Abstract
MiR-21 is a microRNA implicated in cancer, development, and cardiovascular diseases and expressed in the central nervous system (CNS), especially after injury. However, the cellular expression of miR-21 in the adult CNS has not been clearly established either in mice or human subjects, while its alteration in psychiatric disorders is unknown. MiR-21 expression was characterized in reporter mice expressing β–galactosidase (LacZ) under the endogenous miR-21 promoter (miR-21/LacZ). Brain co-localization of miR-21/LacZ with specific neural markers was examined by double immunofluorescence in reporter mice, while extent of immunostaining for myelin basic protein and PDGFRα was determined in miR-21 knockout and wild-type mice. Levels of miR-21, and mRNAs of selected miR-21 targets, miR-21 regulator STAT3 and myelin-related proteins were measured by qRT-PCR in the white matter (WM) adjacent to the left postmortem orbitofrontal cortex (OFC) of human subjects with major depressive disorder (MDD), alcoholism, comorbid MDD plus alcoholism (MDA) and non-psychiatric control subjects. MiR-21/LacZ was highly expressed in cell bodies of WM and myelinated portions of gray matter (GM). Labeled cell bodies were identified as oligodendrocytes, while miR-21/LacZ was barely detectable in other cell types. MiR-21, as well as the mRNAs of several myelin-related proteins, were reduced in the WM of subjects with MDD and alcoholism. MiR-21 positively correlated with mRNA of myelin-related proteins and astrocytic GFAP. High expression of miR-21 in adult oligodendrocytes and the correlation of miR-21 decrease with mRNA of some myelin proteins, regulator STAT3, and oligodendrocyte-related transcription factors suggest an involvement of miR-21 in WM alterations in depression and alcoholism.
Keywords: Glia, prefrontal, miR-21, astrocytes, mRNA, knockout, postmortem, human, mouse
Graphical Abstract
1. INTRODUCTION
MicroRNAs (miRNAs) are small non-coding RNAs, 22-nucleotides long, that are mostly generated from longer RNA precursors (Iwakawa & Tomari, 2015). However, miRNAs are capable of post-transcriptionally regulating the translation of target proteins by binding to specific complementary sequences in protein-encoding mRNAs (Bushati & Cohen, 2007; Valencia-Sanchez, Liu, Hannon, & Parker, 2006).
Some miRNAs are constitutively expressed in differentiated cells, including CNS cells (Krichevsky, King, Donahue, Khrapko, & Kosik, 2003; Nelson et al., 2006; Ziats & Rennert, 2014), but most vary their expression following cell injury, significant physiological alterations or during developmental transitions (Krichevsky et al., 2003; Liu & Xu, 2011). In the CNS, specific miRNAs fluctuate in neurons (Jovicic et al., 2013), astrocytes and oligodendrocytes (Lau et al., 2008) following injury or metabolic stress (Feng & Feng, 2011; Liu & Xu, 2011). More recently, miRNA alterations in particular brain regions have been associated with psychiatric disorders such as depression, schizophrenia (Dwivedi, 2014; Kocerha, Dwivedi, & Brennand, 2015; Smalheiser et al., 2014) or alcoholism (Rajesh C. Miranda, 2014; R. C. Miranda et al., 2010), suggesting pathophysiological involvement of miRNAs. MiRNAs in the GM of relevant brain regions such as the prefrontal cortex (PFC) (Serafini et al., 2014; Smalheiser et al., 2012; Smalheiser et al., 2014) and basal ganglia, or in the blood circulation have been linked to psychopathology or neurodegeneration (Dwivedi, 2016). Less is known, however, about the cellular localization of miRNAs in the WM and possible disturbances of WM miRNAs in psychiatric disorders.
Among pathologically relevant miRNAs, miR-21 has received considerable attention because miR-21 dysregulation is linked to inflammatory diseases (Lu & Rothenberg, 2013) and cancer, including gliomas (Krichevsky & Gabriely, 2009; Kumarswamy, Volkmann, & Thum, 2011). Its role in cancer appears to derive from its ability to interfere with translation of apoptosis-promoting proteins (Buscaglia & Li, 2011). In addition, miR-21 is differentially expressed at various stages during CNS development, where it regulates the expression of proteins involved in cell growth and survival (Sathyan, Golden, & Miranda, 2007). Altered miR-21 expression also occurs in some neurological disorders and their animal models (J. Peng et al., 2013; Yelamanchili, Chaudhuri, Chen, Xiong, & Fox, 2010). Likewise, prolonged alcohol exposure drastically reduces the levels of some miRNA species, including miR-21, in developing neural cells (Balaraman, Winzer-Serhan, & Miranda, 2012). By contrast, increased miR-21 expression may be neuroprotective in animal models of ischemia and traumatic brain injury (Buller et al., 2010; Ge et al., 2014). However, it remains to be established whether detectably high miR-21 expression is conspicuous in specific cell types of adult animals or is related to particular pathological or developmental stages (Kumarswamy et al., 2011).
Most miR-21 studies in the CNS have targeted GM or entire brain regions. However, despite mounting interest in WM glial pathology in psychiatric disorders (Tham, Woon, Sum, Lee, & Sim, 2011), little is known about the expression of miR-21 in these cells or whether miR-21 is changed in psychiatric disorders such depression and alcoholism, in which alterations in myelin proteins and in patterns of white matter-related connectivity have been identified (J.M. Lewohl et al., 2000; J. M. Lewohl, Wixey, Harper, & Dodd, 2005; H. J. Peng et al., 2013; Rajkowska et al., 2015; Rosenbloom, Sullivan, & Pfefferbaum, 2003). Here we describe the rich expression of miR-21 in oligodendrocytes of reporter mice that express the reporter gene β-galactosidase (LacZ) under the endogenous miR-21 promoter, and identify changes in oligodendrocyte markers in miR-21 knockout mice. In addition, we report miR-21 expression levels as well as mRNA levels for miR-21 targets, miR-21 regulator STAT3 and oligodendrocyte-related proteins in the white mater of human subjects with depression, alcoholism or comorbid depression plus alcoholism as compared to non-psychiatric controls.
2. METHODS
2.1 Animals
MiR-21 reporter mice (miR-21-lacZ) were generated by crossing Mir21atm1Mtm mice (Jackson Labs # 036060), a knock-in mouse strain that expresses the β-galactosidase (lacZ) reporter gene under the endogenous miR-21 promoter (Park et al., 2012) with CAG-Cre mouse (Sakai & Miyazaki, 1997) to remove the neomycin-miR-21-loop loxP-flanked cassette to avoid interference in the β-galactosidase expression. miR-21-lacZ mice were backcrossed for more than 10 generations into C57BL/6J genetic background. miR-21-lacZ were maintained as a heterozygous strain carrying one copy of WT miR-21 gene in the complementary chromosome. The generation of miR-21 knockout mice (miR21KO) has been reported (Lu et al., 2011). MiR21KO mice were backcrossed for more than 10 generations into C57BL/6N genetic background, and maintained heterozygous. Homozygous miR21KO and WT littermates were generated by breeding miR21KO heterozygous animals.
2.2 β-galactosidase histochemistry
Twelve-week old male miR-21-lacZ mice were anesthetized and intracardially perfused with saline (0.9% NaCl). Tissues were embedded in OCT and 8 μm sections processed for β-galactosidase histochemistry using standard techniques (Saunders, 2003).
2.3 Immunohistochemistry
Twelve-week old male mice were anesthetized and intracardially perfused with saline (0.9% NaCl) followed by freshly prepared 4% paraformaldehyde in PBS. Tissues were removed, postfixed with 4% paraformaldehyde, loaded with 30% sucrose in PBS by immersion, embedded in OCT and 10-μm thick sections were obtained and mounted on slides.
For LacZ immunodetection we used a primary antibody raised in chicken because distribution of its binding closely matched the X-gal signal for miR-21/LacZ, (see Supplemental materials for information on antibody choice). The cellular phenotype of cells positive for LacZ double-labeling experiments was determined by simultaneous incubation with the LacZ antibody and antibodies to either Neuron Nuclear antigen (NeuN), CNPase, Iba1 or GFAP (all antibodies raised in species different from chicken). After washing in Tris buffer saline (pH 7.6), species-specific fluorescent secondary antibodies were used to identify binding to LacZ and either of the other primary antibodies. Dual fluorescence was assessed at a Nikon NC1 laser-scanning confocal microscope.
Effects of miR-21 absence on PDGFRα and MBP immunohistochemistry were measured in four 12 weeks-old miR21KO and four wild type mice according to immunohistochemical and cell counting procedures described in the Supplemental Information.
