Abstract
Background
Interventions that promote mindfulness consistently show salutary effects on cognition and emotional wellbeing in adults, and more recently, in children and adolescents. However, we lack understanding of the neurobiological mechanisms underlying mindfulness in youth that should allow for more judicious application of these interventions in clinical and educational settings.
Methods
Using multi-echo multi-band fMRI, we examined dynamic (i.e., time-varying) and conventional static resting-state connectivity between core neurocognitive networks (i.e., salience/emotion, default mode, central executive) in 42 children and adolescents (ages 6–17).
Results
We found that trait mindfulness in youth relates to dynamic but not static resting-state connectivity. Specifically, more mindful youth transitioned more between brain states over the course of the scan, spent overall less time in a certain connectivity state, and showed a state-specific reduction in connectivity between salience/emotion and central executive networks. The number of state transitions mediated the link between higher mindfulness and lower anxiety, providing new insights into potential neural mechanisms underlying benefits of mindfulness on psychological health in youth.
Conclusions
Our results provide new evidence that mindfulness in youth relates to functional neural dynamics and interactions between neurocognitive networks, over time.
Keywords: resting-state, default mode network, meditation, intrinsic connectivity, independent components analysis, salience network
1. Introduction
How often do you reach the bottom of a page and realize that you “zoned out”, and were thinking of the past or future instead of the task at hand? The propensity to mind-wander, or to shift attention away from the present moment and toward internal information, appears to be a “default mode” of processing. Incredibly, nearly 50% of our awake life is spent mind-wandering [1]. Yet, mind-wandering is associated with lower levels of happiness [1], possibly through pathological forms of self-referential thought focused on the past or future, such as rumination or worry [2]. These data have prompted interest in understanding present-centered mental states, and ways in which such states can be cultivated.
One such method is mindfulness, which is typically defined as the ability to stay aware of and focus attention on experiences in the present moment, in an accepting, nonjudgmental manner [3]. Mindfulness can be considered a mental capacity that differs between individuals, i.e., trait mindfulness [4, 5], and importantly, can be strengthened through a variety of methods. For instance, interventions that promote mindfulness (e.g., meditation practices) are shown to have broad positive effects on health and wellbeing, including enhanced cognitive functioning, alleviation of pain, and improved mood. Meta-analyses show consistent benefits of mindfulness-based interventions in patients with a variety of conditions, including chronic pain, anxiety disorders, depression, and cancer [6–8], as well as in nonclinical samples [9].
The benefits of mindfulness are firmly established in adults, and exciting emerging data also support the use of mindfulness to improve physical and mental health in children and adolescents. Indeed, recent meta-analyses indicate that mindfulness training relates to improvements in cognitive performance, emotion regulation, and stress resilience, as well as reductions in symptoms of anxiety and depression in clinical and nonclinical youth samples [meta-analysis by 10, 11]. It is not surprising, then, that there has been increased interest in integrating mindfulness practices into education and mainstream pediatric health services. We have recently shown that Kids Kicking Cancer (KKC, www.kidskickingcancer.org), a martial arts therapy that centers around mindfulness based meditative practices, appears to be an effective interventional therapeutic modality for reducing pediatric cancer pain, with over 85% reporting reductions in pain with an average decrease of 40% [12]. KKC and other mindfulness-based interventions are particularly well suited for children because they can be used as a non-pharmacological strategy for early, preventive intervention with little or no adverse side effects. In addition to improving psychological wellbeing, such interventions may reduce morbidity, health care costs, and caregiver and family distress by improving patient adherence to medical procedures.
As the empirical evidence for mindfulness-based approaches continues to grow, there is a critical need to understand the neural correlates underlying positive intervention response. Greater understanding of the underlying neural correlates in youth could enhance the strength and efficacy of mindfulness-based interventions for improving long-term health outcomes, and allow for more judicious application of these techniques in clinical and educational settings. Recent neuroimaging studies in adults suggest that at least three large-scale neural networks play a pivotal role in mindfulness: the default mode network (DMN), the salience and emotion network (SEN), and the central executive networks (CEN), implicated in self-referential processing and mind-wandering, present moment awareness, and shifting and sustaining attentional focus, respectively [13]. Although prior studies in adults find that trait mindfulness relates to altered connectivity between these networks [e.g., 14], such associations are typically measured using static functional connectivity approaches, which treat network interactions as fixed over time. Such an approach is at odds with the increasingly recognized dynamic nature of functional neural networks [15–18], and of mindfulness, which may relate to greater mental and neural flexibility. Indeed, one neuroimaging study in adults [13] identified four accompanying mental or neural “states” associated with mindful meditation: (1) mind-wandering (DMN), (2) attentional awareness of mind-wandering (SEN), (3) shift of attention back to the present moment (CEN), and (4) focus on the present moment (CEN). It is likely that these states correspond with different patterns of connectivity among neural networks, and that mindfulness relates to more flexible transitioning between these brain states and/or altered coordination between networks over time. These functional neural patterns may be better captured with a complementary dynamic, or time-varying approach to neural connectivity [see review by 19].
