Abstract
Purpose
Coexistence of an ocular surface disease can mask the typical features of ocular surface squamous neoplasia (OSSN). The purpose of this study was to evaluate high resolution optical coherence tomography (HR-OCT) as an adjunct in the detection and differentiation of OSSN within coexisting ocular surface pathologies.
Methods
Retrospective study of 16 patients with ocular surface disease and lesions suspicious for OSSN that were evaluated with HR-OCT. HR-OCT images of the lesions were taken to look for evidence of OSSN. Biopsies were performed in all cases, and the HR-OCT findings were compared to the histological results.
Results
Of the 16 patients with OSSN and a coexisting ocular surface disease, 12 were found to have OSSN by HR-OCT and all were subsequently confirmed by biopsy. Two patients had OSSN with rosacea, one with pingueculum, two within pterygia, one with Salzmann’ nodular degeneration, six with limbal stem cell deficiency (LSCD)/scarring. In all 12 cases HR-OCT images revealed classical findings of hyper-reflective, thickened epithelium and an abrupt transition from normal to abnormal epithelium. OSSN was ruled out by HR-OCT in four cases (2 Salzmann’s, 1 mucous membrane pemphigoid, and 1 LSCD). Negative findings were confirmed by biopsy. HR-OCT was used to follow resolution of the OSSN in positive cases, and it detected recurrence in 1 case.
Conclusions
While histopathology is the gold standard in the diagnosis of OSSN, HR-OCT can be used to noninvasively detect the presence of OSSN in patients with coexisting ocular conditions.
Keywords: Cornea, Conjunctiva, Lymphoma, Melanoma, Ocular surface squamous neoplasia (OSSN), Optical coherence tomography (OCT), Pterygium, Tumor
1. Introduction
The term ocular surface squamous neoplasia (OSSN) encompasses neoplasms of squamous cell origin that involve the conjunctiva or the cornea[1]. Individual histopathologic entities include dysplasias, graded I-III, carcinoma in situ (CIS), and invasive carcinoma[1–3]. The lesions are commonly, but not exclusively, located at the limbus. They typically present as elevated lesions with a leukoplakic, gelatinous, or papilliform appearance and can have abnormal blood vessel patterns[4,5].
The diagnosis of OSSN has traditionally relied on detecting the above-noted characteristics on slit lamp examination, which can be challenging when concomitant ocular pathology is present. This is not rare, as OSSN and other ocular surface diseases such as pterygium and pingueculum share common risk factors, including ultra-violet exposure. Furthermore, certain patients, such as those with atopic keratoconjunctivitis, have a higher incidence of OSSN than the general population[3,6–9]. In diseases such as mucus membrane pemphigoid (MMP) and limbal stem cell deficiency (LSCD), the ocular surface can be so altered that it is difficult to identify the classic OSSN features. Therefore, there is a critical need for a reliable, noninvasive diagnostic tool in complex cases to aid with the diagnosis and treatment, and guide localization of biopsy.
Optical coherence tomography (OCT) has recently emerged as a versatile tool to noninvasively study the anterior segment, especially with the introduction of ultrahigh resolution frequency domain techniques[10]. In fact, high resolution OCT (HR- OCT) can attain an axial resolution of less than 5 micra[11]. This elucidates enough detail to allow reliable assessment of OSSN lesions[11–15].
While several studies have investigated the role and reliability of HR-OCT in the diagnosis of OSSN, its usefulness in eye diseases with multiple pathologies needs further evaluation. Previous reports of OSSN in the setting of coexisting ocular surface conditions and masquerading syndromes have emphasized the difficulty in diagnosing OSSN in these circumstances [6,16–29]. The purpose of this study was to evaluate HR-OCT as a tool in detecting and differentiating OSSN in the setting of coexisting ocular surface pathologies.
2. Methods
The study was approved by the University of Miami Institutional Review Board and was conducted in concordance with the tenets of the Declaration of Helsinki and compliant with the Health Insurance Portability and Accountability Act. This retrospective study took place at Bascom Palmer Eye Institute from 2010 through 2016 and included patients with an ambiguous or suspicious ocular surface lesions coexisting with an ocular surface pathology. Patients with no HR-OCT or those with no biopsy confirmation were excluded.
Sixteen eyes of 16 patients with known ocular surface diseases presenting with lesions suspicious for OSSN were evaluated by CLK. HR-OCT images of the lesions were taken at presentation. Findings of OSSN by HR-OCT included a hyper-reflective thickened epithelium, an abrupt transition from normal to abnormal, and sometimes a cleavage plane from underlying tissue[14,15]. The HR-OCT findings were documented, after which biopsies were performed in all cases. This study retrospectively evaluated the OCT findings and compared them with biopsy results. For the cases with confirmed OSSN, follow-up using HR-OCT was done after appropriate treatment. Three patients were part of a previous cohort[11].
