Abstract
The diverse immunomodulatory effects of vitamin D are increasingly being recognized. However, the ability of oral vitamin D to modulate acute inflammation in vivo has not been established in humans. In a double-blinded, placebo-controlled interventional trial, twenty healthy adults were randomized to receive either placebo or a high dose of vitamin D3 (cholecalciferol) one hour after experimental sunburn induced by an erythemogenic dose of ultraviolet radiation. Compared to placebo, participants receiving vitamin D3 (200,000 IU) demonstrated reduced expression of pro-inflammatory mediators TNF-α (p=0.04) and iNOS (p=0.02) in skin biopsy specimens 48 hours after experimental sunburn. A blinded, unsupervised hierarchical clustering of participants based on global gene expression profiles revealed that participants with significantly higher serum vitamin D3 levels after treatment (p=0.007) demonstrated increased skin expression of the anti-inflammatory mediator arginase-1 (p=0.005), and a sustained reduction in skin redness (p=0.02), correlating with significant expression of genes related to skin barrier repair. In contrast, participants with lower serum vitamin D3 levels had significant expression of pro-inflammatory genes. Together the data may have broad implications for the immunotherapeutic properties of vitamin D in skin homeostasis, and implicate arginase-1 up regulation as a previously unreported mechanism by which vitamin D exerts anti-inflammatory effects in humans.
Introduction
Vitamin D is a ubiquitous fat-soluble hormone important in calcium homeostasis and bone metabolism (Jackson et al., 2006). The majority of vitamin D arises from de novo synthesis in the skin triggered by ultraviolet radiation (UVR), with smaller contributions from dietary sources (Bikle, 2011). While considerable attention has been placed on vitamin D deficiency and optimizing supplementation strategies, appreciation for the diverse biological effects and long-term outcomes of vitamin D now include the modulation of immune responses, inflammatory disease, cardiovascular health, and carcinogenesis (Giovannucci et al., 2006, Martins et al., 2007, Rosen, 2011, Sanders et al., 2010, Wobke et al., 2014). However, there is a lack of evidence demonstrating that intervention with vitamin D is capable of resolving acute inflammation in target tissues and organs in humans.
Keratinocytes and macrophages produce active vitamin D within the skin (Baeke et al., 2010, Bikle et al., 1986). Vitamin D has pleiotropic effects on the immune system, including the enhancement of anti-microbial responses, induction of autophagy, and suppression of pro-inflammatory mediators, including tumor necrosis factor-α (TNF-α) (Di Rosa et al., 2012, Liu et al., 2006, Zhang et al., 2012). We recently demonstrated that intervention with a single dose of vitamin D is capable of rapidly attenuating an inflammatory response in a mouse model of chemical induced skin injury through inhibition of inducible nitric oxide synthase (iNOS) and TNF-α by activated macrophages (Au et al., 2015). Therefore, we designed a pilot, proof-of-principle interventional study in humans, modeled after a randomized, double-blinded, placebo-controlled clinical trial, to test the hypothesis that a single high dose of oral vitamin D3 (cholecalciferol) would be capable of rapidly attenuating experimental sunburn induced by simulated solar radiation (SSR).
Results
Dose-dependent response of high dose oral vitamin D3 and UV irradiation
The randomized treatment groups did not differ in their baseline characteristics (Table 1). No participant was taking supplemental vitamin D3 before study initiation. Serum 25-hydroxyvitamin D3 (25(OH)D3), a marker of vitamin D3 stores, increased after treatment in a vitamin D3 dose-dependent fashion (Fig. S1a). Similar trends were observed for the active form of vitamin D3, 1,25(OH)2D3, as well as an inactive breakdown product, 24,25(OH)2D3 (Fig. S1b). No measured vitamin D3 metabolite increased into a toxic range in any of the treatment groups throughout the study period. Furthermore, there were no instances of clinically significant hypercalcemia occuring in any of the treatment groups throughout the study period (Fig S2).
Table 1.
| Placebo (n=6) | 50,000 IU D3 (n=5) | 100,000 IU D3 (n=4) | 200,000 IU D3 (n=5) | |
|---|---|---|---|---|
| Age, median (range) | 24.0 (21–46) |
35.0 (21–58) |
36.5 (22–50) |
27.0 (21–53) |
| Sex, N (%) | ||||
| Male | 3 (50.0) | 3 (60.0) | 3 (75.0) | 4 (80.0) |
| Female | 3 (50.0) | 2 (40.0) | 1 (25.0) | 1 (20.0) |
| BMI, median (range) | 22.8 (16.1–26.8) |
23.1 (21.3–31.9) |
28.6 (22.6–33.1) |
23.0 (21.7–41.6) |
| FST, N (%) | ||||
| I | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) |
| II | 2 (33.3) | 3 (60.0) | 2 (50.0) | 3 (60.0) |
| III | 4 (66.6) | 2 (40.0) | 2 (50.0) | 1 (20.0) |
| Baseline Vitamin D3 Metabolites, mean (95% CI) |
26.8 (23.3,30.4) |
30.9 (21.1,40.6) |
18.6 (10.8,26.4) |
22.4 (15.4,29.4) |
| 25(OH)2D3 (ng/mL) | ||||
| 1,25(OH)2D3 (pg/mL) | 61.8 (47.1,76.6) |
54.5 (50.3,58.8) |
57.7 (46.1,69.4) |
54.8 (42.4,67.2) |
| 24,25(OH)2D3 (ng/mL) | 2.4 (1.8,3.0) |
3.0 (1.5,4.5) |
1.4 (0.9,1.8) |
1.8 (0.9,2.6) |
Abbreviations: D, vitamin D3 (cholecalciferol); BMI, body mass index (the weight in kilograms divided by the square of the height in meters); FST, Fitzpatrick skin type; CI, confidence interval
There were no statistically significant differences in the treatment groups at baseline.