2.4 Human subjects
Collection of human brain samples was performed according to protocols approved by the Institutional Review Boards of the University Hospitals of Cleveland and the University of Mississippi Medical Center. The protocol included obtaining written consent from next-of-kin for retrospective interviews to compile relevant diagnostic information according to procedures detailed in previous publications (Miguel-Hidalgo et al., 2014; Whittom et al., 2014). Blocks of postmortem tissue were collected during autopsy at the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio. Subjects with evidence of neurological injury or disorder, prolonged agonal states or coma were excluded from the study. Weight of the whole brain and postmortem delay (time from death to collection of brain sample) were determined. Subjects included in this study belonged into 4 diagnostic groups: Non-psychiatric comparison subjects (n=10), subjects with alcohol dependence (n=9), subjects with major depressive disorder (MDD, n=10) and subjects with dual diagnosis of MDD and alcohol dependence (MDA, n=10). Table 1 presents summaries of the age at time death, sex, brain pH, brain weight, postmortem delay and mode of death (suicide vs. non-suicide). Estimates of onset and duration of the psychiatric diagnoses are also shown in Table 1.
Table 1.
Characteristics of the human subjects included in this study
| CONT | ALC | MDD | MDA | |
|---|---|---|---|---|
| PMI (hours) | 23.30 ±1.72 | 21.08±4.40 | 24.02 ±1.87 | 26.42 ±2.62 |
| GENDER | 8M, 2F | 7M, 2F | 5M. 5F | 7M, 3F |
| brain pH | 6.54±0.08 | 6.63±0.05 | 6.54 ±0.07 | 6.62±0.08 |
| Age (years) | 51.10±4.03 | 47.33 ±3.02 | 45.80 ±2.63 | 50.40 ±4.14 |
| Brain weight (grams) | 1404.10±58.64 | 1378.44±56.50 | 1427.30±53.67 | 1359.50±47.20 |
| Suicide (S) | NA | 4S, 5Non-S | 8S, 2Non-S | 5S, 5Non-S |
| Depression onset (years) | NA | NA | 34.6 ±4.14 | 31.9 ±3.59 |
| Depression duration (years) | NA | NA | 10.76±3.86 | 12.08±3.56 |
| Alcoholism onset (years) | NA | 25.11±1.80 | NA | 23.7±3.42 |
| Alcoholism duration (years) | NA | 21.67±3.33 | NA | 21.18±3.66 |
2.5 Collection of brain tissue sections for miRNA and RNA determinations
Blocks containing the OFC and the adjacent WM were immediately frozen at autopsy and stored at −80 °C. Two pH values were obtained per brain using frozen cerebellar tissue.
Sections containing OFC WM were cut at a thickness of 50 μm, and 5 mm-diameter punches were collected from the sectioned WM adjacent to cortical area 47. This area was identified according with published morphological criteria (Uylings et al., 2010). Levels of miR-21 and mRNAs of interest were measured in brain tissue as detailed in the Supplemental Information.
2.6 In situ hybridization
Formalin-fixed paraffin embedded 5 μm thick tissue sections were dewaxed in xylenes, and rehydrated through a standard ethanol dilution series. Tissue sections were digested with 5 μg/mL proteinase K for 10 minutes at room temperature, and then loaded onto Ventana Discovery Ultra (Ventana Medical Systems) for in situ hybridization analysis. The tissue slides were hybridized with 5′- and 3′-double digoxygenin-labeled miRCURY LNA microRNA detection probes (Exiqon; miR-21-5p: cat # 619870-360; U6: cat # 699002-360; scramble control: cat # 699004-360) for 2 hours at 50 °C. Hybridized probes were detected with a polyclonal anti-DIG antibody and Alkaline Phosphatase-conjugated secondary antibody (Ventana Medical Systems) using NBT-BCIP as the substrate and counter stained with Nuclear Fast Red.
2.7 Statistical Analysis
The density of immunoreactive cells (cells/mm3) or area fraction of immunoreactivity was statistically compared between wild type and KO mice by using two-tailed t-tests with significance evaluated at p<0.05.
The effect of psychiatric diagnosis on miR-21 and different mRNAs was evaluated in postmortem samples of human subjects using analysis of covariance (ANCOVA) with age at the time of death, postmortem interval (time between death and freezing of brain samples), tissue pH, and brain weight at autopsy as covariates. Among the covariates examined, the mean age appeared to differ the most between controls and MDD subjects. Hence, ANOVA was used to evaluate age between the 4 groups, and for only controls and MDD subjects. There was no significant difference in age among the four groups (F(3, 34)= 0.476, p=0.701) or between controls and MDD subjects alone (F(1,18)=1.123, p= 0.285). The co-variates considered in this study were included because they have been found to variably influence molecular parameters in previous studies of the human brain or WM. When ANCOVA showed significant diagnosis effect (p<0.05) pairwise comparisons to controls were made using Dunnett’s tests.
Pearson analysis of correlations between miR-21 levels and the levels of each of the mRNA species included in this study were Bonferroni-corrected by the number of comparisons (16 comparisons).
3. RESULTS
3.1 EXPRESSION of miR-21/LacZ IN REPORTER MICE
In miR-21 reporter mice expressing LacZ under the control of the endogenous miR-21 promoter (miR-21/LacZ mice) there was abundant detection of miR-21/X-gal signal in the WM and in cell bodies of myelinated parts of the GM all across the brain (Fig. 1). Closer inspection revealed that the strongest intensity of miR-21/LacZ expression corresponded to LacZ-positive cell bodies occurring all throughout the WM and myelinated parts of the GM (Fig. 2). To further confirm that the histochemical reaction catalyzed by X-gal activity corresponded to the localization of miR-21/LacZ we subjected coronal histological sections at several rostrocaudal levels to immunohistochemistry of LacZ using three different antibodies (see Methods section), We based our study on the polyclonal antibody raised in chicken against LacZ, which resulted in a distribution of immunostaining virtually identical to X-gal histochemistry staining in both white and gray matter (Figs. 3–5).
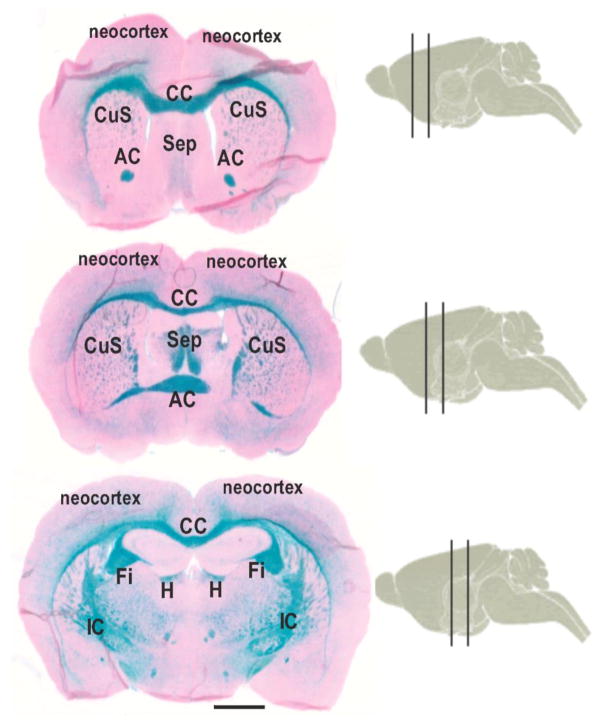
Fig. 1.
X-Gal histochemistry in coronal sections of a miR-21/LacZ reporter mice at three different successive rostro caudal levels each level falling between two vertical lines drawn on sagittal cartoons of the mice brain. Note the abundant X-gal labeling (blue) in the major WM bundles of the mouse brain. CC: Corpus Callosum, AC: Anterior Commissure, OT: Olfactory Tract, CuS: Corpus Striatum, Sep: Septum, IC: Internal capsule.
