Abstract
Purpose
Leber congenital amaurosis (LCA) is a hereditary retinal dystrophy with wide genetic heterogeneity. Next-generation sequencing (NGS) targeting multiple genes can be a good option for the diagnosis of LCA, and we tested a clinical exome panel in patients with LCA.
Methods
A total of nine unrelated Korean patients with LCA were sequenced using the Illumina TruSight One panel, which targets 4,813 clinically associated genes, followed by confirmation using Sanger sequencing. Patients’ clinical information and familial study results were obtained and used for comprehensive interpretation.
Results
In all nine patients, we identified pathogenic variations in LCA-associated genes: NMNAT1 (n=3), GUCY2D (n=2), RPGRIP1 (n=2), CRX (n=1), and CEP290 or SPATA7. Six patients had one or two mutations in accordance with inheritance patterns, all consistent with clinical phenotypes. Two patients had only one pathogenic mutation in recessive genes (NMNAT1 and RPGRIP1), and the clinical features were specific to disorders associated with those genes. Six patients were solved for genetic causes, and it remains unclear for three patients with the clinical exome panel. With subsequent targeted panel sequencing with 113 genes associated with infantile nystagmus syndrome, a likely pathogenic allele in CEP290 was detected in one patient. Interestingly, one pathogenic variant (p.Arg237Cys) in NMNAT1 was present in three patients, and it had a high allele frequency (0.24%) in the general Korean population, suggesting that NMNAT1 could be a major gene responsible for LCA in Koreans.
Conclusions
We confirmed that a commercial clinical exome panel can be effectively used in the diagnosis of LCA. Careful interpretation and clinical correlation could promote the successful implementation of clinical exome panels in routine diagnoses of retinal dystrophies, including LCA.
Introduction
Leber congenital amaurosis (LCA; OMIM 204000) is an inherited ophthalmologic disorder characterized by severe visual impairment in early infancy, nystagmus, absent or sluggish pupillary responses, high hyperopia, and extinguished electroretinogram (ERG) [1,2]. LCA is estimated to affect 1 in every 50,000 to 80,000 individuals and accounts for 20% of blindness in school-age children [3]. To date, mutations in 24 genes have been reported to cause LCA, and more causative genes continue to be identified (assessed September 2016, RetNet) [2,4-7]. These genes encode proteins involved in the processes and pathways associated with phototransduction, retinoid cycle, photoreceptor morphogenesis, guanine synthesis, signal transduction, outer segment phagocytosis, coenzyme NAD synthesis, and intraphotoreceptor ciliary transport.
Genetic testing can definitively diagnose LCA and could provide a basis for future gene therapy. Sequencing analyses of the known causative genes could identify approximately 70% of LCA cases [2,8]. However, the genetic heterogeneity represented by the large number of associated genes leads to difficulties in molecular diagnosis. Moreover, LCA should be differentiated from several other systemic disorders, such as Joubert syndrome, Zellweger syndrome, neuronal ceroid lipofuscinosis, and Senior-Loken syndrome, which exhibit similar ocular phenotypes in early infancy [9,10]. Next-generation sequencing (NGS) technologies have enabled the simultaneous detection of multiple candidate genes, and several laboratories provide gene panel sequencing or whole-exome sequencing (WES) [11].
Disease-specific gene panel sequencing can be designed to sequence almost all coding regions of the genes that cause LCA with high coverage and reliability. However, it cannot detect gene mutations for disorders with overlapping phenotypes for differential diagnosis or detect mutations in other unidentified genes. Such shortcomings can be overcome by WES; however, using WES in diagnostic testing is challenging because of the high burden of interpreting a large number of variants of uncertain significance (VUS), issues with reporting incidental findings, reduced coverage and reliability in some regions of disease-specific genes, increased cost, and prolonged time to obtain final results [12].
Sequencing a few thousand genes with known clinical implications, often called clinical exome sequencing, could reduce the analytical burden of WES. In this report, we evaluate the clinical utility of a commercial clinical exome panel in patients with LCA.
Methods
Patients and families
A total of nine unrelated children with LCA were recruited at Severance Hospital from June 2015 to January 2016. The research protocol was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine. This study adhered to the tenets of the Declaration of Helsinki. All patients were diagnosed with LCA using the following criteria: 1) early onset severe visual impairment during the first year of life, 2) amaurotic pupil accompanied by nystagmus or wandering eye movement, 3) extinguished or severely reduced ERG, and 4) exclusion of other systemic diseases [1]. ERG examination was performed with an oral sedative, chloral hydrate, if patients were uncooperative. ERG protocols adhered to International Society for Clinical Electrophysiology of Vision standards [13], with some modifications for young infants. All patients were offspring of asymptomatic Korean parents, and familial genetic testing of the parents was performed in seven families.
Library construction and targeted sequencing
Briefly, peripheral blood was collected from basilic or cephalic vein in antecubital fossa, with the use of extension tube if needed. Whole blood was stored at room temperature for less than 2 days before it was proceeded to DNA extraction. Genomic DNA was extracted from leukocytes of whole blood samples using the QIAamp Blood DNA mini kit (Qiagen, Venlo, the Netherlands) according to the manufacturer’s instructions which used spin-column procedure with automation on the QIAcube instrument (Qiagen). Intact DNA was quantified and adjusted to a concentration of 5 ng/μl using a Qubit 2.0 fluorometer (Invitrogen, Waltham, MA) and the Qubit dsDNA HS assay kit (Invitrogen). Targeted NGS was performed on the DNA using the Illumina TruSight One sequencing panel on the MiSeq platform (Illumina, San Diego, CA). Briefly, precapture libraries were constructed according to the manufacturer’s sample preparation protocol. Each patient’s genomic DNA was fragmented with a median size of 300 bp. The DNA fragments were end-repaired, phosphorylated, and adenylated on the 3′ ends. The index adaptors were ligated to the repaired ends, DNA fragments were amplified, and fragments of 200 to 500 bp were isolated. Pooled libraries were sequenced on a MiSeq sequencer (Illumina) using the MiSeq Reagent kit v2 (300 cycles).