Although it is now clear that mindfulness has similar salutary effects in youth, the neural correlates of these effects are largely unknown. As a first step, the present study evaluates both dynamic and conventional static neural connectivity to test how mindfulness in children relates to interactions between large-scale neural networks (DMN, SEN, CEN). We focus on trait mindfulness as a broad psychological indicator of health and well-being, associated with increased quality of life, academic competence, and social skills, and decreased somatic complaints and internalizing symptoms in youth [20]. Thus, we will also test for associations among mindfulness, neural connectivity, and indices of psychological health (anxiety, depression) in the sample.
Functional connectivity was measured during the resting-state in order to identify individual differences in trait-like neural patterns that may influence day-to-day life, beyond a meditative state. Indeed, resting-state functional connectivity is thought to reflect habitual network activations [21] that can be remodeled by long-term [e.g., 22] and even brief [e.g., 14] mindfulness training. Further, similar patterns of functional connectivity have been reported during meditation and a non-meditative (resting) state [13], suggesting that the neural substrates underlying trait mindfulness are the same ones that can be strengthened through mindfulness-based practices. We also evaluate amount of present-centered thought during the scan, as measured on a post-scan questionnaire, to assess convergence between state and trait measures and to aid interpretation of observed dynamic connectivity states.
Notably, the study sample was economically and racially diverse, with a large number of youth at risk for emotional psychopathology via socioeconomic disadvantage (i.e., lower income) and/or early threat exposures (i.e. violence, abuse exposure, intensive medical treatments; see Supplemental Material). This served to (1) increase generalizability of findings, and (2) improve our ability to draw initial links among mindfulness, brain connectivity, and indices of psychological health.
2. Material and Methods
2.1 Participants
We report on a functional magnetic resonance imaging (fMRI) study of 42 children and adolescents (23 female), ages 6–17 (M=10.3, SD=2.9). fMRI data were collected in 7 additional youth, but were excluded from the study due to poor fMRI data quality (see Supplemental Material). Although participants were not recruited for race or economic standing, the sample was racially and economically diverse. See Supplemental Material for further information on study procedures and sample characteristics.
2.2 Materials and procedure
All participants were assisted by research staff in completing a validated self-reported measure of trait mindfulness for youth [Child and Adolescent Mindfulness Measure, CAMM; 20]. Possible mindfulness scores range from 0–50, with higher scores corresponding to higher levels of trait mindfulness (see Supplemental Material).
Participants also provided self-reports of pubertal maturation [23] and two indices of psychological health: anxiety [Screen for Child Anxiety-Related Emotional Disorders, SCR; 24] and depressive symptomology [Children’s Depression Inventory - short form, CDI-S; 25]. Three participants were missing data for the anxiety questionnaire. IQ was estimated using the KBIT-2 [26]. The sample was average in IQ (M=101.6, SD=16.3) and average Tanner stage was 2.6 (‘early-mid’ pubertal; SD=1.5). Of note, 40% of study participants exceeded thresholds suggested for detecting pathological anxiety [SCR > 22; 27], and 45% for depression [CDI-S ≥ 3; 28], with 62% of overall participants exceeding thresholds for anxiety and/or depression. Thus, although formal diagnostic testing was not performed here, these standardized measures suggest a significant number of youth at risk for emotional psychopathology.
Immediately following the scan, participants were assisted in completing a brief self-report questionnaire that inquired about their internal experiences during the scan [see 15 for further information]. Participants were not informed that they would be asked about their cognition in advance, and all participants endorsed understanding the questions. Here, we assessed correspondence between trait mindfulness and percent of time children reported present-centered thoughts during the scan. Exploratory analyses evaluated percent of time participants engaged in self-focused, and past- and future-oriented thought during the scan, to ascribe initial significance to observed dynamic connectivity states. Three participants were missing data for this questionnaire.