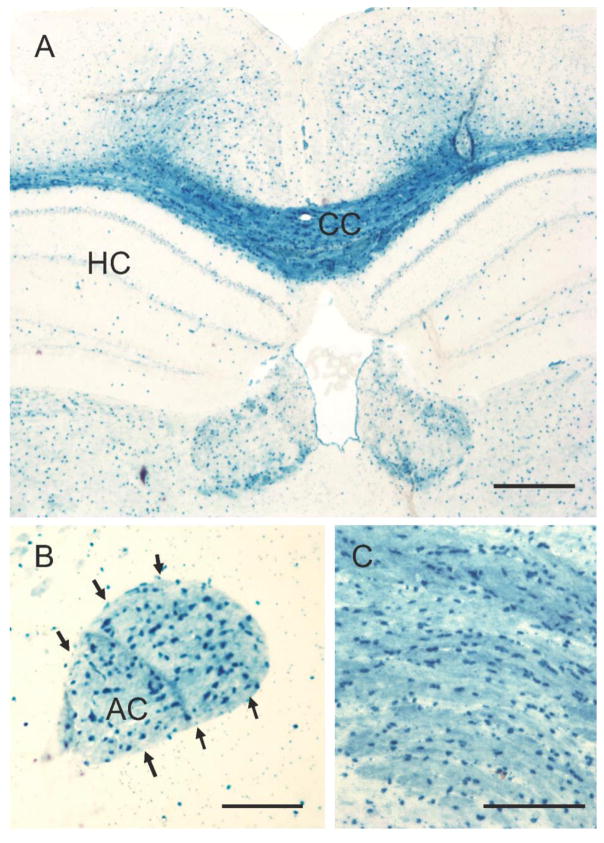
Fig. 2.
High power micrographs of white and GM regions in reporter mice with X-Gal labeling. Note that the corpus callosum (CC in panel A and all panel C), the anterior commissure and some GM in deep cortical layers contain cells intensely labeled with X-Gal reaction. Calibration: 300 μm in A, 100 μm in B and C.
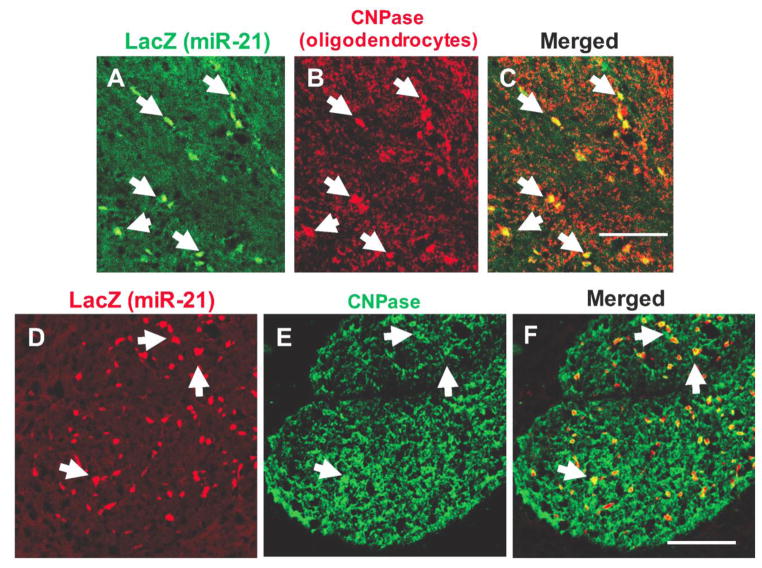
Fig. 3.
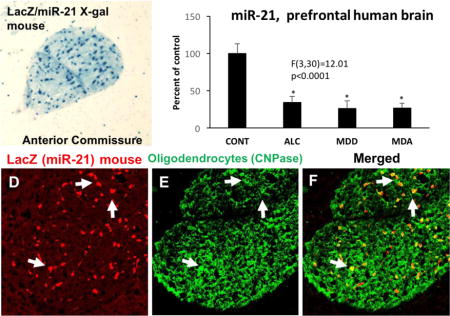
Immunofluorescence labeling of miR-21/LacZ showing co-localization with CNPase immunofluorescence in individual cells of the corpus callosum (A to C) and anterior commissure (D to F). Dual-labeled cells are denoted with arrows in the three panels and appear as yellow structures in panels C (merged picture of A and B) and F (merged picture of D and E). Calibration bars: 100 μm.
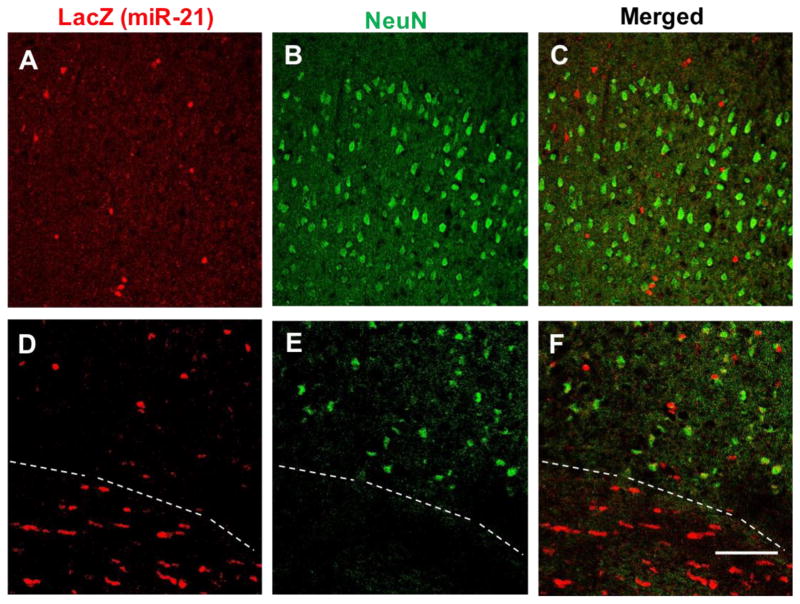
Fig. 5.
Micrographs of dual-labeling immunofluorescence detection of miR-21/LacZ (red) and neuronal marker NeuN (green) in the outer half of the cortical GM of the mouse somatosensory neocortex (A to C) and around the boundary of GM–WM (D to F; GM on top of broken line, WM at the bottom). Calibration bar: 100 μm.
3.2 CELL TYPE IDENTIFICATION OF miR-21/LacZ POSITIVE CELL BODIES
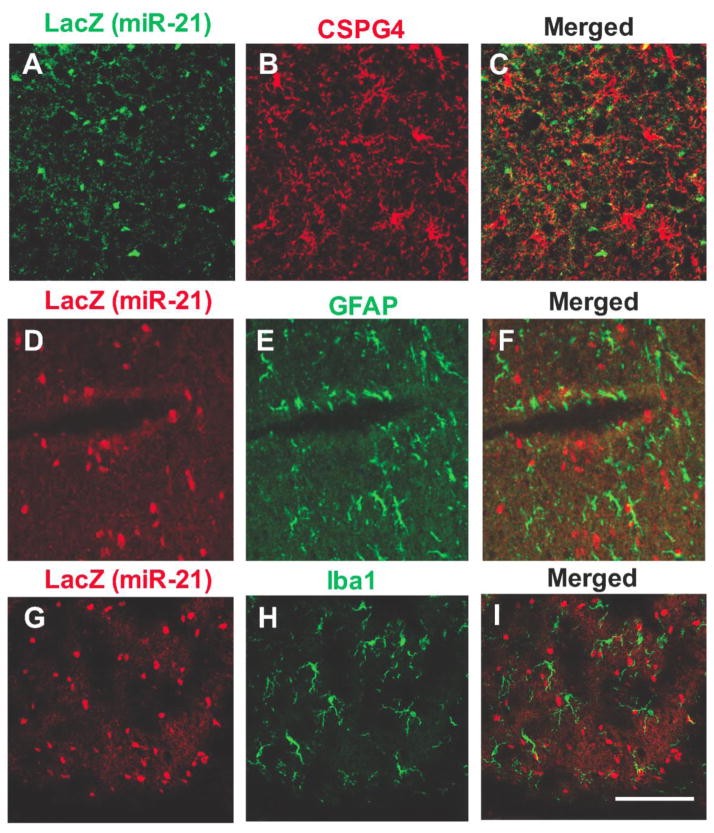
The size and distribution of LacZ positive cell bodies in white and GM suggested that most of them corresponded to oligodendrocytes. To determine whether these cell bodies could also be labeled with antibodies to oligodendrocyte markers, alternating coronal sections at various rostrocaudal levels of the reporter mouse brain were labeled with primary antibodies to LacZ in combination with either antibodies to CNPase (marker of oligodendrocytes)(Fig. 3), CSPG4 (marker of NG2 cells, oligodendrocyte precursors) (Fig. 4A,B,C), GFAP (marker of astrocytes) (Fig. 4C,D,E), Iba1 (marker of microglia) (Fig. 4G,H,I) or NeuN (general marker for neurons) (Fig. 5). These double labeling experiments showed that miR-21/LacZ co-localized with CNPase in the great majority of CNPase positive cell bodies (Fig. 3), while most cells bodies identified with the primary antibodies to the other glial or neuronal markers were mostly devoid of miR-21/LacZ immunoreactivity. (Figs. 4 and 5). The presence of conspicuous miR-21/LacZ immunoreactivity in oligodendrocyte cell bodies was further ascertained with double-fluorescent immunolabeling using the antibody to LacZ in combination with an antibody to glutathione S-transaminase Pi, which is also known for labeling the cytoplasm of mature and early oligodendrocytes with a high degree of specificity (Tamura et al., 2007; Tansey & Cammer, 1991). Examination of sections containing the corpus callosum, anterior commissure or myelinated portions of the medial GM showed that many GSTPi cells were immunopositive for miR-21/LacZ (not shown).