Two HR-OCT machines were used in this study. One is a custom-built device that operates at a wavelength of 840 nm and can achieve an axial resolution of 3 μm. Details are described in previous publications[14]. The second is the commercially available RTVue Premier Scanner (Optovue Inc., Fremont, CA) with a wavelength of 840 nm and an axial resolution of 5μm.
3. Results
Demographic data and diagnostic summary of existing ocular pathologies as well as histopathological findings are provided in Table 1. Four females and 12 males were included in the study with a mean age of 58.5 ± 18.7 years and a range of 28 to 90 years. Eight suspicious lesions involved the right eye and eight involved the left.
Table 1.
Demographic data and diagnostic summary of existing ocular pathologies
| Case# | Gender | Age | Eye | Co-existing | Pathology | HR-OCT | Biopsy result |
|---|---|---|---|---|---|---|---|
| 1 | m | 76 | OS | Rosacea + trichiasis | Corneal CIS | + | + |
| 2 | f | 67 | OS | Rosacea | Conjunctival CIS | + | + |
| 3 | m | 37 | OS | Pingueculum | Conjunctival CIS, melanosis without atypia | + | + |
| 4 | m | 68 | OS | Pseudo PTG | Conjunctival CIS | + | + |
| 5 | m | 67 | OD | PTG | Squamous cell carcinoma | + | + |
| 6 | m | 64 | OS | PTG | Conjunctival CIS | + | + |
| 7 | m | 76 | OD | Salzmann | Corneal CIS, moderate | + | + |
| 8 | f | 57 | OD | Salzmann | Squamous metaplasia, Salzmann’s nodule | − | − |
| 9 | m | 90 | OD | Salzmann | Squamous metaplasia, Salzmann’s nodule | − | − |
| 10 | m | 73 | OD | MMP | Squamous metaplasia, chronic inflammation | − | − |
| 11 | f | 66 | OS | Scar | Chronic inflammation, no malignancy | − | − |
| 12 | m | 28 | OD | Scar | Corneal CIS | + | + |
| 13 | m | 28 | OS | Band | Squamous cell carcinoma, corneal CIS | + | + |
| 14 | m | 35 | OD | LSCD + VKC | Conjunctival neoplasia | + | + |
| 15 | f | 43 | OS | LSCD + PKP | Full thickness dysplasia and corneal CIS | + | + |
| 16 | m | 61 | OD | LSCD + ?recur | Initial: conjunctival intraepithelial neoplasia/squamous cell | + | + |
| Suspicious recurrence: corneolimbal CIS | + | + |
Abbreviations defined: m: male, f: female, OS: left eye, OD: right eye, PTG: pterygium, MMP: mucous membrane pemphigoid, LSCD: limbal stem cell deficiency, VKC: vernal keratoconjunctivitis, PKP: penetrating keratoplasty, CIS: carcinoma in situ, ?recur: suspected recurrence, HR-OCT: high resolution optical coherence tomography, +: positive, −: negative
HR-OCT was positive for OSSN in 12 patients, with classic epithelial layer thickening and an abrupt transition in epithelium suggestive of OSSN. These findings were confirmed by pathology in all cases. Four cases with suspected OSSN were scanned with the HR-OCT and were found to have normal epithelium, ruling out OSSN. HR-OCTs negative for OSSN occurred in one patient with MMP, two with Salzmann’s nodular degeneration, and one with LSCD. As this was a new technology, a biopsy was done to confirm the non-neoplastic status of these patients. Follow-up imaging after appropriate treatment allowed the resolution of the disease to be monitored in all cases and was able to detect recurrence of OSSN in one case. The specific findings are grouped and presented below.
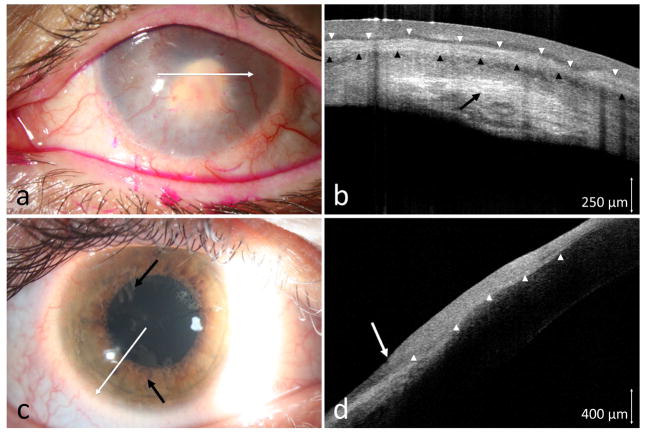
3.1 Ocular rosacea
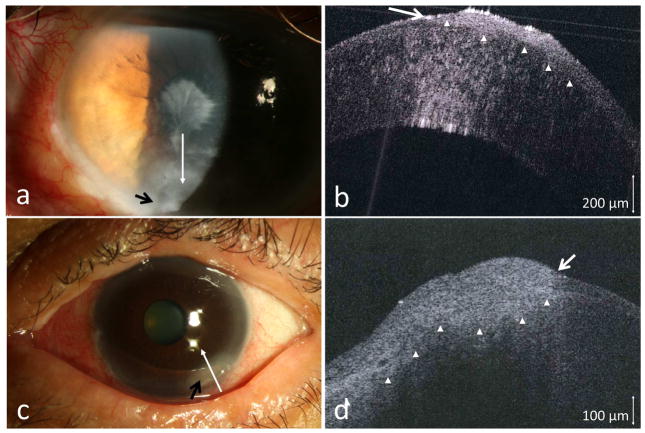
Two patients presented with severe ocular rosacea. Both had ocular surfaces changes of corneal pannus, areas of thinning, and conjunctival injection. A small area with leukoplakia near the corneal scarring (case 1), and an opalescent, ground glass appearance of the pannus with abnormal vasculature pattern (case 2) were suspicious for possible OSSN (Figures 1a and 1c). Due to the overall ocular surface condition in both cases, clinical examination alone was not definitive regarding the presence of OSSN. HR-OCT in both cases showed hyper-reflective thickened epithelium with an abrupt transition from normal to abnormal, features characteristic for OSSN (Figures 1b and 1d). Biopsy confirmed the HR-OCT findings and revealed CIS in both patients.
