Abstract
Background
Using change in estimated glomerular filtration rate (eGFR) based on creatinine as a surrogate outcome in clinical trials of chronic kidney disease has been proposed. Risk of end-stage renal disease (ESRD) and all-cause mortality associated with change in other filtration markers has not been studied in chronic kidney disease populations.
Study Design
Observational analysis of two clinical trials
Setting & Participants
Participants in the MDRD (Modification of Diet in Renal Disease; n=317) Study and AASK (African American Study of Kidney Disease and Hypertension; n=373)
Predictors
Creatinine, cystatin C, β-trace protein (BTP), and β2-microglobulin (B2M) levels were measured in serum samples collected at the 12-month and 24-month follow-up visits, along with measured GFR (mGFR) at these time points.
Outcomes
ESRD and all-cause mortality
Measurements
Poisson regression was used to estimate incidence rate ratios and 95% CIs for ESRD and all-cause mortality during long-term follow-up (10–16 years) per 30% decline in mGFR or eGFR for each filtration marker and the average of all four markers.
Results
One-year decline in mGFR, eGFRcr, eGFRBTP, and the average of the four filtration markers was significantly associated with an increased risk of incident ESRD in both studies (all p≤0.02). Compared to mGFR, only decline in eGFRBTP was statistically significantly more strongly associated with ESRD risk in both studies (both p≤0.03). Decline in eGFRcr, but not mGFR or the other filtration markers, was significantly associated with risk of all-cause mortality in AASK only (incidence rate ratio per 30% decline, 4.17; 95% CI, 1.78–9.74; p<0.001), but this association was not significantly different from decline in mGFR (p=0.2).
Limitations
Small sample size
Conclusions
Declines in mGFR, eGFR based on serum creatinine and BTP, and the average of four filtration markers (creatinine, cystatin C, BTP, and B2M) were consistently associated with progression to ESRD.
Index words: beta-2-microglobulin (B2M), beta trace protein (BTP), creatinine, cystatin C, filtration markers, death, mortality, end-stage renal disease (ESRD), incident ESRD, glomerular filtration rate (GFR), estimated GFR, measured GFR, kidney function decline
Estimated glomerular filtration rate (eGFR) and serum concentrations of filtration markers are strong predictors of adverse outcomes in individuals with chronic kidney disease (CKD). Recent meta-analyses demonstrate the consistency of findings for baseline eGFR across a broad range of clinical outcomes including kidney disease progression, cardiovascular disease, and mortality.1–6 Previous studies have reported differences between baseline levels of filtration markers with respect to their relationships with subsequent development of end-stage renal disease (ESRD) and mortality.7
Assessing filtration markers at multiple time points may improve the prediction of clinical outcomes among individuals with CKD relative to a single measurement. Change in eGFR based on creatinine (eGFRcr) has been proposed as a surrogate outcome of kidney disease progression in clinical trials based on the association with ESRD and all-cause mortality.8,9 Prior studies have reported a U-shaped relationship with both decline and rise in eGFRcr demonstrating adverse health consequences, although there are some inconsistencies for eGFRcr rise.10–14 Risk of ESRD and mortality associated with change in novel filtration markers (cystatin C, β-trace protein [BTP], and β2-microglobulin [B2M]) has not been previously assessed in a CKD study population.
The primary objective of our investigation was to examine the association of one-year change in eGFR based on individual filtration markers compared to mGFR with subsequent risk of developing ESRD and all-cause mortality in adults with CKD from the MDRD (Modification of Diet in Renal Disease) Study and AASK (African American Study of Kidney Disease and Hypertension).
METHODS
Study Design and Participants
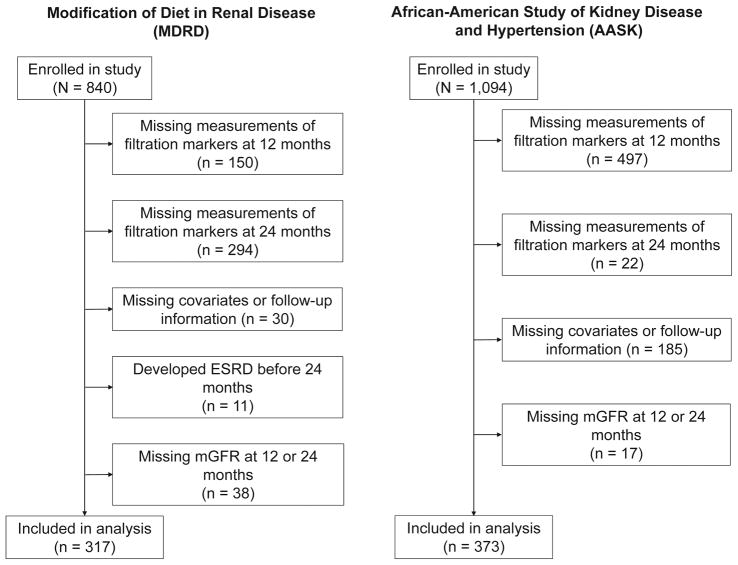
The present study is a prospective analysis of change in mGFR and eGFR using established and novel filtration markers from follow-up visits at 12 and 24 months and subsequent long-term follow-up for clinical outcomes among participants in the MDRD Study and AASK. The MDRD Study was a randomized clinical trial of dietary intake of protein and phosphorus and blood pressure control in individuals with CKD conducted in 1989–1993 with follow-up through 2000.15 The AASK study was a randomized clinical trial of blood pressure lowering in African-Americans with hypertension conducted in 1995–1998 with follow-up through 2001.16 We evaluated MDRD and AASK participants with non-missing data for filtration markers at the 12 month and 24 month follow-up visits, follow-up data for the ascertainment of outcomes, and covariates assessed at the 12 month follow-up visit (Figure 1; MDRD Study: n=317; AASK: n=373). Participants who were included in the present study had slightly higher baseline eGFRcr and were more likely to be male than those who were excluded (Table S1, available as online supplementary material). Approval was provided by the Institutional Review Board (IRB00003782), procedures adhered to the Declaration of Helsinki, and study participants provided informed consent.
Figure 1.
