Abstract
Background
Transthoracic echocardiography is used for assessment of right ventricular (RV) function. Speckle tracking echocardiography (STE) is a new tool to assess myocardial function. The aim of this study was to evaluate RV function using STE in patients with atrial septal defect (ASD) before and the first month after percutaneous closure.
Methods
We prospectively examined 32 consecutive patients (9 male, 23 female) who underwent percutaneous transcatheter closure (PTC) of secundum ASD from June 2013 to December 2015. Echocardiography was initially performed upon admission, prior to cardiac catheterization and then the first month after PTC of secundum ASD. Thereafter, the peak global RV longitudinal strain (RVLSR) was analyzed by two-dimensional STE.
Results
The mean age of the patients was 34.6 ± 8.2 years, and the mean diameter of the occlusive devices was 18.5 ± 7.5 mm. RV end diastolic diameters were significantly larger and decreased significantly after ASD closure (43 ± 5 vs. 38 ± 4 mm, p < 0.05). Left atrium diameters (40 ± 8 vs. 37 ± 6 mm, p < 0.05) decreased significantly after the intervention, whereas left ventricle end-diastolic diameters (45 ± 5 vs. 46 ± 4 mm, nonspecific) remain unchanged. Tricuspid annular plane systolic excursion increased significantly (17.6 ± 5.4 vs. 22.3 ± 8.1 mm, p < 0.05). RV myocardial performance index significantly improved (0.38 ± 0.15 vs. 0.29 ± 0.08, p < 0.05). After interventional closure of the defect, we observed a significant increase of the longitudinal RV strain (28.3 ± 5.6% vs. 22.4 ± 4.3%, p < 0.001).
Conclusions
Two-dimensional strain appears to facilitate the assessment of RV function and its response to correction of volume overload after PTC of secundum ASD.
Keywords: Atrial septal defect, Congenital heart disease, Echocardiography, Right ventricle, Speckle tracking
INTRODUCTION
Atrial septal defect (ASD) accounts for 25-30% of congenital heart defects, which are typically diagnosed during adulthood.1 Ostium secundum ASDs are the most common type of ASDs, constituting 70% of all ASDs and 6-10% of all congenital heart defects. ASDs cause left-to-right shunt, and chronic right chamber volume overload. ASDs can sometimes result in right heart failure; secundum ASDs are centrally located and are generally bounded by the superior and inferior limbic bands, which often make these defects amenable to device closure.2,3
Percutaneous closure of ASDs is associated with improvement in right ventricle (RV) dimension, morphology, function, exercise physiology, and positive remodeling of the RV.4 However, such adaptation may take a long time. Also, this adaptation can be inadequate in adult ASD patients. Left and right atrial diameters and volumes are increased in ASD patients due to volume overload. Also, atrial remodeling after ASD closure have been poorly understood.5
New echocardiographic methods have been developed to quantify global and regional left and RV function. These new echocardiographic methods are important for diagnostic and prognostic evaluation in various cardiovascular diseases.6 A new echocardiographic technique known as two-dimensional speckle tracking echocardiography (2D-STE) is a reliable technique for angle-independent tracking of myocardial deformation. This echocardiographic technique allows noninvasive and quantitative assessment of global or regional myocardial function.7
Myocardial speckle tracking echocardiography measures tissue deformation within the myocardium expressed as a fraction or percentage change. Myocardial tissue lengthening provides a positive and myocardial tissue shortening and gives a negative strain value. Strain rate (SR) measures the local rate of myocardial deformation per time unit. The myocardial global strain and strain rate are measured by averaging the values computed at the segmental values.8 Two-dimensional (2D) strain and SR analyses are new Doppler-independent techniques to obtain these measurements of myocardial movement and deformation. This method has been frequently used to assess LV myocardial function. However, this new echocardiographic method has rarely been used to examine RA and RV myocardial function.9 Myocardial strain and strain rate are able to elucidate RV myocardial function. While this method has been frequently used to assess LV function, it has rarely been used to examine RV function. However, RV myocardial function is very important to determine a prognosis in patients with congenital heart disease.8,9
Aim
The objective of the present study was to quantify RV function in patients with chronic RV volume overload due to an ASD before and after its percutaneous closure.