Fig. 4.
Micrographs of double-labeling immunofluorescence detection of miR-21/LacZ and either of the three glial markers CSPG4 for NG2 cells (A, B, C), GFAP for astrocytes (D, E, F) and Iba1 for microglia (H, I, J). Calibration bar: 100 μm.
3.4 MiR-21 IN SITU HYBRIDIZATION IN MOUSE AND HUMAN BRAIN
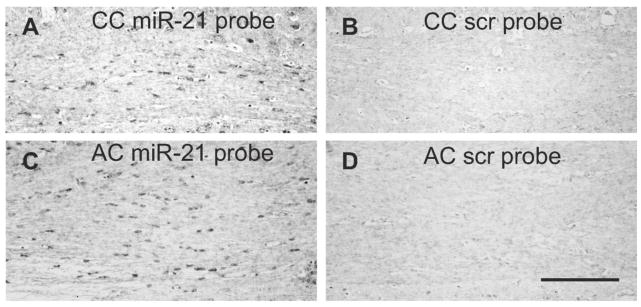
In the brain of wild-type mice, in situ hybridization of miR-21 resulted in the labeling of small size cell bodies in the WM that had morphological features of oligodendrocytes, as exemplified in the corpus callosum and anterior commissure (Fig. 6).
Fig. 6.
In situ hybridization signal for the miR-21-specific probe (A, C) or a scrambled probe (B, D) in coronal sections through the corpus callosum (CC) and the anterior commissure (AC). Calibration: 100 μm.
Using the same protocol as in the mouse brain, histological sections containing WM adjacent to the OFC of a 61 years-old male human subject were subjected to miR-21 in situ hybridization. As compared to hybridization with scrambled RNA probes, sections hybridized with miR-21-complementary probes demonstrated hybridization in relatively small cells in both white and GM as well as at the WM/GM boundary (Supplemental Figure 1 in Supplemental information file). In the human brain, cell bodies of labeled cells were mostly oval or rounded in shape and smaller than 10 μm in diameter, with morphologies consistent with those of oligodendrocytes or astrocytes, although, given that no cell type-specific markers were used, labeling of other cell types such as small-sized neurons cannot be entirely ruled out.
3.5 IMMUNOHISTOCHEMISTRY OF OLIGODENDROCYTE-RELATED PROTEINS IN miR-21 KO MICE
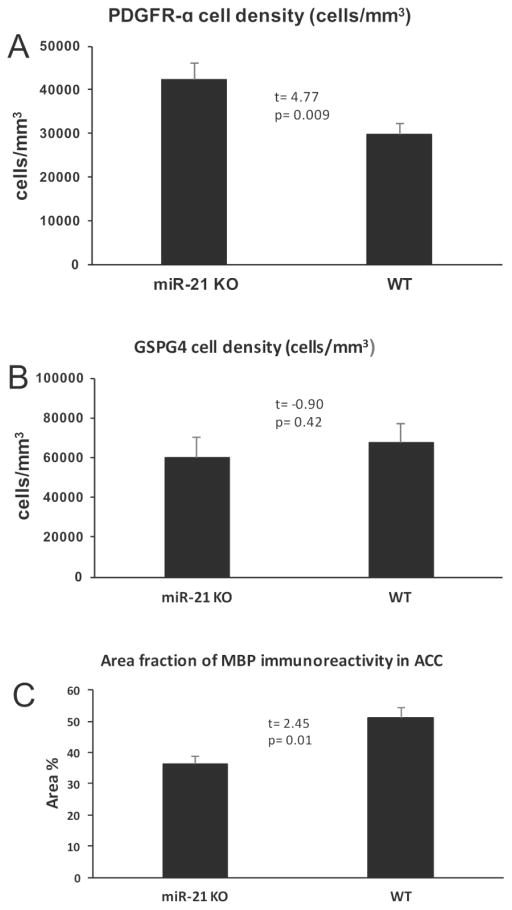
To determine whether miR-21 absence results in changes of myelinated fiber tracts we compared wild-type mice to mice with a full knock out of miR-21 (KO mice) using antibodies to platelet derived growth factor receptor alpha (PDGFRα, a marker of premyelinating oligodendrocytes) (Supplemental Figure 2), chondroitin sulfate proteoglycan 4 (CSPG4, a marker of oligodendrocyte precursor cells and premyelinating oligodendrocytes), and antibodies to myelin basic protein (MBP, the major myelin component of mature oligodendrocytes) (Supplemental figure 3). In sections of the corpus callosum and anterior cingulate cortex, the packing density of PDGFRα immunoreactive cells was significantly higher in miR-21 KO mice as compared to wild type mice (t=4.77, df=6, p=0.009) (Fig. 7A), but density of CSPG4-positive cells in KO mice was not significantly different from controls (t=−0.90, df=6, p=0.42) (Fig. 7B). In contrast, area fraction of MBP immunoreactive fibers localized to the anterior cingulate gyrus (where, unlike in the corpus callosum, fiber bundles can be resolved against a non-immunoreactive background) was significantly lower in KO as compared to wild type mice (t=2.45, df=6, p=0.01) (Fig. 7C).
Fig. 7.
Quantification of the packing density of oligodendrocyte precursor cells using antibodies to markers PDGFRα (A) and CSPG4 (B) in the corpus callosum as well as of the area fraction of immunoreactivity of myelin marker MBP (C) in the anterior cingulate cortex in miR-21 KO and wild type (WT) mice.
3.6 DOUBLE-LABELING FOR miR-21/LacZ and PROTEIN TMEM49 (VMP1)
The 22-nt long miR-21 is generated from a longer pri-miR-21 sequence regulated by its own promoter. Pri-miR-21 DNA sequence and its promoter overlap with the TMEM49 sequence (also known as VMP1) both in the human and mice, with the promoter sequences for pri-miR-21 being located in intron 10 of the same protein (Cai, Hagedorn, & Cullen, 2004; Kumarswamy et al., 2011). We found very scarce to no evidence for co-localization of miR-21/LacZ with TMEM49 (see Supplemental information file).
3.7 miR-21 LEVEL IN THE OFC WM OF HUMAN NON-PSYCHIATRIC CONTROL SUBJECTS AND SUBJECTS WITH MAJOR DEPRESSION OR ALCOHOLISM
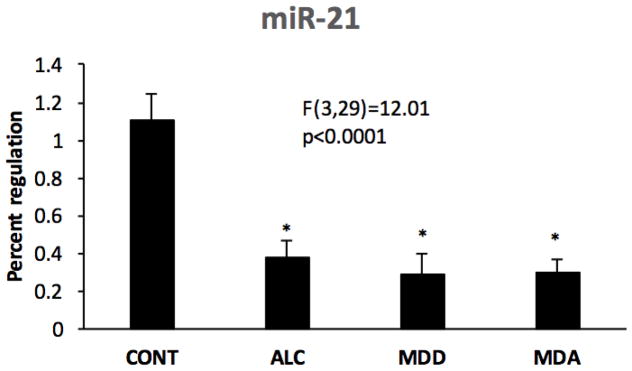
ANCOVA of RT-qPCR data revealed a significant effect of psychiatric diagnosis on miR-21 levels (Fig. 8) among human subjects with diagnoses of MDD, alcoholism (ALC), comorbid alcoholism plus MDD (MDA), and non-psychiatric comparison subjects (F(3,29)=12.01, p<0.0001). Pairwise contrasts revealed that miR-21 was significantly lower in each of the psychiatric groups as compared to controls (ALC vs control: d= 0.723, p<0.001; MDD vs control: d= −0.816, p<0.001; MDA vs. control: d=-0.807, p<001).
Fig. 8.
miR-21 levels in human postmortem WM adjacent to the OFC in non-psychiatric controls (CONT) as compared to subjects diagnosed with alcohol dependence (ALC), major depressive disorder (MDD) or comorbid major depression and alcoholism (MDA). Asterisks indicate significant differences as compared to the control groups (ALC vs control: d= −0.723, p<0.001; MDD vs control: d= −0.816, p<0.001; MDA vs. control: d= −0.807, p<001).