Figure 1.
(a) A 76-year-old male with longstanding scarring, trichiasis and chronic inflammation. An inferior leukoplakic opacification (black arrow) appeared suspicious. (b) HR-OCT (custom device, white line scan in a) demonstrated hyper-reflective thickened epithelium (above arrow heads) with an abrupt transition from normal to abnormal (white arrow). (c) A 67-year-old female with ocular surface inflammation and a suspicious area of elevation at the 5 o’clock position (black arrow). (d) HR-OCT (custom device, white line scan in c) demonstrated hyper-reflective thickened epithelium (above arrowheads) with an abrupt transition from normal to abnormal (white arrow). OSSN confirmed by biopsy in both cases.
3.2. Pterygia and pinguecula
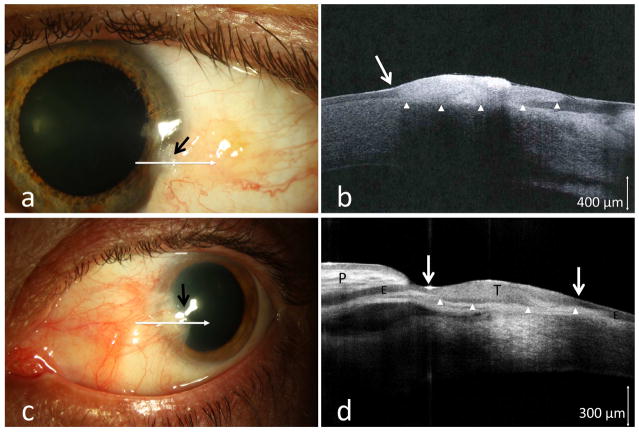
One patient with pingueculum and two patients with existing pterygia presented with atypical features. Neoplastic foci within the existing lesions were suspected due to areas of leukoplakia (case 3, Figure 2a), and a subtle thickening and opalescence at the pteryigum head in two cases (cases 4 and 5). Such subtle findings could be easily missed clinically given the coexisting pterygium (Figure 2c). HR-OCT images performed demonstrated the classic findings of OSSN in all of these cases (Figures 2b and 2d). Biopsy revealed conjunctival CIS in cases 3, 4, and 5, thus confirming HR-OCT findings.
Figure 2.
(a) A 37-year-old male boat captain with a longstanding conjunctival lesion presented with a subtle area of leukoplakia (black arrow) at the corneal margin of the lesion. (b) HR-OCT image (commercial device, white line scan in a) demonstrated hyper-reflective thickened epithelium (above arrow heads) and an abrupt transition zone (white arrow). (c) Similarly, a subtle opacification and elevation was noted at the head of a longstanding pterygium of a 64-year-old male (black arrow). (d) HR-OCT (commercial device, white line scan in c) revealed the anatomy of the folded pterygium tissue (P) with normal epithelium (E), and an adjacent area of thickened epithelium (above arrow heads, T), with two abrupt transition zones (white arrows) suspicious for OSSN. Biopsy confirmed OSSN in both cases.