Gene targets and NGS data analysis
The TruSight One sequencing panel provides targeted sequencing for 4,813 genes associated with known clinical phenotypes and includes 22 causative genes for LCA (AIPL1 Gene ID 23746, OMIM 604392; CABP4, Gene ID 64802,OMIM 608700; CEP290 Gene ID 80184, OMIM 610142; CRB1 Gene ID 23418, OMIM 604210; CRX Gene ID 1406, OMIM 602225; GDF6 Gene ID 392255, OMIM 601147; GUCY2D Gene ID 3000, OMIM 600179; IFT140 Gene ID 9742, OMIM 614620; IQCB1 Gene ID 9657, OMIM 609237; KCNJ13 Gene ID 3769, OMIM 603208; LCA5 Gene ID 167691, OMIM 611408; LRAT Gene ID 9227, OMIM 604863; NMNAT1 Gene ID 64802, OMIM 608700; PRPH2 Gene ID 5961, OMIM 179605; RD3 Gene ID 343035, OMIM 180040; RDH12 Gene ID 145226, OMIM 608830; RPE65 Gene ID 6121, OMIM 180069; RPGRIP1 Gene ID 57096, OMIM 605446; SPATA7 Gene ID 55812, OMIM 609868; TULP1 Gene ID 7287, OMIM 602280; IMPDH1 Gene ID 3614, OMIM 146690, and OTX2 Gene ID 5015,OMIM 600037) listed in RetNet (last accessed September 2016). Two recently discovered genes, DTHD1 (Gene ID 401124, OMIM 616979) and CLUAP1 (Gene ID 23059, OMIM 616787) [14,15], are not included in the panel.
Data analysis was performed according to the default parameters of the Illumina MiSeq Reporter software. The Burrows-Wheeler Aligner (BWA)-MEM algorithm was used to read and map the raw sequence data, and the Genome Analysis Toolkit (GATK) was used for variant calling. For each subject, a vcf file containing variant calls was generated, reviewed, and filtered. For every equivocal call in the vcf files, a visual inspection of the mapped data was performed using the Integrated Genomics Viewer 2.3 software (IGV; Broad Institute, Cambridge, MA). For an overview of the sequencing coverage, position-wise coverage values were calculated from each bam file using the SAMtools software. Coverage information was calculated and plotted using the R statistics software.
GRCh37 (hg19) was used as the reference sequence for mapping and variant calling. Databases used for analysis and variant annotation include the Online Mendelian Inheritance in Man (OMIM), Human Gene Mutation Database, ClinVar, Single Nucleotide Polymorphism database (dbSNP), 1000 Genome, Exome Aggregation Consortium (ExAC), Exome Sequencing Project, and Korean Reference Genome Database, with a minor allele frequency cut-off of 0.5% [16]. The pathogenicity of missense variants was predicted using the following in silico prediction algorithms: Sorting Tolerant from Intolerant (SIFT), Polymorphism Phenotyping v2 (PolyPhen-2), and Mutation Taster.
Validation with Sanger sequencing
All pathogenic variations or VUS with high priority were validated with Sanger sequencing, for the proband and parent samples.
Results
Pathogenic variants identified with NGS analysis using TruSight One panel
Quality metrics of the NGS runs performed in the nine patients are summarized in Table 1. More than 14 million reads were sequenced per sample, and approximately 10 million reads were mapped on the target regions. Horizontal coverage, the percentage of regions with more than 20X coverage, was 92.8%.
Table 1. Quality control metrics of next generation sequencing runs.
| Patient | Total aligned reads | Targeted aligned reads | Targeted aligned bases (bp) | Mean coverage | >=Q30 base pair | Median fragment length (bp) |
|---|---|---|---|---|---|---|
| P1 | 12,248,376 | 8,305,029 | 887,342,110 | 74.3× | 91.8% | 308 |
| P2 | 12,514,079 | 8,734,402 | 927,395,332 | 77.6× | 92.5% | 279 |
| P3 | 16,477,550 | 11,991,255 | 1,235,952,620 | 103.5× | 93.3% | 256 |
| P4 | 14,490,845 | 10,323,509 | 1,074,284,233 | 89.9× | 92.9% | 267 |
| P5 | 19,383,130 | 13,549,987 | 1,411,822,212 | 118.2× | 88.1% | 271 |
| P6 | 14,609,502 | 10,076,663 | 1,039,201,848 | 87.0× | 94.9% | 279 |
| P7 | 13,891,487 | 9,961,705 | 1,026,703,521 | 85.9× | 90.0% | 259 |
| P8 | 13,675,583 | 10,242,904 | 1,022,195,973 | 85.6× | 90.9% | 225 |
| P9 | 17,416,972 | 13,005,976 | 1,312,819,546 | 109.9× | 93.6% | 233 |
| Average | 14,967,503 | 10,687,937 | 1,104,190,822 | 92.4× | 92.0% | 264 |
Abbreviation: bp, base-pair
In six of the nine patients, pathogenic variants in LCA-associated genes were detected in accordance with inheritance patterns. In the remaining three patients, only a single pathogenic variant for each gene was identified (Table 2). P1 had a single pathogenic variant in CRX, and a trio study revealed a de novo occurrence. Five patients were compound heterozygous for recessive genes: GUCY2D (P2 and P3), NMNAT1 (P4 and P5), and RPGRIP1 (P9). For patients with available parental samples (P2, P3, P4, and P5), all compound heterozygous mutations were confirmed to be inherited each from the carrier mother and the carrier father.