2.3 MRI Data Acquisition, Preprocessing, and Denoising
See Supplemental Material for overview of data acquisition, preprocessing, and denoising steps. Of note, resting-state fMRI data were acquired using a using a multi-echo/multi-band (ME/MB) echo-planar imaging sequence. MB fMRI is associated with improvements in temporal resolution [29], and ME fMRI allows for more specific removal of non-BOLD artifacts that has been shown to improve effect size estimates and statistical power [30, 31]. Enhanced artifact elimination is particularly relevant for pediatric imaging where artifact due to excess head motion poses significant challenges [32].
2.4 Group ICA and Dynamic and Static Connectivity Estimation
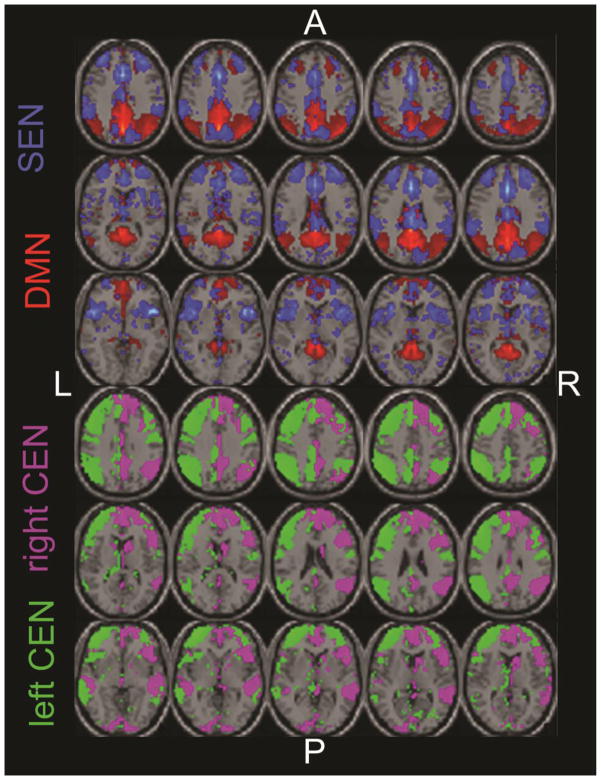
Individual participants’ datasets were submitted to a group-level spatial independent components analysis (ICA) to identify four networks of interest: DMN, SEN, and left and right CEN (see Supplemental Material). Individual participant component network maps were used to estimate dynamic and conventional static connectivity between network components, following our prior work [15]. Static connectivity was measured using estimates of covariance between network components, averaged across the entire scan. Dynamic connectivity was estimated using a sliding windows approach (see Supplemental Material for additional information).
2.5 Statistical Analysis
Using Pearson Bivariate correlation in IBM SPSS v.23, we tested for associations between mindfulness scores and measures of dynamic and conventional static connectivity. Age was controlled for in all analyses, and follow-up analyses examined effects of age, and potential age x mindfulness interactions. For static connectivity, we evaluated strength of connectivity between network components, averaged across the experiment. For dynamic connectivity, we investigated (1) the number of state transitions, as well as (2) mean dwell time (i.e., how long a participant is in each state), (3) fraction of (total) time spent in each state, and (4) strength of between-network connectivity, for each of the five connectivity states. Although for each state, dwell time was positively associated with fraction of time spent in that state (r’s>0.7, p’s<0.001), these measures capture unique properties of time-varying connectivity. All results were considered significant using a p <0.05 threshold. Given that five connectivity states were assessed for each measure, we indicate in Results the effects that additionally survive false discovery rate [FDR; 33] correction for multiple comparisons (α=0.01). All statistical tests were two-tailed.
2.6 Relation to self-reported indices of psychological health
Associations between mindfulness and anxiety (SCR) and depressive symptomology (CDI) were evaluated using Pearson bivariate correlation. For significant associations (p<0.05), PROCESS software [2.11; 34] implemented in SPSS was then used to test for the mediating effects of neural connectivity in the association between mindfulness and symptomology. Indirect effects are considered significant when confidence intervals do not overlap zero [34].
3. Results
3.1 Mindfulness
Mindfulness scores in the sample ranged from 14 to 50 (M=35, SD=8.9). Consistent with previous reported benefits of mindfulness on psychological health in children [20], higher mindfulness was associated with lower symptoms of anxiety, r(39)=−0.49, p=0.004. This effect remained significant when controlling for age, p=0.005. Controlling for age, mindfulness was not associated with income, parental education, IQ, puberty, gender, or depressive symptoms, p’s>0.4, and did not differ between threat-exposed and unexposed youth, or when split by type of threat exposure (i.e., violence/abuse vs. intensive medical treatment), p’s>0.1.