Flow Diagram of Study Participant Selection in the Modification of Diet in Renal Disease Study and the African American Study of Kidney Disease and Hypertension
Measurement of GFR and Endogenous Filtration Markers
In the MDRD Study and AASK, mGFR was assessed as four period urinary clearance of 125I-iothalamate.17,18 Concentrations of creatinine, cystatin C, BTP, and B2M were measured in serum specimens collected at the 12-month and 24-month follow-up visits. Using the Roche Cobas 6000 chemistry auto-analyzer, creatinine was measured by the enzymatic method and calibrated to the isotope-dilution mass spectrometry (IDMS) standard (coefficient of variation [CV], 2.8%–2.9%), cystatin C was measured by a particle-enhanced turbidimetric immunoassay (Gentian; CV, 3.2%–4.3%), and B2M was measured immunoturbidimetrically by quantifying absorbance due to agglutination of latex particles consisting of B2M antibody bound to B2M in the specimens (Roche Tina-Quant β2-microglobulin reagent; CV, 3.2%–4.3%).19 The marker BTP was measured by the immunonephelometric method (Siemens) and quantified using the Siemens ProSpec nephelometer (CV, 7.4%–10.6%). Assays were completed at the University of Minnesota Advanced Research and Diagnostic Laboratory in 2014–2015. Specimens from both visits were run mixed together (i.e. not sorted by study visit) to minimize the likelihood of a systematic bias due to batch effect.
Calculation of Change in mGFR and eGFR
The main exposure was one-year percent change in mGFR and eGFR, which was calculated as the difference between two measurements as a proportion of the first measurement. Change in mGFR was compared to change in eGFR for each of the four filtration markers alone and in combination. eGFR was calculated using the CKD-EPI (CKD Epidemiology Collaboration) equations for creatinine (eGFRcr), cystatin C (eGFRcys), β-trace protein (eGFRBTP), and β2-microglobulin (eGFRB2M).20–22 The average of four markers was calculated by summing percent change in eGFR for the four individual filtration markers and dividing by four: average of four markers = (% Δ eGFRcr + % Δ eGFRcys + % Δ eGFRBTP + % Δ eGFRB2M)/4. Percent change in mGFR and eGFR was expressed continuously using two linear spline terms with a knot at 0% change.
Outcome Ascertainment
Incident ESRD and all-cause mortality were ascertained after the one-year change period through December 31, 2010, in the MDRD Study and through June 30, 2007 in AASK. In the MDRD Study, incident ESRD was defined as initiation of dialysis or receipt of a kidney transplant as determined by entry into the US Renal Data System registry, and participants who died from any cause were identified by linkage to the National Death Index, as previously described.23,24 Deaths that occurred both prior to and subsequent to ESRD were ascertained in the MDRD Study. In AASK, incident ESRD was defined as self-reported transplantation or initiation of dialysis.17,25 In AASK, deaths prior to ESRD were ascertained and were identified by phase, and deaths were identified by review of hospitalization records and confirmed with autopsy forms and death certificates during the trial and by proxy self-report during the cohort phase.25
Assessment of Other Covariates
Demographic and clinical characteristics known to be risk factors for ESRD and mortality were collected according to the protocol during study visits for these two clinical trials. Sociodemographic factors (age, sex, racial group) and health history (diabetes status) were ascertained through structured questionnaires. Health metrics including weight and height for the calculation of body mass index (kg/m2) and systolic blood pressure were determined by physical examination. Total cholesterol (mg/dL) was measured in blood specimens according to the original study protocols. Study design characteristics, i.e. randomized treatment group and study group (for MDRD Study only), were also included in our analysis. We used covariates ascertained at the 12 month follow-up visit (baseline for this analysis).
Statistical Analysis
Baseline demographics, clinical characteristics, and eGFR levels were described using means, standard deviations, and proportions. Kernel density plots were created for the two studies to display the distribution of percent change in mGFR, eGFR for each individual filtration marker, and eGFR for the average of all four filtration markers. Spearman correlation coefficients were calculated between change in mGFR and change in eGFR for the filtration markers separately and in combination. Poisson regression was used to estimate incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for the association between one-year percent change in mGFR and eGFR for each filtration marker, separately and in combination, with subsequent risk of developing ESRD and all-cause mortality, incorporating time to event or censoring. We evaluated risk associated with both decline and rise in mGFR and eGFR given prior reports of a U-shaped relationship. In addition to examining the unadjusted associations, we used multivariable regression to estimate the independent associations between change in GFR and outcomes after accounting for participant characteristics, risk factors, and design features that could partially explain the change in GFR. To examine whether mGFR and eGFR change was independently associated with ESRD and death, we used multivariable regression models adjusted for baseline age, sex, race, body mass index, systolic blood pressure, diabetes status, total cholesterol, randomized treatment group, study group (for MDRD Study only), and first measurement of mGFR or eGFR for the respective filtration marker (“first” refers to the baseline visit for the present analysis, i.e., the 12 month follow-up visit). We conducted several sensitivity analyses: 1) adjusted for the first measurement of mGFR in lieu of the first measurement of eGFR based on the respective filtration marker; 2) adjusted for proteinuria; and 3) adjusted for eGFR for the respective filtration marker and covariates ascertained at the “last” visit (or second time point for the change period, i.e. the 24 month follow-up visit).
Adjusted IRRs were calculated per 30% decline and per 30% rise in mGFR and eGFR using linear spline terms with one knot at 0%. Although 30% decline over a one-year period is large, this quantity is relevant since it has been proposed as a surrogate of kidney disease progression in clinical trials.8 To visualize ESRD and mortality risk associated with decline and rise in mGFR and eGFR, we plotted adjusted risk estimates across the spectrum of percent change in mGFR and eGFR using the same linear spline terms and centered the graph at 0% as the reference point. Seemingly unrelated regression was used to formally test for differences in risk estimates from separate regression models and account for correlated error terms.26 Competing risk regression models were used to evaluate the association between change in mGFR and eGFR and ESRD while accounting for the competing risk of death prior to the development of ESRD using the Fine and Gray method.27 Analyses were conducted separately for the MDRD Study and AASK given the inherent differences in the study populations and study designs.
RESULTS
Baseline Characteristics at 12-Month Follow-up Visit
In both studies, participants were middle-aged and approximately one third were men (Table 1). In AASK, all study participants were African-American and none had diabetes, whereas in the MDRD Study, only 5.0% were African-American and 4.4% had type 2 diabetes. Systolic blood pressure levels and mGFR levels were higher in AASK than in the MDRD Study.
