Abstract
Background
Clinical studies have suggested predictive parameters in mortality risk assessment for pulmonary arterial hypertension (PAH) patients. However, these studies predominantly include Caucasian population; information in Asian population is relatively deficient. In this study, we investigated the long-term survival of PAH patients and the predictors of mortality in our population.
Methods
We prospectively collected 70 patients with PAH at the Chang Gung Memorial Hospital between March 2002 and February 2015. Baseline data including functional class (FC), 6-minute walk distance (6MWD), hematological and biochemical data, echocardiography and cardiac catheterization were obtained before commencing PAH- targeted treatment. The follow-up period for analyses of survivors ended in October 2015.
Results
The mean age at diagnosis was 40.7 ± 15.2 years. Mean follow-up period was 4.6 ± 3.4 years, with 1-, 2-, 3-, and 5-year survival rates of 93%, 88%, 84%, and 77%, respectively. The baseline FC was worse in non-survivors than in survivors. More frequent presence of pericardial effusion, higher serum glucose levels, higher estimated systolic pulmonary artery pressure (SPAP) by echocardiography, and higher right atrial pressure (RAP) were found in non-survivors. Higher FC, lower 6MWD, and presence of pericardial effusion were associated with risk of mortality. Patients with worsening FC and increased serum uric acid had an increased risk of mortality during follow-up.
Conclusions
The overall survival remained unsatisfactory in PAH patients. Baseline FC, 6MWD, pericardial effusion, RAP, and a worsening FC and an increased serum uric acid levels during follow-up were significant prognostic parameters.
Keywords: Functional class, Pericardial effusion, Pulmonary arterial hypertension, Serum glucose, Uric acid
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a serious chronic pulmonary arteriole disorder that results in increased pulmonary vascular resistance. It affects functional capacity and the quality of life, and it leads to right heart failure and significantly decreases life expectancy.1,2 Numerous effective treatments have evolved in the past two decades;3 however, the overall survival remains unsatisfactory.4-6
According to recent guidelines, the treatment goal is to achieve a low-risk status.7 A number of clinical studies have identified predictive parameters for mortality including WHO functional class (FC), 6-minute walk distance (6MWD), brain natriuretic peptide (BNP) serum level, right atrial pressure (RAP), and mixed-venous oxygen saturation (SvO2).8,9 These factors are suggested to be risk assessment parameters in the 2015 guidelines for the diagnosis and treatment of pulmonary hypertension.7 However, most of these studies were based on Caucasian patients, and information about predictive parameters in Asian patients is relatively insufficient.10 This study aimed to investigate the long-term survival of patients with PAH and the predictors of mortality at our institute.
MATERIALS AND METHODS
Study subjects
We prospectively collected 70 patients with PAH who received at least one medical treatment or outpatient department follow-up visit at Chang Gung Memorial Hospital (CGMH) between March 2002 and February 2015.
Baseline data including FC, functional capacity of 6MWD, hematological and biochemical data, echocardiography of all patients, and cardiac catheterization of 38 patients were obtained before commencing PAH-targeted treatment. Cardiac catheterization was performed on the same day or the day after the echocardiography examination. The catheterization data were obtained and confirmed by at least two experts.
The follow-up period for analysis of the survivors ended in October 2015. The end-point for survival analysis was disease-related death. Each patient received examinations during follow-up visits. The follow-up data of the survivors were retrieved from their latest examinations; data of the non-survivors were based on their last examinations before their last admission to hospital. All of the patients gave informed consent to participate in the study, which was reviewed and approved by the Institutional Review Board of CGMH.
Statistical analysis
Results were expressed as absolute numbers, percentages, or mean ± standard deviation (SD). Differences in continuous variables between the survivors and non-survivors were compared using the one sample t-test, whereas differences in categorical variables between these two groups were compared using Pearson’s chi-squared test or Fisher’s exact test. A nonparametric Wilcoxon signed-rank test was used for comparisons of FC between baseline and follow-up.
To evaluate the predictive value of the parameters with the risk of mortality, we used a sequential approach. Values obtained at baseline assessment were analyzed using univariate Cox regression analysis. Identical analysis was performed to analyze changes in the variables between baseline and follow-up. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Their predictive value was then confirmed by testing all of the statistically significant variables from the univariate analysis in a multivariate model.
Analysis of overall survival was performed using a prospective approach. Patients lost to follow-up were censored as of the date of the last outpatient follow-up visit. Survival analysis was conducted using the Kaplan-Meier method. Differences between survival curves were assessed using the log-rank test. The cut-off value of RAP was derived from receiver operator characteristic curve analysis. All analyses were performed with IBM SPSS version 19. A p-value < 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
We recruited 70 patients [57 women (81%) and 13 men (19%)], with a mean age of 40.7 ± 15.2 years. Of the 70 patients, 14 (20%) had idiopathic pulmonary arterial hypertension (IPAH), 33 (47%) had connective tissue disease (CTD), and 18 (26%) had congenital heart disease (CHD)-associated PAH. Five (7%) had other etiologies, including three with chronic liver disease, one with human immunodeficiency virus (HIV) infection, and one with amphetamine-related PAH. A significant difference in FC was found between baseline and follow-up (Figure 1). At the time of enrollment, 14 patients had FC class II, 51 class III, and 5 class IV. During follow-up, 51% of the patients improved at least 1 FC class, 36% were unchanged, and 13% deteriorated. Three patients preliminarily with class II, 29 with class III, and 4 with class IV had improvements in FC, whereas 2 patients with class II and 7 patients with class III deteriorated. The other 25 patients remained at their baseline FC class.
Figure 1.