3.2 Static connectivity across the sample
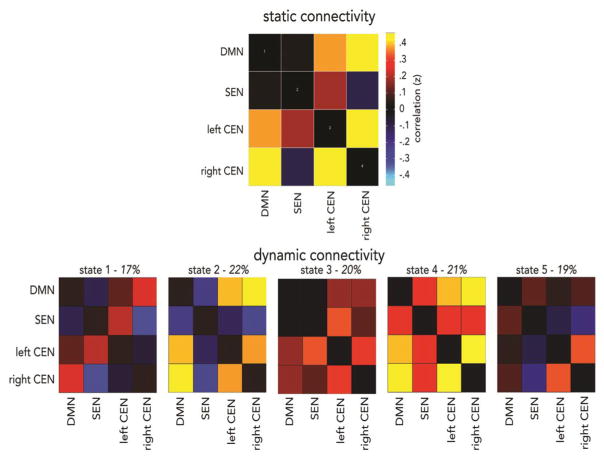
The static functional connectivity analysis showed positive connectivity between left and right CEN, between SEN and left CEN, and between DMN and both CEN components (see Figure 2, top). Negative connectivity was observed between SEN and right CEN, and DMN and SEN were weakly correlated. Overall, these patterns are consistent with prior work [35].
Figure 2. Static (top) and dynamic (bottom) functional neural connectivity across the entire youth sample.
Conventional static connectivity is shown as the correlation between core neurocognitive network components, averaged across the entire resting-state scan. Using sliding windows analysis and k-means clustering, five dynamic connectivity states were identified that reoccur across the scan and over all participants. Percentage of occurrence is listed for each state, over the course of the scan. Abbreviations: default mode network, DMN; salience and emotion network, SEN; central executive network, CEN.
3.3 Dynamic connectivity across the sample
Dynamic connectivity analysis identified five connectivity states that re-occurred throughout the scan and across participants (see Figure 2, bottom). These states diverged, in part, from static connectivity averaged across the experiment. State 1 was characterized by negative connectivity between SEN-right CEN, DMN-SEN, and left CEN-right CEN, and positive DMN connectivity with left and right CEN, as well as positive SEN-left CEN connectivity. State 2 was characterized by positive DMN connectivity with left and right CEN components, positive connectivity between left and right CEN, negative SEN connectivity with left and right CEN, and negative DMN-SEN connectivity. State 3 was characterized by positive DMN connectivity with left and right CEN, positive SEN connectivity with left and right CEN, weak DMN-SEN connectivity, and positive connectivity between left and right CEN. State 4 was characterized by positive connectivity between all network components, and was similar to a state observed in our prior study in an independent youth sample [15]. State 5 was characterized by weak connectivity among all components, except positive connectivity between left and right CEN. This state was also similar to one previously observed in children [15].
3.4 Mindfulness and static connectivity
Controlling for age, there was a negative association between trait mindfulness and DMN-left CEN connectivity, but this effect did not reach significance, r(39)=−0.294, p=0.062. No effects of mindfulness, age, or the age x mindfulness interaction on static connectivity reached significance.
3.5 Mindfulness and dynamic connectivity
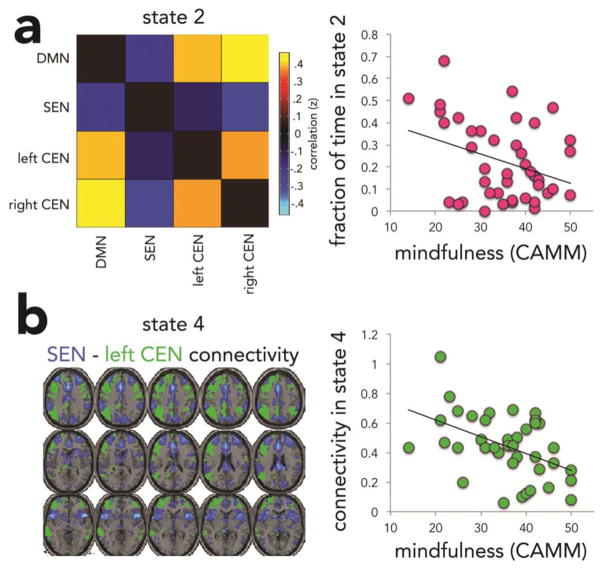
Controlling for age, we found that more mindful children spent less time in state 2, r(39)=−0.36, p=0.021 (Figure 3A), and demonstrated a greater number of state transitions over the course of the scan, r(39)=0.342, p=0.029 (Figure 4A). Of note, although the former did not pass FDR correction for multiple comparisons (adjusted p=0.06), results using a separate measure of present-focused thought were in agreement with this result, as described below. In addition, although mindfulness was not related to network interactions when averaged across the experiment, we observed a negative association between mindfulness and SEN-left CEN connectivity during state 4 only, r(36)=−0.475, p=0.003 (Figure 3B). This finding passed FDR correction for multiple comparisons. Thus, mindfulness was associated with the number of transitions between connectivity states, how frequently certain states are expressed, and state-dependent changes in strength of network interactions. There was also no effect of age or age x mindfulness on dynamic connectivity measures.