Table 1.
Study Participant Characteristics Assessed at Baseline in MDRD Study and AASK
| MDRD (n=317) | AASK (n=373) | |
|---|---|---|
| Age, years | 52.1 (11.9) | 55.8 (10.3) |
| Male sex | 64.0 (203) | 62.2 (232) |
| African-American race | 5.0 (16) | 100.0 (373) |
| Body mass index, kg/m2 | 27.1 (4.2) | 30.5 (6.6) |
| Systolic blood pressure, mmHg | 130.5 (17.2) | 149.3 (24.3) |
| Diabetes | 4.4 (14) | 0.0 (0) |
| Total cholesterol, mg/dL | 214.3 (43.0) | 209.9 (41.5) |
| mGFR, mL/min/1.73 m2 | 29.6 (12.6) | 46.3 (16.4) |
| eGFRcr, mL/min/1.73 m2 | 31.9 (14.7) | 48.6 (17.4) |
| eGFRcys, mL/min/1.73 m2 | 28.8 (12.8) | 43.9 (16.5) |
| eGFRBTP, mL/min/1.73 m2 | 31.4 (9.5) | 42.5 (12.4) |
| eGFRB2M, mL/min/1.73 m2 | 34.4 (13.0) | 49.4 (15.6) |
| Average of 4 markersb, mL/min/1.73 m2 | 31.6 (11.8) | 46.1 (14.1) |
Note: Baseline for present study is 12-month follow-up visit. Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation. Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586
Average of 4 markers = (eGFRcr + eGFRcys + eGFRBTP + eGFRB2M)/4
AASK, African American Study of Kidney Disease and Hypertension; eGFR, estimated glomerular filtration rate; eGFRcr, creatinine-based eGFR; eGFRcys, cystatin C–based eGFR; eGFRBTP, β-trace protein–based eGFR; eGFRB2M, β2-microglobulin-based eGFR; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate
Distribution of Percent Change in mGFR and eGFR From 12- to 24-Month Follow-up
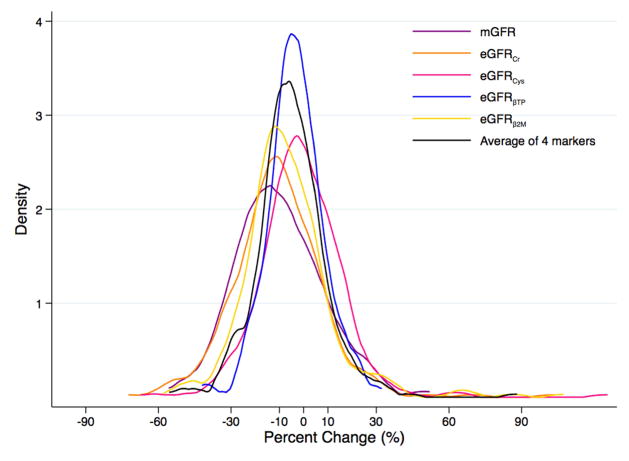
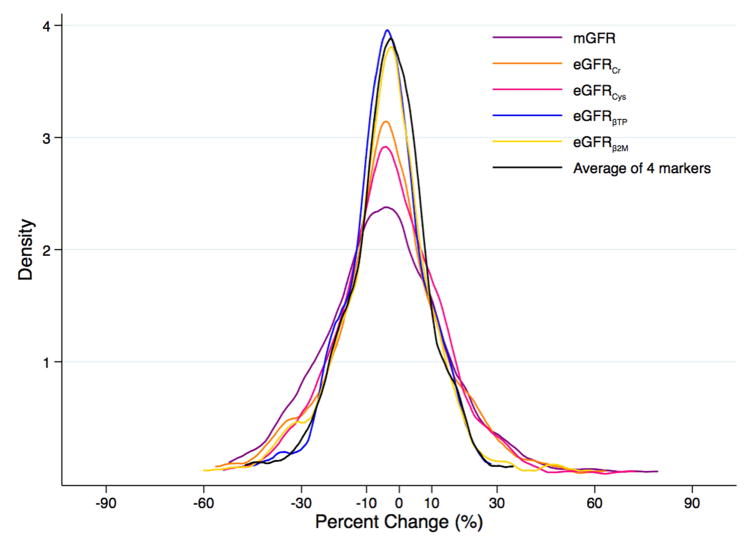
For percent change in mGFR and eGFRcr in the MDRD Study, the distribution was wider and shifted toward greater decline relative to the other filtration markers (Figure 2A). There was less variability in the distributions of percent change in mGFR and eGFR for the filtration markers in AASK (Figure 2B) compared to the MDRD Study, which could be due to differences in demographic characteristics such as race/ethnicity between the two studies (Table 1). Change in mGFR had a moderate to strong positive correlation with change in eGFR for the individual filtration markers and the combination of the filtration markers in both studies (Table S2).
Figure 2.
Kernel Density Plot of Change in Measured and Estimated Glomerular Filtration Ratea,b (A) in the Modification of Diet in Renal Disease Study and (B) in the African American Study of Kidney Disease and Hypertension
amGFR in purple; eGFRcr in orange; eGFRcys in pink; eGFRBTP in blue; eGFRB2M in yellow; average of four filtration markers in black
bMean (standard deviation) percent change over one year in the MDRD Study and AASK, respectively, was −10.0% (18.2%) and −4.2% (18.8%) for mGFR, −10.5% (19.0%) and −3.2% (16.9%) for eGFRcr, −1.0% (17.5%) and −2.6% (16.1%) for eGFRcys, −3.3% (11.6%) and −2.5% (22.5%) for eGFRBTP, −6.6% (18.9%) and −3.8% (14.7%) for eGFRB2M, and −5.4% (14.6%) and −3.0% (13.0%) for the average of the four filtration markers.