3.8 mRNA LEVELS OF miR-21 TARGETS, STAT3 AND MYELIN-RELATED PROTEINS IN THE OFC WM IN DEPRESSION AND ALCOHOLISM
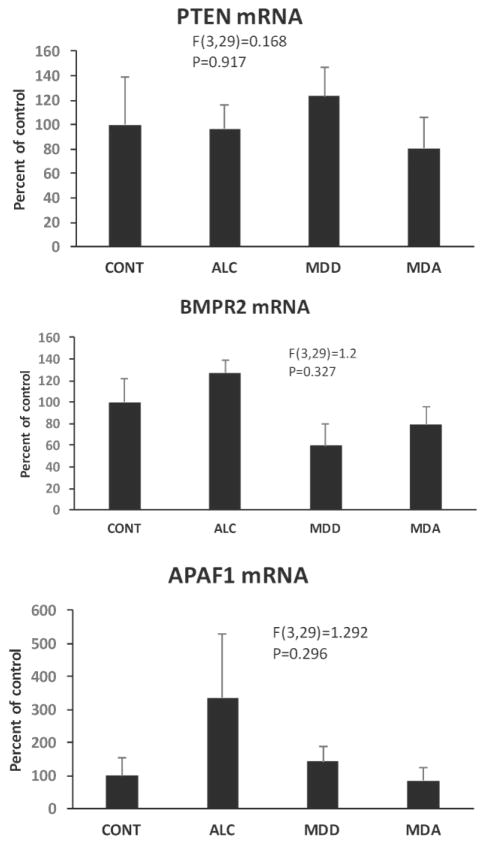
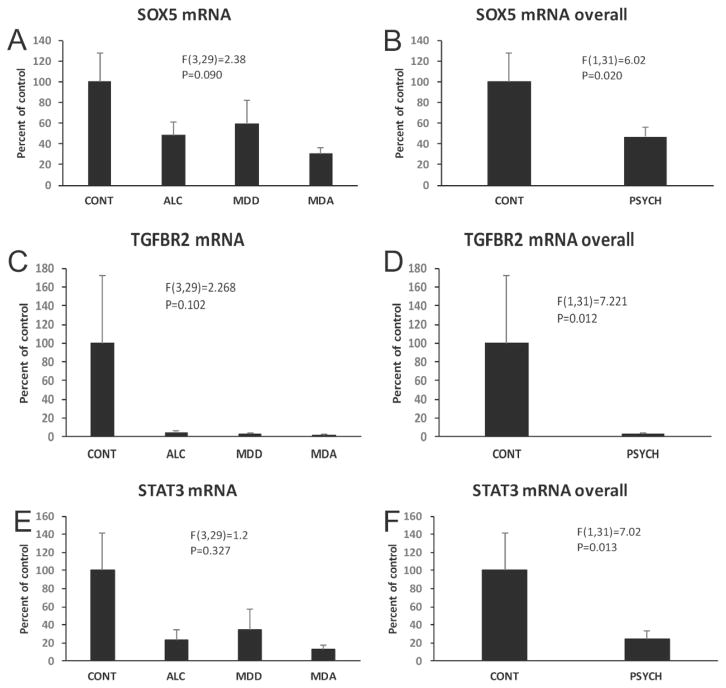
Among validated miR-21 targets, PTEN, BMFR, and APAF1 did not differ between control and diseased subjects (F(3,29)=0.168, p=0.971; F(3,29)=1.3, p=0.327; F(3,29)=1.292, p=0.296, respectively) (Fig. 9). MiR-21 levels were inversely correlated with levels of either APAF1 mRNA or PTEN mRNA when including all subjects (Table 2). Levels of mRNA of miR-21 targets SOX5 and TGFβR2 were reduced in the overall population of psychiatrically-diagnosed subjects (F(1,31)=6.02, p=0.02; F(1,31)=7.221, p=0.012, respectively) as compared to controls (Fig. 10A–D).
Fig. 9.
Relative levels of mRNA for miR-21 targets PTEN (top), BMPR2 (middle), and APAF1 in CONT, ALC, MDD and MDA subjects. Diagnostic group abbreviations as in Fig. 8.
Table 2.
| Correlations of miR-21 with mRNA levels of target and other relevant proteins
|
||||||
|---|---|---|---|---|---|---|
| ALL SUBJECTS IN THE STUDY
|
SUBJECTS WITH PSYCHIATRIC DIAGNOSES
|
|||||
| Corr. Coef. | p | p (Bonf. adj.) | Corr. Coef. | p | p (Bonf. adj.) | |
| PLP1 | 0.678 | 0.0001 | <0.005a | 0.638 | 0.0001 | <0.005 |
|
| ||||||
| MBP | 0.071 | 0.679 | 1 | 0.329 | 0.094 | 1 |
|
| ||||||
| MAG | 0.563 | 0.0001 | <0.05 | 0.382 | 0.045 | 0.72 |
|
| ||||||
| MOG | 0.516 | 0.001 | <0.05 | 0.505 | 0.006 | 0.096 |
|
| ||||||
| OLIG1 | 0.636 | 0.0001 | <0.005 a | 0.456 | 0.015 | 0.24 |
|
| ||||||
| OLIG2 | 0.315 | 0.074 | 1 | 0.128 | 0.532 | 1 |
|
| ||||||
| CSPG4 | 0.562 | 0.0001 | <0.005 | 0.648 | 0.0001 | <0.005 |
|
| ||||||
| GJC2 | 0.535 | 0.001 | <0.05 | 0.23 | 0.238 | 1 |
|
| ||||||
| GFAP | 0.586 | 0.0001 | <0.005 | 0.163 | 0.408 | 1 |
|
| ||||||
| GJA1 | −0.029 | 0.871 | 1 | 0.376 | 0.058 | 0.928 |
|
| ||||||
| STAT3 | 0.612 | 0.0001 | <0.005 | 0.21 | 0.292 | 1 |
|
| ||||||
| BMPR2 | −0.27 | 0.106 | 1 | −0.329 | 0.087 | 1 |
|
| ||||||
| SOX5 | 0.689 | 0.0001 | <0.005 | 0.704 | 0.0001 | <0.005 |
|
| ||||||
| APAF1 | −0.07 | 0.679 | 1 | 0.122 | 0.535 | 1 |
|
| ||||||
| TGFBR | 0.322 | 0.055 | 0.88 | −0.026 | 0.896 | 1 |
|
| ||||||
| PTEN | −0.236 | 0.159 | 1 | −0.523 | 0.004 | 0.064 |
Corr. Coef: Correlation coefficient; Bonf. Adj: After Bonferroni adjustment
Fig. 10.
Relative levels of mRNA for miR-21 targets SOX5 (top) and TGFBR2 (middle), as well as for major regulator of miR-21expression STAT3 (bottom) in CONT, ALC, MDD and MDA subjects. Graphs on the right compare levels of the three mRNAs in controls subjects to the overall group of psychiatric subjects. Diagnostic group abbreviations as in Fig. 8.
We also determined STAT3 mRNA levels in WM because STAT3 is a well-known upstream regulator of miR-21 (Ou, Li, & Kang, 2014). STAT3 was considerably decreased in psychiatric patients when all diseased subjects were combined as compared to non-psychiatric subjects (F(1,31)=7.02, p=0.013) (Fig. 10E, F). A positive correlation existed between STAT3 mRNA and miR-21 when including either all subjects or only subjects with psychiatric diagnoses (Table 2).