Sunburn is a stereotypical inflammatory response induced by exposure to an erythemogenic dose of UVR. Sunburn is characterized clinically by redness, mediated by dermal vasodilatation, and edema, mediated by increased vascular permeability and inflammatory cell infiltration (Clydesdale et al., 2001, Cooper et al., 1993, Ouhtit et al., 2000). While skin redness peaks early after UVR exposure, skin thickness increases steadily for up to two weeks after irradiation (Clydesdale et al., 2001, Ouhtit et al., 2000). Compared to one minimal erythema dose (MED), exposure to 2MED increased skin erythema 24hr and 48hr after irradiation, and exposure to 3MED increased skin thickness 72hr and 1 week after irradiation (p<0.05 for all) (Fig S3a, S3b). We observed saturation of skin redness after exposure to 3MED, limiting the ability to discern subtle differences among treatment groups with this high dose of UVR.
High dose oral vitamin D3 attenuates skin inflammation
Clinically, irradiated skin appeared red and swollen 48hr after UVR exposure (Fig. 2a). Irradiated skin also displayed histologic evidence of structural damage as compared to non-irradiated skin, including epidermal vesiculation and edema formation, which improved in a vitamin D3 dose-dependent fashion (Fig. 2b). Skin expression of TNF-α and iNOS was lower in participants receiving 200,000 international units (IU) D3 as compared to placebo 48hr after irradiation (p=0.04 for TNF-α; p=0.02 for iNOS) (Fig. 2c). With higher doses of vitamin D3, there was a trend for decreased skin thickness after irradiation, which reached significance in the 100,000 IU D3 group at both 72hr (p=0.03) and 1 week (p=0.02) after irradiation (Table S1). Comparison of global gene expression profiles among the treatment groups revealed that the 200,000 IU D3 group had a distinct gene expression profile that was very different from the placebo group, and most closely related to the 100,000 IU D3 group (Fig. 2d).
Fig. 2. Primary Outcomes of Randomized Treatment Groups.
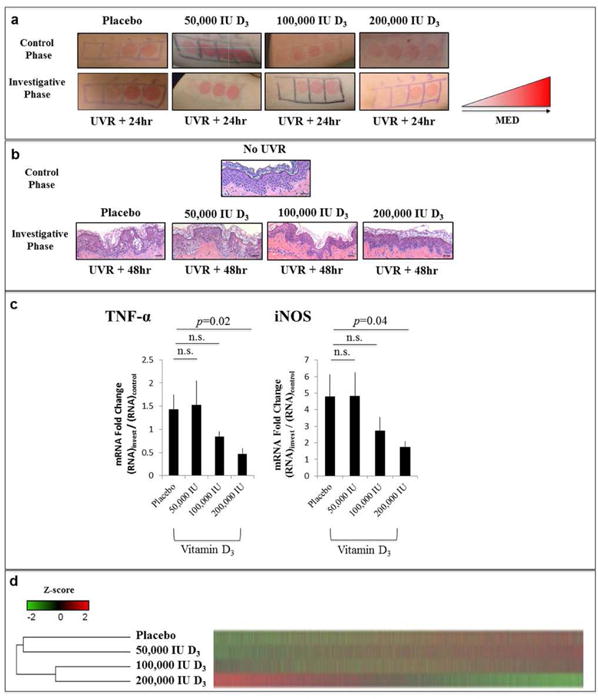
Panel A shows representative clinical images of irradiation sites of participants in each treatment group in the control and investigative phases of the study. Panel B shows representative hematoxylin and eosin stained histological images obtained from punch biopsies from participants in each treatment group 48hr after irradiation with 3MED. Panel C presents the difference in TNF-α and iNOS mRNA expression obtained from punch biopsies between the investigative and control phases of the study [(RNA48hr)invest/(RNA48hr)control]. Bars represent the mean, and error bars represent the standard error of the mean for the placebo (n=4), 50,000 IU D3 (n=5), 100,000 IU D3 (n=4), and 200,000 IU D3 (n=5) groups. Two participants were excluded from the placebo analysis given poor RNA sample quality. Panel D presents a heat map depicting global gene expression averages for each treatment group, with dendrogram depicting the unbiased hierarchical clustering of treatment groups based on similarities in gene expression profiles. Red indicates increased gene expression and green indicates decreased gene expression, correlating to a row-wise z-score. Statistical comparisons are made between vitamin D3 treatment groups and the placebo group. Abbreviations: n.s., non-significant. Scale bar 100 μm.