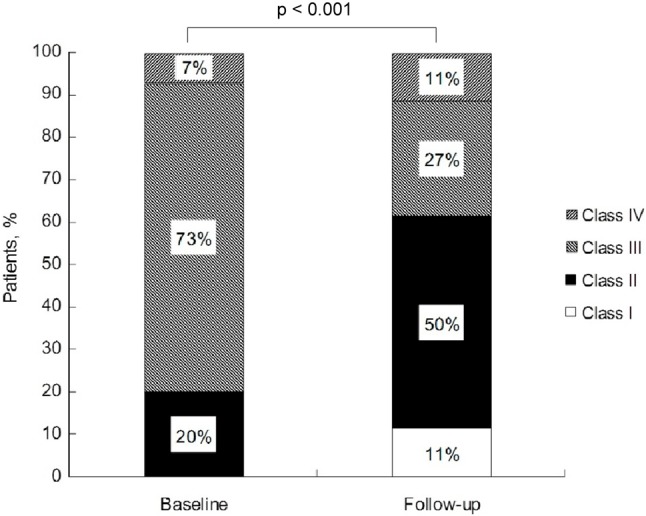
Functional class in patients with pulmonary arterial hypertension at baseline and follow-up.
During the study period, 59 patients received PAH-targeted therapy, and the remaining 11 (16%) did not receive therapy due to a lack of reimbursement from the National Health Insurance program or patients’ unwillingness. The targeted therapies included phosphodiesterase-5 inhibitors (PDE5i) (79%), endothelin receptor antagonists (ERA) (26%), and prostanoids (17%). Eighteen patients (26%) were treated with combination therapy.
Overall survival
A total of 20 patients died during the study period, including 16 patients of right heart failure, 2 from sepsis, 1 from respiratory failure due to pneumonia, and 1 from small cell lung cancer. Four patients were treated for less than 1 year. One patient whose treatment duration was also less than a year was lost to follow-up. Patients were censored if they were lost to follow-up or alive on October 31, 2015. The mean follow-up period was 4.6 ± 3.4 years (0.2-13.6 years), with 1-, 2-, 3-, and 5-year survival rates of 93%, 88%, 84%, and 77%, respectively (Figure 2).
Figure 2.
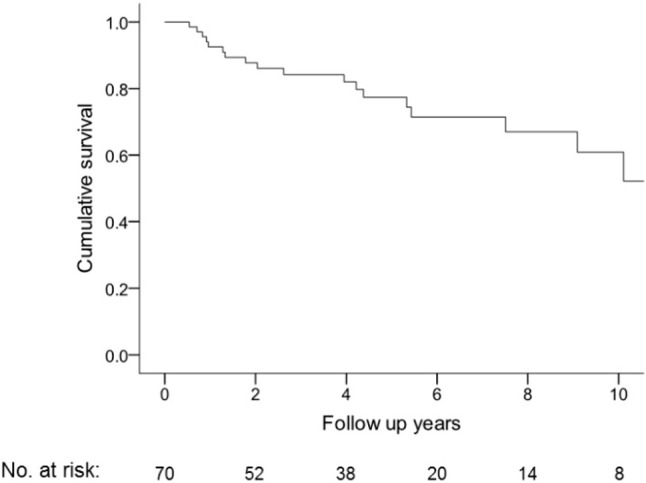
Kaplan-Meier estimates of overall survival in 70 patients with pulmonary arterial hypertension. The 1-, 2-, 3-, and 5-year survival rates were 93%, 88%, 84%, and 77%, respectively.
Baseline data of the survivors and non-survivors
Table 1 shows the demographic and baseline laboratory data of the patients, and Table 2 presents the clinical and hemodynamic data of the 38 patients who received cardiac catheterization. Table 3 shows the baseline parameters related to the risk of mortality.
Table 1. Demographic characteristics and baseline laboratory data of patients with pulmonary arterial hypertension.
| Variables | Survivor (n = 50) | Non-survivor (n = 20) | p-value |
| Demographics | |||
| Age, years | 41.1 ± 15.3 | 40.0 ± 15.3 | 0.777 |
| Female, n (%) | 41 (82) | 16 (80) | 1.000 |
| BMI, kg/m2 | 21.5 ± 4.2 | 22.5 ± 3.7 | 0.356 |
| SBP, mmHg | 119 ± 19 | 112 ± 14 | 0.162 |
| DBP, mmHg | 74 ± 14 | 74 ± 17 | 0.977 |
| HB, beats/min | 85 ± 18 | 92 ± 16 | 0.141 |
| FC (II/III/IV), n | 14/33/3 | 0/18/2 | 0.012 |
| 6MWD, m | 331 ± 116 | 296 ± 97 | 0.254 |
| Etiology of PAH | |||
| Idiopathic, n (%) | 10 (20) | 4 (20) | 1.000 |
| CTD, n (%) | 23 (46) | 10 (50) | 0.762 |
| CHD, n (%) | 14 (28) | 4 (20) | 0.489 |
| Others*, n (%) | 3 (6) | 2 (10) | 0.619 |
| Hematological and biochemical data | |||
| Hemoglobin, g/dL | 14.1 ± 3.5 | 13.4 ± 2.2 | 0.415 |
| WBC, 1000/μL | 7.4 ± 2.6 | 8.6 ± 3.1 | 0.136 |
| Platelet, 1000/μL | 188 ± 63 | 167 ± 78 | 0.263 |
| AST, U/L | 31 ± 16 | 29 ± 16 | 0.781 |
| ALT, U/L | 24 ± 12 | 24 ± 19 | 0.857 |
| Bilirubin, mg/dL | 1.0 ± 0.7 | 0.9 ± 0.4 | 0.836 |
| Creatinine, mg/dL | 0.84 ± 0.37 | 0.77 ± 0.25 | 0.416 |
| Fasting glucose, mg/dL | 93 ± 18 | 115 ± 48 | 0.026 |
| Cholesterol, mg/dL | 159 ± 46 | 156 ± 31 | 0.850 |
| Uric acid, mg/dL | 7.6 ± 2.4 | 7.0 ± 2.9 | 0.527 |
| Troponin-I, ng/mL | 0.08 ± 0.16 | 0.11 ± 0.15 | 0.655 |
| BNP, pg/mL | 271 ± 329 | 220 ± 233 | 0.596 |
| Echocardiographic data | |||
| Pericardial effusion, n (%) | 11 (22) | 12 (60) | 0.004 |
| Tei index | 0.62 ± 0.29 | 0.76 ± 0.46 | 0.148 |
| SPAP, mmHg | 81 ± 21 | 97 ± 33 | 0.014 |
| TAPSE, cm | 1.89 ± 1.14 | 1.72 ± 0.54 | 0.548 |
| Diastolic LVeI | 1.49 ± 0.34 | 1.67 ± 0.44 | 0.076 |
| Systolic LVeI | 1.59 ± 0.55 | 1.86 ± 0.58 | 0.072 |
| RA, cm2 | 22.6 ± 7.4 | 26.7 ± 9.8 | 0.083 |
| PAH-targeted treatment | |||
| PDE5i, n (%) | 38 (76) | 17 (85) | 0.528 |
| ERAs, n (%) | 12 (24) | 6 (30) | 0.604 |
| Prostanoids, n (%) | 7 (14) | 5 (25) | 0.304 |
| Monotherapy, n (%) | 30 (60) | 11 (55) | 0.701 |
| Combination therapy, n (%) | 11 (22) | 7 (35) | 0.261 |
| Two combinations, n (%) | 6 (12) | 4 (20) | 0.456 |
| Three combinations, n (%) | 5 (10) | 3 (15) | 0.680 |
| No PAH-targeted drug, n (%) | 9 (18) | 2 (10) | 0.494 |
* Others included 3 chronic liver disease, 1 HIV infection, and 1 amphetamine-related PAH.
