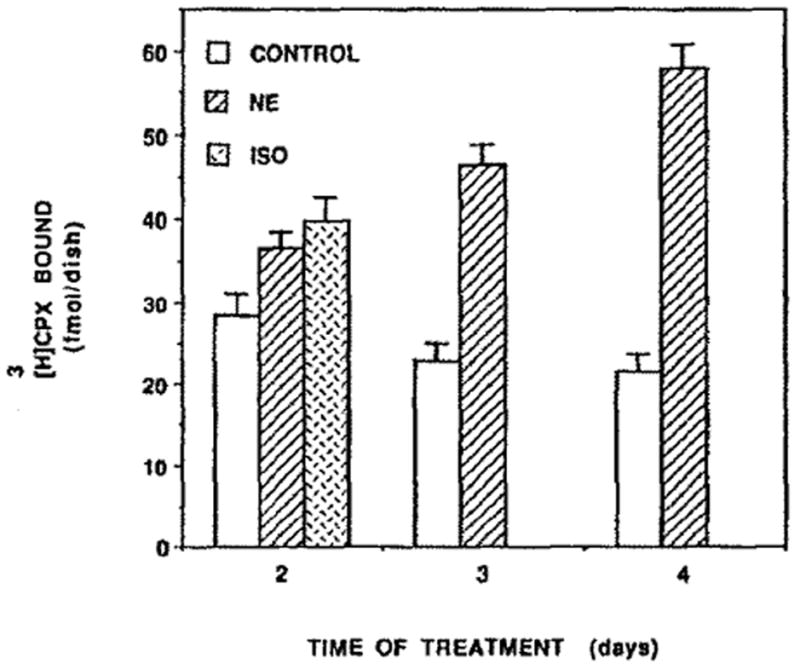
Time course of NE effect on adenosine receptors. Four-day-old cardiocytes were treated with 20 μM NE for 2, 3 and 4 days and with 10 μM Iso for 2 days. Then, the levels of specific [3H]CPX binding were determined. The results are expressed as means ± SE of triplicate determinations from a representative experiment.
Fig. 8.
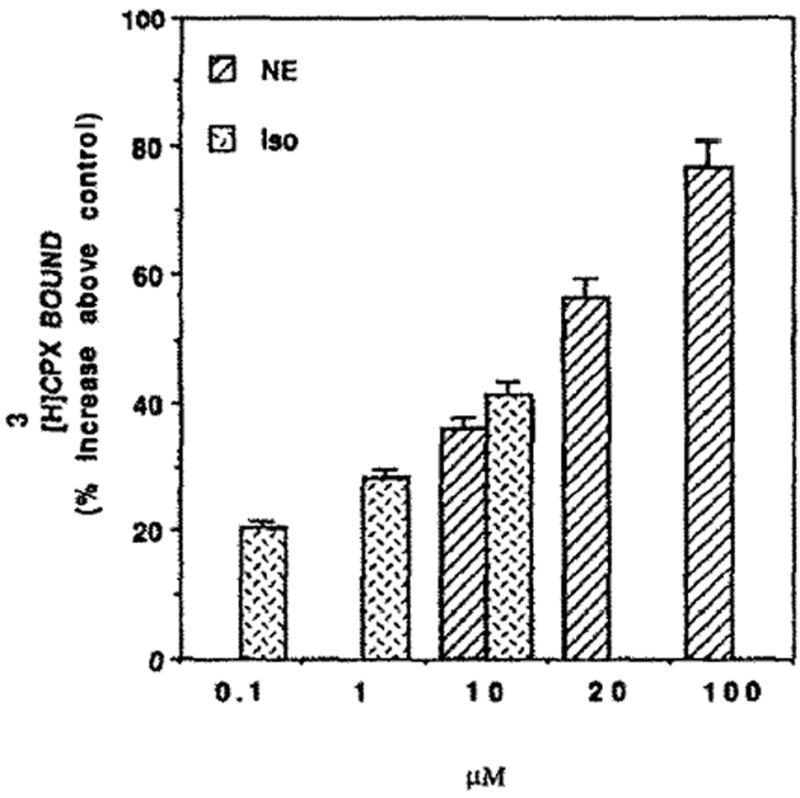
Dose–response of NE or Iso on [3H]CPX binding. Four-day-old cardiocytes were treated with the indicated concentrations of NE for 60 hr, or Iso for 48 hr. Specific binding of [3H]CPX was determined.