Elevated serum levels of 25(OH)D3 correlate with decreased skin redness
To further investigate a potential link between vitamin D3 and gene expression, we analyzed the global gene expression profiles of all participants, blinded to their allocated treatment groups. The dendrogram resulting from this analysis produced two clusters of participants (Fig. 3a), representing an unbiased, unsupervised hierarchical clustering of all individuals based on similarities in gene expression profiles.
Fig. 3. Unsupervised Clustering of Participants Based on Gene Expression Profiles.
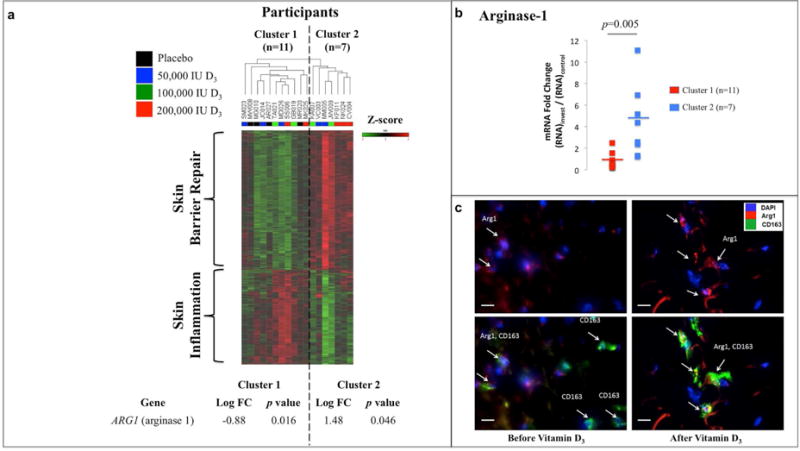
Panel A presents a heat map depicting global gene expression profiles for individual participants, with the resulting dendrogram depicting the unsupervised hierarchical clustering of participants based only on similarities in gene expression. Red indicates increased gene expression and green indicates decreased gene expression, correlating to a row-wise z-score. Two unique clusters emerged from this unbiased and blinded analysis. Cluster 1 was characterized by down regulation of arginase-1, and up regulation of genes involved in skin inflammation. Cluster 2 was characterized by up regulation of arginase-1 and genes involved in skin barrier repair. Panel B presents the fold change difference in skin arginase-1 mRNA expression from punch biopsies obtained in the investigative and control phases of the study [(RNA48hr)invest/(RNA48hr)control]. Data points represent arginase-1 fold change differences for individual participants at each time point. Horizontal lines represent the mean for cluster 1 (n=11) and cluster 2 (n=7). Panel C presents immunofluorescently stained sections from a representative participant receiving 200,000 IU D3 before and after vitamin D3 intervention. Nuclear DNA is depicted in blue (DAPI), arginase-1 protein expression is depicted in red, and CD163, a marker of macrophages, is depicted in green. Arginase-1 protein levels are increased primarily within CD163+ macrophages after vitamin D3 intervention. White scale bar represents 20 micrometers. Abbreviations: FC, fold change.
Of note, arginase-1, known to enhance tissue repair and inhibit inflammation through the utilization of iNOS precursors, was significantly down regulated in cluster 1 (p=0.016) and up regulated in cluster 2 (p=0.046) (Fig. 3a) (Bronte and Zanovello, 2005). Increased arginase-1 expression observed in cluster 2 compared to cluster 1 was subsequently validated with qRT-PCR (p=0.005) (Fig. 3b). Additionally, confocal microscopy analysis of a representative participant from cluster 2 revealed increased expression of the arginase-1 protein localized to CD163+ macrophages after vitamin D3 treatment (Fig. 3c).
Unblinding of the participants’ demographics and treatment group allocation revealed that the two clusters of participants did not differ in their baseline characteristics (Table S2). However, cluster 1 contained all participants randomized to receive placebo, as well as a mixture of participants from the various vitamin D3 treatment groups (Fig. 3a). Cluster 2 predominately contained participants who had received higher doses of vitamin D3, and notably none of the participants who had received placebo. While the two clusters had similar baseline serum 25(OH)D3 levels, participants in cluster 2 had significantly higher 25(OH)D3 levels after treatment as compared to participants in clusters 1 (p<0.05 for all time points) (Fig. 4a). When subjects receiving placebo were excluded from this analysis, serum 25(OH)D3 levels after treatment remained lower for participants in cluster 1 as compared to participants in cluster 2 (p<0.05 for 24hr, 48hr and 1 week) (Fig. 4b). We will now refer to participants from cluster 2 as vitamin D3 responders and participants from cluster 1 as vitamin D3 non-responders.
Fig. 4. Serum Vitamin D3 and Skin Erythema Following Experimental Sunburn in Cluster 1 and Cluster 2.