Figure 3. Effects of mindfulness on fraction of time youth spent in state 2 (a), and SEN-left CEN connectivity in state 4 (b).
Abbreviation: default mode network, DMN; salience and emotion network, SEN; central executive network, CEN; Child and Adolescent Mindfulness Measure, CAMM.
Figure 4. Number of dynamic connectivity state-transitions mediates the link between higher mindfulness and lower anxiety.
A: Higher mindfulness was associated with a greater number of dynamic state-transitions. Higher anxiety, in contrast, was associated with fewer state-transitions (see text). B: More mindful youth reported lower anxiety. C: A mediation model was tested, indicating significant indirect and direct effects. Abbreviations: number, n; confidence interval, CI; Child and Adolescent Mindfulness Measure, CAMM. *p<0.05, **p<0.01.
3.6 Relation to state measures
Not surprisingly, children with higher levels of trait mindfulness reported more present-focused thoughts during the scan, as reported on a post-scan questionnaire, r(36)=0.363, p=0.025. In addition, consistent with observed effects of trait mindfulness reported above, a higher percent of time spent thinking about the experience in the scanner was associated with a lower fraction of time and shorter dwell time in state 2, r(40)=−0.32, p=0.043 and r(40)=−0.35, p=0.027, respectively. These were independent of level of trait mindfulness. Together, these findings suggest that youth who are more inclined to have present-centered thoughts have greater flexibility in transitioning between connectivity states, and spend less time in state 2.
To ascribe initial significance to other observed connectivity states, we tested for correlations with other dimensions of internal experience reported on the post-scan questionnaire, namely self-focused and past- and future-oriented thought during the scan (measured as percent of time for each). Youth who report more self-focused thoughts had a longer dwell time in state 5, r(39)=0.337, p=0.036. In addition, youth reporting more thoughts about the past spent a greater fraction of time in state 3, r(39)=0.338, p=0.036, suggesting that state 3 might be associated with ruminative thought or episodic memory. An association between past-focused thoughts and fraction of time spent in a similar connectivity state was observed in our previous dynamic connectivity study in an independent youth sample [15].
3.7 Relation to indices of psychological health
Given the observed negative relation between trait mindfulness and anxiety symptomology, we tested whether anxiety had opposite effects on dynamic neural connectivity. Indeed, we found that more anxious children showed fewer state transitions during the scan, r(39)=−0.54, p=0.0004, and showed a longer dwell time in state 2, r(39)=0.336, p=0.037. Anxiety was not related to static connectivity between networks. Given that effects of anxiety on dynamic connectivity were opposite to those observed for mindfulness, we tested whether dynamic connectivity mediated the association between mindfulness and anxiety. We found that, controlling for age, the number of state transitions mediated the association between mindfulness and anxiety symptomology (β=−0.24, SE=0.14, lower limit confidence interval [LLCI]=−0.62, upper limit confidence interval [ULCI]=−0.03). Direct effects of mindfulness on anxiety were also significant (β=−0.56, SE=0.25, LLCI=−1.07, ULCI=−0.05), suggesting that number of state transitions partially mediated the pathway between mindfulness and anxiety. Overall, the model explained 39% of the variance in anxiety scores, and mindfulness and number of state transitions were significant predictors in the model (age was a nonsignificant predictor). Reversal of this model (mindfulness→anxiety→state-transitions) yielded nonsignificant indirect effects, implying that time-varying brain patterns mediate anxiety symptoms but not the reverse. In addition, a variant on this model predicting mindfulness from anxiety also yielded nonsignificant indirect effects, suggesting that neural patterns observed in this sample may be driven by mindfulness rather than anxiety.