Table 2. Results of mutation analysis in 9 patients using TruSight One panel and targeted panel sequencing.
| Patients | Gene | Inherit-ance | Nucleotide change | Amino acid change | Depth | Zygosity | Origin of mutation | SIFT | ACMG classification | Allele frequency | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | CRX | AD | c.442delG | p.Gly148AlafsTer39 | 40 | Hetero | De novo | - | P | Not found | Novel |
| P2 | GUCY2D | AR | c.2649delT | p.Phe883LeufsTer13 | 111 | Hetero | Paternal | - | P | Not found | Novel |
| c.3038G>A | p.Gly1013Glu | 68 | Hetero | Maternal | DT(0) | LP | Not found | Novel | |||
| P3 | GUCY2D | AR | c.1991A>C | p.His664Pro | 53 | Hetero | Paternal | DT(0) | LP | Not found | Novel |
| c.2649delT | p.Phe883LeufsTer13 | 105 | Hetero | Maternal | - | P | Not found | Novel | |||
| P4 | NMNAT1 | AR | c.196C>T | p.Arg66Trp | 81 | Hetero | Maternal | DT(0) | LP | 0.00008529 | 2012 Nat Genet [43], 2015 J Biol Chem [44] |
| c.709C>T | p.Arg237Cys | 30 | Hetero | Paternal | DT(0) | LP | 0.00005052 | 2012 Nat Genet [43], 2015 J Biol Chem [44], 2012 Nat Genet [45] | |||
| P5 | NMNAT1 | AR | c.196C>T | p.Arg66Trp | 96 | Hetero | Maternal | DT(0) | LP | 0.00008529 | 2012 Nat Genet [43], 2015 J Biol Chem [44] |
| c.709C>T | p.Arg237Cys | 48 | Hetero | Paternal | DT(0) | LP | 0.00005052 | 2012 Nat Genet [43], 2015 J Biol Chem [44], 2012 Nat Genet [45] | |||
| P6 | NMNAT1 | AR | c.709C>T | p.Arg237Cys | 25 | Hetero | Paternal | DT(0) | LP | 0.00005052 | 2012 Nat Genet [43], 2015 J Biol Chem [44], 2012 Nat Genet [45] |
| P7 | CEP290 | AR | c.-1G>A | _ | 110 | Hetero | Paternal | - | VUS | 0.00003291 | Novel |
| c.4661_4663delAAG | p.Glu1554del | 83 | Hetero | Paternal | - | VUS | 0.00002529 | 2007 Hum Mutat [17] | |||
| c.6012-12T>A | _ | 1252 | Hetero | Maternal | - | LP | 0.00002369 | 2013 J Hum Genet [19], 2016 Exp Mol Med [20] | |||
| SPATA7 | AR | c.20_23delTCAG | _ | 44 | Hetero | Maternal | - | VUS | 0.0004735 | 2011 PLoS One [18] | |
| P8 | RPGRIP1 | AR | c.3565_3571delCGAAGGC | p.Arg1189GlyfsTer7 | 163 | Hetero | Unknown | - | LP | 0.00001625 | 2008 Mol Vis [41] |
| P9 | RPGRIP1 | AR | c.2079C>G | p.Tyr693Ter | 231 | Hetero | Unknown | - | LP | Not found | Novel |
| c.2209_2215+18del | _ | 13 | Hetero | Unknown | - | LP | Not found | Novel |
Mutations in bolded characters indicate the detection by targeted panel sequencing. Abbreviation: ACMG, American College of Medical Genetics; VUS, variant of unknown significance; AD, autosomal dominant; AR, autosomal recessive; DT, deleterious; Hetero, heterozygous; M, materal; P, paternal.
In P7, two VUSs in CEP290 and one pathogenic variant in SPATA7 were observed. Both CEP290 VUSs were inherited from the father, and one VUS (c.4661_4663delAAG) was previously reported as a disease-causing mutation [17], but further validation is needed. Because another VUS 1 bp upstream of the ATG start site (c.-1G>A) of CEP290 might affect gene transcription, the pathogenicity of the VUS could not be excluded. Additional sequencing was performed for c.2991+1655A>G, a well-known deep intronic mutation in CEP290, but the results were negative. The frameshift variant in SPATA7 was confirmed to be inherited from the mother. Although the same heterozygous variant was reported as pathogenic in a Chinese patient with LCA with an additional mutation in GUCY2D as digenic manner [18], we concluded that this frameshift deletion should be classified as VUS based on the results of maternal inheritance and another likely pathogenic mutation detected in CEP290 after additional targeted panel sequencing.
In P6 and P8, only a single mutated allele was found in NMNAT1 and RPGRIP1, respectively. Additional copy number analyses using the ExomeDepth algorithm and our preestablished pipeline failed to find any pathogenic deletion or duplication.
Additional targeted panel sequencing with copy number variation analysis in three unsolved patients
We performed additional targeted panel next-generation sequencing analysis with 113 genes responsible for infantile nystagmus syndrome in three unsolved patients (Appendix 1). These 113 genes included two recently discovered genes (CLUAP1 and DTHD1) and are listed in a Appendix 1. In P6, additional analysis found a nonsense mutation c.3946C>T, p.Gln1316Ter in the RP1L1 gene (Gene ID 94137, OMIM 608581). Because RP1L1 causes occult macular dystrophy as an autosomal dominant inheritance pattern, this novel nonsense variation is not related to the patient’s phenotype. In P7, additional analysis of targeted NGS revealed a new intronic variant c.6012–12T>A CEP290 was found. This mutation was previously found in Joubert syndrome [19,20] and was classified as a likely pathogenic mutation based on the result of trans-detection for recessive disorder in the trio study. In P8, no additional variants including copy number variation (CNV) were discovered other than the same frameshift mutation in RPGRIP1. Because copy number changes might be missed by exome-depth algorithms, multiplex ligation-dependent probe amplification (MLPA) assay for RPGRIP1 using the SALSA MLPA P222 LCA kit (MRC Holland, Amsterdam, the Netherlands) was performed. However, the assay also failed to discover any deletion or duplication, including the exon 17 deletion previously reported in Japanese patients with LCA [21].