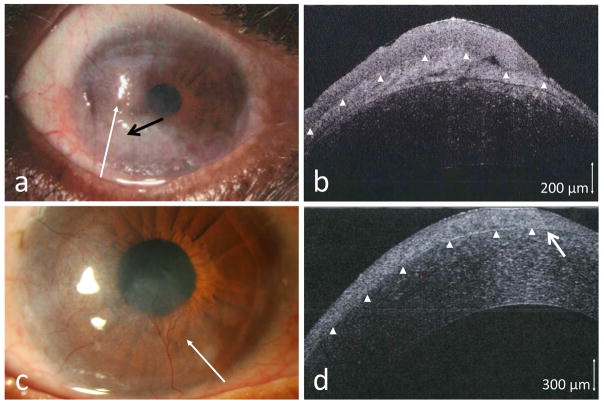
3.3. Salzmann’s nodular degeneration
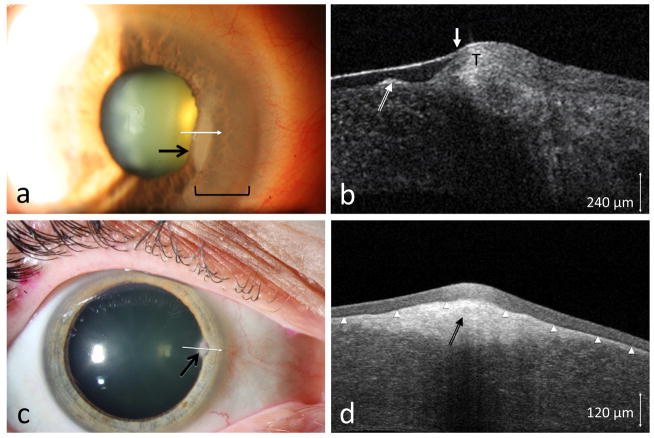
Three patients with paracentral nodular elevations were referred for possible OSSN. In case 6, a nodular corneal lesion was seen, but, in addition, there was epithelial opacification emanating from the limbus (Figure 3a). As Salzmann’s often has an opaque non-elevated adjacent scarring, it was clinically difficult to determine the nature of this opacification.
Figure 3.
(a) A 76-year-old male seen with an opacity (area within bracket) adjacent to a long standing nodule (black arrow). (b) HR-OCT (custom device, white line scan in a) showed the subepithelial opacities (long white arrow) consistent with Salzmann’s nodular changes but also subtle adjacent epithelial thickening (T) and a transition zone (short white arrow) consistent with OSSN. Biopsy confirmed OSSN. (c) A 57-year-old female referred for a corneal opacity thought to be OSSN (black arrow). (d) HR-OCT (commercial device, white line scan in c) demonstrated thin, dark epithelium (above the arrowheads) with a hyper-reflective subepithelial lesion (black arrow) consistent with Salzmann’s, which was confirmed by biopsy.
On HR-OCT, a Salzmann’s nodule is characterized by a subepithelial hyper-reflective lesion with an overlying non-thickened or even thin epithelium. HR-OCT imaging of case 6 showed a subepithelial Salzmann’s nodule, as well as epithelial thickening consistent with OSSN adjacent to the nodular changes (Figure 3b). Biopsy of this case confirmed corneal intraepithelial neoplasia coexistent with a Salzmann’s nodule. In cases 7 and 8, no epithelial thickening was noted on HR-OCT and only a subepithelial hyper-reflective lesion was seen (Figure 3d), consistent with Salzmann’s and ruling out OSSN. Biopsy confirmed the HR-OCT findings and was negative for neoplasia in both.
3.4. Mucous membrane pemphigoid
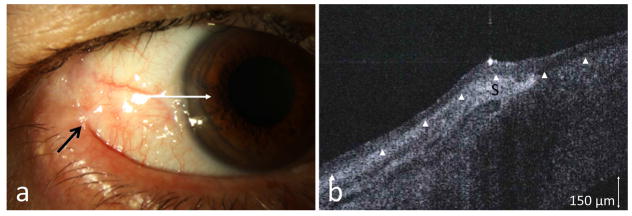
One patient (case 9) with a history of OSSN (CIS) developed biopsy-proven Stage III MMP by Foster classification. He was referred for a new suspicious conjunctival lesion. The clinical evaluation for OSSN was challenging, as the ocular surface had areas of symblephara, keratinization, and a gelatinous appearance at the limbus (Figure 4a). HR-OCT revealed normal thickness epithelium and no evidence of OSSN (Figure 4b). A biopsy confirmed that no malignancy was present in the area of limbus or lateral canthus.
Figure 4.
(a) A 73-year-old male with a history of OSSN in the lateral canthus 7 years prior to presentation developed Stage III MMP (Foster classification) with symblepharon formation (black arrow) and presented for possible OSSN recurrence at the limbus. (b) HR-OCT (commercial device) along the white line, however, demonstrated thin epithelium (above arrowheads) with a subepithelial hyper reflectivity consistent with scarring (S) and no OSSN. Biopsy was negative for OSSN.