4. Discussion
This is the first study to investigate neural correlates underlying trait mindfulness, and its associated salutary effects on psychological health, in children and adolescents. We investigated these links in a racially and economically diverse sample of at-risk youth, with 62% of participants exceeding thresholds for pathological anxiety and/or depression. Using resting-state functional connectivity MRI, we found no associations between trait mindfulness in youth and measures of conventional static connectivity between “core” neurocognitive networks implicated in mindfulness in adults (DMN, SEN, CEN). Interestingly, associations with trait mindfulness in youth did emerge, however, when a sliding windows approach was employed to elucidate how network interactions vary over time (i.e., dynamic connectivity). We found that more mindful children transitioned more between brain states over the course of the scan, spent overall less time in a certain connectivity state (state 2), and showed a state-dependent reduction in strength of SEN-left CEN connectivity (state 4). Importantly, results of trait mindfulness were consistent with state-measures of present-focused thought during the scan, as reported in a post-scan questionnaire, suggesting state-and trait convergence in our results. Finally, we observed a significant mediation model, suggesting that flexibility in transitioning between neural states bridges the well-established link between higher mindfulness and lower anxiety in children [meta-analysis by 10, 11].
Dynamic connectivity analyses revealed five connectivity states that differed significantly from conventional, static connectivity, measured across the experiment. Prior research suggests that evaluation of time-varying connectivity provides additional, complementary information that may relate to important individual differences [e.g., 36, 37]. This is consistent with the present findings. We found effects of mindfulness on ongoing functional network dynamics over time. Of note, for static connectivity, we observed a nonsignificant trend for reduced DMN-CEN connectivity in more mindful children. A similar effect was observed for static DMN-CEN connectivity in adults; although in that study, this effect did not survive correction for multiple comparisons [14]. The study by Doll and colleagues (2015) also found associations between mindfulness scores and static connectivity between the DMN and the SEN, while we did not find similar associations in our pediatric sample, in static or dynamic analyses. Given that that dynamic connectivity has not been evaluated as it relates to mindfulness in adults, dynamic neural correlates of mindfulness may be similar in children and adults. For example, the association between trait mindfulness and DMN-CEN connectivity may be stronger in certain states (e.g., state 5) rather than when averaged across the scan, as described below. Taken together, these findings suggest that neural correlates of mindfulness differ among adults and youth, that there are differences in how these traits are measured or expressed across develop [see 20], or that we may be underpowered to detect smaller effect sizes on static connectivity,
Our dynamic analyses revealed that more mindful children showed a greater number of state-transitions during the resting-state scan. This may reflect greater flexibility in transitioning between functional neural states and their corresponding network configurations. This interpretation is fitting with conceptual ideas of mindfulness; more mindful individuals are thought to have greater awareness of and/or greater capacity to volitionally switch attention from “narrative” forms of self-referential states, to a more mindful “experiencing” of sensations, emotions, and interoception [38]. In addition to more state-transitions, youth more inclined to have present-centered thoughts spent less overall time in state 2, suggesting that state 2 might support mind-wandering or another form of past- or future-oriented cognition. In support of this hypothesis, state 2 was characterized by high positive DMN connectivity with left and right CEN components, a pattern previously linked to mind-wandering in adults [e.g., 39]. Of note, the form of mind-wandering supported by state 2 might differ from that supported by other states. For example, state 5 was associated with self-focused thoughts during the scan, and, similar to state 2, was characterized by high positive connectivity between DMN and CEN components. Unlike state 2, however, state 5 was characterized by high connectivity between DMN and SEN, involved in emotional and bodily awareness. Thus, state 5 might reflect a more self-focused form of mind-wandering relative to state 2. In addition, it is interesting to note that mindfulness was not associated with amount of time children spent in state 3, which was associated with past-focused thought. Although speculative, our results might indicate that mindfulness may be more strongly related to the flexibility in transitioning between states, rather than amount of time spent in past-focused states.
We also found that more mindful children showed lower SEN-left CEN connectivity in state 4, which was characterized by positive connectivity among all network components. Reduced SEN-CEN connectivity in more mindful individuals was reported in a prior study of adults, averaged across the scan [14], although this effect did not survive correction for multiple comparisons. Given the role of the SEN in emotional processing [40, 41] and the CEN in redirection of attention [42], Doll et al. [14] speculated that lower connectivity might reflect preferential conscious attentional processing over emotional value-based evaluation of stimuli. For the first time, we suggest that this neural pattern may be present in youth, in certain neural configurations that reoccur over time.