MATERIAL AND METHODS
Patients
Patients with secundum ASDs admitted to our centre for percutaneous closure between June 2013 and December 2015 were included in this prospective controlled study. We investigated a total of 38 consecutive patients with secundum type ASD. Of that total, six patients with insufficient rims, sinus venosus ASD, and large ASD were not suitable for percutaneous ASD closure. The study consisted of 32 patients (23 female, 9 male, mean age: 41 ± 13 years) with secundum type ASD and normal sinus rhythm who underwent successful percutaneous ASD closure procedure. Clinical indication for ASD closure was haemodynamically significant left-to-right shunt (Qp/Qs > 2.0) or echocardiographic signs of right heart dilation or shunt related symptoms. Patients with a stretched secundum ASD larger than 36 mm, those with inadequate atrial septal rims to permit stable device deployment, or those with proximity to the defect to the atrioventricular valves, the coronary sinus, or the vena cavae, sinus venosus or primum type ASD, pulmonary vascular resistance greater than 8 Woods despite 100% oxygen inhalation, other concomitant congenital heart disease, valvular heart disease, coronary artery disease, LV systolic dysfunction, atrial fibrillation, or hypertension were excluded from the study.
The secundum ASD patients were evaluated by clinical and echocardiographic examinations before and a month after the percutaneous closure ASD procedure. The study was approved by the local ethics committee of our hospital.
Echocardiographic examination
All patients underwent comprehensive transthoracic echocardiography examinations at rest, according to the American Society of Echocardiography guidelines using an ultrasound system (Philips EPIQ 7C, Philips Healthcare, Andover, MA, USA) equipped with a multifrequency transducer (3-8 MHz) and tissue harmonic imaging capability.10 Single lead electrocardiogram was recorded continuously. Pulmonary artery systolic pressure (PASP) was calculated by measuring maximal tricuspid regurgitation velocity, and applying the modified Bernoulli equation to convert this value into pressure values. Estimated right atrial pressure (RAP) was added to this obtained value [PASP = tricuspid regurgitation gradient + RA-pressure (RAP)].
Additionally, 2D echocardiographic images of the RA and RV were obtained in the apical four-chamber view at the end of expiration. These echocardiographic images were obtained while taking care to capture the entire RA, allowing for more reliable delineation of the atrial endocardial border. The frame rate was set at between 40 and 80 Hz. At least three consecutive cardiac cycles of 2D echocardiographic images recorded at each plane were stored in order to select the images with the best quality for off-line speckle tracking analysis.11
Calculation of RV myocardial performance index (MPI)
The RV inflow was recorded with the transducer in the apical 4-chamber view, aligning the Doppler beam as perpendicular as possible to the plane of the tricuspid annulus. The sample volume was placed at the tips of the tricuspid leaflets during diastole. The RV outflow velocity curve was recorded from the parasternal short-axis view, with the Doppler sample volume positioned just below the pulmonary valve. There were time intervals used for calculating the Doppler index, which were measured from the tricuspid inflow and RV outflow recordings.
The RV MPI was calculated as (a – b/b). The interval "a" from cessation to onset tricuspid valve inflow. Ejection time "b" is derived from the duration of RV outflow Doppler velocity profile. Three consecutive beats were measured and averaged for each measurement.12
Two-dimensional speckle tracking analysis
Speckle tracking analysis was performed by a single experienced and independent investigator, who was blinded to the clinical data, using commercially available semiautomated 2D-STE software for a Philips system (2D Wall Motion Tracking, Philips Healthcare Systems). To assess the RV myocardial function with 2D-STE, RV basal septal, basal lateral and apical borders were manually traced in the four-chamber view, followed by automatic tracing of the endocardial and epicardial borders, thus delineating a region of interest composed of six segments. After analysis of segmental tracking quality and manual adjustment of the region of interest, longitudinal strain curves were generated for each atrial segment by the software. A cine loop preview feature allowed visual confirmation that the internal line followed the RV endocardium movements throughout the cardiac cycle. If tracking of the RV endocardium was unsatisfactory, manual adjustments of the region of interest size were performed to ensure optimal tracking. RV longitudinal strain (RVLS) was measured in six segments of the RV. Once approved by the reading analyst, the software displayed longitudinal strain [peak systolic strain (PSS)]. The RV speckle tracking echocardiography imaging before and after percutaneous ASD closure was demonstrated in Figure 1 and Figure 2.9,10,13
Figure 1.