Effect of thyroid hormones on [3H]CPX binding
Thyroid hormones like catecholamines stimulate the contraction of cardiomyocytes [25]. Therefore, the effect of access thyroid hormones on [3H]CPX binding was studied. Three days of treatment with T3 increased the number of adenosine receptors by 56% (Fig. 9) without a significant change in the affinity of the receptors to [3H]CPX (Fig. 9, inset). The maximum number of binding sites (Bmax) in T3 treatment was 5.0 ± 0.23 fmol/dish and 3.2 ± 0.14 fmol/dish in the control (the experiment was carried out in multi wells and not in the ordinary 35 mm dishes). There were no significant differences between T3 and the control on the affinity of the receptors. The Kd was 0.11 nM (Fig. 9, inset). The dose effect on T3 on [3H]CPX binding is shown in Fig. 10. The maximum increase of adenosine receptors was obtained at 10 nM of T3. Higher concentrations than 100 nM causes toxic effects as revealed by a decrease in CK and LDH activity in the homogenates (data not shown). Protein content and cell number in T3-treated cells were similar to the control (data not shown).
Fig. 9.
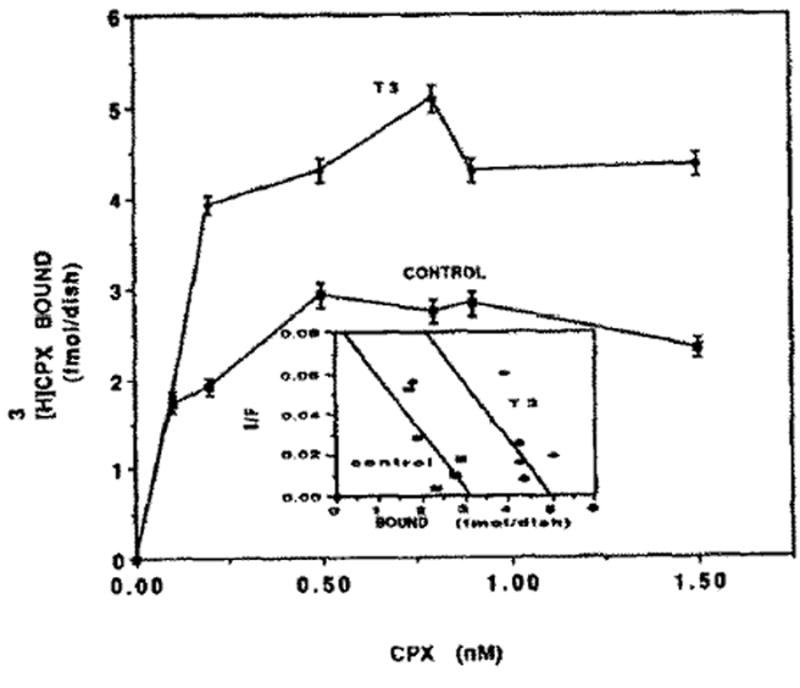
Effect of TH on [3H]CPX binding. Four-day-old cardiocytes were treated with 10nM T3. Specific binding of [3H]CPX was determined after 72 hr. (The experiment was carried out in multi wells.) Inset: Scatchard plot of specific [3H]CPX binding of Fig, 9. The Kd for [3H]CPX was 0.11 nM. Bmax was 3.2 and 5.0 fmol/dish in control group and T3-treated cells, respectively.
Fig. 10.