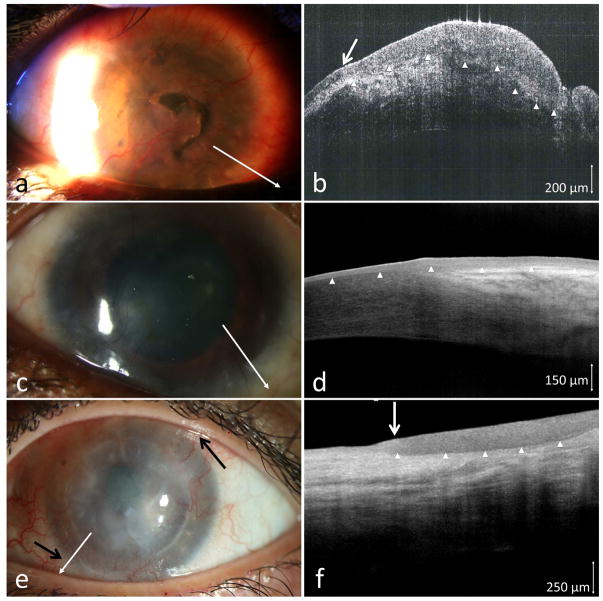
3.5. Corneal scarring and limbal stem cell deficiency
Case 10 presented with a gelatinous atypical-looking lesion adjacent to an iatrogenic pseudopterygium/focal area of LSCD. The lesion could have been easily attributed to the scarring; however, a HR-OCT showed a hyper-reflective, thickened epithelium. A biopsy demonstrated conjunctival CIS.
Case 11 had a history of OSSN treated with interferon and presented with total LSCD and lipid keratopathy (Figure 5a). Given the presence of scaring and pannus, OSSN recurrence could not be determined clinically. HR-OCT demonstrated dark, uniform epithelium with no transition zone (Figure 5b), ruling out OSSN. A subepithelial band of hyper-reflectivity suggestive of scarring/opacity was seen. This HR-OCT was consistent with conjunctival pannus formation, not OSSN. Biopsy confirmed the absence of OSSN.
Figure 5.
(a) A 66-year-old female with history of lipid keratopathy, stem cell deficiency and a prior history of OSSN was referred for possible OSSN recurrence. (b) HR-OCT (commercial device, white line scan in a) demonstrated a uniform, only mildly thickened epithelium (above white arrow tips) but without a transition zone and a diffuse subepithelial scar (above black arrow heads) and stromal scar (black arrow) ruling out OSSN. Biopsy was negative for OSSN. (c) A 28-year-old male with diffuse corneal scarring presumed to be herpetic keratitis. The opacity had a whirling opalescent appearance (black arrows). (d) HR-OCT (commercial device, white line scan in c) revealed hyper-reflective epithelial thickening (above arrow heads) with an abrupt transition from normal epithelium (white arrow) suggestive of OSSN, which was confirmed on biopsy.
Case 12 had diffuse subtle corneal scarring that was initially thought to be scarring from a herpetic infection (Figure 5c). HR-OCT was indicative of suspicious for OSSN (Figure 5d). Biopsy confirmed CIS.
Case 13 had partial LSCD and a band-like corneal scar with calcium deposits. A thickening at the para-central area was noted, and the patient was sent for evaluation of possible OSSN. HR-OCT of this case showed a subtle thickened, hyper-reflective epithelium consistent with OSSN, which was overlying the scar tissue and adjacent to the calcific deposits. Biopsy verified the presence of CIS.
Case 14 had human immunodeficiency virus (HIV) and extensive LSCD in the setting of vernal keratoconjunctivitis (Figure 6a). Evaluation of this ocular surface was very challenging, given the complicated condition of the cornea. A HR-OCT scan demonstrated epithelial thickening overlying subepithelial scarring (Figure 6b). A biopsy directed to the area seen on HR-OCT confirmed the presence of conjunctival intraepithelial neoplasia overlying subepithelial inflammation/scarring. The patient was followed up after topical interferon treatment with serial HR-OCT’s, which monitored resolution of the disease and the recovering ocular surface (Figures 6c and 6d).
Figure 6.
(a) A 35-year-old male with HIV, vernal keratoconjunctivitis, and extensive LSCD with chronic epithelial defects presented for evaluation of possible OSSN. (b) HR-OCT (custom device, white line scan in a) demonstrated epithelial thickening (above arrow heads), an abrupt transition (arrow) and subepithelial scarring (below arrow heads) consistent with OSSN, which was confirmed by biopsy. (c) Slit lamp photograph showing resolution after treatment with topical interferon and (d) HR-OCT (commercial device) demonstrating thin, normalized epithelium on OCT (white arrowheads). (e) A 43-year-old female who presented with neovascularization and opacification of her penetrating keratoplasty implant. A subtle gelatinous change was noted in the limbal conjunctiva (black arrows) suspicious of OSSN. (f) HR-OCT (commercial device, white line scan in e) was consistent with OSSN showing a thickened mildly hyper reflective epithelium (above arrow heads) and a transition zone (white arrow). Biopsy confirmed OSSN.
Case 15 had penetrating keratoplasty for progressive opacification secondary to herpes simplex virus (HSV) keratitis. Her graft failed, with neovascularization and opacification of the corneal transplant consistent with LSCD and a subtle gelatinous change (Figure 6e). HR-OCT showed the typical OSSN features and confirmed the clinical suspicion (Figure 6f). Biopsy verified the presence of a CIS with severe dysplasia (1 o’clock) and corneal intraepithelial neoplasia (7 o’clock).