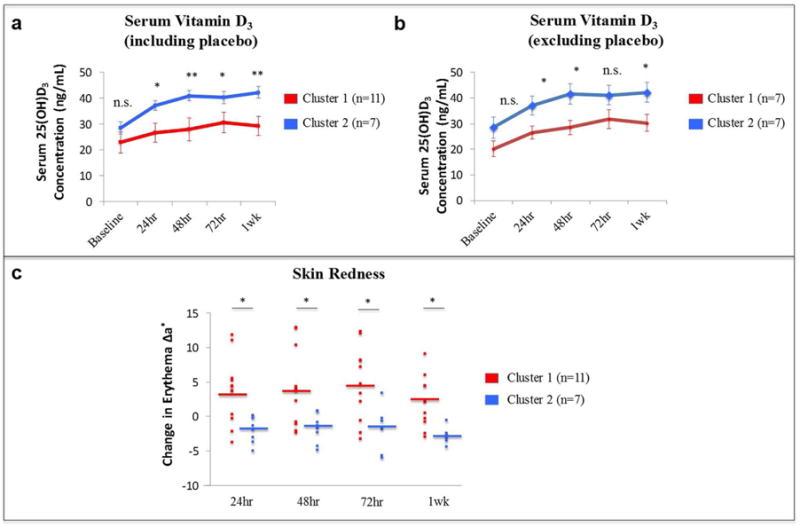
Panel A presents the serum 25(OH)D3 levels over time after study drug administration for cluster 1 (n=11) and cluster 2 (n=7). Panel B presents the serum 25(OH)D3 levels over time after study drug administration for cluster 1 1 (n=7) and cluster 2 (n=7), excluding the participants from cluster 1 who received placebo (n=4). Error bars represent the standard error of the mean for each group at each time point. Panel c presents the change in erythema over time after experimental sunburn with 2MED for each cluster. Data points represent erythema values for individual participants at each time point. Horizontal lines represent the mean for cluster 1 (n=11) and cluster 2 (n=7) at each time point. Statistical comparisons are between cluster 1 and cluster 2 at each time point. * p<0.05; **p<0.01.
As indicated above, body-mass index (BMI), age, gender, and baseline 25(OH)D3 stores had no effect on the serum response to oral vitamin D3. Furthermore, along with higher serum 25(OH)D3 levels, vitamin D3 responders demonstrated a statistically significant sustained reduction in skin redness at all time points after irradiation as compared to vitamin D3 non-responders (p<0.05 for all) (Fig. 4c), and a trend for reduced skin thickness 1 week after irradiation (p=0.09; data not shown).
Differential gene expression profiles characterize vitamin D3 responders and vitamin D3 non-responders
Vitamin D3 non-responders displayed up regulation of various pro-inflammatory genes not observed in the gene expression profiles of vitamin D3 responders, including matrix metalloproteinases (MMP1, MMP3), interleukin-1 alpha (IL-1A), and monocyte chemokines (CCL2) (Fig. S4a). Likewise, canonical pathways related to leukocyte migration and IL-6 signaling were significantly activated in vitamin D3 non-responders, including TNF-α as a predicted up-stream regulator (p<0.0001 for all) (Fig. S4b). Conversely, vitamin D3 non-responders displayed a strikingly different gene expression profile, characterized by up regulation of genes implicated in skin barrier repair, including tissue transglutaminases (TGM3, TGM5), keratins (KRT78, KRT80), corneodesmosin (CDSN), and calmodulin-like 5 (CALML5) (Fig. S4a).
Discussion
In this pilot, proof-of-principle human interventional study modeled after a randomized, double-blinded, placebo-controlled trial, we provide in vivo evidence that a single high dose of oral vitamin D3 is capable of rapidly attenuating a local inflammatory response to UVR. Participants responding to high doses of vitamin D3 demonstrated a sustained reduction in skin redness following experimental sunburn, as well as less epidermal structural damage, reduced expression of pro-inflammatory markers in the skin, and a gene expression profile characterized by up regulation of skin barrier repair genes. This study also demonstrates that regardless of baseline serum vitamin D3 levels, a single high dose of oral vitamin D3 is safe, with serum vitamin D3 and calcium concentrations remaining within a normal reference range (Rosen, 2011). The simplicity and safety of high dose oral vitamin D3 treatment, combined with its rapid and sustained therapeutic efficacy, suggest that these proof-of-concept findings may ultimately be translated to routine clinical use once larger studies are performed on diverse populations of subjects (Ilahi et al., 2008, Sanders et al., 2010).
Moreover, up regulation of arginase-1 is associated with the anti-inflammatory effects of vitamin D3 in humans (Fig. 5). While arginase has been identified to be present at physiological levels in inflammatory skin diseases, tumors, and chronic wounds, to our knowledge the induction of arginase-1 expression by vitamin D3 in human skin in vivo is previously unreported (Abd-El-Aleem et al., 2000, Bruch-Gerharz et al., 2003, Gokmen et al., 2001). These findings suggest that arginase-1 may also be a clinically useful tissue biomarker for monitoring the immunomodulatory effects of vitamin D3 in humans. Given the presence of a putative vitamin D3 response element upstream of the arginase-1 promoter, future studies should be aimed at defining the mechanism by which vitamin D3 treatment activates the arginase-1 pathway (Andrukhova et al., 2014).
Fig. 5. The Effect of Oral Vitamin D3 Intervention on Skin Inflammation.