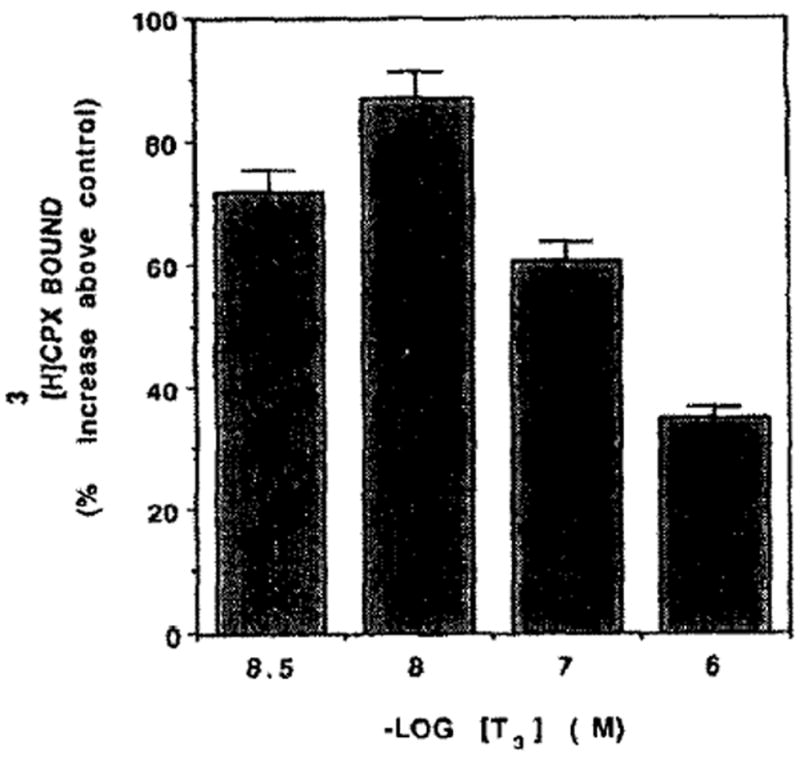
Dose–response of TH on [3H]CPX binding. Five-day-old cardiocytes were treated with various concentrations of T3. [3H]CPX binding was determined 84 hr later.
Effect of carbamylcholine on [3H]CPX binding
Carb is known as an inhibitor of contractile force and heart rate [27]. Therefore we examined the influence of Carb on adenosine receptors. It was found that [3H]CPX binding was decreased by 26% following 72 hr of Carb treatment (Fig. 11), whereas the rate of contractions was reduced from 91 ± 16 to 30 ± 18 beats/min. Scatchard analysis (Fig. 11, inset) indicates that the Bmax decreased from 25.5 fmol/dish to 18.7 fmol/dish. Kd levels were unchanged significantly (0.16 and 0.17 nM in control and Carb-treated cells). The Carb influence on adenosine receptors was dose dependent (Fig. 12). [3H]CPX binding was reduced by 2, 17,45 and 53% following 84 hr of 10−3, 10−4, 10−5 and 10−6 M of Carb treatment, respectively. Protein content and cell number in Carb-treated cells were similar to that of the control (0.8 mg/dish and 82 × 104 cells/dish, respectively).
Fig. 11.
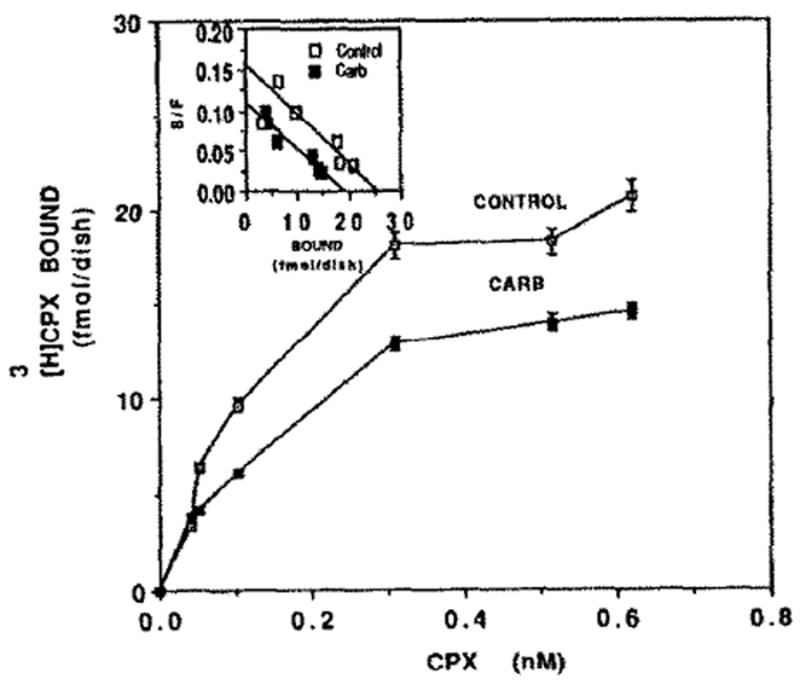
Effect of Carb on [3H]CPX binding. Four-day-old cardiocytes were treated with 5 μM Carb for 72 hr before specific binding of [3H]CPX was determined. Inset: Scatchard plot of specific [3H]CPX binding of Fig. 11. Kd = 0.16 and 0.17 nM, and Bmax = 25.5 and 18.7 fmol/dish in control and Carb-treated cells, respectively.