Case 16 presented with LSCD and a suspicious gray lesion (Figure 7a). HR-OCT demonstrated epithelial thickening overlying dense non-epithelial tissue consistent with OSSN and underlying scaring (Figure 7b). OSSN was confirmed by biopsy. The patient responded well to combined medical and surgical treatment. Nineteen months after treatment, a subtle new opacity was detected (Figure 7c). Clinically it was difficult to determine if the opacity was OSSN or simply scarring. HR-OCT, however, revealed epithelial thickening with marked hyper-reflectivity (Figure 7d) suggesting recurrence. A biopsy confirmed the HR-OCT findings of OSSN.
Figure 7.
(a) 61-year-old male with LSCD presented with a gray lesion (black arrow). (b) HR-OCT (custom device, white line scan in a) revealed thickened and hyper reflective epithelium (above arrow heads) consistent with OSSN. A band of scar/inflammation was seen (between Bowman’s and arrowheads). Biopsy confirmed the OSSN. (c) Nineteen months after resolution, a new opacity was detected at the 4 o’clock area. (d) HR-OCT (custom device, white line scan in c) revealed epithelial thickening, hyper reflectivity (above arrow heads) and a visible transition zone (white arrow) suggestive of OSSN recurrence. OCT finding was confirmed on biopsy.
4. Discussion
While OSSN is often clinically easy to identify, its coexistence with other ocular surface disease can make it difficult to determine what is occurring on the ocular surface. OSSN can occur with corneal ulcer [16], chronic blepharoconjuctivitis [17], pterygium [6,19,20], necrotizing scleritis [21,22], sclerokeratitis[18,29], orbital cellulitis [23], superior limbic keratoconjuctivitis [24], ocular prosthesis [25,26], fibroxanthoma[27], and MMP [28]. In the published literature, several cases of the OSSN were not recognized clinically, but were found when biopsies were done for other presumed diagnoses. This emphasizes the need for better noninvasive diagnostic tools to survey the ocular surface.
In the current study, we evaluated the role of HR-OCT in 16 patients with OSSN and a coexisting ocular surface disease and lesions potentially suspicious for OSSN. HR-OCT was able to consistently discern OSSN features that were otherwise difficult to assess clinically. While the custom device was 2–3 μm and the commercially available one was 5–7 μm, both were found to provide excellent resolution in order to visualize the features of OSSN. Our series encompassed a diverse group of ocular surface pathologies, including MMP, LSCD, rosacea, HSV keratitis, and pterygia. Characteristic features of OSSN by OCT, namely epithelial thickening, hyper-reflectivity, and abrupt transition zone, were identified in the 12 patients with OSSN and were ruled out in 4 cases. All findings were confirmed by biopsy.
Other technologies that can be used to identify OSSN in the setting of coexistent ocular pathology include exfoliative cell cytology [30,31], papanicolau [32], impression cytology [33–35]), in vivo staining [36,37], and in vivo confocal microscopy [38–41]. While all can play a role in the diagnosis of OSSN, HR-OCT stands out with several advantages in that it is noninvasive, easily scans the entire ocular surface, and requires minimal operator training and no need for laboratory processing.
While histopathology remains the gold standard, HR-OCT can be an important adjunct in surveillance and follow-up of treated lesions. It is especially helpful when multiple biopsies can have adverse effects, as in patients with MMP or LSCD. HR-OCT can also direct the biopsy location to potentially decrease the risk of false negative results. Furthermore, patients can be noninvasively scanned over the whole ocular surface to assess for OSSN resolution and detect subclinical recurrence. In medical treatment of OSSN patients, HR-OCT can help to monitor resolution and avoid premature termination of therapy.
It is important, however, to recognize the limitations of HR-OCT. For instance, the current HR-OCT devices cannot yet detect invasion of the tumor or the grade. Shadowing can also limit penetration and full view of the lesion. Ideal images of areas such as the inferior fornix and caruncle are not provided with current technology. Our study was a retrospective one designed to evaluate agreement between HR-OCT and biopsy findings in patients with coexisting ocular surface conditions. A prospective, masked study with a larger sample size would best be able to determine the predictive value of this diagnostic technique and to uncover any false positive or false negative results not detected with our sample size.
5. Conclusions
We demonstrated that HR-OCT is a powerful tool for detecting OSSN in patients with coexisting ocular conditions. In these cases, clinical determination of neoplasia can be challenging due to extensive ocular surface pathologies. With HR-OCT, subtle or suspicious lesions can be scanned at the office visit and decisions pertaining to management can be made promptly.
Acknowledgments
Supported by the NIH Center Core Grant P30EY014801, RPB Unrestricted Award and Career Development Awards, Department of Defense (DOD- Grant#W81XWH-09-1-0675), Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, The Azar Family Grant, The Robert Baer Family Grant, The Gordon Charitable Foundation, The H. Scott Huizenga Grant, The Grant and Diana Stanton-Thornbrough Grant, The Mark Feldberg Family Grant, and supported in part by an unrestricted grant from Research to Prevent Blindness (all institutional grants).