Data are expressed as mean ± SD for continuous variables and as a number with percentage for categorical variables.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CHD, congenital heart disease; CTD, connective tissue disease; DBP, diastolic blood pressure; ERA, endothelin receptor antagonist; FC, WHO functional class; HB, heart beat; LVeI, left ventricular eccentricity index; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase-5 inhibitor; RA, right atrial area; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell; 6MWD, 6-minute walk distance.
Table 2. Clinical and hemodynamic data of 38 pulmonary arterial hypertension patients with cardiac catheterization examination.
| Variables | Survivor (n = 26) | Non-survivor (n = 12) | p-value |
| Demographics | |||
| Age, years | 41.2 ± 16.2 | 37.7 ± 18.3 | 0.549 |
| Female, n (%) | 23 (88) | 9 (75) | 0.357 |
| BMI, kg/m2 | 21.4 ± 3.9 | 23.4 ± 3.2 | 0.124 |
| SBP, mmHg | 117 ± 15 | 114 ± 12 | 0.538 |
| DBP, mmHg | 72 ± 12 | 73 ± 17 | 0.885 |
| HB, beats/min | 87 ± 18 | 91 ± 16 | 0.502 |
| FC (II/III/IV), n | 5/18/3 | 0/12/0 | 0.108 |
| 6MWD, m | 326 ± 109 | 305 ± 101 | 0.567 |
| Etiology of PAH | |||
| Idiopathic, n (%) | 10 (38) | 4 (33) | 1.000 |
| CTD, n (%) | 11 (42) | 4 (33) | 0.728 |
| CHD, n (%) | 5 (19) | 2 (17) | 1.000 |
| Others*, n (%) | 0 (0) | 2 (17) | 0.940 |
| Hematological and biochemical data | |||
| Hemoglobin, g/dL | 13.9 ± 2.1 | 13.7 ± 2.0 | 0.792 |
| WBC, 1000/μL | 7.3 ± 2.0 | 7.6 ± 2.8 | 0.751 |
| Platelet, 1000/μL | 189 ± 50 | 167 ± 78 | 0.263 |
| AST, U/L | 27 ± 8 | 31 ± 20 | 0.410 |
| ALT, U/L | 24 ± 12 | 24 ± 19 | 0.857 |
| Bilirubin, mg/dL | 1.0 ± 0.5 | 1.0 ± 0.5 | 0.829 |
| Creatinine, mg/dL | 0.75 ± 0.29 | 0.79 ± 0.29 | 0.673 |
| Fasting glucose, mg/dL | 91 ± 16 | 126 ± 59 | 0.040 |
| Cholesterol, mg/dL | 155 ± 42 | 149 ± 35 | 0.735 |
| Uric acid, mg/dL | 7.2 ± 2.3 | 7.5 ± 3.2 | 0.815 |
| Troponin-I, ng/mL | 0.03 ± 0.02 | 0.11 ± 0.15 | 0.089 |
| BNP, pg/mL | 327 ± 368 | 226 ± 198 | 0.470 |
| Echocardiographic data | |||
| Pericardial effusion, n (%) | 6 (23) | 9 (75) | 0.004 |
| Tei index | 0.71 ± 0.29 | 0.84 ± 0.54 | 0.332 |
| SPAP, mmHg | 82 ± 22 | 93 ± 26 | 0.212 |
| TAPSE, cm | 1.88 ± 1.48 | 1.46 ± 0.43 | 0.342 |
| Diastolic LVeI | 1.54 ± 0.37 | 1.70 ± 0.49 | 0.265 |
| Systolic LVeI | 1.73 ± 0.61 | 1.97 ± 0.61 | 0.283 |
| RA, cm2 | 23.7 ± 7.9 | 26.6 ± 9.8 | 0.353 |
| Hemodynamics | |||
| RAP, mmHg | 10 ± 5 | 15 ± 7 | 0.040 |
| SPAP, mmHg | 85 ± 23 | 100 ± 26 | 0.094 |
| DPAP, mmHg | 40 ± 12 | 45 ± 11 | 0.229 |
| MPAP, mmHg | 58 ± 15 | 66 ± 15 | 0.119 |
| PAWP, mmHg | 11 ± 4 | 10 ± 3 | 0.294 |
| Cardiac output, L/min | 3.6 ± 1.0 | 3.6 ± 1.0 | 0.814 |
| Cardiac index, L/min/m2 | 2.5 ± 0.8 | 2.3 ± 0.5 | 0.573 |
| PVR, Wood units | 16.2 ± 8.4 | 15.1 ± 5.3 | 0.714 |
| SVR, Wood units | 27.7 ± 13.7 | 22.1 ± 4.5 | 0.277 |
| SvO2, % | 62 ± 9 | 56 ± 13 | 0.093 |
| SaO2, % | 93 ± 3 | 94 ± 4 | 0.263 |
| PAH-targeted treatment | |||
| PDE5i, n (%) | 23 (89) | 10 (83) | 0.643 |
| ERAs, n (%) | 11 (42) | 5 (42) | 0.970 |
| Prostanoids, n (%) | 7 (27) | 5 (42) | 0.460 |
| Monotherapy, n (%) | 14 (54) | 5 (42) | 0.485 |
| Combination therapy, n (%) | 11 (42) | 6 (50) | 0.658 |
| Two combinations, n (%) | 6 (23) | 3 (25) | 1.000 |
| Three combinations, n (%) | 5 (19) | 3 (25) | 0.689 |
| No PAH-targeted drug, n (%) | 1 (4) | 1 (8) | 0.538 |
* 1 chronic liver disease and 1 HIV infection related PAH.