Fig. 12.
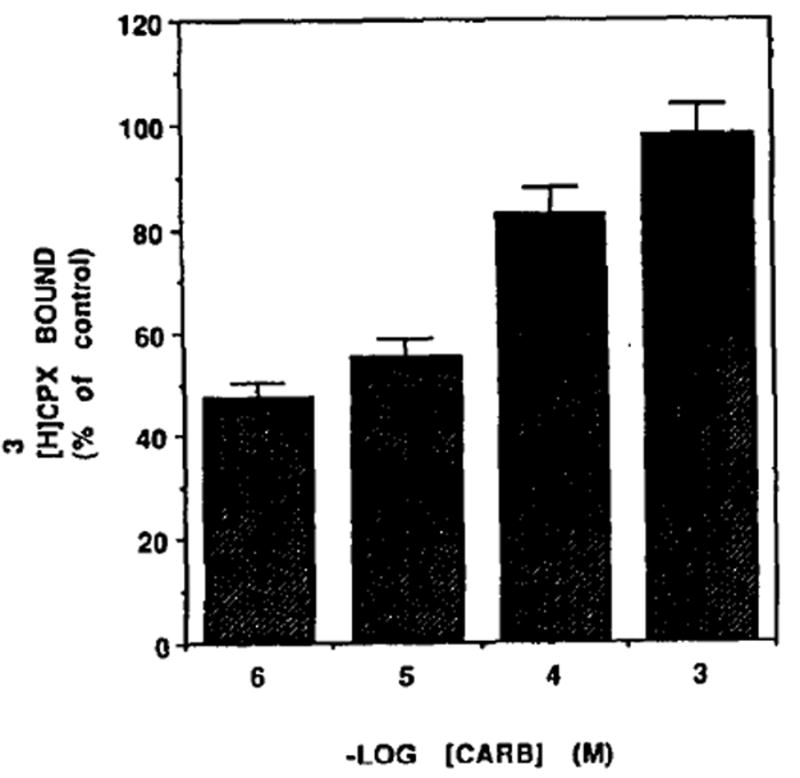
Dose–response of Carb [3H]CPX binding. Five-day-old cardiocytes were treated with various concentrations of Carb and [3H]CPX binding was determined 84 hr later.
DISCUSSION
We have characterized and quantified the surface A1 adenosine receptors in intact cardiomyocytes grown in cultures, using the antagonist [3H]CPX. The binding of [3H]CPX is saturable, reversible and of high affinity, The dissociation constant of this ligand is Kd = 0.13 nM (Fig. 3). This value is lower than that obtained by Liang [21], who found in the membranes of chick atrial cultured cardiomyocytes to be 2.1 nM. The difference may be related to species differences, or due to differences in experimental conditions: intact cells versus a membrane preparation. When both of these differences were eliminated, like in the Martens experiment, who measured adenosine receptors on intact rat cultured ventricutar cells, then the Kd was 0.48 nM, which is closer to our results [32]. A recent study using [3H]CPX binding to bovine cardiac membranes [33] reported a Kd of 0.12 nM, which is similar to our study.