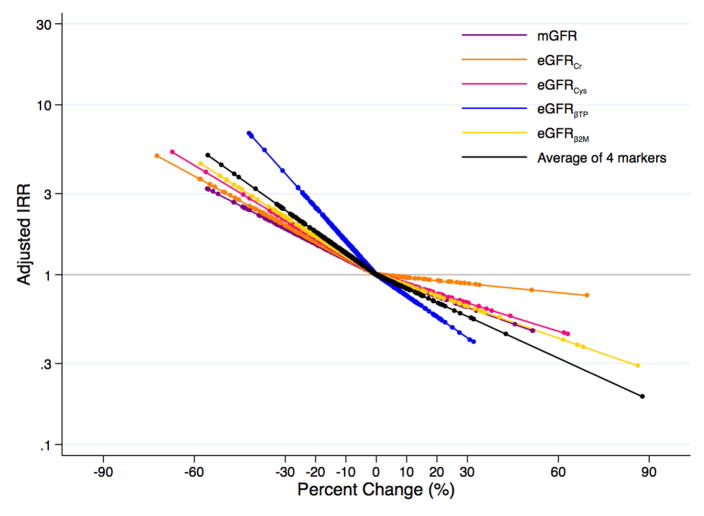
Decline in eGFR and mGFR From 12- to 24-Month Follow-up and Subsequent ESRD
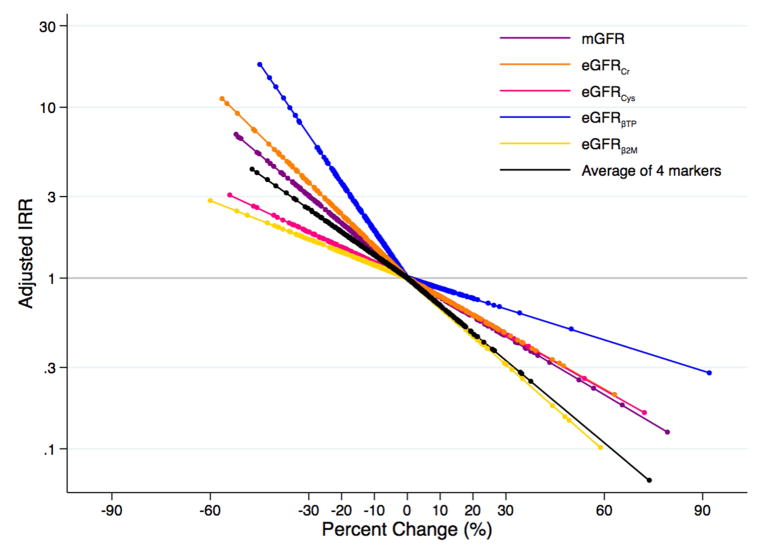
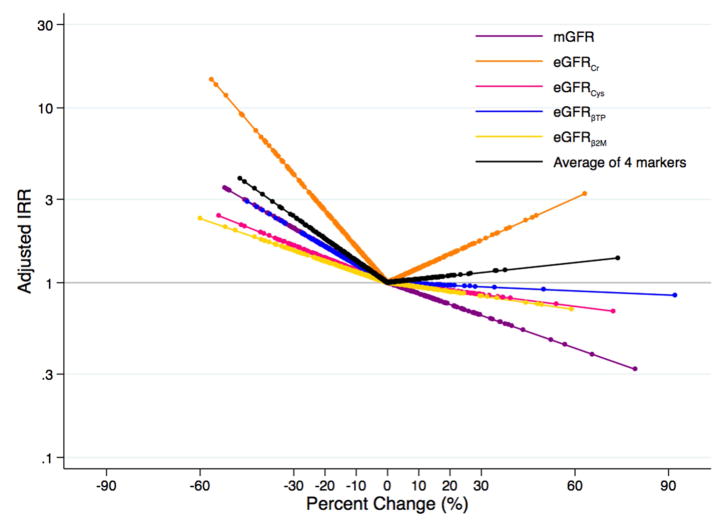
In the MDRD Study and AASK, respectively, there were 237 and 92 incident ESRD cases during a median follow-up of 5 (range, 0–16) and 7 (range, 0–10) years. Decline in mGFR and eGFR decline for all filtration markers over the one-year period was associated with an increased risk of incident ESRD in the MDRD Study (Table 2; Figure 3A), and decline in mGFR, eGFRcr, eGFRBTP, and the average of four markers was associated with increased risk of ESRD in AASK (Table 2; Figure 3B). Per 30% decline in mGFR over a one year change period, there was a 1.91 (95% CI, 1.39–2.61) times (p<0.001) and 3.03 (95% CI, 1.76–5.23) times (p<0.001) increased risk of ESRD for the MDRD Study and AASK, respectively. Decline in eGFRBTP, but not the other filtration markers, was more strongly associated with ESRD risk than decline in mGFR for both studies (IRRs per 30% decline were 4.06 [95% CI, 2.39–6.90; p<0.001; p=0.01 for comparison to mGFR] and 6.88 [95% CI, 3.43–13.80; p<0.001; p=0.03 for comparison to mGFR] for MDRD Study and AASK, respectively). The association between percent change in eGFRcr and ESRD was similar to that for mGFR in the MDRD Study and AASK (IRRs per 30% eGFRcr decline were 1.98 [95% CI, 1.52–2.59; p=0.9 for comparison to mGFR] and 3.62 [95% CI, 2.05–6.39; p=0.5 for comparison to mGFR], respectively).
Table 2.