Abbreviations
- CIS
carcinoma in situ
- HR-OCT
high resolution optical coherence tomography
- LSCD
limbal stem cell deficiency
- MMP
mucous membrane pemphigoid
- OCT
optical coherence tomography
- OSSN
ocular surface squamous neoplasia
Footnotes
The authors have no proprietary or commercial interest in any materials or concepts discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–50. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Basti S, Macsai MS. Ocular surface squamous neoplasia. Cornea. 2003;22:687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Blodi FC. Squamous cell carcinoma of the conjunctiva. Doc Ophthalmol. 1973;34:93–108. doi: 10.1007/BF00151799. [DOI] [PubMed] [Google Scholar]
- 5.Kao AA, Galor A, Karp CL, Abdelaziz A, Feuer WJ, Dubovy SR. Clinicopathologic correlation of ocular surface squamous neoplasms at Bascom Palmer Eye Institute: 2001 to 2010. Ophthalmology. 2012;119:1773–6. doi: 10.1016/j.ophtha.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Oellers P, Karp CL, Sheth A, Kao AA, Abdelaziz A, Matthews JL, et al. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology. 2013;120:445–50. doi: 10.1016/j.ophtha.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreira H, Coutinho F, Carrilho C, Lunet N. HIV and HPV infections and ocular surface squamous neoplasia: systematic review and meta-analysis. Br J Cancer. 2013;109:1981–8. doi: 10.1038/bjc.2013.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pe’er J. Ocular surface squamous neoplasia. Ophthalmol Clin North Am. 2005;18:1–13. vii. doi: 10.1016/j.ohc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Rundle P, Mudhar HS, Rennie I. Conjunctival intra-epithelial neoplasia occurring in young patients with asthma. Eye (Lond) 2010;24:1182–5. doi: 10.1038/eye.2009.296. [DOI] [PubMed] [Google Scholar]
- 10.Simpson T, Fonn D. Optical coherence tomography of the anterior segment. Ocul Surf. 2008;6:117–27. doi: 10.1016/s1542-0124(12)70280-x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas BJ, Galor A, Nanji Aa, El Sayyad F, Wang J, Dubovy SR, et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12:46–58. doi: 10.1016/j.jtos.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shousha MA, Karp CL, Perez VL, Hoffmann R, Ventura R, Chang V, et al. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology. 2011;118:1531–7. doi: 10.1016/j.ophtha.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Kieval JZ, Karp CL, Abou Shousha M, Galor A, Hoffman RA, Dubovy SR, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 2012;119:481–6. doi: 10.1016/j.ophtha.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Shousha MA, Karp CL, Canto AP, Hodson K, Oellers P, Kao AA, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013;120:883–91. doi: 10.1016/j.ophtha.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanji AA, Sayyad FE, Galor A, Dubovy S, Karp CL. High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology. Ocul Surf. 2015;13:226–35. doi: 10.1016/j.jtos.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sridhar MS, Honavar SG, Vemuganti G, Rao GN. Conjunctival intraepithelial neoplasia presenting as corneal ulcer. Am J Ophthalmol. 2000;129:92–4. doi: 10.1016/s0002-9394(99)00286-x. [DOI] [PubMed] [Google Scholar]
- 17.Akpek EK, Polcharoen W, Chan R, Foster CS. Ocular surface neoplasia masquerading as chronic blepharoconjunctivitis. Cornea. 1999;18:282–8. doi: 10.1097/00003226-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood MA, Al-Rajhi A, Riley F, Karcioglu ZA. Sclerokeratitis: an unusual presentation of squamous cell carcinoma of the conjunctiva. Ophthalmology. 2001;108:553–8. doi: 10.1016/s0161-6420(00)00585-6. [DOI] [PubMed] [Google Scholar]
- 19.Mirza E, Gumus K, Evereklioglu C, Arda H, Oner A, Canoz O, et al. Invasive squamous cell carcinoma of the conjunctiva first misdiagnosed as a pterygium: a clinicopathologic case report. Eye Contact Lens. 2008;34:188–90. doi: 10.1097/ICL.0b013e31815700af. [DOI] [PubMed] [Google Scholar]
- 20.Rootman DB, McGowan HD, Yücel YH, Pavlin CJ, Simpson ER. Intraocular extension of conjunctival invasive squamous cell carcinoma after pterygium surgery and cataract extraction. Eye Contact Lens. 2012;38:133–6. doi: 10.1097/ICL.0b013e318235c4d3. [DOI] [PubMed] [Google Scholar]