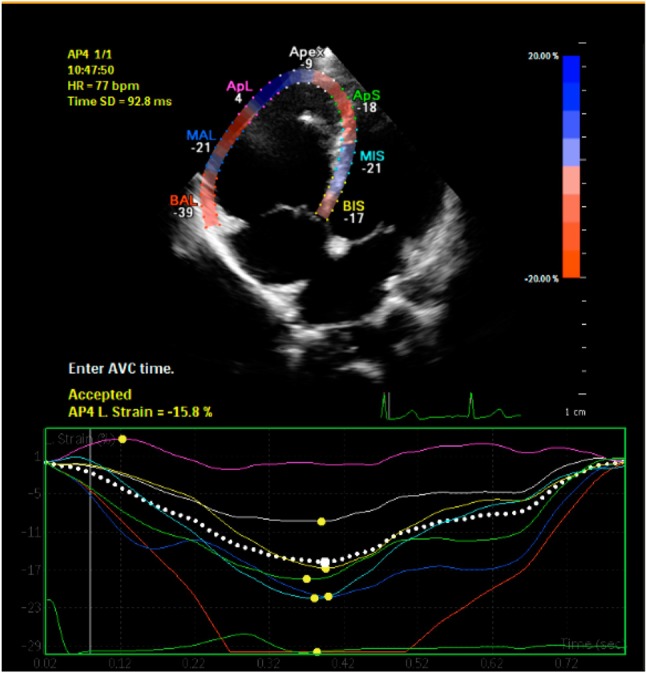
Right ventricle speckle tracking echocardiography imaging before percutaneous ASD closure.
Figure 2.
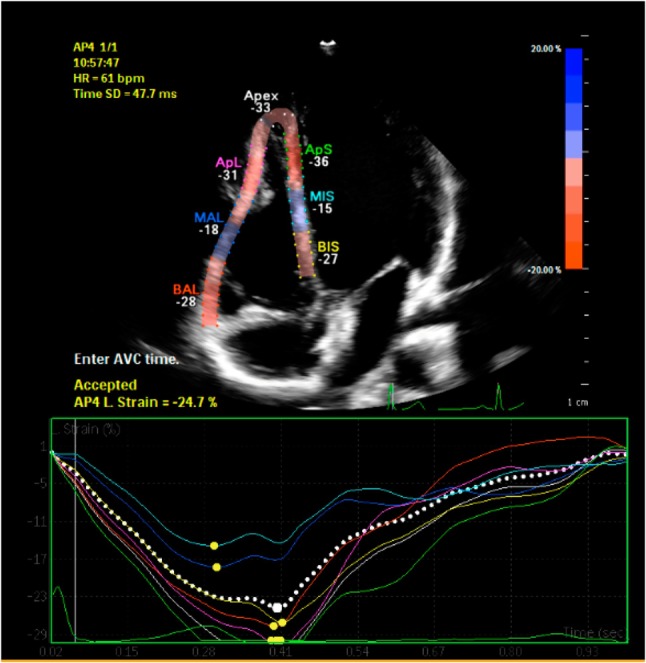
Right ventricle speckle tracking echocardiography imaging after percutaneous ASD closure.
Percutaneous ASD closure procedure
Percutaneous closure of the ASD was performed under general anaesthesia utilizing fluoroscopic and multiplane transesophageal echocardiographic (TEE) guidance. Larger diameters of ASD were measured in different angles of 2D and 3D TEE. Percutaneous closure of ASD was carried out using devices by Occlutech Figulla Flex II ASD (Jena, Germany). Device size was determined by adding 2-4 mm larger ASD diameter. In addition, the distance to the defect is required to be at least 5 mm away from the mitral valve, right upper pulmonary vein, coronary sinus, tricuspid valve, inferior vena cava, and superior vena cava. In the case of deficient aortic rim (aortic rim < 5 mm), percutaneous closure was performed if the other rims had enough length. However, balloon measurements were not performed routinely.11,13 Post-interventional treatment included 100 mg/d of acetylsalicylic acid and 75 mg/d of clopidogrel for 6 months.
Statistical analysis
Statistical analysis was carried out with the SPSS statistical package (Version 12.0; SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as percentages. Intra-observer variability was calculated as the absolute difference between the two measurements as a percent of their mean. The Student’s t-test and the Chi-square test were used for comparison of data as appropriate. Differences between baseline and follow-up were analysed by the paired sample t-test. A p value < 0.05 was considered statistically significant. A Pearson’s correlation was used to determine the relationship between RV STE and other echocardiographic parameters.