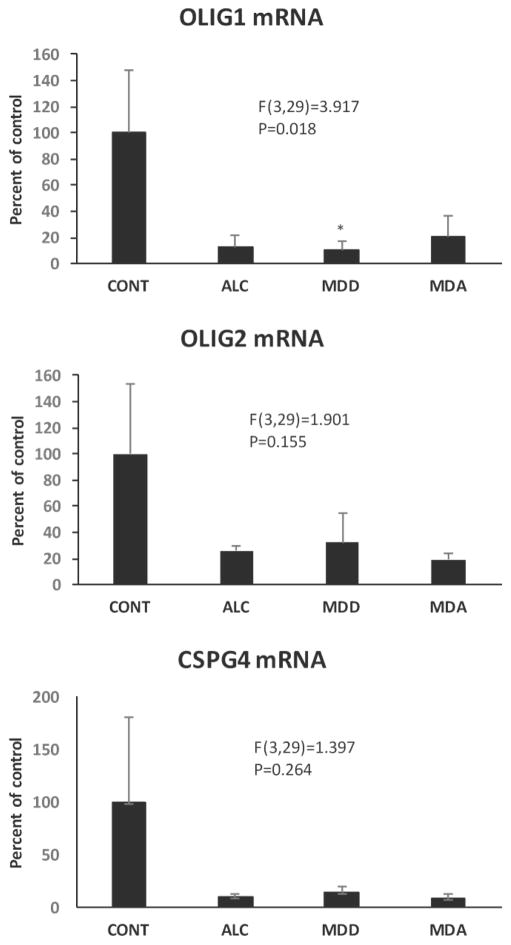
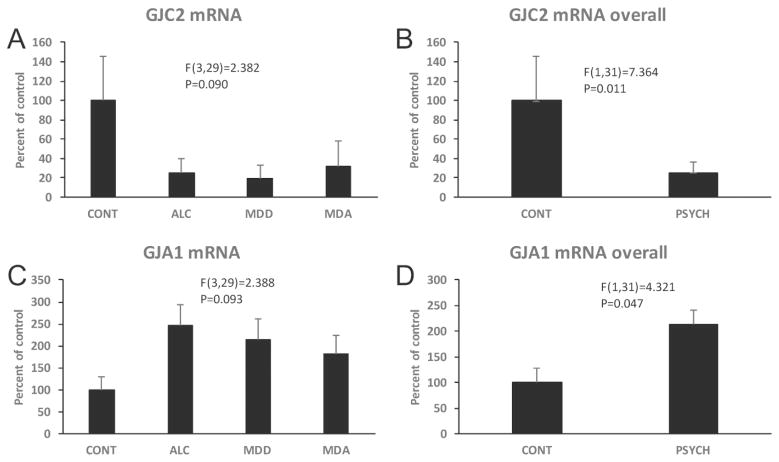
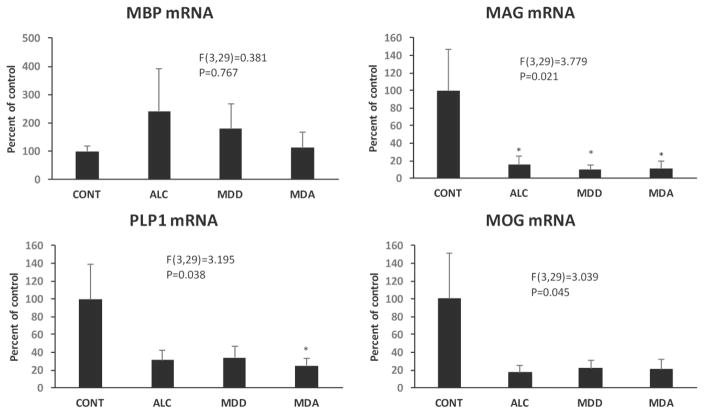
mRNA of MBP, PLP1, MAG, MOG, GJC2 (connexin 47), OLIG1, OLIG2 and CSPG4 (NG2), all proteins of either oligodendrocytes or their precursors, were also quantified in the OFC WM. ANCOVA showed that there was no significant effect of diagnosis on MBP, CSPG4 and OLIG 2 mRNA levels, but significant reduction of PLP1, MAG, MOG, and OLIG1 mRNA (PLP1 F(3,29)=3.195, p=0.038; MAG F(3,29)=3.779, p=0.021; MOG F(3,29)=3.039, p=0.045; OLIG1 i F(3,29)=3.917, p=0.018) in the psychiatric groups (Figs. 10–12). Comparisons to controls (Dunnett’s tests) showed significantly lower level of PLP1 mRNA in MDA subjects, and lower MAG mRNA in each of the psychiatrically diagnosed groups. GJC2 mRNA levels were lower in the overall group of psychiatric subjects (F(1,31)=7.364, p=0.011) (Fig. 13A, B).
Fig. 12.
Levels mRNA for oligodendrocyte-related transcription factors OLIG1 and OLIG2 as well as for CSPG4 in the four diagnostic groups. Diagnostic group abbreviations as in Fig. 8.
Fig. 13.
Levels of mRNAs for gap junction proteins GJC2 (A, B) and GJA1 (C.D) in the WM of that four diagnostic groups. Graphs on the right compare levels of the three mRNAs in controls subjects to the overall group of psychiatric subjects. Diagnostic group abbreviations as in Fig. 8.
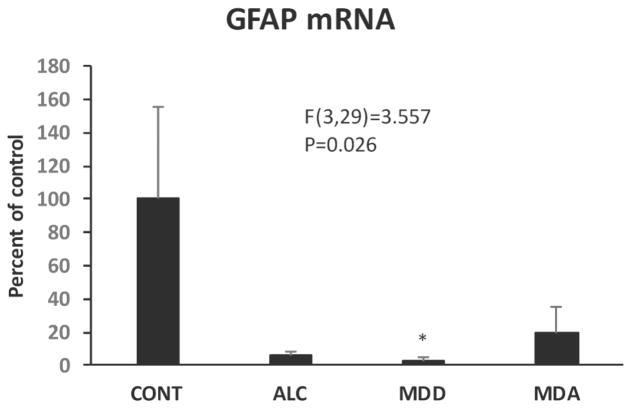
Since astrocytes are abundant in the WM and have important roles in myelin maintenance, mRNA levels of astrocyte-related GJA1 (also known as connexin-43, which pairs GJC2 to form heterologous gap junctions with oligodendrocytes) and glial fibrillary acidic protein (GFAP) were also determined. ANCOVA revealed that the combined psychiatric subjects had significantly higher GJA1 mRNA than control subjects (F(1,31)=4.321, p=0.047) (Fig. 13C, D). GFAP mRNA was significantly different among the 4 diagnostic groups (F(3,29)=3.557, p=0.026) (Fig. 14), and Dunnett’s tests demonstrated lower GFAP mRNA in MDD subjects as compared to controls (Fig. 14).
Fig. 14.
GFAP mRNA levels in the human postmortem WM adjacent to the OFC in the four diagnostic groups. Diagnostic group abbreviations as in Fig. 8.
miR-21 mRNA levels in all subjects were positively and significantly correlated with GFAP mRNA but not with GJA1 mRNA levels (Table 2).
4. DISCUSSION
4.1 miR-21 expression in oligodendrocytes
Several microRNAs play crucial roles during the development of oligodendrocytes as well as in the formation of myelin (Fitzpatrick, Anderson, & McDermott, 2015; Jovicic et al., 2013). Dicer deletion causes oligodendrocyte damage and disturbs myelin formation (Shin, Shin, McManus, Ptacek, & Fu, 2009). MiRNAs miR-335 and miR-9 are important for the differentiation of glial progenitors into oligodendrocyte precursors, immature and myelinating oligodendrocytes (Lau et al., 2008). However, less is known about miRNAs expressed in differentiated oligodendrocytes. The present data suggest that there are high miR-21 expression levels in the WM and myelinated portions of GM of the mouse CNS. Likewise, double immunofluorescence indicates that miR-21 is expressed in mature oligodendrocytes, but much less abundantly in other cell types. Nevertheless, it cannot be ruled out that smaller amounts of miR-21 are also present in other cell types, because miR-21 has been shown to be significantly induced in astrocytes, microglia or some neurons after specific treatments in cell cultures or in models of brain injury and neurodegeneration (Buller et al., 2010; Ge et al., 2014; Montalban et al., 2014; J. Peng et al., 2013; Sahni et al., 2010; Yelamanchili et al., 2010).
Consistent with the observations of high miR-21 expression in oligodendrocytes is that cell bodies with oligodendrocyte morphology were patent in the WM after miR-21 in situ hybridization in mice and, although sparser, in a non-psychiatric human subject. Thus, possible constitutive expression of miR-21 in oligodendrocytes of adult rodents and humans may lead to a degree of enduring regulation of miR-21 targets at the translational level. However, it is difficult to determine the precise impact of such a regulation in the expression of miR-21 target proteins without further investigating the concurrent activity of other factors that control the transcription of specific mRNAs and their translation into target proteins.
MiR-21 expression during development, in cancerous cells, or after brain injury has been linked to increases in cell survival or resilience (Buller et al., 2010; Buscaglia & Li, 2011; Ge et al., 2014; Ou et al., 2014). Since abundant miR-21 expression in adult oligodendrocytes apparently occurs in the absence of developmental or pathogenic challenges, there is the possibility that the normal function of oligodendrocytes or the interactions of these with their environment require continuous activation of survival-related mechanisms involving miR-21. However, as shown in miR21KO mice, mere absence of miR-21 might be insufficient to dramatically affect development, myelin formation and general behavior of mice. The possibility still remains that under specific challenges constitutive miR-21 may buffer the effects of deleterious alterations of impulse conduction caused by oligodendrocyte pathology. In the same vein, it is possible that putative behavioral alterations caused by miR-21 reduction are only detectable after specific physiological challenges or exposure to stressing circumstances. Thus, it remains to be ascertained whether miR-21 depletion alters behavior or whether challenging cognitive or behavioral conditions in the absence of oligodendrocyte miR-21 may lead to significant cognitive or behavioral abnormalities. Studies using such challenges will be necessary to determine the role of elevated miR-21 expression in CNS oligodendrocytes.