Risk of Incident ESRD and All-Cause Mortality Associated with Decline in Measured and Estimated GFR Over a One-Year Period
| MDRD | AASK | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome and Filtration Marker | IRRa (95% CI) per 30% decline | P | P vs mGFRb | IRRa (95% CI) per 30% decline | P | P vs mGFRb |
| ESRD | ||||||
|
| ||||||
| mGFR | 1.91 (1.39–2.61) | <0.001 | -- | 3.03 (1.76–5.23) | <0.001 | -- |
| eGFRcr | 1.98 (1.52–2.59) | <0.001 | 0.9 | 3.62 (2.05–6.39) | <0.001 | 0.5 |
| eGFRcys | 2.25 (1.58–3.21) | <0.001 | 0.5 | 1.86 (0.99–3.49) | 0.05 | 0.1 |
| eGFRBTP | 4.06 (2.39–6.90) | <0.001 | 0.01 | 6.88 (3.43–13.80) | <0.001 | 0.03 |
| eGFRB2M | 2.12 (1.51–2.97) | <0.001 | 0.6 | 1.68 (0.90–3.17) | 0.1 | 0.08 |
| Average of 4 markersc | 2.36 (1.63–3.41) | <0.001 | 0.4 | 2.54 (1.19–5.39) | 0.02 | 0.6 |
|
| ||||||
| Mortality | ||||||
|
| ||||||
| mGFR | 1.09 (0.69–1.72) | 0.7 | -- | 2.05 (0.86–4.91) | 0.1 | -- |
| eGFRcr | 1.00 (0.66–1.51) | 0.9 | 0.7 | 4.17 (1.78–9.74) | <0.001 | 0.2 |
| eGFRcys | 1.09 (0.61–1.92) | 0.8 | 0.9 | 1.63 (0.62–4.32) | 0.3 | 0.7 |
| eGFRBTP | 1.68 (0.80–3.55) | 0.2 | 0.2 | 2.04 (0.68–6.14) | 0.2 | 0.9 |
| eGFRB2M | 0.91 (0.54–1.54) | 0.7 | 0.4 | 1.53 (0.58–4.00) | 0.4 | 0.6 |
| Average of 4 markersc | 0.99 (0.55–1.78) | 0.9 | 0.7 | 2.39 (0.76–7.54) | 0.1 | 0.8 |
IRR expressed per 30% decline in mGFR or eGFR calculated by modeling percent change in mGFR or eGFR below 0% (linear spline term with a knot at 0%); adjusted for age, sex, race, body mass index, systolic blood pressure, diabetes, total cholesterol, randomized treatment group, study group (for MDRD Study only), and first assessment of mGFR or eGFR for the respective marker
P-value from seemingly unrelated regression comparing IRR for decline in respective filtration marker vs IRR for decline in mGFR
Average of 4 markers = (% Δ eGFRcr + % Δ eGFRcys + % Δ eGFRBTP + % Δ eGFRB2M)/4
AASK, African American Study of Kidney Disease and Hypertension; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IRR, incidence rate ratio; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based eGFR; eGFRcys, cystatin C–based eGFR; eGFRBTP, β-trace protein–based eGFR; eGFRB2M, β2-microglobulin-based eGFR
Figure 3.
Adjusteda Risk of Incident End-Stage Renal Disease According to Changeb in Measured and Estimated Glomerular Filtration Ratec (A) in the Modification of Diet in Renal Disease Study and (B) in the African American Study of Kidney Disease and Hypertension
aAdjusted for age, sex, race, body mass index, systolic blood pressure, diabetes, total cholesterol, randomized treatment group, study group (for MDRD Study only), and first measurement of mGFR or eGFR for the respective marker
bPercent change modeled as linear spline terms with one knot at 0%
cmGFR in purple; eGFRcr in orange; eGFRcys in pink; eGFRBTP in blue; eGFRB2M in yellow; average of four filtration markers in black
In a sensitivity analysis, risk estimates were attenuated after adjustment for first determination of mGFR in lieu of adjusting for first assessment of eGFR based on the respective filtration marker (Table 3). In unadjusted models, risk estimates were stronger and decline in mGFR and eGFR for all filtration markers, separately and in combination, were associated with ESRD (Table S3). After adjusting for proteinuria, the magnitude of the results was similar to that in the main analysis, but less precise in AASK (Table S4). After adjusting for covariates and GFR ascertained at the 24 month follow-up visit, decline in mGFR and eGFR was no longer significantly associated with ESRD (Table S5). After accounting for the competing risk of death prior to ESRD, results were similar to those in the main analysis for decline in mGFR and eGFR (Table S6).
Table 3.
Risk of Incident ESRD and All-Cause Mortality Associated with Decline in Measured and Estimated GFR Over a One-Year Period Adjusted for First Measurement of mGFR
| MDRD | AASK | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome and Filtration Marker | IRRa (95% CI) per 30% decline | P | P vs mGFRb | IRRa (95% CI) per 30% decline | P | P vs mGFRb |
| ESRD | ||||||
|
| ||||||
| mGFR | 1.91 (1.39–2.61) | <0.001 | -- | 3.03 (1.76–5.23) | <0.001 | -- |
| eGFRcr | 2.33 (1.78–3.04) | <0.001 | 0.3 | 3.06 (1.70–5.53) | <0.001 | 0.9 |
| eGFRcys | 2.23 (1.59–3.15) | <0.001 | 0.5 | 1.76 (0.91–3.41) | 0.09 | 0.09 |
| eGFRBTP | 2.78 (1.66–4.67) | <0.001 | 0.1 | 2.79 (1.45–5.37) | 0.002 | 0.8 |
| eGFRB2M | 2.17 (1.55–3.04) | <0.001 | 0.5 | 1.81 (0.95–3.43) | 0.07 | 0.1 |
| Average of 4 markersc | 2.76 (1.92–3.97) | <0.001 | 0.1 | 3.30 (1.56–6.97) | 0.002 | 0.8 |
|
| ||||||
| Mortality | ||||||
|
| ||||||
| mGFR | 1.09 (0.69–1.72) | 0.7 | -- | 2.05 (0.86–4.91) | 0.1 | -- |
| eGFRcr | 1.01 (0.67–1.53) | 0.9 | 0.7 | 3.74 (1.57–8.87) | 0.003 | 0.2 |
| eGFRcys | 1.07 (0.61–1.86) | 0.8 | 0.9 | 1.59 (0.58–4.34) | 0.4 | 0.7 |
| eGFRBTP | 1.50 (0.71–3.18) | 0.3 | 0.3 | 1.58 (0.55–4.56) | 0.4 | 0.7 |
| eGFRB2M | 0.90 (0.53–1.51) | 0.7 | 0.4 | 1.77 (0.68–4.65) | 0.3 | 0.8 |
| Average of 4 markersc | 1.02 (0.57–1.83) | 0.9 | 0.8 | 2.65 (0.84–8.33) | 0.1 | 0.7 |
IRR expressed per 30% decline in mGFR or eGFR calculated by modeling percent change in mGFR or eGFR below 0% (linear spline term with a knot at 0%); adjusted for age, sex, race, body mass index, systolic blood pressure, diabetes, total cholesterol, randomized treatment group, study group (for MDRD Study only), and first assessment of mGFR
P-value from seemingly unrelated regression comparing IRR for decline in the respective filtration marker vs. IRR for decline in mGFR
Average of 4 markers = (% Δ eGFRcr + % Δ eGFRcys + % Δ eGFRBTP + % Δ eGFRB2M)/4