RESULTS
The mean native diameter of secundum ASD on colour Doppler echocardiographic evaluation was 14 ± 5 mm (ranging from 10 to 22 mm). The mean diameter of the ASD devices used in these patients was 18.5 ± 7.5 mm (ranging 14-28 mm). Percutaneous secundum ASD closure was performed successfully in all 32 patients. Basic demographic characteristics and percutaneous ASD closure-related clinical parameters of the study population are summarized in Table 1. There was no residual ASD observed in any of the patients on echocardiographic evaluations at the first month after the procedure. The echocardiographic measurements are provided in Table 2. RV end diastolic diameters were significantly larger before ASD closure, and RV end diastolic diameters were decreased significantly after ASD closure (43 ± 5 vs. 38 ± 4 mm, p < 0.05). LA diameters (40 ± 8 vs. 37 ± 6 mm, p < 0.05) decreased significantly after the intervention, whereas LV end-diastolic diameters (45 ± 5 vs. 46 ± 4 mm, nonspecific) remained unchanged. Tricuspid annular plane systolic excursion (TAPSE) increased significantly (17.6 ± 5.4 vs. 22.3 ± 8.1 mm, p < 0.05). Also, RV MPI was significantly increased before ASD closure, and RV MPI was significantly decreased after ASD closure (0.38 ± 0.15 vs. 0.29 ± 0.08, p < 0.05).
Table 1. Clinical characteristics and echocardiographic measurements in patients with ASD.
| Variable | Parameters |
| Age (years) | 34.6 ± 8.2 |
| Gender (female/male) | 23/9 |
| Systolic blood pressure, mmHg | 123 ± 15 |
| Diastolic blood pressure, mmHg | 69 ± 9 |
| Heart rate, beats/min | 93 ± 11 |
| ASD diameter, mm | 14 ± 5 |
| Device diameter, mm | 18.5 ± 7.5 |
| Qp/Qs ratio | 2.6 ± 0.7 |
| BMI, kg/m2 | 1.89 ± 0.16 |
ASD, atrial septal defect; BMI, body mass index.
Table 2. Effect of ASD device closure on echocardiographic parameters.
| Variable | Pre-procedure | Post-procedure first month | p value |
| LV end-diastolic diameter, mm | 45 ± 5 | 47 ± 4 | 0.683 |
| LV end-systolic diameter, mm | 28 ± 5 | 29 ± 4 | 0.784 |
| LV EF, % | 58 ± 3 | 63 ± 4 | 0.036 |
| LA diameters, mm | |||
| Antero-posterior | 37 ± 8 | 34 ± 5 | 0.041 |
| Medio-lateral | 40 ± 8 | 37 ± 6 | 0.039 |
| Apico-basal | 48 ± 5 | 47 ± 6 | 0.044 |
| RA diameters, mm | |||
| Medio-lateral | 43 ± 7 | 37 ± 6 | 0.015 |
| Apico-basal | 50 ± 6 | 46 ± 5 | 0.026 |
| RV end-diastolic diameter, mm | 43 ± 5 | 38 ± 4 | 0.017 |
| PASP, mmHg | 51.4 ± 16.3 | 37.3 ± 12.7 | 0.030 |
| TAPSE, mm | 17.6 ± 5.4 | 22.3 ± 8.1 | 0.025 |
| Pulsed Doppler RV MPI | 0.38 ± 0.15 | 0.29 ± 0.08 | 0.040 |
| RV longitudinal strain (%) | 22.4 ± 4.3 | 28.3 ± 5.6 | 0.0008 |
ASD, atrial septal defect; EF, ejection fraction; LA, left atrium; LV, left ventricle; MPI, myocardial performance index; PASP, pulmonary artery systolic pressure; RA, right atrium; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
After interventional closure of the defect, we observed a significant increase of the longitudinal RV strain (28.3 ± 5.6% vs. 22.4 ± 4.3%, p < 0.001) (Table 2). Intra-observer variability for RV longitudinal strain was 3.4 + 1.5%.
Correlation analysis were measured utilizing those changes post- and pre-ASD parameters. Delta value is calculated as post-ASD parameter minus pre-ASD parameter. The correlation analysis showed a negative correlation among delta RV STE and delta RV MPI, delta RV diameter, delta RA diameter, and delta PASP. Also, we found a positive correlation between delta RV STE and delta TAPSE. However, there was no correlation among delta RV STE and delta LV ejection fraction (LVEF), delta LV diameter, and delta LA diameter (Table 3).