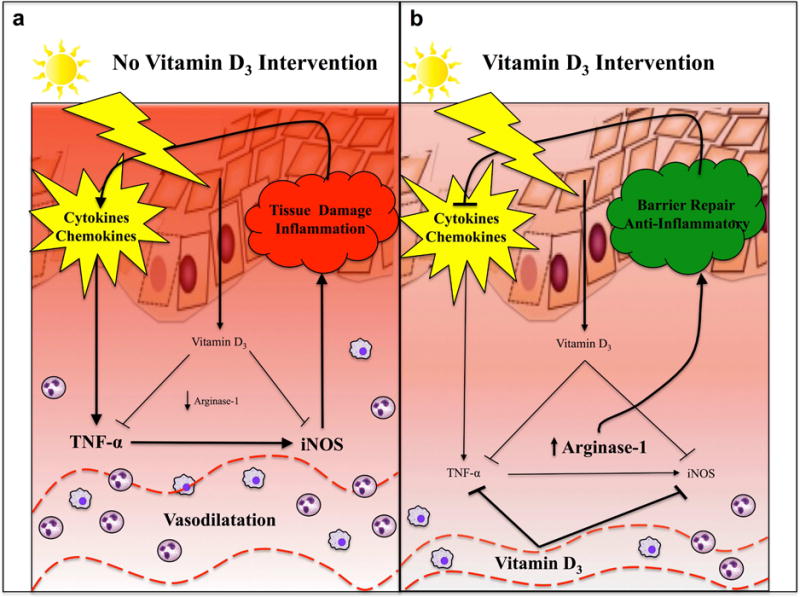
As depicted in panel A, levels of vitamin D3 are at baseline levels in the absence of high dose oral vitamin D3 intervention. In this context, exposure to erythemogenic doses of UVR results in sunburn and the release of pro-inflammatory cytokines and chemokines in the skin, including TNF-α and iNOS, which further propagate tissue inflammation. Increased skin redness and thickness are mediated by vasodilation, an influx of inflammatory cells, and vascular congestion within the skin. The gene expression profile of skin at this time is characterized by increased expression of various pro-inflammatory genes. As depicted in panel B, levels of vitamin D3 rapidly rise within the serum after high dose oral vitamin D3 intervention. Arginase-1 is up regulated within the skin, and production of the pro-inflammatory mediators TNF-α and iNOS are attenuated after sunburn. Reduced skin erythema and thickness are observed clinically. The gene expression profile of skin at this time is characterized by increased expression of skin barrier genes, which help to repair the epidermal barrier and attenuate the inflammatory insult.
Exploratory analyses suggest that the host’s response to vitamin D3 intervention plays a critical role in the modulation of inflammation. Participants segregated into two clusters based on similarities in global gene expression profiles, and these two clusters differed significantly in their serum vitamin D3 levels after treatment. The pharmacokinetic properties of oral vitamin D3 are complex, however, and an individual’s serum response to oral vitamin D3 depends on the dose of vitamin D3, age, BMI, baseline vitamin D3 stores, and genetic polymorphisms (Didriksen et al., 2013, Ilahi et al., 2008). Large randomized, double-blinded, placebo-controlled trials will be required to elucidate factors responsible for the therapeutic variability of vitamin D3 treatment within populations and to determine whether individuals with lower baseline vitamin D3 levels require higher treatment doses to achieve immunomodulation.
Exposure to erythemogenic doses of UVR initiates an influx of inflammatory cells into the skin, generating a microenvironment rich in inflammatory mediators (Cooper et al., 1993, Ouhtit et al., 2000). Specifically, release of TNF-α by damaged keratinocytes and other inflammatory cells plays a crucial role in initiating and sustaining UVR-induced inflammation (Clydesdale et al., 2001, Di Rosa et al., 2012). Following an inflammatory insult, classically activated M1-polarized macrophages infiltrate the skin and produce iNOS as part of an oxidative burst in an evolutionarily conserved attempt to prevent infection (Bronte and Zanovello, 2005, Mills, 2012). However, excessive production of iNOS perpetuates tissue damage, retards the resolution of inflammation, and prevents tissue repair (Bronte and Zanovello, 2005, Mills, 2012). We and others have shown using murine models that vitamin D3 inhibits the production of TNF-α, and is capable of attenuating skin inflammation by reducing macrophage-specific iNOS production (Au et al., 2015, Zhang et al., 2012).
In the presence of retinoic acid, vitamin D3 induces the in vitro differentiation of monocytes into alternatively activated, M2-polarized CD163+ macrophages expressing arginase-1 (Di Rosa et al., 2012, Takahashi et al., 2014). Furthermore, it has been shown that exposure to acute UVR increases endogenous retinoids in the skin of mice (Gressel et al., 2015). Taken together, our results combined with these data suggest that a potential mechanism by which vitamin D3 mediates resolution of experimental sunburn is via the up regulation of arginase-1 by endogenous repair molecules, leading to the selective induction of anti-inflammatory, M2-polarized CD163+ macrophages. Additionally, vitamin D3 may have other protective mechanisms in skin, including reducing DNA damage and keratinocyte apoptosis following experimental sunburn, as was shown in mice treated topically with the active form of vitamin D3 immediately after exposure to UVR (Dixon et al., 2007).