The Bmax value in chick atria1 myocytes was 26.2 fmol/mg protein [21]. Considering that this value was obtained in membranes preparation, it is higher than our Bmax(20–30 fmol/mg. protein). We have calculated the number of receptors per cell, assuming that the receptor distribution is uniform among the cells, to be 14–15 × 103 receptors/cardiomyocyte. This number is lower than the number of adenosine receptors obtained by Martens [32] in adult heart cells (40,000). This difference may reflect age dependence, as can be seen in Fig. 5, that during the development of the cultures the receptor level continues to increase with time. The number of adenosine receptors is about 10–20 times lower than the muscarinic acetylcholine receptors on the same cells [27], or in cardiac atrium [10]. The number of adenosine A1 receptors is similar to the number of β adrenoreceptors, e.g. 24,000 [26].
Estimates of adenosine A1 receptor density in cardiac cell membranes are in the range of 15–30 fmol/mg protein in rat [34], pig [35], guinea pig [22,37], chick embryos [21,36], bovine [38] and diseased human cardiac preparations [39]. There are differences in the density of atria1 adenosine receptors (A1) according to the species: guinea pig > rat > rabbit [37]. This may explain the different sensitivity of the AV node to adenosine which has been reported as: guinea pig > man > rabbit > dog [37,40].
The present study shows an increase in the [3H]-CPX binding (Bmax) with no significant change in the Kd upon exposure of the cardiomyocytes to NE or Iso for 48–96 hr. Although this treatment also caused an elevation in CK and LDH activities (Table 1), nevertheless, the increase in [3H]CPX binding is 7–8-fold higher than the elevation of these enzymes. Thus, we can regard this stimulation by catecholamines as specific for A1 receptors.
The increase in the level of adenosine receptors following catecholamine treatment might be reflected by increased sensitivity to adenosine linked to a functional response. These responses include negative chronotropic, dromotropic and ionotropic effects on the heart [7,8]. A1 adenosine receptors in the heart also appear to mediate the attenuation of the positive inotropic effects of catecholamine [12, 13, 41-45]. Thus, the increased level of adenosine receptors following catecholamine treatment might act as an inhibitory feedback to protect heart cells from excessive contractions.
Many of the physiological and biochemical responses characteristic of hyperthyroidism are similar to those induced by adrenergic stimulation. We have previously demonstrated that thyroxine induces an increase in contraction rate in cultured heart cells [25]. Therefore, we analysed the effect of TH on adenosine receptors. When cultured heart cells were exposed to T3 for 72 hr, a 56% increase in the number of adenosine receptors was observed (Figs 9 and 10). The increase in the number of adenosine receptors is not accompanied by a significant change in binding affinity, nor by a change in total protein content. This increase in adenosine receptors is probably a result of increased receptor synthesis, since the protein synthesis inhibitor, cycloheximide (5 μg/mL) prevented T3 stimulation (data not shown). Its worth mentioning that the opposite result was obtained in adipocytes, in which T3 reduced the level of adenosine receptors by 65% [46].
Again we find that factors that accelerated the rate of heart contractions brought about an increase in the number of adenosine receptors, which increase the cardiac cell sensitivity to the attenuating effect of adenosine. This is probably the mechanism by which heart cells adapt to stress and compensate for excessive contractions. Further support for this mechanism of adaptation is demonstrated in the experiments with Carb. This transmitter, which like adenosine reduces the heart rate, caused a 28% reduction of adenosine receptors following 2–3 days of treatment (Fig. 12).
It is worth mentioning that similar results were obtained by Liang and Donovan [36], in which sustained exposure of cultured chick myocytes to adenosine agonists produced a 40% decrease in the force of contractions, accompanied by a 40% reduction in the receptor number.
It is possible that the changes obtained on the level of the A1 receptors are related to cAMP and not to cardiac contractions. Indeed, cAMP or theophylline caused an increase of A1 receptors (data not shown). However, these drugs also accelerated the rate of cardiac contractions. Thus, it is impossible to distinguish between these two possibilities. On the other hand, when direct measurements of cAMP level were performed following Iso treatment, it caused an elevation of the level of cAMP during the first 30 min. But the values of cAMP returned to normal in the cardiomyocytes after 24 and 48 hr. Furthermore, when the cells were treated with T3, for 48 hr, the basal level of cAMP was 10–12% higher than the control, insignificant difference [26]. These results support our proposal that contractile activity plays a major role in regulation of adenosine receptor’s synthesis although the possibility that cAMP exerts a second messenger in this process is not excluded.