Data are expressed as mean ± SD for continuous variables and as a number with percentage for categorical variables.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CHD, congenital heart disease; CTD, connective tissue disease; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; ERA, endothelin receptor antagonist; FC, WHO functional class; HB, heart beat; LVeI, left ventricular eccentricity index; MPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE5i, phosphodiesterase-5 inhibitor; PVR, pulmonary vascular resistance; RA, right atrial area; RAP, right atrial pressure; SaO2, systemic arterial oxygen saturation; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; SvO2, mixed venous oxygen saturation; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell; 6MWD, 6-minute walk distance.
Table 3. Results of univariate and multivariate proportional hazard modeling of baseline variables.
| Variables | Univariate model | Multivariate model | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Demographics | ||||
| Age, years | 1.01 (0.98-1.04) | 0.696 | ||
| Male | 1.46 (0.49-4.43) | 0.509 | ||
| BMI, kg/m2 | 1.07 (0.97-1.19) | 0.181 | ||
| SBP, mmHg | 0.99 (0.96-1.01) | 0.267 | ||
| DBP, mmHg | 1.00 (0.97-1.03) | 1.000 | ||
| HB, beats/min | 1.03 (1.00-1.06) | 0.031 | ||
| FC (I/II/III/IV) | 2.48 (1.04-5.90) | 0.041 | 3.55 (1.19-10.55) | 0.023 |
| 6MWD, m | 0.99 (0.99-1.00) | 0.041 | 0.99 (0.99-1.00) | 0.007 |
| Etiology of PAH | ||||
| Idiopathic | 1.15 (0.38-3.49) | 0.812 | ||
| CTD | 1.11 (0.45-2.78) | 0.819 | ||
| CHD | 0.57 (0.19-1.74) | 0.325 | ||
| Others | 2.47 (0.54-11.18) | 0.242 | ||
| Hematological and biochemical data | ||||
| Hemoglobin, g/dL | 0.94 (0.82-1.08) | 0.366 | ||
| WBC, 1000/μL | 1.12 (0.95-1.32) | 0.171 | ||
| Platelet, 1000/μL | 1.00 (0.99-1.01) | 0.666 | ||
| AST, U/L | 1.01 (0.98-1.04) | 0.370 | ||
| ALT, U/L | 1.01 (0.97-1.04) | 0.656 | ||
| Bilirubin, mg/dL | 1.22 (0.46-3.23) | 0.687 | ||
| Creatinine, mg/dL | 0.46 (0.06-3.46) | 0.454 | ||
| Glucose, mg/dL | 1.01 (1.00-1.02) | 0.088 | ||
| Cholesterol, mg/dL | 1.00 (0.99-1.01) | 0.943 | ||
| Uric acid, mg/dL | 1.02 (0.77-1.35) | 0.893 | ||
| Troponin-I, ng/mL | 0.01 (0.01-16.34) | 0.173 | ||
| BNP, pg/mL | 1.00 (0.998-1.002) | 0.665 | ||
| Echocardiographic data | ||||
| Pericardial effusion | 5.29 (2.05-13.65) | 0.001 | 9.15 (3.08-27.19) | < 0.001 |
| Tei index | 1.47 (0.51-4.23) | 0.48 | ||
| SPAP, mmHg | 1.01 (1.00-1.03) | 0.105 | ||
| TAPSE, cm | 0.84 (0.42-1.69) | 0.626 | ||
| Diastolic LVeI | 1.49 (0.56-4.00) | 0.430 | ||
| Systolic LVeI | 1.73 (0.84-3.57) | 0.139 | ||
| RA, cm2 | 1.04 (0.99-1.09) | 0.164 | ||
| Hemodynamics | ||||
| SPAP, mmHg | 1.02 (0.99-1.05) | 0.152 | ||
| DPAP, mmHg | 1.04 (0.98-1.09) | 0.195 | ||
| MPAP, mmHg | 1.03 (0.99-1.07) | 0.163 | ||
| RAP, mmHg | 1.10 (1.02-1.19) | 0.011 | ||
| PAWP, mmHg | 0.97 (0.82-1.15) | 0.716 | ||
| CI, L/min/m2 | 0.84 (0.27-2.65) | 0.772 | ||
| CO (L/min) | 1.12 (0.55-2.28) | 0.753 | ||
| PVR, Wood units | 0.94 (0.84-1.05) | 0.252 | ||
| SVR, Wood units | 0.95 (0.83-1.09) | 0.437 | ||
| SvO2, % | 0.98 (0.93-1.03) | 0.332 | ||
| SaO2, % | 1.02 (0.84-1.24) | 0.835 | ||
| PAH-targeted treatment | ||||
| PDE5i | 1.96 (0.57-6.70) | 0.286 | ||
| ERAs | 0.86 (0.32-2.28) | 0.759 | ||
| Prostanoids | 1.64 (0.58-4.60) | 0.351 | ||
| Monotherapy | 0.84 (0.35-2.03) | 0.694 | ||
| Combination therapy | 1.56 (0.62-3.91) | 0.347 | ||
| Two combinations | 1.93 (0.63-5.96) | 0.251 | ||
| Three combinations | 1.09 (0.31-3.78) | 0.895 | ||
| No PAH-targeted drug | 1.60 (0.37-6.95) | 0.534 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CHD, congenital heart disease; CI, cardiac index; CO, cardiac output, CTD, connective tissue disease; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; ERA, endothelin receptor antagonist; FC, WHO functional class; HB, heart beat; HR, hazard ratio; LVeI, left ventricular eccentricity index; MPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE5i, phosphodiesterase-5 inhibitor; PVR, pulmonary vascular resistance; RA, right atrial area; RAP, right atrial pressure; SaO2, systemic arterial oxygen saturation; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; SvO2, mixed venous oxygen saturation; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell; 6MWD, 6-minute walk distance 6MWD; 95% CI, 95% confidence intervals.