AASK, African American Study of Kidney Disease and Hypertension; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IRR, incidence rate ratio; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based eGFR; eGFRcys, cystatin C–based eGFR; eGFRBTP, β-trace protein–based eGFR; eGFRB2M, β2-microglobulin-based eGFR
Decline in mGFR and eGFR From 12- to 24-Month Follow-up and Subsequent Mortality
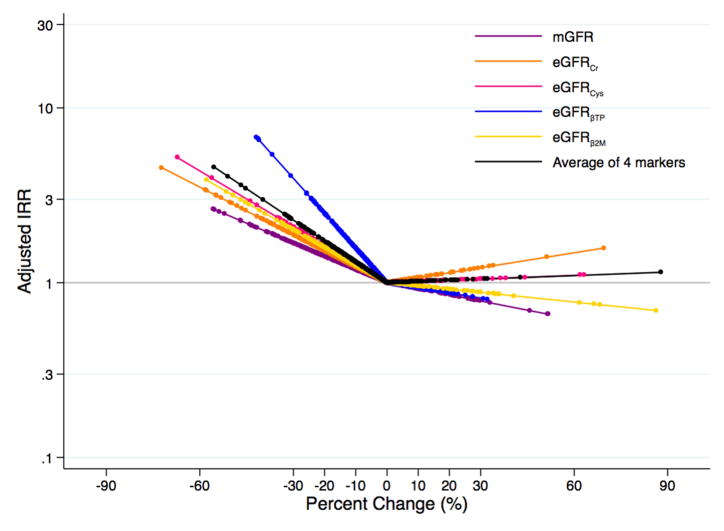
In the MDRD study and AASK, respectively, there were 140 and 48 deaths during a median follow-up of 15 (range, 0–16) and 7 (range, 0–10) years. Decline in mGFR was not significantly associated with risk of all-cause mortality in either study (IRRs per 30% decline were 1.09 [95% CI, 0.69–1.72; p=0.7] and 2.05 [95% CI, 0.86–4.91; p=0.1] for the MDRD Study and AASK, respectively; Table 2). Decline in eGFRcr was significantly associated with risk of all-cause mortality in AASK only (IRR per 30% decline, 4.17; 95% CI, 1.78–9.74; p<0.001; Table 2; Figure 4A; Figure 4B), but this association was not significantly different from mGFR (p=0.2). For the other filtration markers, eGFR decline was not associated with risk of all-cause mortality. Results were similar after adjusting for the first measurement of mGFR (Table 3) and after adjusting for covariates and GFR ascertained at the 24 month follow-up visit (Table S5).
Figure 4.
Adjusteda Risk of All-Cause Mortality According to Changeb in Measured and Estimated Glomerular Filtration Ratec (A) in the Modification of Diet in Renal Disease Study and (B) in the African American Study of Kidney Disease and Hypertension
aAdjusted for age, sex, race, body mass index, systolic blood pressure, diabetes, total cholesterol, randomized treatment group, study group (for MDRD Study only), and first measurement of mGFR or eGFR for the respective marker
bPercent change modeled as linear spline terms with one knot at 0%
cmGFR in purple; eGFRcr in orange; eGFRcys in pink; eGFRBTP in blue; eGFRB2M in yellow; average of four filtration markers in black
Increase in mGFR and eGFR From 12- to 24-Month Follow-up and Subsequent Outcomes
It appeared that the risk of ESRD was lower for higher percent change in eGFR (Figure 3A; Figure 3B). There did not seem to be an association with the risk of all-cause mortality with higher percent change in eGFR with the possible exception of eGFRcr (Figure 4A; Figure 4B). However, the only statistically significant finding with eGFR rise was observed for eGFRB2M and ESRD risk in the MDRD Study and AASK (IRRs per 30% increase were 0.63 [95% CI, 0.43–0.93; p=0.02] and 0.31 [95% CI, 0.10–0.94; p=0.04], respectively; Table 4). In unadjusted models, a rise in eGFRBTP and the average of 4 markers was significantly associated with a higher risk of all-cause mortality (Table S7). Using competing risk regression, the risk estimates for a rise in eGFR and ESRD were stronger and statistically significant for eGFRBTP, eGFRB2M, and the average of all four filtration markers in both studies as well as for eGFRcys in the MDRD Study (Table S6).
Table 4.
Risk of Incident ESRD and All-Cause Mortality Associated with a Rise in Measured and Estimated GFR Over a One-Year Period
| MDRD | AASK | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome and Filtration Marker | IRRa (95% CI) per 30% increase | P | P vs mGFRb | IRRa (95% CI) per 30% increase | P | P vs mGFRb |
| ESRD | ||||||
|
| ||||||
| mGFR | 0.65 (0.37–1.13) | 0.1 | -- | 0.46 (0.18–1.13) | 0.09 | -- |
| eGFRcr | 0.87 (0.53–1.43) | 0.6 | 0.3 | 0.47 (0.17–1.28) | 0.1 | 0.9 |
| eGFRcys | 0.72 (0.47–1.10) | 0.1 | 0.8 | 0.47 (0.17–1.28) | 0.1 | 0.9 |
| eGFRBTP | 0.46 (0.21–1.03) | 0.06 | 0.4 | 0.66 (0.40–1.08) | 0.1 | 0.4 |
| eGFRB2M | 0.63 (0.43–0.93) | 0.02 | 0.9 | 0.31 (0.10–0.94) | 0.04 | 0.5 |
| Average of 4 markersc | 0.54 (0.29–1.02) | 0.06 | 0.7 | 0.33 (0.09–1.23) | 0.1 | 0.6 |
|
| ||||||
| Mortality | ||||||
|
| ||||||
| mGFR | 1.30 (0.70–2.42) | 0.4 | -- | 0.65 (0.20–2.13) | 0.5 | -- |
| eGFRcr | 1.06 (0.68–1.65) | 0.8 | 0.4 | 1.74 (0.71–4.31) | 0.2 | 0.2 |
| eGFRcys | 1.28 (0.93–1.77) | 0.1 | 0.9 | 0.86 (0.27–2.72) | 0.8 | 0.8 |
| eGFRBTP | 1.87 (0.82–4.29) | 0.1 | 0.3 | ,0.95 (0.52–1.74) | 0.9 | 0.6 |
| eGFRB2M | 1.13 (0.79–1.61) | 0.5 | 0.6 | 0.84 (0.29–2.45) | 0.8 | 0.7 |
| Average of 4 markersc | 1.31 (0.82–2.12) | 0.3 | 0.9 | 1.14 (0.28–4.60) | 0.9 | 0.5 |
IRR expressed per 30% increase in mGFR or eGFR calculated by modeling percent change in mGFR or 0% (linear spline term with a knot at 0%); adjusted for age, sex, race, body mass index, systolic blood total cholesterol, randomized treatment group, study group (for MDRD Study only), and first assessment of eGFR for the respective marker
P-value from seemingly unrelated regression comparing IRR for rise in the respective filtration marker vs. mGFR
Average of 4 markers = (% Δ eGFRcr + % Δ eGFRcys + % Δ eGFRBTP + % Δ eGFRB2M)/4
AASK, African American Study of Kidney Disease and Hypertension; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IRR, incidence rate ratio; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based eGFR; eGFRcys, cystatin C–based eGFR; eGFRBTP, β-trace protein–based eGFR; eGFRB2M, β2-microglobulin-based eGFR
DISCUSSION
Decline in mGFR and eGFR, using serum concentrations of creatinine, BTP, and the average of four filtration markers, was associated with increased risk of developing ESRD during long-term follow-up in two clinical trials of kidney disease: the MDRD Study and AASK. These associations were independent of demographics, established risk factors, and first determination of GFR. Only decline in eGFRBTP was more strongly associated with ESRD than mGFR decline. Decline in eGFRcr in AASK only, but not mGFR, any of the other filtration markers, or the average of four filtration markers, was associated with all-cause mortality in fully-adjusted models, but was not more strongly associated with mortality than mGFR decline.