We have previously demonstrated, that any treatment which stimulates heart contractions or inhibits heart contractions causes a reduction in the level of muscarinic acetylcholine receptors [27]. We reasoned that we could not get up regulation in the muscarinic receptor level; either because we did not find the required experimental conditions for the induction process, or the cardiac cells used were saturated with these receptors and no further up regulation was possible [27]. Now, when we make comparison between these two receptors, which have similar functions on the heart, we find that: (A) the ratio of muscarinic to A1 receptors is 10: 1; (B) under the same experimental conditions, such as TH treatment, the muscarinic receptors are down regulated [27], whereas A1 adenosine receptors are up regulated (Figs 9 and 10). Thus, a different mechanism probably regulates the biosynthesis of these two receptors, but only the A1 receptors respond to environmental conditions (stress) so as to restore the basal rate of heart contractions.
Acknowledgments
We are indebted to Mrs T. Zinman and A. Isaac for their valuable technical assistance. This work was partially supported by funds from Israeli Ministry of Health and from Otto Meyerhoff Drug Receptor Center, at Bar-Ilan University.
Footnotes
Abbreviations: Carb, carbamylcholine; CK, creatine kinase; [3H]CPX, [3H]-8-cyclopentyl-1, 3-dipropylxanthine; DMEM, Dulbecco’s modified Eagle Medium; Iso, isoproterenol; LDH, lactate dehydrogenase; NE, norepinephrine; RDB, fig tree extract; R-PIA, R-N6-(2-phenylisopropyl)-adenosine; S-PIA, S-N6-(2-phenylisopropyl)-adenosine; TH, thyroid hormones; T3 tri-iodothyronine.
References
- 1.Evoniuk G, Jacobson KA, Shamim MT, Daly JW, Wurtman RJ. Al and A2-selective adenosine antagonist, in vivo characterization of cardiovascular effects. J Pharmacol Exp Ther. 1987;242:882–887. [PMC free article] [PubMed] [Google Scholar]
- 2.Bellardinelli L, Klockner U, Isenberg G. Modulation of potassium and calcium current in atria1 and nodal cells. In: Piper HM, Isenberg G, editors. Isolation Adult Cardiomyocytes. Vol. 2. CRC Press; Boca Raton: 1989. pp. 155–180. [Google Scholar]
- 3.Jacobson KA, Van Galen PJM, Williams M. Adenosine receptors: Pharmacology, structure-activity relationshius and theraueutic notential. J Med Chem. 1992;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berne RM. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- 5.Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992;85:893–904. doi: 10.1161/01.cir.85.3.893. [DOI] [PubMed] [Google Scholar]
- 6.Forman MB, Velasco CE. Adenosine attenuates reperfusion injury following regional myocardial ischaemia. Cardio Res. 1993;27:9–17. doi: 10.1093/cvr/27.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Drury AN, Szent-Gyorgi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol (Land) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belardinelli L, Wu SN, Visentin S. Adenosine regulation of cardiac electrical activity. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. WB Saunders; 1990. pp. 284–290. [Google Scholar]
- 9.Ogilby JD, Jaekyeong H, Iskandrian AS. Effect of adenosine on coronary blood flow and its use as diagnostic test for coronary artery disease. Cardio Res. 1993;27:48–54. doi: 10.1093/cvr/27.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Linden J, Holleu CE, Patel A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium: correlation with cyclic adenosine monophosphates and receptor densities. Circ Res. 1985;56:728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- 11.Baumann G, Schrader J, Gerlach E. Inhibitory action of adenosine on histamine and dopamine-stimulated cardiac contractility and adenylate cyclase in guinea pigs. Circ Res. 1981;48:259–266. doi: 10.1161/01.res.48.2.259. [DOI] [PubMed] [Google Scholar]