Genotype-phenotype correlation
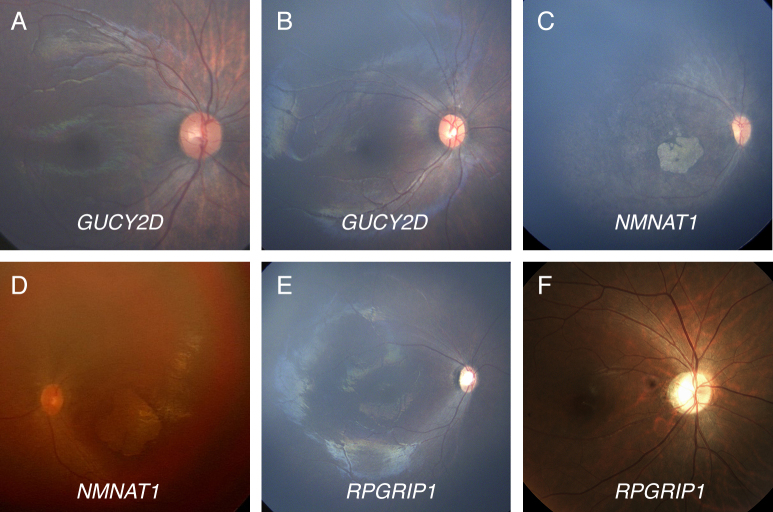
Demographic and clinical features of the enrolled patients are summarized in Table 3. None of the nine patients had syndromic features such as hearing loss, renal failure, or gross motor delay. All patients were babies around 1 year of age except P9 who was advised for genetic testing at the age of 29 years. Considering the association between affected genes and fundus findings, the patients carrying GUCY2D mutations (P2 and P3) showed grossly normal retinal appearances without noticeable retinal vessel caliber changes or pigmentary changes. In addition, patients carrying NMNAT1 mutations (P4, P5, and P6) displayed macular coloboma-like atrophic lesions and retinal vessel narrowing that were discovered in early infancy (Figure 1). Although we found only a single pathogenic variant in NMNAT1 (p.Arg237Cys) in P6, the retinal examination of that patient showed a typical macular coloboma-like lesion in early infancy. None of these patients had overt optic atrophy at the time of examination.
Table 3. Demographics and clinical features of patients suspected with Leber congenital amaurosis.
| Patients | Sex | Age at testing | Gene | Main symptom | Fundus finding | ERG | Oculodigital sign | Cycloplegic refraction |
|---|---|---|---|---|---|---|---|---|
| P1 | F | 7.5 months | CRX | Poor eye contact | Grossly normal, OU | Extinguished | + | OD: +sph3.00 |
| OS: +sph2.50 | ||||||||
| P2 | M | 6 months | GUCY2D | Poor eye contact | Grossly normal, OU | Extinguished | - | OD: +sph5.50 |
| OS: +sph5.50 | ||||||||
| P3 | F | 6 months | GUCY2D | Nystagmus | Grossly normal, OU | Extinguished | - | OD: +sph8.50 |
| Poor eye contact | OS: +sph8.50 -cyl100 axis180 | |||||||
| P4 | M | 13 months | NMNAT1 | Eye poking, rubbing | Bony speculated pigmentation | Extinguished | + | R: +sph5.50 |
| Macular coloboma, OU | L: +sph5.50 | |||||||
| P5 | F | 7 months | NMNAT1 | Poor eye contact | Bony speculated pigmentation | Extinguished | + | R: +sph4.00 |
| Macular coloboma, OU | L: +sph5.00 | |||||||
| P6 | M | 12 months | NMNAT1 | Nystagmus | Macular coloboma | Extinguished | + | OD: +sph5.50 -cyl3.00 axis180 |
| Marbled fundus, OU | OS: +sph5.00 -cyl3.00 axis180 | |||||||
| P7 | F | 7 months | CEP290 | Poor eye contact | Grossly normal, OU | Extinguished | + | OD: +sph6.00 |
| OS: +sph6.00 | ||||||||
| P8 | F | 8 months | RPGRIP1 | Poor eye contact | Pale disc, OU | Extinguished | - | OD: +sph6.00 -cyl0.75 axis170 |
| OS: +sph6.00 -cyl0.50 axis10 | ||||||||
| P9 | M | 29 years | RPGRIP1 | Nystagmus | Pale disc, OU | Extinguished | - | OD: -sph6.50 |
| OS: -sph6.50 |
Abbreviation: ERG, electroretinogram, OD, oculus dexter; OS, oculus sinister, OU, oculus uterque
Figure 1.
Color photographs of the retinas of six patients with LCA. A, B: Two 6-month-old babies (P2 and P3) with compound heterozygous mutations in GUCY2D (p.[Phe993LeufsTer13]+[Gly1013Glu] and p.[His664Pro]+[Phe883LeufsTer13], respectively) have no definite abnormal finding (OD) in the retina. C: A 7-month-old baby (P5) with compound heterozygous missense mutations in NMNAT1 (p.[Arg66Trp]+[Arg237Cys]) has marked chorioretinal atrophic changes in the macula with vessel narrowing and mild pigmentary changes (OD). D: A 1-year-old baby (P6) with only one pathogenic mutation in NMNAT1 (p.[Arg237Cys]+[?]) shows nystagmus, hyperopia, and a characteristic macular coloboma-like lesion (OS). E: An 8-month-old baby (P8) presented with poor eye contact, extinguished electroretinogram, +6.00 diopters hyperopia, slight optic disc pallor, and mild vessel narrowing (OD). We identified one frameshift mutation (p.Arg1189GlyfsTer7) in RPGRIP1. F: A 29-year-old male (P9) presented with nystagmus developed at the age of 6 years, 20/200 acuity, optic atrophy, and mild vessel attenuation (OD). Compound heterozygous mutations were identified in RPGRIP1 (p.[Tyr693Ter]+[c.2212_2215+21del]).