4.2 Downregulation of miR-21 and myelin-related proteins in the human OFC WM in depression and alcoholism
Low miR-21 in the human prefrontal WM appears to be associated with the diagnoses of depression and alcoholism. Low miR-21 in these disorders would be consistent with miR-21 involvement in mechanisms of myelin dysregulation, and possibly with connectivity alterations such as those observed in functional and DTI neuroimaging studies of both depression (Fox, Buckner, White, Greicius, & Pascual-Leone, 2012; Korgaonkar, Cooper, Williams, & Grieve, 2012; H. J. Peng et al., 2013; Tham et al., 2011; Zeng et al., 2012; Zhu et al., 2012) and alcoholism (McQueeny et al., 2009; Rosenbloom et al., 2003; Schulte, Muller-Oehring, Pfefferbaum, & Sullivan, 2010). Consistent with this suggestion, several myelin-related mRNAs were also reduced in the WM of psychiatric groups, while significant positive correlations of miR-21 levels and levels of oligodendrocyte proteins such as the major myelin structural protein PLP1, were observed even when the correlation only included psychiatric subjects. Low PLP1 was also found in a different cohort of MDD and control subjects (Rajkowska et al., 2015), indicating that PLP1 mRNA dysregulation in the OFC WM may be a feature of WM pathology in MDD and possibly in alcoholism.
The reduced area fraction of MBP-immunoreactive fibers in the anterior cingulate cortex and the increased density of PDGFRα-positive cells in the corpus callosum of the miR21 KO mice are consistent with an involvement of miR-21 in myelin disturbances. Interestingly, the level of MBP mRNA appears to be unchanged in the OFC WM of human diseased subjects as compared to controls in this study, which agrees with unchanged MBP mRNA found also in the OFC WM of a different cohort MDD subjects (58). Since the levels of PLP1, but not of MBP, were found to be significantly and strongly correlated with miR-21 levels in the human OFC WM, it is possible that miR-21 is associated with downregulation of PLP1 and not MBP. In miR-21 KO mice, we used MBP as a marker for myelin, finding that miR-21 knockout resulted in a reduction in the area fraction of MBP-immunoreactivity. It remains to be determined how the structural alteration of MBP-positive fibers may relate to changes in the levels of MBP mRNA and protein or to PLP1 changes in miR21 KO mice and the human prefrontal WM and how miR-21 could be related to the regulation of those two main myelin proteins.
Low mRNA levels of miR-21 targets SOX-5 and TGFBR2 in the aggregated psychiatric subjects may indicate disrupted regulation of myelin formation (Palazuelos, Klingener, & Aguirre, 2014; Wegner, 2008), particularly on the case of Sox5. Sox5 together with other Sox-family factors regulates oligodendrocyte differentiation (Wegner, 2008). In this process, the expression of PDGFRα in oligodendrocyte precursors and of MBP and PLP1 in differentiated astrocytes is affected by SOX5, SOX 6 and SOX10 (Stolt et al., 2006). SOX5 and SOX6 activity results in delayed MBP and PLP1 expression and the persistence of oligodendrocyte precursors as undifferentiated, while absence of those factors rather favors premature oligodendrocyte differentiation. It remains to be determined whether a putative SOX5 decrease in depression and alcoholism affects oligodendrocyte differentiation, and how the low SOX5 level may interact with low transcription factor OLIG1 (important positive factor in oligodendrocyte differentiation)(Snaidero & Simons, 2017), and with low miR-21 to result in oligodendrocyte pathology.
miRNAs other than miR21 may also regulate oligodendrocyte markers and transcription factors and could additively or synergistically act with miR-21 in the regulation of oligodendrocyte pathology. For example, miR-219 and miR-338 suppress the expression of PDGFRα and SOX5 (Simons & Nave, 2015), which is decreased in the human white matter (mRNA), and white matter of the miR-21 KO mice (protein), respectively. Since SOX5 is also a target of miR-21 and the knock out of miR-21 alters PDGFRα positive cell numbers, it will important to determine the relative contribution of these other miRNAs and miR-21 in the regulation of myelin-related changes in psychiatric disorders.
Differences between psychiatric and control subjects, although suggestive, do not necessarily demonstrate that miR-21 is a mediator in myelin and oligodendrocyte alterations because both myelin and miR-21 disturbances may depend on other upstream pathogenic changes, as suggested by the lack of differences among groups in some of the major miR-21 targets. However, since several other miR-21 targets were not examined here, further study of these targets may be necessary to establish mechanisms affected by miR-21 reduction and their link to myelin maintenance.
Determining the mechanisms ultimately responsible for low miR-21 in depression will require further research in animal models and postmortem tissue. However, low WM miR-21 in alcoholism may be related to direct actions of prolonged alcohol exposure, as suggested by the effects of ethanol on cultured neocortical neurospheres (Balaraman et al., 2012; Sathyan et al., 2007). Alcoholism is also associated with significant reductions in the expression of myelin-related proteins and their mRNAs in human subjects (J.M. Lewohl et al., 2000; J. M. Lewohl et al., 2005). Hence, given the correlations between mRNAs of myelin-related proteins and miR-21 there may be a mechanistic link between miR-21 deficits and myelin dysregulation in alcoholism. The molecular factors responsible for reduced miR-21 in subjects with MDD (and without alcoholism) remain to be elucidated.
4.3 Correlation of miR-21 and mRNA levels of astrocyte proteins
Overall, there was strong correlation between miR-21 and GFAP mRNA, the major astrocyte intermediate filament protein, while GFAP mRNA was significantly lower in the psychiatric subjects than in controls. In addition, GJA1 mRNA level, which is expressed mainly by astrocytes in neocortical areas (Yamamoto, Ochalski, Hertzberg, & Nagy, 1990), was higher in the combined psychiatric subjects than in controls, but not correlated with miR-21. Differences between control and psychiatric subjects regarding GFAP and GJA1 mRNA levels in WM, although in opposite directions, are consistent with the known roles of astrocytes in support of oligodendrocyte maturation and physiology, and the known myelin-destabilizing effects of GFAP alterations or astrocyte gap junction disturbance (Li, Giaume, & Xiao, 2014; Liedtke et al., 1996; Magnotti, Goodenough, & Paul, 2011; Sargiannidou, Markoullis, & Kleopa, 2010; Stumpf et al., 2003). Whether the correlation of GFAP mRNA and miR-21 levels reflects a causal relationship between these two factors or just a dependence on other depression related alterations remains to be determined. In addition, since other studies have found miR-21 upregulation in astrocytes after brain injury (Bhalala et al., 2012; Ge et al., 2014), the exact contribution of astrocytes to miR-21 changes and its relevance to oligodendrocyte physiology remains to be established. Future studies in animal models and the human brain should determine the exact roles of miR-21 in WM oligodendrocytes, astrocytes or other glial cells and how they may contribute to connectivity alterations in depression and alcoholism.
5. Conclusion
MiR-21 was highly expressed in adult oligodendrocytes in the white matter and myelinated portions of the gray matter. Significantly low levels of miR-21 were found in the white matter adjacent to the orbitofrontal cortex of human subjects with depression and alcoholism as compared to non-psychiatric controls. The correlation of the miR-21 decrease with some mRNAs of myelin proteins, regulator STAT3, and oligodendrocyte-related transcription factors suggest an involvement of miR-21 in WM alterations in depression and alcoholism.
Supplementary Material
Fig. 11.
Levels of mRNA for myelin-related proteins MBP, MAG, PLP1 and MOG in the four diagnostic groups. Diagnostic group abbreviations as in Fig. 8.
HIGHLIGHTS.
miR-21 is highly expressed in adult oligodendrocytes
miR-21 level in orbitofrontal white matter is low in depression and alcoholism
miR-21 correlates with low mRNA of PLP1, transcription factor OLIG1 and STAT3
miR-21 may be implicated in the white matter pathology of depression and alcoholism
Acknowledgments
This work was supported by grants from NIH, numbers R21MH82297, P30GM103328, R21DK113500, R56MH113828; and by American Heart Association Grant 12SDG8980032. We are grateful for the support of the Postmortem Brain and Imaging Cores of the Center for Psychiatric Neuroscience at the University of Mississippi Medical Center and for the support of INBRE for HPB.