- 12.Belardinelli L, Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1983;53:287–297. doi: 10.1161/01.res.53.3.287. [DOI] [PubMed] [Google Scholar]
- 13.Isenberg G, Belardinelli L. Ionic basis for the antagonism between adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1984;55:309–325. doi: 10.1161/01.res.55.3.309. [DOI] [PubMed] [Google Scholar]
- 14.West GA, Isenberg G, Belardinelli L. Antagonism of forskolin effects by adenosine in isolated hearts and ventricular myocytes. Am J Physiol. 1986;250:H769–H777. doi: 10.1152/ajpheart.1986.250.5.H769. [DOI] [PubMed] [Google Scholar]
- 15.Jochem G, Nawrath H. Adenosine activates a potassium conductance in guinea-pig atria1 heart 16, muscle. Experientia. 1983;39:1347–1349. doi: 10.1007/BF01990096. [DOI] [PubMed] [Google Scholar]
- 16.Kurachi Y, Nakaiima T, Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atria1 cells: Involvement of GTP-binding proteins. Pflugers Arch. 1986;407:264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffinger PJ, Martin JM, Hunter DD. GTP-binding proteins couple cardiac cellular muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 18.Belardinelli L, Linden J, Berne RM. The cardiac effects of Adenosine. Prog Cardiov Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- 19.Hescheler J, Trautwein W. Modulation of Calcium Isolated Adult Cardiomycytes. Vol. 2. CRC Press; Boca Raton: 1989. pp. 129–154. [Google Scholar]
- 20.Johnson EA, Mckinnson MG. Effects of acetylcholine and adenosine on cardiac cellular potentials. Nature. 1956;178:1174–1175. doi: 10.1038/1781174a0. [DOI] [PubMed] [Google Scholar]
- 21.Liang BT. Characterization of the adenosine receptor in cultured embryonic chick atria1 myocytes: coupling to modulation of contractility and adenylate cyclase activity and identification by direct radioligand binding. J Pharmacol Exp Ther. 1989;249:775–785. [PubMed] [Google Scholar]
- 22.Wu SN, Linden J, Visentin S, Boykin M, Belardinelli L. Enhanced sensitivity of heart cells to adenosine and up regulation of receptor number after treatment of guinea pigs with theophylline. Circ Res. 1989;65:1066–1077. doi: 10.1161/01.res.65.4.1066. [DOI] [PubMed] [Google Scholar]
- 23.Liang BT, Hirsch AJ. Homologous sensitization of embryonic chick atria1 myocytes to adenosine: mediation by adenosine A1 receptor and guanine nucleotide binding protein. Cardiol Res. 1993;27:102–110. doi: 10.1093/cvr/27.1.102. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson KA. Molecular probes for adenosine receptors. In: Jacobson KA, Daly JW, Manganiello V, editors. Purines in Cellular Signaling: Targets for New Drugs. Springer; New York: 1990. pp. 54–64. [Google Scholar]
- 25.Brik H, Shainberg A. Thyroxine induces transition of red towards white muscle in cultured heart cells. Basic Res Cardio1. 1990;85:237–246. doi: 10.1007/BF01907112. [DOI] [PubMed] [Google Scholar]
- 26.Disatnik MH, Shainberg A. Regulation of β-adrenoceptors by thyroid hormone and Amiodarone in rat myocardiac cells in culture. Biochem Pharmacol. 1991;41:1039–1044. doi: 10.1016/0006-2952(91)90212-n. [DOI] [PubMed] [Google Scholar]
- 27.Waisberg M, Shainberg A. Characterization of muscarinic cholinergic receptors in intact myocardial cells in vitro. Biochem Pharmacol. 1992;43:2327–2334. doi: 10.1016/0006-2952(92)90310-f. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YC, Prusoff WH. Relationship between the inhibition constant Ki and the concentration of inhibition which caused 50% inhibition of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 30.Scatchard G. The attraction of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 31.Bylund DB, Yamamura H. In: Methods for receptor binding in methods in neurotransmitter receptor analysis. Yamamura IH, et al., editors. Raven Press Ltd; New York: 1990. pp. l–35. [Google Scholar]