No developmental delay or systemic abnormalities were found in the patient with suspected CEP290 mutations (P7). This 7-month-old female had oculodigital sign and wandering eye movement. The retinal examination showed a normal optic disc, no pigmentary retinopathy, and only mild vessel narrowing.
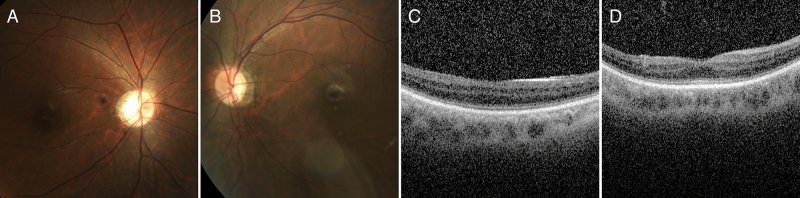
Interestingly, P9 was initially misdiagnosed with idiopathic infantile nystagmus because the initial fundus finding was grossly normal in early infancy. The patient underwent Anderson-Kestenbaum surgery at the age of 6 years for significant left head turn. His best-corrected visual acuity remained 20/200 bilaterally. At the age of 29 years, targeted NGS revealed a compound heterozygous RPGRIP1 mutation in this patient. Dilated fundus examination showed mild vessel attenuation and temporal optic atrophy without overt retinal pigmentary changes. Optical coherence tomography showed a relatively preserved inner segment and outer segment line (Figure 2).
Figure 2.
Fundus photographs of a 29-year-old male with mutations in RPGRIP1 who was initially misdiagnosed with idiopathic infantile nystagmus. He had sustained left head turn posture with left beating jerk nystagmus. Anderson-Kestenbaum surgery was performed at the age of 6 years. At the age of 29 years, his best visual acuity was 20/200 bilaterally. A, B: Fundus photography shows mild optic atrophy with retinal vessel attenuations. C, D: Spectral domain optical coherence tomography shows thinning of the inner segment and outer segment line.
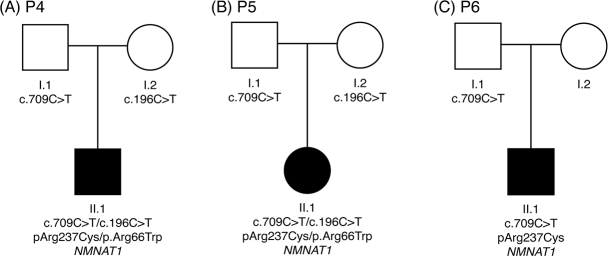
The c.709C>T, p.Arg237Cys variant in the NMNAT1 gene is known as a pathologic variant. This variant was found in all three individuals with macular coloboma-like degeneration, including one heterozygous individual (Figure 3). The allele frequency is 0.000074 (nine alleles out of 121,284 alleles are reported) in the ExAC browser and 0.00031 (two alleles out of 6,501 alleles) in the Exome Variant Server. However, the c.709C>T allele frequency was 0.0024 in the Korean Reference Genome Database (KRGDB), which consists of the whole exome sequencing results for 622 Korean individuals, suggesting that this mutation is relatively common in the Korean population.
Figure 3.
Pedigrees of three patients with mutations in NMNAT1. These patients were nonconsanguineous, and all patients harbored a pathogenic mutation in NMNAT1 (c.709C>T; p. Arg237Cys), which was supposed to be a founder mutation in Koreans.
Discussion
Several custom NGS panels [22,23] and commercial panels [24] have been reported to show high diagnostic utility in patients with LCA. We evaluated a commercial clinical exome kit that encompasses 4,813 genes associated with known clinical phenotypes, including 22 genes associated with LCA. The panel also includes genes for other retinal disorders and can help make a differential diagnosis. For example, differentiation of LCA from achromatopsia or syndromic retinal disorders is practically impossible, especially in young infants. These disease entities not only present similar phenotypes in early infancy but also genetically overlap each other (e.g., mutations in CEP290 cause either Joubert syndrome or LCA). Another strength of using commercial kits is the feasibility of standardization and inter-laboratory harmonization. However, the list of disease-related genes is rapidly expanding [15], which suggests that using a fixed set could miss candidate genes. Therefore, new genes like CLUAP1 can be missed with a clinical exome kit that targets already known genes that cause LCA. Another drawback is that targeting too many genes could lead to a reduced average depth of coverage and more regions with low depth. In a missense RPGRIP1 variant (P9), the depth of coverage was 13, and further confirmation with Sanger sequencing was needed.
LCA can be diagnosed at an early age with careful clinical examination and ERG testing. Considering the progressive nature of retinal degeneration in LCA, early molecular diagnosis could open up an opportunity to initiate a gene therapy trial [25]. Although still in clinical trials, recent gene therapy studies have shown that great improvements in visual acuity can be achieved in young patients with better baseline visual acuity [26]. Initial phase 1 and 2 trials of a gene therapy for RPE65 have been accomplished [27], and those for GUCY2D and gene editing for CEP290 are under investigation [28,29].
Genotype–phenotype correlation is important in narrowing down causative genes, particularly in genetically heterogeneous disorders. The present study showed that all three patients with coloboma-like macular atrophic lesions had NMNAT1 mutations, whereas those with grossly normal retinal appearances had mutations in GUCY2D, CRX, and CEP290, consistent with previous reports [30,31]. The other types of LCA with macular atrophic lesions present in infancy or later in life have been reported to occur in individuals carrying mutations in AIPL1, CRB1, RDH12, RPGRIP1, TULP1, and RPE65 [32-34]. Those other types show progressive macular degeneration in the natural course, whereas macular coloboma-like degeneration in early infancy could be specific to mutations in NMNAT1. Therefore, single-gene sequencing of NMNAT1 could be a cost-effective strategy in patients with macular coloboma-like degeneration in early infancy, especially when NGS testing is unavailable.