It is likely that the generation of vitamin D3 from cholesterol precursors in the skin after UVR evolved to perform vital homeostatic functions (Bikle, 2011). Moreover, skin-resident cells are capable of locally converting vitamin D3 into its active form, which can then signal in an intracrine, autocrine, and paracrine fashion to exert diverse biological effects (Bikle, 2011, Di Rosa et al., 2012). It is worthwhile to conjecture that vitamin D3 may provide an “endocrine barrier” within the skin, utilizing energy derived from sunlight to reduce inflammation, and promote wound healing, tissue repair, and an enhanced epidermal barrier. This would provide the host with additional protection against environmental insults by complementing the classically described brick and mortar mechanical, melanin pigment, and Langerhans cell immunologic barriers.
Materials and methods
Screening, randomization, and study design
The Institutional Review Board at University Hospitals Cleveland Medical Center approved this pilot study, which was conducted between March 2013 and February 2015. The study was modeled after a randomized, double-blinded, placebo-controlled trial. The trial is registered with clinicaltrials.gov (NCT02920502). Twenty-seven healthy adults 18 years and older were screened for eligibility and provided written informed consent (Fig. 1a). A total of twenty-five participants were randomized to receive, in a double-blinded fashion, either placebo or a single oral dose of vitamin D3 (cholecalciferol) at 50,000, 100,000, or 200,000 IU one hour after SSR exposure. UVR was administrated as SSR emitted from a 1000 watt Xenon arc lamp (Newport, Stratford, CT), a full spectrum light source that closely resembles natural sunlight (Clydesdale et al., 2001).
Fig. 1. Study Design and Baseline Characteristics.
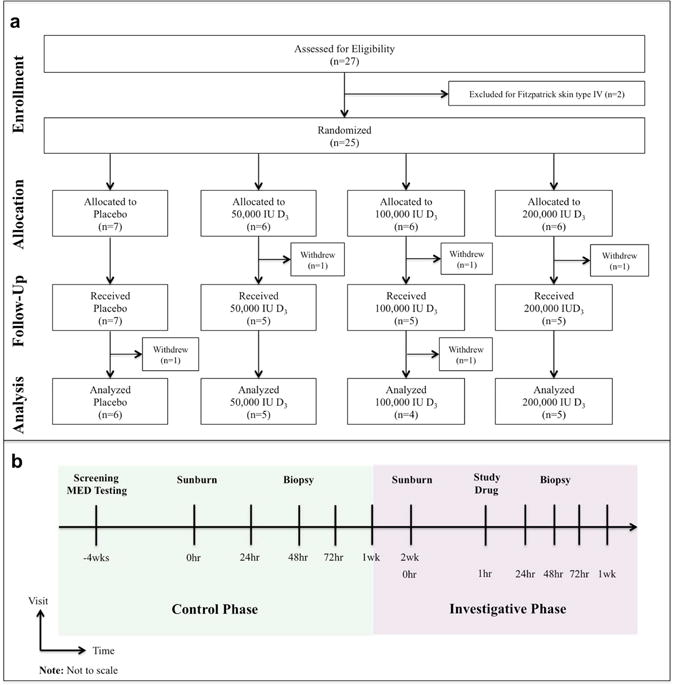
Panel A depicts the enrollment, allocation, follow-up, and analysis of participants. Panel B depicts a schematic of the parallel phase study design. Skin erythema and thickness were measured 24hr, 48hr, 72hr, and 1 week after experimental sunburn induced by an erythemogenic dose of simulated solar radiation. Participants returned two weeks after conclusion of the control phase of the study for the investigative phase, and were subsequently randomized to receive the study drug as a post-exposure treatment 1hr after experimental sunburn on the contralateral arm. Punch biopsies for tissue TNF-α, iNOS, and microarray analyses were obtained 48hr after experimental sunburn in both phases of the study.
This study was designed in a parallel fashion with exposure to SSR occurring on one arm without study drug administration (control phase), followed two weeks later by exposure to SSR on the contralateral arm with study drug administration (investigative phase) (Fig. 1b). The MED to experimentally induce sunburn was determined for each participant during the initial screening visit as previously described (Heckman et al., 2013). Participants were irradiated with one, two, and three times the MED on the sun-shielded, upper arm using plastic holed templates to ensure that adjacent skin was not exposed. A total of twenty participants completed both phases of the study and were included in the per-protocol analysis.
Quantification of vitamin D3 metabolites and calcium
The concentrations of the vitamin D3 metabolites 25(OH)D3, 1,25(OH)2D3, and 24,25(OH)2D3, as well as total serum calcium, were measured from freshly frozen serum obtained during the screening visit, as well as 24hr, 48hr, 72hr, and 1 week after receiving the study drug. Serum levels of 25(OH)D3 (ng/mL) and 1,25(OH)2D3 (pg/mL) were measured by Liaison assay, and serum levels of 24,25(OH)2D3 (ng/mL) were measured by liquid chromatography-mass spectrometry (Heartland Assays, Ames, IA). Toxic serum 25(OH)D3 levels were defined as those greater than 150 ng/mL (Holick, 2003). Total serum calcium was measured by the University Hospitals Cleveland Medical Center core laboratory (Cleveland, OH), and the normal reference range was considered 8.8 to 10.7 mg/dL.