FC and functional capacity
FC was significantly worse in the non-survivors than in the survivors (p = 0.012). Higher FC (HR 2.48, 95% CI 1.04-5.90; p = 0.041), higher heart beat (HR 1.03, 95% CI 1.00-1.06; p = 0.031), and lower 6MWD (HR 0.995, 95% CI 0.991-1.000; p = 0.041) were associated with the risk of mortality in the univariate analysis. In addition, higher FC (HR 3.55, 95% CI 1.19-10.55; p = 0.023) and lower 6MWD (HR 0.99, 95% CI 0.99-1.00; p = 0.007) were also statistically significant in multivariate modeling. The patients with FC I and II exhibited better survival than those with FC III and IV (Figure 3A).
Figure 3.
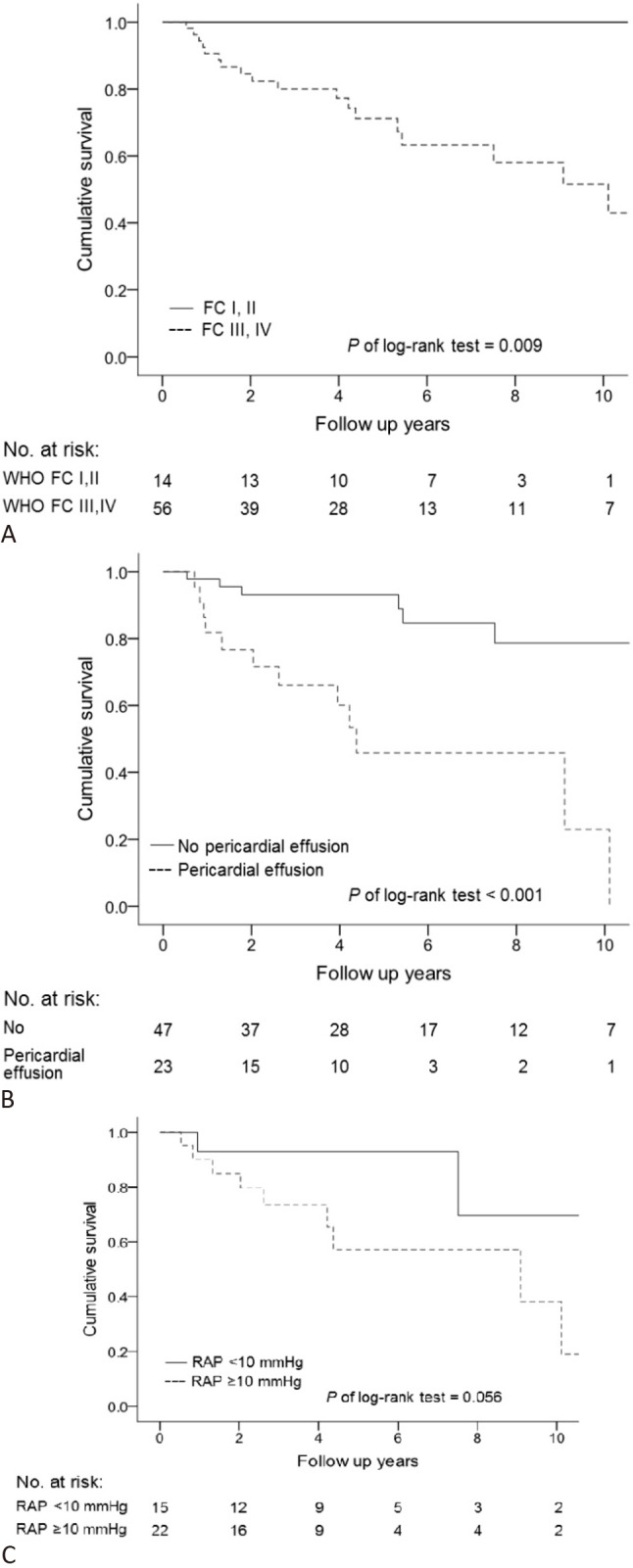
Survival rate of patients stratified by baseline parameters. (A) WHO functional class (FC), (B) pericardial effusion, (C) right atrial pressure.
Hematological and biochemical variables
There were no significant difference in hematological and biochemical parameters except for serum glucose between the survivors and non-survivors. The non-survivors had higher serum glucose levels than the survivors (p = 0.026). However, there was no significant association with risk of mortality in univariate or multivariate analysis.