- 21.Comstock CP. Invasive squamous cell carcinoma of the conjunctiva presenting as necrotizing scleritis with scleral perforation and uveal prolapse. Surv Ophthalmol. n.d;33:50–4. doi: 10.1016/0039-6257(88)90072-0. [DOI] [PubMed] [Google Scholar]
- 22.Younan N, McClellan K. Squamous cell carcinoma with necrotizing scleritis. Aust N Z J Ophthalmol. 1999;27:149–51. doi: 10.1046/j.1440-1606.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- 23.Rootman J, Roth AM, Crawford JB, Fox LP, Patel S. Extensive squamous cell carcinoma of the conjunctiva presenting as orbital cellulitis: the hermit syndrome. Can J Ophthalmol. 1987;22:40–4. [PubMed] [Google Scholar]
- 24.Moshirfar M, Khalifa YM, Kuo A, Davis D, Mamalis N. Ocular surface squamous neoplasia masquerading as superior limbic keratoconjunctivitis. Middle East Afr J Ophthalmol. 2011;18:74–6. doi: 10.4103/0974-9233.75895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campanella PC, Goldberg SH, Erlichman K, Abendroth C. Squamous cell tumors and ocular prostheses. Ophthal Plast Reconstr Surg. 1998;14:45–9. doi: 10.1097/00002341-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker KW, Trivedi D, Bridger J, Sandramouli S. Ocular surface squamous neoplasia: report of an unusual case and review of the literature. Orbit. 2002;21:209–15. doi: 10.1076/orbi.21.3.209.7173. [DOI] [PubMed] [Google Scholar]
- 27.Engelbrecht NE, Ford JG, White WL, Yeatts RP. Combined intraepithelial squamous neoplasia and atypical fibroxanthoma of the cornea and limbus. Am J Ophthalmol. 2000;129:94–6. doi: 10.1016/s0002-9394(99)00275-5. [DOI] [PubMed] [Google Scholar]
- 28.Sivalingam V, Shields CL, Shields JA, Pearah JD. Squamous cell carcinoma of the conjunctiva associated with benign mucous membrane pemphigoid. Ann Ophthalmol. 1990;22:106–9. [PubMed] [Google Scholar]
- 29.Arteaga-Sánchez A, Toledano-Fernández N, Díaz-Valle D, Fernández-Aceñero MJ, Hijós-Gastón M. Sclerokeratitis and invasive conjunctival squamous cell carcinoma. Arch Soc Esp Oftalmol. 2007 Apr;82(4):237–40. doi: 10.4321/s0365-66912007000400010. (Spanish) [DOI] [PubMed] [Google Scholar]
- 30.Semenova EA, Milman T, Finger PT, Natesh S, Kurli M, Schneider S, et al. The diagnostic value of exfoliative cytology vs histopathology for ocular surface squamous neoplasia. Am J Ophthalmol. 2009;148:772–8. e1. doi: 10.1016/j.ajo.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Larmande A, Timsit E. Importance of cytodiagnosis in ophthalmology: preliminary report of 8 cases of tumors of the sclero-corneal limbus. Bull Soc Ophtalmol Fr. 1954;5:415–9. [PubMed] [Google Scholar]
- 32.Gelender H, Forster RK. Papanicolaou cytology in the diagnosis and management of external ocular tumors. Arch Ophthalmol. 1980;98:909–12. doi: 10.1001/archopht.1980.01020030903020. [DOI] [PubMed] [Google Scholar]
- 33.Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–33. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- 34.Nolan GR, Hirst LW, Wright RG, Bancroft BJ. Application of impression cytology to the diagnosis of conjunctival neoplasms. Diagn Cytopathol. 1994;11:246–9. doi: 10.1002/dc.2840110310. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89:1655–9. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero IL, de Barros JN, Martins MC, Ballalai PL. The use of 1% toluidine blue eye drops in the diagnosis of ocular surface squamous neoplasia. Cornea. 2013;32:36–9. doi: 10.1097/ICO.0b013e318243f61f. [DOI] [PubMed] [Google Scholar]
- 37.Steffen J, Rice J, Lecuona K, Carrara H. Identification of ocular surface squamous neoplasia by in vivo staining with methylene blue. Br J Ophthalmol. 2014;98:13–5. doi: 10.1136/bjophthalmol-2013-303956. [DOI] [PubMed] [Google Scholar]
- 38.Parrozzani R, Lazzarini D, Dario A, Midena E. In vivo confocal microscopy of ocular surface squamous neoplasia. Eye (Lond) 2011;25:455–60. doi: 10.1038/eye.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Zhou Z, Wang M, Liu F, Qu H, Hong J. The clinical value of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Eye (Lond) 2012;26:781–7. doi: 10.1038/eye.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguena MB, van den Tweel JG, Makupa W, Hu VH, Weiss HA, Gichuhi S, et al. Diagnosing ocular surface squamous neoplasia in East Africa: case-control study of clinical and in vivo confocal microscopy assessment. Ophthalmology. 2014;121:484–91. doi: 10.1016/j.ophtha.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res. 2014;39:213–31. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]