Primary outcomes of randomized participants
Primary outcomes included non-invasive measurements of skin erythema and thickness 24hr, 48hr, 72hr, and 1 week after irradiation, as well as tissue expression of TNF-α and iNOS 48hr after irradiation. Skin erythema (redness) was quantified using a CR300 chromameter (Minolta, Ramsey, NJ). The difference in erythema (Δa*) between irradiated and non-irradiated skin (a*irrad − a*non-irrad) was calculated for each time point after SSR exposure (Δa*time). The difference in Δa*time between the investigative and control phases of the study was calculated to determine the effect of the study drug on skin redness after SSR exposure [(Δa*time)invest − (Δa*time)control].
Skin thickness, an acute measure of edema, was quantified using a Mitutoyo 9mm dial caliper (Northamptonshire, UK). Thickness measurements were repeated in triplicate and the mean was used for all calculations. The difference in thickness (Δth) between irradiated and non-irradiated skin (thirrad − thnon-irrad) was calculated for each time point after SSR exposure (Δthtime). The difference in Δthtime between the investigative and control phases of the study was calculated to determine the effect of the study drug on skin thickness after UVR exposure [(Δthtime)invest − (Δthtime)control].
A six millimeter punch biopsy specimen was obtained from the 3MED site 48hr after irradiation in both the control and investigative phases of the study. RNA was extracted from fresh frozen tissue using the RNeasy Lipid Mini Kit (Qiagen, Redwood City, CA), and tissue mRNA expression of TNF-α and iNOS were quantified as previously described (Au et al., 2015). A fold change representing the difference in RNA expression between the investigative and control phases of the study was calculated for TNF-α and iNOS [(RNA48hr)invest/(RNA48hr)control].
Unsupervised hierarchical clustering of participants
An exploratory analysis was performed utilizing clusters obtained from the unsupervised hierarchical clustering of individual participants based on similarities in their gene expression profiles, regardless of allocated treatment group. A minimum of three micrograms of total RNA was submitted to the Gene Expression and Genotyping facility at Case Western Reserve University for microarray analysis (Cleveland, OH). Human Genome 2.0 ST arrays (Affymetrix, Santa Clara, CA) were the chosen platform to analyze transcriptomic changes incurred by treatment. For microarray analyses, normalized linear data post- (Tinterv) and pre- (Tcontrol) study drug intervention were averaged amongst the eighteen participants. The microarray raw data for two participants was not interpretable given poor sample quality. A fold change representing the difference in RNA log expression between the investigative and control phases of the study was determined for each gene for each participant [(RNA48hr)invest − (RNA48hr)control)]. Data analysis was performed using Rv3.2.2/Bioconductor (R Studio, Boston, MA). The oligo package was used to read, background correct, and normalize the raw sample data using the Robust Multiarray Average algorithm (Bolstad et al., 2003). The limma package was used to create a paired design matrix and conduct differential gene expression analysis based on a linear model fit and empirical Bayes methodology (Ritchie et al., 2015).
Heatmaps and dendrograms were generated utilizing 26,599 transcripts with unique gene names using GENE-E software (http://www.broadinstitute.org/cancer/software/GENE-E/index.html, Accessed June 14, 2016). Hierarchical clustering was used to recursively merge samples based on pairwise distance, determined using the 1-Pearson correlation coefficient and average linkage methods. Ingenuity Pathway Analysis (QIAGEN, Redwood City, CA, www.qiagen.com/ingenuity) was used to determine statistically significant canonical pathways, predicted up-stream regulators, and biological networks most likely affected by the set of genes differentially expressed for each group of participants.
Tissue arginase-1 mRNA expression was quantified using qRT-PCR as described above. To analyze the expression of the arginase-1 protein in skin, freshly frozen biopsy samples embedded in optimal cutting temperature compound were cut into eight micrometer sections and stained immunofluorescently as previously described (Au et al., 2015). CD163 was used as a marker for skin macrophages, and 4′-6-diamidino-2-phenylindole (DAPI) was used to stain nuclear DNA.
Statistical analysis
Given the pilot nature of the study design, power calculations were not performed. To control for type I error rate, hierarchical closed-testing procedures were utilized for analysis of primary outcomes. Unpaired t-tests were used to compare the means of groups with respect to changes in the primary outcomes at each time point. Fisher’s exact test was used for inter-group comparisons of categorical data and for determining significance of the canonical pathways. The Benjamini-Hochberg correction was used to determine differentially expressed genes in the microarray analysis, adjusting for multiple comparisons (Benjamini and Hochberg, 1995). A two-sided p value of 0.05 or less was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
The authors would like to thank T.S. McCormick for critical discussions and reading of the manuscript; K. Honda for hematoxylin and eosin tissue slide analysis; and the Skin Disease Research Center at Case Western Reserve University for their recruitment of participants and execution of the study protocol.
Grant Support: National Institute of Arthritis Musculoskeletal and Skin Diseases (NIAMS) (P30-AR039750); National Institute of Health (U01-AR064144).
Abbreviations
- iNOS
- TNF-α
- 25(OH)D3
- 1, 25(OH)2D3
- 24,25(OH)2D3
- IU
- MED
- CI
- BMI
- FST
- D3
- UVR
- DAPI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Microarray data: Repository name: GEO Omnibus; Accession Number GSE86406 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE86406
username: sahsanuddin; password: lavalamp
Conflict of Interest: The authors report no conflicts of interest relevant to this work.