Echocardiographic parameters
The non-survivors had more frequent pericardial effusion (p = 0.004) and higher estimated systolic pulmonary artery pressure (SPAP) in echocardiography (p = 0.014) than the survivors. The presence of pericardial effusion was a significant predictor of mortality in both univariate (HR 5.29, 95% CI 2.05-13.65; p = 0.001) and multivariate (HR 9.15, 95% CI 3.08-27.19; p < 0.001) analyses. The patients with pericardial effusion had worse survival than those without effusion (Figure 3B).
Right heart catheterization
Among the 38 patients who received cardiac catheterization, the non-survivors had higher serum glucose levels (p = 0.040) and more frequent pericardial effusion (p = 0.004) (Table 2). This was similar to the results in the study population overall (Table 1). Pericardial effusion (HR 5.53, 95% CI 1.49-20.62; p = 0.011) was a predictor of survival in univariate analysis but not in multivariate analysis. A worsening functional class (HR 3.05, 95% CI 1.39-6.67; p = 0.005) and increased alanine aminotransferase (HR 1.04, 95% CI 1.00-1.07; p = 0.037) during follow-up were predictors of survival in univariate analysis but not in multivariate analysis. RAP was significantly higher in the non-survivors than in the survivors (p = 0.040), and it was a significant risk factor of mortality in univariate analysis (HR 1.10, 95% CI 1.02-1.19; p = 0.011) but not in multivariate analysis. The patients with RAP ≥ 10 mmHg had a trend of poor outcomes (Figure 3C).
Etiologies and treatment
There were no significant differences in the etiologies of PAH including IPAH, CTD, CHD, and other associated PAH between the survivors and non-survivors (Table 1). There was also no significant difference in survival curves among the IPAH, CTD-PAH and CHD-PAH groups (log-rank test, p = 0.281) (Figure 4). Regarding PAH-targeted therapy, there were no significant differences among PDE5i, ERA, and prostanoids between the two groups. Moreover, there were no significant differences in monotherapy or combination therapy between the survivors and non-survivors, or in survival curves between those who received PAH-targeted treatment and those who did not receive treatment (log-rank test, p = 0.723) (Figure 5).
Figure 4.
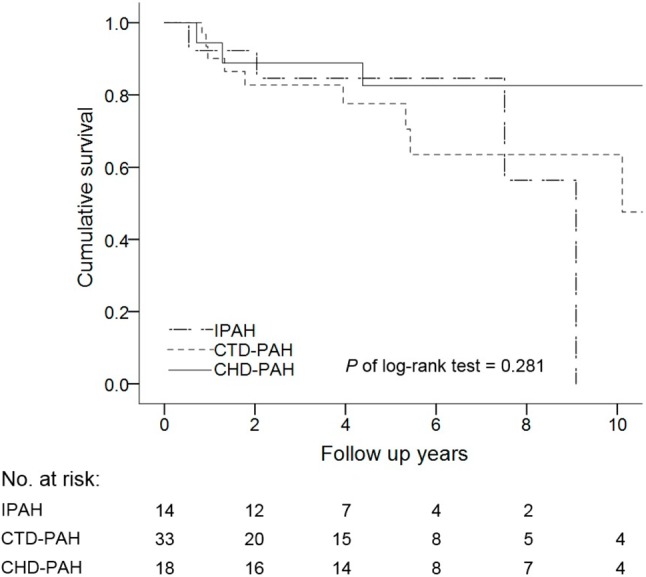
Survival rate of different PAH subgroups including IPAH, CTD-PAH and CHD-PAH. CHD, congenital heart disease; CTD, connective tissue disease; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension.
Figure 5.
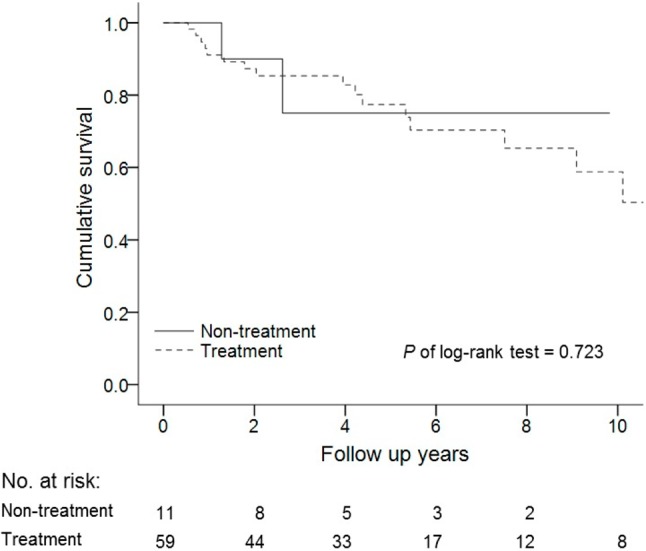
Survival rates of the patients who did and did not receive PAH-targeted treatment. PAH, pulmonary arterial hypertension.
Prognostic importance of changes between baseline and follow-up
The changes between baseline and follow-up parameters related to the risk of mortality are shown in Table 4. A deterioration in FC during follow-up increased the risk of mortality in both univariate (HR 2.21, 95% CI 1.28-3.79; p = 0.004) and multivariate (HR 3.25, 95% CI 1.33-7.91; p = 0.009) analyses. However, a change in 6MWD was an insignificant predictor of mortality. An increase in serum uric acid level was a significant predictor of a poor outcome in univariate analysis (HR 1.36, 95% CI 1.01-1.81; p = 0.041), however it had a trend of association with mortality in multivariate analysis (HR 1.26, 95% CI 0.99-1.59; p = 0.059) during follow-up.