One patient with mutations in RPGRIP1 (P9) was initially misdiagnosed with an idiopathic form of infantile nystagmus, but the diagnosis changed because of the NGS results. Most patients with mutations in RPGRIP1 have a grossly normal fundus in early infancy [35]. Khan et al. reported a 6-year-old girl with a mutation in RPGRIP1 whose nystagmus abated when she adapted a moderate right face turn [35]. Thus, careful clinical examination, including of the peripheral retina, is essential in the diagnosis of LCA, and meticulous ERG testing should be conducted in patients who exhibit poor visual acuity.
One patient (P7) had two heterozygous VUSs in CEP290, of paternal origin, and one heterozygous pathogenic variant in SPATA7 of maternal origin. It is possible that mutations in those genes work together as “digenic inheritance” [36]. However, additional targeted panel sequencing revealed another intronic variant in CEP290 that could be classified as likely pathogenic and was confirmed to be inherited from the mother. Although LCA has been known to be inherited as digenic pattern in some reports [36-38], it is important to be cautious about the possibilities of a digenic inheritance because genetic testing might miss mutations in the affected gene.
Several genetic studies of patients with LCA have been performed in different ethnic groups [39,40]. Seong et al. [22,41,42] performed consecutive genetic studies on a few genes in Korean patients with LCA but failed to find any founder mutation. Through comprehensive testing on several genes, we found three common alleles associated with LCA: GUCY2D c.2649delT, NMNAT1 c.196C>T, and NMNAT1 c.709C>T. NMNAT1 was only recently identified as a causative gene for LCA. The minor allele frequency of NMNAT1 c.709C>T (p.Arg237Cys; rs375110174) is estimated to be as high as 0.24% (Korean Reference Genome DB). Therefore, mutations in NMNAT1 and GUCY2D might be major genes responsible for LCA in Koreans, which contrasts with the high frequency of mutations in CEP290 in Caucasian populations [2]. Additionally, one frameshift mutation found in RPGRIP1 in P8 has also been reported in another Korean patient [41].
In conclusion, the present study supports the utility of clinical exome sequencing in LCA diagnosis and differential diagnosis from other syndromic diseases with overlapping ocular phenotypes. Understanding the technology, applying careful interpretation, supplementing with other testing, and correlating with clinical phenotypes could lead to the successful implementation of clinical exome sequencing in the routine diagnosis of inherited retinal disorders, such as LCA.
Acknowledgments
We thank the patients and the family members for their participation. This study was supported by a faculty research grant of Yonsei University College of Medicine for 2015 (6–2015–0077) and the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A01059210).
Appendix 1. Target genes were listed.
To access the data, click or select the words “Appendix 1.” Genes included in infantile nystagmus syndrome target enrichment. Listed are the genes included in the custom designed target enrichment along with the disease or phenotype associated with the gene according to Online Medelian Inheritance in Man (OMIM), OMIM phenotype identification number, and OMIM or Gene Cards gene identification number. Genes are named according HUGO Gene Nomenclature Committee (HUGO, http://www.genenames.org/) approved nomenclature.
References
- 1.De Laey JJ. Leber’s congenital amaurosis. Bull Soc Belge Ophtalmol. 1991;241:41–50. [PubMed] [Google Scholar]
- 2.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Schappert-Kimmijser J, Henkes HE, Van Den Bosch J. Amaurosis congenita (Leber). AMA Arch Opthalmol. 1959;61:211–8. doi: 10.1001/archopht.1959.00940090213003. [DOI] [PubMed] [Google Scholar]
- 4.Estrada-Cuzcano A, Koenekoop RK, Coppieters F, Kohl S, Lopez I, Collin RW, De Baere EB, Roeleveld D, Marek J, Bernd A, Rohrschneider K, van den Born LI, Meire F, Maumenee IH, Jacobson SG, Hoyng CB, Zrenner E, Cremers FP, den Hollander AI. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011;52:834–9. doi: 10.1167/iovs.10-5221. [DOI] [PubMed] [Google Scholar]
- 5.Koenekoop RK, Wang H, Majewski J, Wang X, Lopez I, Ren H, Chen Y, Li Y, Fishman GA, Genead M, Schwartzentruber J, Solanki N, Traboulsi EI, Cheng J, Logan CV, McKibbin M, Hayward BE, Parry DA, Johnson CA, Nageeb M, Poulter JA, Mohamed MD, Jafri H, Rashid Y, Taylor GR, Keser V, Mardon G, Xu H, Inglehearn CF, Fu Q, Toomes C, Chen R. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012;44:1035–9. doi: 10.1038/ng.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, den Hollander AI, Moayedi Y, Abulimiti A, Li Y, Collin RW, Hoyng CB, Lopez I, Abboud EB, Al-Rajhi AA, Bray M, Lewis RA, Lupski JR, Mardon G, Koenekoop RK, Chen R. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am J Hum Genet. 2009;84:380–7. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sergouniotis PI, Davidson AE, Mackay DS, Li Z, Yang X, Plagnol V, Moore AT, Webster AR. Recessive mutations in KCNJ13, encoding an inwardly rectifying potassium channel subunit, cause leber congenital amaurosis. Am J Hum Genet. 2011;89:183–90. doi: 10.1016/j.ajhg.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Wang H, Sun V, Tuan HF, Keser V, Wang K, Ren H, Lopez I, Zaneveld JE, Siddiqui S, Bowles S, Khan A, Salvo J, Jacobson SG, Iannaccone A, Wang F, Birch D, Heckenlively JR, Fishman GA, Traboulsi EI, Li Y, Wheaton D, Koenekoop RK, Chen R. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet. 2013;50:674–88. doi: 10.1136/jmedgenet-2013-101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert SR, Kriss A, Taylor D, Coffey R, Pembrey M. Follow-up and diagnostic reappraisal of 75 patients with Leber’s congenital amaurosis. Am J Ophthalmol. 1989;107:624–31. doi: 10.1016/0002-9394(89)90259-6. [DOI] [PubMed] [Google Scholar]