Consistent with prior pediatric research [20], more mindful youth reported lower symptoms of anxiety. Our results suggest that functional neural flexibility, particularly flexibility in state-transitions over time, mediates this link. Greater neural flexibility associated with mindfulness may protect individuals against getting “stuck in a rut” of repetitive and uncontrollable worry about potential future threats, a hallmark of anxiety [43]. More mindful individuals may be less prone to these anxious cognitions, more aware of or more able to challenge them, or better able to switch to another mode of awareness (e.g., present moment). Although not a mediator of the relationship between mindfulness and anxiety symptoms, both higher anxiety and lower mindfulness were each also related to time spent in state 2 (in opposing directions). This suggests another possible interpretation of state 2, as supporting anxious cognitions. Together, these findings indicate that mindfulness may alter neural substrates involved in risk, providing new insights into the potential protective effects and clinical benefits of mindfulness in children. It is striking that mindfulness in youth related to ongoing interactions among three neural networks (DMN, SEN, CEN) shown to play a critical role in risk for various psychopathologies [44], many of which show a sharp increase in incidence during childhood and adolescence [45].
Limitations of this study should be considered. First, our approach to identify neural correlates of mindfulness is a correlation-based approach. Future research using prospective intervention studies in children will help to address a causal link between neural connectivity and mindfulness, and also, to determine whether the neural correlates altered by mindfulness-based interventions in are the same ones identified here for trait mindfulness. Such studies might also consider an experience sampling approach to more closely link dynamic neural states to subjective mental states [13]. Second, younger children may be less capable of reporting on their internal experience relative to older children. However, we took several steps to reduce the likelihood that responses from younger children, including assistance in completing questionnaires and examination of potential age or age x mindfulness interactions (see Methods and Results). Next, focus on DMN, SEN, and CEN here does not exclude other networks that may be relevant for mindfulness in children. We focus on these neurocognitive “core” networks (1) to limit the number of comparisons, (2) because they contribute critically to self-, emotion-, and cognitive control-related processes [44] that relate conceptually to mindfulness, and (3) because interactions between these networks vary with mindfulness scores in adults [14]. Future studies building on this work could examine whole-brain organization or the role of other networks, and how network interactions and their relations with mindfulness may shift with age.
4.1 Conclusions
This study reveals that functional neural dynamics relate to mindfulness scores in youth. Further, our results suggest that greater flexibility in transitioning between neural states may bridge the well-established link between higher mindfulness and lower anxiety. These data lay the groundwork for understanding how mindfulness-based interventions exert positive cognitive, psychological, and physical effects in children. In particular, increased capacity for volitional shifting of mental states and corresponding neural network configurations may be a therapeutic mechanism of mindfulness-based therapies in youth. Future studies should provide greater mechanistic understanding of trait mindfulness and mindfulness training. Such interventions may be particularly well suited for youth exposed to stress and adversity, who are at risk for cognitive and emotional impairment.
Figure 1. Spatial maps of the four components of interest, corresponding to core neurocognitive networks.
Abbreviations: default mode network, DMN; salience and emotion network, SEN; and left and right central executive network, CEN; right, R; left, L; posterior, P; anterior, A.
Acknowledgments
Funding
Research reported in this publication was supported, in part, by the Department of Pharmacy Practice, the Department of Pediatrics, and the Merrill Palmer Skillman Institute of WSU, NIH National Institute of Biomedical Imaging and Bioengineering awards P20GM103472, R01EB006841 (VDC), and R01EB020407 (VDC), National Science Foundation grant 1539067 (VDC), and American Cancer Society and Karmanos Cancer Institute Institutional Research Grant 14-238-04-IRG (CAR). Dr. Marusak is supported by American Cancer Society award 129368-PF-16-057-01-PCSM. Dr. Rabinak is supported by NIH National Institute of Mental Health grant K01MH101123 and R61MH111935.
The authors thank Pavan Jella, Laura Crespo, Kelsey Sala-Hamrick, Shelley Paulisin, Sajah Fakhoury, Allesandra Iadipaolo, Limi Sharif, Farah Sheikh, Brian Silverstein, Suzanne Brown, Klaramari Gellci, Maria Tocco, Andrea Bedway, and Angela Vila of Wayne State-University (WSU) and Kristopher Dulay of Children’s Hospital of Michigan, for assistance in participant recruitment and data collection. The authors would like to thank Dr. Moriah Thomason for sharing some of the included data and for comments on an earlier version of this paper. Thanks also to the children and families who generously shared their time to participate in this study, and to the children of Kids Kicking Cancer (www.kidskickingcancer.org) for their teaching and inspiration.