The authors deeply appreciate the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also gratefully acknowledge the support of the staff of the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio. We acknowledge the expert assistance of Drs. James C. Overholser, George Jurjus, and Lisa C. Konick, and of Lesa Dieter in establishing the psychiatric diagnoses, acquiring written consent and in collecting the tissues.
We thank Dr. Jun-ichi Miyazaki (Osaka University, Japan) for generously providing the CAG-Cre mice.
The monoclonal antibodies JIE7 and 40-1a developed by T.L. Matson, J.A. Partadelis and J.R. Sanes were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaraman S, Winzer-Serhan UH, Miranda RC. Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alc Clin Exp Res. 2012;36(10):1669–1677. doi: 10.1111/j.1530-0277.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neuroscience. 2012;32(50):17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, … Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. 2011;30(6):371–380. doi: 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10(12):1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y. Emerging role of microRNAs in major depressive disorder: diagnosis and therapeutic implications. Dialogues Clin Neurosci. 2014;16(1):43–61. doi: 10.31887/DCNS.2014.16.1/ydwivedi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y. Pathogenetic and therapeutic applications of microRNAs in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:341–348. doi: 10.1016/j.pnpbp.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Feng Y. MicroRNAs in neural cell development and brain diseases. Sci China Life Sci. 2011;54(12):1103–1112. doi: 10.1007/s11427-011-4249-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Anderson RC, McDermott KW. MicroRNA: Key regulators of oligodendrocyte development and pathobiology. Int J Biochem Cell Biol. 2015;65:134–138. doi: 10.1016/j.biocel.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, … Zhang JN. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;4:6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa HO, Tomari Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol. 2015;25(11):651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Jovicic A, Roshan R, Moisoi N, Pradervand S, Moser R, Pillai B, Luthi-Carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J Neurosci. 2013;33(12):5127–5137. doi: 10.1523/JNEUROSCI.0600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Dwivedi Y, Brennand KJ. Noncoding RNAs and neurobehavioral mechanisms in psychiatric disease. Mol Psychiatry. 2015;20:677–684. doi: 10.1038/mp.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Cooper NJ, Williams LM, Grieve SM. Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: a whole-brain diffusion tensor imaging tractography study. Neuroreport. 2012;23(9):566–571. doi: 10.1097/WNR.0b013e3283546264. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9(10):1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8(5):706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 2008;28(45):11720–11730. doi: 10.1523/JNEUROSCI.1932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alc Clin Exp Res. 2000;24(12):1873–1882. [PubMed] [Google Scholar]
- Lewohl JM, Wixey J, Harper CG, Dodd PR. Expression of MBP, PLP, MAG, CNP, and GFAP in the Human Alcoholic Brain. Alc Clin Exp Res. 2005;29(9):1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- Li T, Giaume C, Xiao L. Connexins-Mediated Glia Networking Impacts Myelination and Remyelination in the Central Nervous System. Mol Neurobiol. 2014;49(3):1460–1471. doi: 10.1007/s12035-013-8625-1. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17(4):607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Liu NK, Xu XM. MicroRNA in central nervous system trauma and degenerative disorders. Physiol Genomics. 2011;43(10):571–580. doi: 10.1152/physiolgenomics.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, … Rothenberg ME. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187(6):3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013;132(1):3–13. doi: 10.1016/j.jaci.2013.04.039. quiz 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti LM, Goodenough DA, Paul DL. Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia. 2011;59(7):1064–1074. doi: 10.1002/glia.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alc Clin Exp Res. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Whittom A, Villarreal A, Soni M, Meshram A, Pickett JC, … Stockmeier CA. Apoptosis-related proteins and proliferation markers in the orbitofrontal cortex in major depressive disorder. J Affect Disord. 2014;158:62–70. doi: 10.1016/j.jad.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC. Chapter Seven - MicroRNAs and Ethanol Toxicity. In: Subhash CP, editor. Int Rev Neurobiol. Vol. 115. Academic Press; 2014. pp. 245–284. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Pietrzykowski AZ, Tang Y, Sathyan P, Mayfield D, Keshavarzian A, … Hereld D. MicroRNAs: master regulators of ethanol abuse and toxicity? Alc Clin Exp Res. 2010;34(4):575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban E, Mattugini N, Ciarapica R, Provenzano C, Savino M, Scagnoli F, … Nasi S. MiR-21 is an Ngf-modulated microRNA that supports Ngf signaling and regulates neuronal degeneration in PC12 cells. Neuromolecular Med. 2014;16(2):415–430. doi: 10.1007/s12017-014-8292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z. RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA. 2006;12(2):187–191. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou H, Li Y, Kang M. Activation of miR-21 by STAT3 Induces Proliferation and Suppresses Apoptosis in Nasopharyngeal Carcinoma by Targeting PTEN Gene. PLoS One. 2014;9(11):e109929. doi: 10.1371/journal.pone.0109929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, Aguirre A. TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014;34(23):7917–7930. doi: 10.1523/JNEUROSCI.0363-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Jeker LT, Carver-Moore K, Oh A, Liu HJ, Cameron R, … McManus MT. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 2012;1(4):385–391. doi: 10.1016/j.celrep.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HJ, Zheng HR, Ning YP, Zhang Y, Shan BC, Zhang L, … Li LJ. Abnormalities of cortical-limbic-cerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry. 2013;13:72. doi: 10.1186/1471-244X-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F. Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;50(2):291–297. doi: 10.1007/s12031-013-9953-3. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, … Newton SS. Oligodendrocyte morphometry and expression of myelin-related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res. 2015;65:53–62. doi: 10.1016/j.jpsychires.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health. 2003;27(2):146–152. [PMC free article] [PubMed] [Google Scholar]
- Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, … Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30(5):1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237(2):318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Sargiannidou I, Markoullis K, Kleopa KA. Molecular mechanisms of gap junction mutations in myelinating cells. Histol Histopathol. 2010;25(9):1191–1206. doi: 10.14670/HH-25.1191. [DOI] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27(32):8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders TL. Reporter molecules in genetically engineered mice. Meth Mol Biol. 2003;209:125–143. doi: 10.1385/1-59259-340-2:125. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues Clin Neurosci. 2010;12(4):554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Hansen KF, Obrietan K, Dwivedi Y, Shomron N, Girardi P. The involvement of microRNAs in major depression, suicidal behavior, and related disorders: a focus on miR-185 and miR-491-3p. Cell Mol Neurobiol. 2014;34(1):17–30. doi: 10.1007/s10571-013-9997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Shin JY, McManus MT, Ptacek LJ, Fu YH. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol. 2009;66(6):843–857. doi: 10.1002/ana.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Nave KA. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol. 2015;8(1):a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS One. 2012;7(3):e33201. doi: 10.1371/journal.pone.0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9(1):e86469. doi: 10.1371/journal.pone.0086469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero N, Simons M. The logistics of myelin biogenesis in the central nervous system. Glia. 2017;65(7):1021–1031. doi: 10.1002/glia.23116. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, … Wegner M. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11(5):697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Stumpf E, Masson H, Duquette A, Berthelet F, McNabb J, Lortie A, … Cossette P. Adult Alexander disease with autosomal dominant transmission: a distinct entity caused by mutation in the glial fibrillary acid protein gene. Arch Neurol. 2003;60(9):1307–1312. doi: 10.1001/archneur.60.9.1307. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience. 2007;148(2):535–540. doi: 10.1016/j.neuroscience.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Tansey FA, Cammer W. A Pi Form of Glutathione-S-Transferase Is a Myelin-and Oligodendrocyte-Associated Enzyme in Mouse Brain. J Neurochem. 1991;57(1):95–102. doi: 10.1111/j.1471-4159.1991.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132(1–2):26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res. 2010;183(1):1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Wegner M. A Matter of Identity: Transcriptional Control in Oligodendrocytes. J Mol Neurosci. 2008;35(1):3–12. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- Whittom A, Villarreal A, Soni M, Owusu-Duku B, Meshram A, Rajkowska G, … Miguel-Hidalgo JJ. Markers of apoptosis induction and proliferation in the orbitofrontal cortex in alcohol dependence. Alc Clin Exp Res. 2014;38(11):2790–2799. doi: 10.1111/acer.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J Comp Neurol. 1990;302(4):853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 2010;1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, … Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(Pt 5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol Psychiatry. 2014;19(7):848–852. doi: 10.1038/mp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.