- 10.Casteels I, Spileers W, Demaerel P, Casaer P, De Cock P, Dralands L, Missotten L. Leber congenital amaurosis--differential diagnosis, ophthalmological and neuroradiological report of 18 patients. Neuropediatrics. 1996;27:189–93. doi: 10.1055/s-2007-973785. [DOI] [PubMed] [Google Scholar]
- 11.Singleton AB. Exome sequencing: a transformative technology. Lancet Neurol. 2011;10:942–6. doi: 10.1016/S1474-4422(11)70196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang JP, Trzupek K. The current status of molecular diagnosis of inherited retinal dystrophies. Curr Opin Ophthalmol. 2015;26:346–51. doi: 10.1097/ICU.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, Al-Zahrani J, Al-Abdi L, Hashem M, Al-Tarimi S, Sebai MA, Shamia A, Ray-Zack MD, Nassan M, Al-Hassnan ZN, Rahbeeni Z, Waheeb S, Alkharashi A, Abboud E, Al-Hazzaa SA, Alkuraya FS. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23:236–47. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soens ZT, Li Y, Zhao L, Eblimit A, Dharmat R, Li Y, Chen Y, Naqeeb M, Fajardo N, Lopez I, Sun Z, Koenekoop RK, Chen R. Hypomorphic mutations identified in the candidate Leber congenital amaurosis gene CLUAP1. Genet Med. 2016 doi: 10.1038/gim.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenson PD, Mort M, Ball EV, Howells K, Phillips AD, Thomas NS, Cooper DN. The Human Gene Mutation Database: 2008 update. Genome Med. 2009;1:13. doi: 10.1186/gm13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrault I, Delphin N, Hanein S, Gerber S, Dufier JL, Roche O, Defoort-Dhellemmes S, Dollfus H, Fazzi E, Munnich A, Kaplan J, Rozet JM. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007;28:416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Xiao X, Li S, Jia X, Wang P, Guo X, Jiao X, Zhang Q, Hejtmancik JF. Detection of variants in 15 genes in 87 unrelated Chinese patients with Leber congenital amaurosis. PLoS One. 2011;6:e19458. doi: 10.1371/journal.pone.0019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsurusaki Y, Kobayashi Y, Hisano M, Ito S, Doi H, Nakashima M, Saitsu H, Matsumoto N, Miyake N. The diagnostic utility of exome sequencing in Joubert syndrome and related disorders. J Hum Genet. 2013;58:113–5. doi: 10.1038/jhg.2012.117. [DOI] [PubMed] [Google Scholar]
- 20.Kang HG, Lee HK, Ahn YH, Joung JG, Nam J, Kim NK, Ko JM, Cho MH, Shin JI, Kim J, Park HW, Park YS, Ha IS, Chung WY, Lee DY, Kim SY, Park WY, Cheong HI. Targeted exome sequencing resolves allelic and the genetic heterogeneity in the genetic diagnosis of nephronophthisis-related ciliopathy. Exp Mol Med. 2016;48:e251. doi: 10.1038/emm.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki T, Fujimaki T, Yanagawa A, Arai E, Fujiki K, Wada Y, Murakami A. A novel exon 17 deletion mutation of RPGRIP1 gene in two siblings with Leber congenital amaurosis. Jpn J Ophthalmol. 2014;58:528–35. doi: 10.1007/s10384-014-0339-z. [DOI] [PubMed] [Google Scholar]
- 22.Seong MW, Seo SH, Yu YS, Hwang JM, Cho SI, Ra EK, Park H, Lee SJ, Kim JY, Park SS. Diagnostic application of an extensive gene panel for Leber congenital amaurosis with severe genetic heterogeneity. J Mol Diagn. 2015;17:100–5. doi: 10.1016/j.jmoldx.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Huang H, Wang Y, Liu Z, Li N, Chen Y, Li X, Li M, Zhou X, Mu D, Zhong J, Wu J, Su Y, Yi X, Zhu J. A novel missense NMNAT1 mutation identified in a consanguineous family with Leber congenital amaurosis by targeted next generation sequencing. Gene. 2015;569:104–8. doi: 10.1016/j.gene.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Vamos R, Kulm M, Szabo V, Ahman A, Lesch B, Schneider M, Varsanyi B, Nagy ZZ, Nemeth J, Farkas A. Leber congenital amaurosis: first genotyped Hungarian patients and report of 2 novel mutations in the CRB1 and CEP290 genes. Eur J Ophthalmol. 2015;26:78–84. doi: 10.5301/ejo.5000643. [DOI] [PubMed] [Google Scholar]
- 25.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–97. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, McBride MT, Flotte TR, Humphries M, Calcedo R, Hauswirth WW, Chulay JD, Stout JT. Results at 2 Years after Gene Therapy for RPE65-Deficient Leber Congenital Amaurosis and Severe Early-Childhood-Onset Retinal Dystrophy. Ophthalmology. 2016;123:1606–20. doi: 10.1016/j.ophtha.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Rakoczy EP, Narfstrom K. Gene therapy for eye as regenerative medicine? Lessons from RPE65 gene therapy for Leber’s Congenital Amaurosis. Int J Biochem Cell Biol. 2014;56:153–7. doi: 10.1016/j.biocel.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Genzyme Investment Boosts Development of Sight-Saving Gene Therapy. Foundation Fighting Blindness 2014. http://www.blindness.org/foundation-news/genzyme-investment-boosts-development-sight-saving-gene-therapy