Several prior studies have examined the risk of ESRD and all-cause mortality in association with eGFRcr decline.9–13,28–31 The CKD Prognosis Consortium, a meta-analysis of 1.7 million study participants, 12,344 ESRD cases, and 223,944 deaths, reported that 30% eGFRcr decline over two years was associated with 5.4 (95% CI, 4.6–6.4) times higher risk of ESRD and 1.77 (95% CI, 1.65–1.89) times higher risk of all-cause mortality among those with baseline eGFR <60 mL/min/1.73 m2.9 These effect estimates, which included change in eGFRcr from the MDRD Study and AASK in addition to 33 other studies, were generally similar to or slightly stronger than our results. Given that the analytic sample sizes of the two clinical trials (MDRD Study and AASK) were relatively small compared to those of previously published studies, it is possible that the present study was not sufficiently statistically powered to detect the weaker associations between change in filtration markers and all-cause mortality. Another potential reason for the slightly different results is the duration of the change period (two years in the CKD Prognosis Consortium versus one year in the present study). Sustained decline over a longer period of time is more likely to be true change rather than transient change and it is less likely to be due to measurement error.
We observed that eGFRBTP decline was more strongly associated with ESRD risk than mGFR decline. To the best of our knowledge, no other studies have examined change in eGFRBTP. In our study, fewer participants had decline in eGFRBTP than decline in eGFRcr. Decline in eGFRBTP may represent unique aspects of ESRD risk, but the biological explanation for this finding is unclear. Since mGFR is thought to be unbiased, change in eGFRBTP may reflect some pathophysiologic pathway leading to ESRD that is distinct from GFR.
We also observed that an increase in mGFR and eGFR was not significantly associated with risk of ESRD or all-cause mortality, except for an increase in eGFRB2M, which was associated with a reduced risk of ESRD. Prior studies on eGFR increase have largely not detected significant associations with ESRD, similar to our findings. Some studies have reported significant associations between a rise in eGFR and mortality risk.10–14 In the CKD Prognosis Consortium, a eGFRcr slope of +6 mL/min/1.73 m2 per year was associated with 1.58 (95% CI, 1.29–1.95) times higher risk of all-cause mortality in a meta-analysis of CKD cohorts.14 We would expect an increase in mGFR to be associated with better health outcomes. Alternatively, a rise in mGFR might reflect glomerular hyperfiltration or recovery from an acute kidney injury episode, which may be associated with adverse health outcomes. A rise in eGFRcr could also be due to change in factors unrelated to mGFR such as malnutrition and muscle wasting.
Strengths of our study are the assessment of mGFR and multiple filtration markers, ascertainment of two major clinical outcomes, and inclusion of individuals with kidney disease from two clinical trials. The results of our study may not be broadly generalizable since the study participants who enrolled in these two clinical trials may differ from other individuals with CKD in the United States, and, given the smaller analytic sample in the present analysis, these results may not be representative of the overall MDRD Study and AASK populations. Inclusion in the present analysis on change in mGFR and eGFR was conditional on attending the study visits at the two time points (12 months and 24 months) and the availability of an adequate amount of stored serum for the measurement of filtration markers. Those who died or developed ESRD before the second time point for the calculation of change in filtration markers were not included in the analysis. The relatively small sample size was a study limitation, resulting in wide CIs around the effect estimates. There may not have been sufficient statistical power to detect significant differences across filtration markers with respect to the strength of their associations with ESRD despite wide variability in the risk estimates. Further research is needed in a larger study in order to more precisely estimate the association between change in GFR and outcomes. Standardization of assays for novel filtration markers will be necessary before assessment of GFR change can be translated from the research setting to clinical practice. Furthermore, since BTP is not currently available for clinical practice, its use outside the research setting is limited. Our study is an improvement upon prior studies which typically rely on a single measurement of kidney function for the estimation of risk of future clinical outcomes. Nonetheless, more repeated measurements would allow for a detailed characterization of GFR trajectory over time which may be warranted for future research. The one year time window for assessing eGFR change provides a range that is relevant for clinical practice since kidney disease patients are often monitored for kidney disease progression for a similar period. Another strength of our study is that the measurements were conducted altogether for all specimens collected at the two time points for both of the clinical trials, thereby eliminating the possibility of measurement error due to drift in the laboratory assays over time. There was limited variability detected using blind replicates (CV <11%).
In conclusion, declines in eGFRcr and decline in the average of the four filtration markers (creatinine, cystatin C, BTP, and B2M) were consistently associated with higher risk of incident ESRD relative to decline in mGFR, but only decline in eGFRBTP was significantly more strongly associated with ESRD risk than decline in mGFR. Measurement of BTP over time may offer additional information about future ESRD risk.