- 32.Martens D, Lohse MJ, Schwabe U. [3H]-8-Cyclopentyl-1,3-dipropylxanthine binding to A1 adenosine receptors of intact rat ventricular myocytes. Circ Res. 1988;63:613–620. doi: 10.1161/01.res.63.3.613. [DOI] [PubMed] [Google Scholar]
- 33.Leung E, Jacobson KA, Green RD. Apparent heterogeneity of cardiac A1 adenosine receptors as revealed by radioligand binding experiments on N-ethylmaleimide-treated membranes. Nuunyn-Schmiedebergs Arch Pharmacol. 1991;344:639–644. doi: 10.1007/BF00174747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens D, Loshe MJ, Rauch B, Schwabe U. Pharmacological characterization of A1 adenosine receptors in isolated rat ventricular myocytes. Nnunyn Schmiedebergs Arch Pharmacol. 1987;336:342–348. doi: 10.1007/BF00172688. [DOI] [PubMed] [Google Scholar]
- 35.Leid M, Franklin PH, Murray TF. Labelling of A1 adenosine receptors in porcine atria with the antagonist radioligand [3H]-8-cyclopentyl-l,3-dipropylxanthine. Eur J Pharmacol. 1988;147:141–144. doi: 10.1016/0014-2999(88)90644-9. [DOI] [PubMed] [Google Scholar]
- 36.Liang BT, Donovan LA. Differential desensitization of adenosine receptor-mediated inhibition of cardiac myocyte contractility and adenylate cyclase activitv. Circ Res. 1990;67:406–414. doi: 10.1161/01.res.67.2.406. [DOI] [PubMed] [Google Scholar]
- 37.Froli G, Belardinelli I. Species dependent effects of adenosme on heart rate and atrioventricular nodal conduction, Mechanism and physiological implications. Circ Res. 1990;67:960–981. doi: 10.1161/01.res.67.4.960. [DOI] [PubMed] [Google Scholar]
- 38.Lohse MJ, Ukena D, Schwabe U. Demonstration of Ri-type adenosine receptors in bovine myocardium by radioligand binding. Naunyn-Schmiedebergs Arch Pharmacol. 1985;328:310–316. doi: 10.1007/BF00515559. [DOI] [PubMed] [Google Scholar]
- 39.Bohm M, Pieske B, Ungerer M, Erdman E. Characterization of A1 adenosine receptors in atria1 and ventricular myocardium from diseased human hearts. Circ Res. 1989;65:1201–1211. doi: 10.1161/01.res.65.5.1201. [DOI] [PubMed] [Google Scholar]
- 40.Ueeda M, Thompson RD, Padgett WL, Secunda S, Daly JW, Olsson RA. Cardiovascular actions of adenosine, but not adenosine receptors, differ in rat and guinea pig. Life Sci. 1991;49:1351–1358. doi: 10.1016/0024-3205(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 41.Romano FD, Naimi TS, Dobson JG., Jr Adenosine attenuation of catecholamine-enhanced contractility of rat heart in vivo. Am J Physiol. 1991;260:1635–1639. doi: 10.1152/ajpheart.1991.260.5.H1635. [DOI] [PubMed] [Google Scholar]
- 42.Shyrock JC, Travagli HC, Belardinelli L. Evaluation of N-0861, (+ −)-N6-endonorbornan-2-yl-9-methyladenine, as an A1 subtype selective adenosine receptor antagonist in the guinea pig isolated heart. J Pharmacol Exp Ther. 1992;260:1292–1299. [PubMed] [Google Scholar]
- 43.Dobson JG., Jr Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circ Res. 1983;52:151–160. doi: 10.1161/01.res.52.2.151. [DOI] [PubMed] [Google Scholar]
- 44.Rardon DP, Bailey JC. Adenosine attenuation of the electrophysiological effects of isoproterenol on canine Purkinje fibers. J Pharmacol Exp Ther. 1984;228:792–798. [PubMed] [Google Scholar]
- 45.Song YJ, Thedford S, Lerman BB, Belardinelli L. Adenosine sensitive after depolarizations and triggered activity in guinea-pig ventricular myocytes. J Physiol (Lond) 1987;392:523–542. [Google Scholar]
- 46.Rapiejko PJ, Malbon C. Short-term hyperthyroid- ism modulates adenosine receptors and catalytic activity of adenylate cyclase in adipocytes. Biochem J. 1987;241:765–771. doi: 10.1042/bj2410765. [DOI] [PMC free article] [PubMed] [Google Scholar]