- 29.Editas Developing Gene-Editing Therapy for Leber Congenital Amaurosis (CEP290 Mutations). Foundation Fight Blindness 2015. http://www.blindness.org/foundation-news/editas-developing-gene-editing-therapy-leber-congenital-amaurosis-cep290-mutations
- 30.Hedergott A, Volk AE, Herkenrath P, Thiele H, Fricke J, Altmuller J, Nurnberg P, Kubisch C, Neugebauer A. Clinical and genetic findings in a family with NMNAT1-associated Leber congenital amaurosis: case report and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2015;253:2239–46. doi: 10.1007/s00417-015-3174-0. [DOI] [PubMed] [Google Scholar]
- 31.Traboulsi EI. The Marshall M. Parks memorial lecture: making sense of early-onset childhood retinal dystrophies--the clinical phenotype of Leber congenital amaurosis. Br J Ophthalmol. 2010;94:1281–7. doi: 10.1136/bjo.2009.165654. [DOI] [PubMed] [Google Scholar]
- 32.Weleber RG, Francis PJ, Trzupek KM, Beattie C. Leber Congenital Amaurosis. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews®. Seattle, WA: University of Washington, Seattle; 1993-2017. [Google Scholar]
- 33.Dharmaraj S, Leroy BP, Sohocki MM, Koenekoop RK, Perrault I, Anwar K, Khaliq S, Devi RS, Birch DG, De Pool E, Izquierdo N, Van Maldergem L, Ismail M, Payne AM, Holder GE, Bhattacharya SS, Bird AC, Kaplan J, Maumenee IH. The phenotype of Leber congenital amaurosis in patients with AIPL1 mutations. Arch Ophthalmol. 2004;122:1029–37. doi: 10.1001/archopht.122.7.1029. [DOI] [PubMed] [Google Scholar]
- 34.McKibbin M, Ali M, Mohamed MD, Booth AP, Bishop F, Pal B, Springell K, Raashid Y, Jafri H, Inglehearn CF. Genotype-phenotype correlation for leber congenital amaurosis in Northern Pakistan. Arch Ophthalmol. 2010;128:107–13. doi: 10.1001/archophthalmol.2010.309. [DOI] [PubMed] [Google Scholar]
- 35.Khan AO, Abu-Safieh L, Eisenberger T, Bolz HJ, Alkuraya FS. The RPGRIP1-related retinal phenotype in children. Br J Ophthalmol. 2013;97:760–4. doi: 10.1136/bjophthalmol-2012-303050. [DOI] [PubMed] [Google Scholar]
- 36.Schaffer AA. Digenic inheritance in medical genetics. J Med Genet. 2013;50:641–52. doi: 10.1136/jmedgenet-2013-101713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rachel RA, May-Simera HL, Veleri S, Gotoh N, Choi BY, Murga-Zamalloa C, McIntyre JC, Marek J, Lopez I, Hackett AN, Zhang J, Brooks M, den Hollander AI, Beales PL, Li T, Jacobson SG, Sood R, Martens JR, Liu P, Friedman TB, Khanna H, Koenekoop RK, Kelley MW, Swaroop A. Combining Cep290 and Mkks ciliopathy alleles in mice rescues sensory defects and restores ciliogenesis. J Clin Invest. 2012;122:1233–45. doi: 10.1172/JCI60981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzo AM, Daneels D, Cilia E, Bonduelle M, Abramowicz M, Van Dooren S, Smits G, Lenaerts T. DIDA: A curated and annotated digenic diseases database. Nucleic Acids Res. 2016;44:D900–7. doi: 10.1093/nar/gkv1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astuti GD, Bertelsen M, Preising MN, Ajmal M, Lorenz B, Faradz SM, Qamar R, Collin RW, Rosenberg T, Cremers FP. Comprehensive genotyping reveals RPE65 as the most frequently mutated gene in Leber congenital amaurosis in Denmark. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Zhang Q, Shen T, Xiao X, Li S, Guan L, Zhang J, Zhu Z, Yin Y, Wang P, Guo X, Wang J, Zhang Q. Comprehensive mutation analysis by whole-exome sequencing in 41 Chinese families with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2013;54:4351–7. doi: 10.1167/iovs.13-11606. [DOI] [PubMed] [Google Scholar]
- 41.Seong MW, Kim SY, Yu YS, Hwang JM, Kim JY, Park SS. Molecular characterization of Leber congenital amaurosis in Koreans. Mol Vis. 2008;14:1429–36. [PMC free article] [PubMed] [Google Scholar]
- 42.Seong MW, Kim SY, Yu YS, Hwang JM, Kim JY, Park SS. LCA5, a rare genetic cause of leber congenital amaurosis in Koreans. Ophthalmic Genet. 2009;30:54–5. doi: 10.1080/13816810802592567. [DOI] [PubMed] [Google Scholar]
- 43.Falk MJ, Zhang Q, Nakamaru-Ogiso E, Kannabiran C, Fonseca-Kelly Z, Chakarova C, Audo I, Mackay DS, Zeitz C, Borman AD, Staniszewska M, Shukla R, Palavalli L, Mohand-Said S, Waseem NH, Jalali S, Perin JC, Place E, Ostrovsky J, Xiao R, Bhattacharya SS, Consugar M, Webster AR, Sahel JA, Moore AT, Berson EL, Liu Q, Gai X, Pierce EA. NMNAT1 mutations cause Leber congenital amaurosis. Nat Genet. 2012;44:1040–5. doi: 10.1038/ng.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki Y, Margolin Z, Borgo B, Havranek JJ, Milbrandt J. Characterization of Leber Congenital Amaurosis-associated NMNAT1 Mutants. J Biol Chem. 2015;290:17228–38. doi: 10.1074/jbc.M115.637850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrault I, Hanein S, Zanlonghi X, Serre V, Nicouleau M, Defoort-Delhemmes S, Delphin N, Fares-Taie L, Gerber S, Xerri O, Edelson C, Goldenberg A, Duncombe A, Le Meur G, Hamel C, Silva E, Nitschke P, Calvas P, Munnich A, Roche O, Dollfus H, Kaplan J, Rozet JM. Mutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophy. Nat Genet. 2012;44:975–7. doi: 10.1038/ng.2357. [DOI] [PubMed] [Google Scholar]