Supplementary Material
Supplementary Table S1 (PDF). Baseline characteristics of included and excluded participants in MDRD Study and AASK.
Supplementary Table S2 (PDF). Spearman correlation coefficients for percent change in mGFR and eGFR.
Supplementary Table S3 (PDF). Unadjusted risk of incident ESRD and all-cause mortality associated with eGFR decline over 1 y.
Supplementary Table S4 (PDF). Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for proteinuria.
Supplementary Table S5 (PDF). Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for covariates at 24-mo.
Supplementary Table S6 (PDF). Risk of incident ESRD associated with decline and rise in mGFR and eGFR over 1 y, accounting for competing risk of death.
Supplementary Table S7 (PDF). Unadjusted risk of incident ESRD and all-cause mortality associated with mGFR and eGFR increase over 1 y.
Acknowledgments
Support: Research was supported by the CKD Biomarkers Consortium funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; U01 DK085689). Dr Rebholz is supported by a grant from the NIDDK (K01 DK107782). Some of the data reported here have been supplied by the US Renal Data System. The funders had no role in the study designs; collection, analysis, and interpretation of the data; writing the report; and the decision to submit the report for publication.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: LAI, MCF, PLK, RSV, HIF, CYH, ASL, JC; data acquisition: JHE, MJS; statistical analysis: CMR, YC, ML; data interpretation: CMR, LAI, ASL, JC: CMR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by 2 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Footnotes
Table S1: Baseline characteristics of included and excluded participants in MDRD Study and AASK.
Table S2: Spearman correlation coefficients for percent change in mGFR and eGFR.
Table S3: Unadjusted risk of incident ESRD and all-cause mortality associated with eGFR decline over 1 y.
Table S4: Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for proteinuria.
Table S5: Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for covariates at 24 mo.
Table S6: Risk of incident ESRD associated with decline and rise in mGFR and eGFR over 1 y, accounting for competing risk of death.
Table S7: Unadjusted risk of incident ESRD and all-cause mortality associated with mGFR and eGFR increase over 1 y.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 7.Inker LA, Coresh J, Sang Y, et al. Filtration Markers as Predictors of ESRD and Mortality: Individual Participant Data Meta-Analysis. Clin J Am Soc Nephrol. 2017;12(1):69–78. doi: 10.2215/CJN.03660316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turin TC, Coresh J, Tonelli M, et al. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int. 2013;83(4):684–691. doi: 10.1038/ki.2012.443. [DOI] [PubMed] [Google Scholar]
- 12.Turin TC, Coresh J, Tonelli M, et al. One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol. 2012;36(1):41–49. doi: 10.1159/000339289. [DOI] [PubMed] [Google Scholar]
- 13.Perkins RM, Bucaloiu ID, Kirchner HL, Ashouian N, Hartle JE, Yahya T. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1879–1886. doi: 10.2215/CJN.00470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naimark DM, Grams ME, Matsushita K, et al. Past Decline Versus Current eGFR and Subsequent Mortality Risk. J Am Soc Nephrol. 2016;27(8):2456–2466. doi: 10.1681/ASN.2015060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck GJ, Berg RL, Coggins CH, et al. Design and statistical issues of the Modification of Diet in Renal Disease Trial. The Modification of Diet in Renal Disease Study Group. Control Clin Trials. 1991;12(5):566–586. doi: 10.1016/0197-2456(91)90069-x. [DOI] [PubMed] [Google Scholar]
- 16.Wright JT, Jr, Kusek JW, Toto RD, et al. Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Control Clin Trials. 1996;17(4 Suppl):3S–16S. doi: 10.1016/s0197-2456(96)00081-5. [DOI] [PubMed] [Google Scholar]
- 17.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(7 Suppl 2):S154–165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Greene T, Schluchter MD, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol. 1993;4(5):1159–1171. doi: 10.1681/asn.v451159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan PC, Kulasingam V, Lem-Ragosnig B. Validating urinary measurement of beta-2-microglobulin with a Roche reagent kit designed for serum measurements. Clin Biochem. 2012;45(16–17):1533–1535. doi: 10.1016/j.clinbiochem.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–48. doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87(5):1055–1060. doi: 10.1038/ki.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonberg-Yoo AK, Tighiouart H, Levey AS, Beck GJ, Sarnak MJ. Urine Potassium Excretion, Kidney Failure, and Mortality in CKD. Am J Kidney Dis. 2017;69(3):341–349. doi: 10.1053/j.ajkd.2016.03.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appel LJ, Middleton J, Miller ER, 3rd, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14(7 Suppl 2):S166–172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 26.Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. Journal of the American Statistical Association. 1962;57(298):348–368. [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 28.Turin TC, Coresh J, Tonelli M, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant. 2012;27(10):3835–3843. doi: 10.1093/ndt/gfs263. [DOI] [PubMed] [Google Scholar]
- 29.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebholz CM, Grams ME, Matsushita K, et al. Change in Multiple Filtration Markers and Subsequent Risk of Cardiovascular Disease and Mortality. Clin J Am Soc Nephrol. 2015;10(6):941–948. doi: 10.2215/CJN.10101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebholz CM, Grams ME, Matsushita K, Selvin E, Coresh J. Change in novel filtration markers and risk of ESRD. Am J Kidney Dis. 2015;66(1):47–54. doi: 10.1053/j.ajkd.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 (PDF). Baseline characteristics of included and excluded participants in MDRD Study and AASK.
Supplementary Table S2 (PDF). Spearman correlation coefficients for percent change in mGFR and eGFR.
Supplementary Table S3 (PDF). Unadjusted risk of incident ESRD and all-cause mortality associated with eGFR decline over 1 y.
Supplementary Table S4 (PDF). Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for proteinuria.
Supplementary Table S5 (PDF). Risk of incident ESRD and all-cause mortality associated with mGFR and eGFR decline over 1 y, adjusted for covariates at 24-mo.
Supplementary Table S6 (PDF). Risk of incident ESRD associated with decline and rise in mGFR and eGFR over 1 y, accounting for competing risk of death.
Supplementary Table S7 (PDF). Unadjusted risk of incident ESRD and all-cause mortality associated with mGFR and eGFR increase over 1 y.