Table 4. Results of univariate and multivariate proportional hazard modeling of changes in variables between baseline and follow-up.
| Variables | Univariate model | Multivariate model | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Demographics | ||||
| ΔBMI, kg/m2 | 0.87 (0.73-1.04) | 0.122 | ||
| ΔSBP, mmHg | 1.00 (0.97-1.02) | 0.697 | ||
| ΔDBP, mmHg | 1.00 (0.97-1.03) | 0.919 | ||
| ΔHB, beats/min | 0.99 (0.97-1.02) | 0.658 | ||
| ΔFC (I/II/III/IV) | 2.21 (1.28-3.79) | 0.004 | 3.25 (1.33-7.91) | 0.009 |
| Δ6MWD, m | 1.00 (0.99-1.00) | 0.061 | ||
| Hematological and biochemical data | ||||
| ΔHemoglobin, g/dL | 0.86 (0.72-1.03) | 0.104 | ||
| ΔWBC, 1000/μL | 1.07 (0.93-1.23) | 0.347 | ||
| ΔPlatelet, 1000/μL | 1.00 (0.99-1.01) | 0.795 | ||
| ΔAST, U/L | 1.02 (0.99-1.04) | 0.253 | ||
| ΔALT, U/L | 1.01 (0.99-1.02) | 0.295 | ||
| ΔBilirubin, mg/dL | 1.11 (0.79-1.55) | 0.546 | ||
| ΔCreatinine, mg/dL | 1.34 (0.91-1.97) | 0.143 | ||
| ΔGlucose, mg/dL | 1.00 (0.98-1.01) | 0.669 | ||
| ΔCholesterol, mg/dL | 0.86 (0.51-1.46) | 0.578 | ||
| ΔUric acid, mg/dL | 1.36 (1.01-1.81) | 0.041 | 1.26 (0.99-1.59) | 0.059 |
| ΔBNP, pg/mL | 1.00 (1.00-1.00) | 0.125 | ||
| Echocardiographic data | ||||
| ΔPericardial effusion | 0.65 (0.28-1.51) | 0.315 | ||
| ΔTei index | 0.97 (0.35-2.64) | 0.945 | ||
| ΔSPAP, mmHg | 0.99 (0.97-1.01) | 0.298 | ||
| ΔTAPSE, cm | 0.97 (0.60-1.58) | 0.905 | ||
| ΔDiastolic LVeI | 1.09 (0.42-2.83) | 0.858 | ||
| ΔSystolic LVeI | 0.56 (0.27-1.16) | 0.116 | ||
| ΔRA, cm2 | 1.02 (0.98-1.06) | 0.325 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; FC, WHO functional class; HB, heart beat; HR, hazard ratio; LVeI, left ventricular eccentricity index; RA, right atrial area; SBP; systolic blood pressure; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell; 6MWD, 6-minute walk distance; 95% CI, 95% confidence intervals.
DISCUSSION
The long-term survival of patients with PAH in this study is comparable with previous reports.4-6 The 1-, 2-, 3- and 5-year survival rates were 93%, 88%, 84%, and 77%, respectively. Since PAH-targeted therapy remains expensive, reimbursement is required for these patients. In addition, the choices of medication may be limited to the etiologies of PAH as in the present study. Several prognostic parameters of PAH, including baseline FC, 6MWD, serum glucose levels, pericardial effusion, and RAP, were identified in this study. A worsening FC and an increased serum uric acid level were predictors of mortality during follow-up.
FC is a subjective but easily obtainable parameter, and it has been shown to be an important prognostic indicator in previous studies.11,12 FC III and IV are associated with more intensive cardiopulmonary involvement and higher mortality than FC I and II.13 Similarly, the patients with FC I and II had better survival than those with FC III and IV in this study. 6MWD is an objective parameter used to evaluate functional capacity and as a prognostic factor.7,14,15 This is consistent with our results in that baseline 6MWD was a predictor of mortality. However, changes in 6MWD after treatment was not significantly related to survival. This is consistent with a recent meta-analysis which showed that improvements in 6MWD were not associated with a beneficial effect on long-term clinical outcomes in patients with PAH.16
An elevated level of serum uric acid significantly affected mortality in the present study. Previous studies have demonstrated that tissue ischemia depletes adenosine triphosphate levels and activates the purine nucleotide degradation pathway to uric acid, which reflects increased xanthine oxidase activity and results in uric acid overproduction in the heart, lungs, liver, and skeletal muscles.17,18 Nagaya et al. found that serum uric acid increased in proportion to the clinical severity of primary pulmonary hypertension, and that this was independently associated with the long-term mortality of patients with primary pulmonary hypertension.19 Our results revealed that although no significant difference existed between the survivors and non-survivors at baseline, the risk of mortality significantly increased in univariate analysis, and a trend of association in multivariate analysis was found in the patients with an elevated level of serum uric acid during follow-up. Therefore, monitoring serum uric acid is important in the clinical follow-up of patients with PAH. Interestingly, the non-survivors appeared to have significantly higher levels of serum glucose than the survivors in the present study. Hyperglycemia is associated with pulmonary hypertension, and it has an adverse effect on survival.20 Among patients with no history of diabetes mellitus, hyperglycemia may reflect undiagnosed diabetes, carbohydrate intolerance, or stress-related carbohydrate intolerance.21 Our results showed a similar tendency in that hyperglycemia adversely affected the outcomes of the patients with PAH.
BNP levels have been reported to be closely related to functional impairment in patients with PAH parallel to the extent of pulmonary hemodynamic changes and right heart failure.22-24 In addition, elevated serum BNP levels have been associated with increased mortality in patients with PAH, and decreased BNP levels after therapy have been associated with improved survival.25 However, there were no significant differences in serum BNP levels in this study between the survivors and non-survivors at baseline or follow-up. This may be due to the relatively high levels of baseline BNP in our patients, who also had high BNP levels as their clinical condition deteriorated.