Footnotes
The authors declare no conflict of interest or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science (New York, NY) 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 2.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on psychological science: a journal of the Association for Psychological Science. 2008;3(5):400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 3.Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice. 2004;11(3):230–241. [Google Scholar]
- 4.Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakash SR, De Leon AA, Klatt M, Malarkey W, Patterson B. Mindfulness disposition and default-mode network connectivity in older adults. Social cognitive and affective neuroscience. 2013;8(1):112–7. doi: 10.1093/scan/nss115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clinical psychology review. 2011;31(6):1032–1040. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of consulting and clinical psychology. 2010;78(2):169. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA internal medicine. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. The journal of alternative and complementary medicine. 2009;15(5):593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 10.Zenner C, Herrnleben-Kurz S, Walach H. Mindfulness-based interventions in schools-a systematic review and meta-analysis. Frontiers in psychology. 2014;5:603. doi: 10.3389/fpsyg.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoogman S, Goldberg SB, Hoyt WT, Miller L. Mindfulness interventions with youth: A meta-analysis. Mindfulness. 2015;6(2):290–302. [Google Scholar]
- 12.Bluth M, Thomas R, Cohen C, Bluth A, Goldberg RE. Martial arts intervention decreases pain scores in children with malignancy. Pediatric Health, Medicine and Therapeutics. 2016;7:79–87. doi: 10.2147/PHMT.S104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2012;59(1):750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Doll A, Holzel BK, Boucard CC, Wohlschlager AM, Sorg C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Frontiers in human neuroscience. 2015;9:461. doi: 10.3389/fnhum.2015.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, Thomason ME. Dynamic functional connectivity of neurocognitive networks in children. Human brain mapping. 2016 doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cerebral cortex. 2014;24(3):663–76. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma (New York, NY) 2010;23(5–6):351–66. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84(2):262–74. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greco LA, Baer RA, Smith GT. Assessing mindfulness in children and adolescents: development and validation of the Child and Adolescent Mindfulness Measure (CAMM) Psychological assessment. 2011;23(3):606–14. doi: 10.1037/a0022819. [DOI] [PubMed] [Google Scholar]
- 21.Harmelech T, Malach R. Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends in cognitive sciences. 2013;17(12):606–15. doi: 10.1016/j.tics.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, Courtemanche J, Lavarenne AS, Marrelec G, Benali H, Beauregard M. Impact of meditation training on the default mode network during a restful state. Social cognitive and affective neuroscience. 2013;8(1):4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual review of medicine. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 24.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–53. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs M. Children’s Depression Inventory. Multi-Health Systems; 1992. Incorporated. [Google Scholar]
- 26.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test: KBIT 2; Manual. Pearson; 2004. [Google Scholar]
- 27.Desousa DA, Salum GA, Isolan LR, Manfro GG. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child psychiatry and human development. 2013;44(3):391–9. doi: 10.1007/s10578-012-0333-y. [DOI] [PubMed] [Google Scholar]
- 28.Allgaier AK, Fruhe B, Pietsch K, Saravo B, Baethmann M, Schulte-Korne G. Is the Children’s Depression Inventory Short version a valid screening tool in pediatric care? A comparison to its full-length version. Journal of psychosomatic research. 2012;73(5):369–74. doi: 10.1016/j.jpsychores.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magnetic resonance in medicine. 2010;63(5):1144–53. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kundu P, Benson BE, Baldwin KL, Rosen D, Luh WM, Bandettini PA, Pine DS, Ernst M. Robust resting state fMRI processing for studies on typical brain development based on multi-echo EPI acquisition. Brain imaging and behavior. 2015;9(1):56–73. doi: 10.1007/s11682-014-9346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombardo MV, Auyeung B, Holt RJ, Waldman J, Ruigrok AN, Mooney N, Bullmore ET, Baron-Cohen S, Kundu P. Improving effect size estimation and statistical power with multi-echo fMRI and its impact on understanding the neural systems supporting mentalizing. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotsoni E, Byrd D, Casey BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. Journal of magnetic resonance imaging: JMRI. 2006;23(6):877–86. doi: 10.1002/jmri.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 34.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Publications; 2013. [Google Scholar]
- 35.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthoffer D, Zimmer C, Forstl H, Bauml J, Riedl V, Wohlschlager AM, Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in human neuroscience. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaffke L, Schweizer L, Rüther NN, Luerding R, Tegenthoff M, Bellebaum C, Schmidt-Wilcke T. Dynamic changes of resting state connectivity related to the acquisition of a lexico-semantic skill. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 37.Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in human neuroscience. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in human neuroscience. 2012;6:296. doi: 10.3389/fnhum.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; 2000. text revision - DSM-IV-TR. [Google Scholar]
- 44.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Kessler RC, Angermeyer M, Anthony JC, DEGR, Demyttenaere K, Gasquet I, DEGG, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World psychiatry: official journal of the World Psychiatric Association (WPA) 2007;6(3):168–76. [PMC free article] [PubMed] [Google Scholar]