EDITOR’S NOTE
During the review of this article, questions arose concerning the post-enrollment trial registration date reported herein. The authors have explained this as follows:
“Our aim was to conduct a proof-of-concept study demonstrating that vitamin D can modulate acute inflammation in target tissues and, secondarily, to discover new skin repair genes in vivo. No established studies were available to inform on dosing to correlate with biologic activity in the skin. Therefore dose-finding was also incorporated in conceptualizing this study. To achieve rigor in the data and remove potential bias, the pilot study was modeled after a randomized double-blinded placebo-controlled trial design. The findings in this study and the results from exploratory analysis with gene array will help define parameters for primary and secondary outcomes in larger clinical trials. In preparation of the manuscript, we learned that medical journals have a broader definition of clinical trials than the FDA. Pre-registration for this pilot study was not done because our understanding was that non-regulated supplements such as Vitamin D did not constitute an applicable clinical trial. Since learning of the International Committee of Medical Journal Editors guidelines, the study was retrospectively registered and the data were made available.”
The JID requires pre-registration of all clinical trials as part of its broad interest in assuring that all published data are free of bias of any kind. In this case, however, it is the Editor’s judgment that sharing these results has priority and the investigators’ misunderstanding of what constitutes a clinical trial should not preclude publication.
References
- Abd-El-Aleem SA, Ferguson MW, Appleton I, Kairsingh S, Jude EB, Jones K, et al. Expression of nitric oxide synthase isoforms and arginase in normal human skin and chronic venous leg ulcers. The Journal of pathology. 2000;191(4):434–42. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH654>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Molecular endocrinology (Baltimore, Md) 2014;28(1):53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au L, Meisch JP, Das LM, Binko AM, Boxer RS, Wen AM, et al. Suppression of Hyperactive Immune Responses Protects against Nitrogen Mustard Injury. The Journal of investigative dermatology. 2015;135(12):2971–81. doi: 10.1038/jid.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Current opinion in pharmacology. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bikle DD. Vitamin D metabolism and function in the skin. Molecular and cellular endocrinology. 2011;347(1–2):80–9. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25(7):1545–8. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics (Oxford, England) 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews Immunology. 2005;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Bruch-Gerharz D, Schnorr O, Suschek C, Beck KF, Pfeilschifter J, Ruzicka T, et al. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. The American journal of pathology. 2003;162(1):203–11. doi: 10.1016/S0002-9440(10)63811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunology and cell biology. 2001;79(6):547–68. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- Cooper KD, Duraiswamy N, Hammerberg C, Allen E, Kimbrough-Green C, Dillon W, et al. Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. The Journal of investigative dermatology. 1993;101(2):155–63. doi: 10.1111/1523-1747.ep12363639. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cellular immunology. 2012;280(1):36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Didriksen A, Grimnes G, Hutchinson MS, Kjaergaard M, Svartberg J, Joakimsen RM, et al. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. European journal of endocrinology/European Federation of Endocrine Societies. 2013;169(5):559–67. doi: 10.1530/EJE-13-0233. [DOI] [PubMed] [Google Scholar]
- Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. The Journal of steroid biochemistry and molecular biology. 2007;103(3–5):451–6. doi: 10.1016/j.jsbmb.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Gokmen SS, Aygit AC, Ayhan MS, Yorulmaz F, Gulen S. Significance of arginase and ornithine in malignant tumors of the human skin. The Journal of laboratory and clinical medicine. 2001;137(5):340–4. doi: 10.1067/mlc.2001.114543. [DOI] [PubMed] [Google Scholar]
- Gressel KL, Duncan FJ, Oberyszyn TM, La Perle KM, Everts HB. Endogenous Retinoic Acid Required to Maintain the Epidermis Following Ultraviolet Light Exposure in SKH-1 Hairless Mice. Photochemistry and photobiology. 2015;91(4):901–8. doi: 10.1111/php.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Chandler R, Kloss JD, Benson A, Rooney D, Munshi T, et al. Minimal Erythema Dose (MED) testing. Journal of visualized experiments. JoVE. (75) 2013:e50175. doi: 10.3791/50175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Evolution and function of vitamin D. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. The American journal of clinical nutrition. 2008;87(3):688–91. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. The New England journal of medicine. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (New York, NY) 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine. 2007;167(11):1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Critical reviews in immunology. 2012;32(6):463–88. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. The American journal of pathology. 2000;156(1):201–7. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ. Clinical practice. Vitamin D insufficiency. The New England journal of medicine. 2011;364(3):248–54. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. Jama. 2010;303(18):1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hatta Y, Iriyama N, Hasegawa Y, Uchida H, Nakagawa M, et al. Induced differentiation of human myeloid leukemia cells into M2 macrophages by combined treatment with retinoic acid and 1alpha,25-dihydroxyvitamin D3. PloS one. 2014;9(11):e113722. doi: 10.1371/journal.pone.0113722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Frontiers in physiology. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of immunology (Baltimore, Md: 1950) 2012;188(5):2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