Echocardiography is an important tool for diagnostic and follow-up studies in patients with PAH. The presence of pericardial effusion is an indicator of right heart failure associated with poor outcomes.26,27 Our results demonstrated that pericardial effusion was more frequently observed in the non-survivors than in the survivors. Moreover, pericardial effusion was a significant predictor of mortality in both univariate and multivariate analyses, suggesting that aggressive treatment strategies are mandatory in these patients. SPAP can be estimated by echocardiography or directly measured via right heart catheterization (RHC).28 There was a significant difference in SPAP between the survivors and non-survivors in echocardiography in this study, but not in RHC. However, not all patients in the present study received RHC, and this may have caused the inconsistent results. Nevertheless, SPAP was not a predictor of survival at baseline or during follow-up in this study. Increased RAP, which can be reflected by an enlarged right atrium and dysfunction of the right ventricle, is an established risk factor for poor outcomes.29,30 RAP was significantly higher in the non-survivors than in the survivors in the present study, which is consistent with the results of previous studies.
No significant differences between the survivors and non-survivors were found in the type of PAH-targeted drug class or mono versus combination therapy in this study. This is in line with many clinical trials in which symptoms of dyspnea, 6MWD, and hemodynamics were improved by PAH-targeted drugs, but not mortality.31-33 Studies with larger populations and longer follow-up periods may be necessary to clarify the effectiveness of such drugs for reducing mortality.
The limitations of this study are as follows. First, the data were collected from a single center, and the number of cases was small. Thus, our findings may not be representative of the entire population of Taiwan. Second, only 38 patients underwent RHC. RHC was not performed because of patients’ or their relatives’ unwillingness, which is most common in our population of "cardiac catheterization phobia". Lastly, the choices of PAH-targeted drugs depended on the reimbursement of the National Health Insurance program in Taiwan. Patients with IPAH can choose from all three classes of drugs, patients with CHD can choose PDE5i and ERA, whereas patients with CTD can only use PDE5i. Combination therapy other than for IPAH is often not permitted. These limitations could have affected the results of the study.
CONCLUSIONS
PAH is a serious disease that remains incurable but manageable. The overall patient survival remained unsatisfactory in this study. Hit "early-and-hard" is the current treatment strategy for this disease.9 The long-term outcomes in the present study may help in planning treatment for patients with PAH. Several prognostic parameters including baseline FC, 6MWD, pericardial effusion, RAP, worsening FC and increased serum uric acid during follow-up were important. Further multicenter investigations are mandatory to determine the prognostic factors in patients with PAH.
Acknowledgments
The authors thank Alfred Hsing-Fen Lin for assistance with the statistical analysis. This study was supported by grants from the Chang Gung Research Grant Foundation (CMRPG3B1021, CMRPG3B1022, and CMRPG3B1023).
CONFLICT OF INTEREST STATEMENT
All the authors declare no conflict of interest.
REFERENCES
- 1.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J. 2010;31:2080–2086. doi: 10.1093/eurheartj/ehq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montani D, Chaumais MC, Guignabert C, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141:172–191. doi: 10.1016/j.pharmthera.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 5.Lee WT, Ling Y, Sheares KK, et al. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40:604–611. doi: 10.1183/09031936.00196611. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS); endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CH, Ho WJ, Huang WC, et al. 2014 guidelines of Taiwan Society of Cardiology (TSOC) for management of pulmonary arterial hypertension. Acta Cardiol Sin. 2014;30:401–444. [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D73–D81. doi: 10.1016/j.jacc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 10.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 12.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39:589–596. doi: 10.1183/09031936.00092311. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Chung L, Zamanian RT, et al. Functional class improvement and 3-year survival outcomes in patients with pulmonary arterial hypertension in the REVEAL registry. Chest. 2013;144:160–168. doi: 10.1378/chest.12-2417. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 15.Fritz JS, Blair C, Oudiz RJ, et al. Baseline and follow-up 6-min walk distance and brain natriuretic peptide predict 2-year mortality in pulmonary arterial hypertension. Chest. 2013;143:315–323. doi: 10.1378/chest.12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savarese G, Paolillo S, Costanzo P, et al. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol. 2012;60:1192–1201. doi: 10.1016/j.jacc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CY, Ma LL, Wang LX. Relationship between serum uric acid levels and ventricular function in patients with idiopathic pulmonary hypertension. Exp Clin Cardiol. 2013;18:e37–e39. [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int. 2011;31:263–267. doi: 10.1007/s00296-010-1557-4. [DOI] [PubMed] [Google Scholar]
- 19.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 20.Abernethy AD, Stackhouse K, Hart S, et al. Impact of diabetes in patients with pulmonary hypertension. Pulm Circ. 2015;5:117–123. doi: 10.1086/679705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grinnan D, Farr G, Fox A, Sweeney L. The role of hyperglycemia and insulin resistance in the development and progression of pulmonary arterial hypertension. J Diabetes Res. 2016;article ID 2481659, 7 pages, doi:10.1155/2016/2481659 doi: 10.1155/2016/2481659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32:503–512. doi: 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 24.Ho WJ, Hsu TS, Tsay PK, et al. Serial plasma brain natriuretic peptide testing in clinical management of pulmonary arterial hypertension. Acta Cardiol Sin. 2009;25:147–153. [Google Scholar]
- 25.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 26.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 27.Sahay S, Tonelli AR. Pericardial effusion in pulmonary arterial hypertension. Pulm Circ. 2013;3:467–477. doi: 10.1086/674302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56–64. doi: 10.1111/j.1751-7133.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 29.Chemla D, Castelain V, Hervé P, et al. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20:1314–1331. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- 30.Austin C, Alassas K, Burger C, et al. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest. 2015;147:198–208. doi: 10.1378/chest.13-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galiè N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox BD, Shimony A, Langleben D. Meta-analysis of monotherapy versus combination therapy for pulmonary arterial hypertension. Am J Cardiol. 2011;108:1177–1182. doi: 10.1016/j.amjcard.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Coeytaux RR, Schmit KM, Kraft BD, et al. Comparative effectiveness and safety of drug therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Chest. 2014;145:1055–1063. doi: 10.1378/chest.13-1864. [DOI] [PubMed] [Google Scholar]