Table 3. Correlation among RV STE and other echocardiographic parameters in patients with ASD.
| Parameters | Delta value (Post ASD parameters – pre ASD parameters) | Pearson’s correlation coefficient (r value) | p value |
| RV MPI | -0.09 ± 0.011 | -0.481 | 0.028 |
| RV end-diastolic diameter | -4.9 ± 4.23 | -0.569 | 0.021 |
| RA diameter | -5.23 ± 5.76 | -0.154 | 0.042 |
| LV end-diastolic diameter | +2.19 ± 4.45 | 0.126 | 0.058 |
| LA diameter | -2.25 ± 4.9 | -0.132 | 0.064 |
| LV EF | +5.13 ± 3.38 | 0.119 | 0.067 |
| PASP | -14.1 ± 13.8 | -0.497 | 0.035 |
| TAPSE | +4.7 ± 6.9 | 0.514 | 0.029 |
ASD, atrial septal defect; EF, ejection fraction; LA, left atrium; LV, left ventricle; MPI, myocardial performance index; PASP, pulmonary artery systolic pressure; RA, right atrium; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
DISCUSSION
Assesment of the RV myocardial function is important to determine prognosis, and is an independent predictor of cardiovascular morbidity and mortalityin patients with left to right intracardiac shunts and pulmonary arterial hypertension.14 2D echocardiography is frequently used evaluation of RV function. However, the 2D assessment of the RV function is very challenging due to complex RV morphology.15 In the past decade, a novel echocardiographic technique known as strain has been used to make more accurate assessments of the myocardial function.16 Speckle tracking echocardiography has been used in a wide range of cardiovascular conditions, including hypertension, heart failure and myocardial infarction. Conventional techniques of determining RV systolic function often rely on visual assessment of wall motion and changes in volume.15 However, 3D echocardiography is a reliable quantitation of RV volumes, function, and mass.17 Also, STE measures actual tissue deformation within the myocardium. STE measures global and regional myocardial function.18
In our study, an improvement of multiple echocardiographic parameters such as the RVLS, RVEDD, right atrial diameter, RV/LV EDD ratio, left atrial diameter, RV MPI, and PASP was noted in the first month post-procedure period. LVDD and LVSD remained unchanged in the first month post-procedure period. However, LVEF and TAPSE were significantly increased in the first month post-procedure period. In our study, we found a positive correlation among delta RVSTE and delta LVEF and TAPSE. Also, we found a negative correlation among delta RVSTE and delta RV MPI, RV diameter, RA diameter, and PASP. Previous studies have also demonstrated similar findings of decreased right atrial and ventricular dimensions after percutaneous ASD closure. Akula et al. showed that RV volumes decreased significantly in the first month after ASD device closure and continued up to 6 months.17 Veldtman et al. found that right heart morphology undergoes rapid improvement within one month of percutaneous ASD closure.16 Atashband et al. showed that percutaneous ASD closure in adults is effective with reverse remodeling and better functional capacity.18 Thilén et al. found that cardiac remodeling after ASD closure in adults is common and completed within the first half-year after closure.19
We demonstrated that peak RVLS was shown to be significantly increasing after percutaneous closure of ASD. However, Elsheikh et al. found that volume overload induced by ASD is associated with increased strain values, which return to normal after closure.20 Also, Jategaonkar et al. found that volume overload in patients with ASD is associated with increased strain values.5 Strain values return to normal after abolishment of the volume overload with percutaneous ASD closure. On the other hand, Teo et al. found that a significant reduction in RV volumes at 6 months after ASD closure and RV ejection fraction (RVEF) was significantly increased in cardiovascular magnetic resonance (CMR).21 CMR is an accurate and reproducible imaging modality for the assessment of cardiac function and volumes. Study results of Teo et al. are similar to our study findings. In accordance with the study by Teo et al, we observed a significant reduction in RV volumes at the first month post ASD closure, and significantly improved RV function with speckle tracking and RV MPI.
RV function is difficult to assess because of its complex structures. MPI does not depend on any geometric assumption. Also, MPI is not age or heart rate dependent. In previous studies, MPI has been used to assess RV function in patients with congenital heart disease. Ding et al. found that MPI increased significantly in patients with ASD compared to the control subjects.22 Also, they found that after transcatheter closure, MPI decreased markedly in patients with ASD. Also, study results of Ding et al. are similar to our study findings. In accordance with the study by Ding et al, we observed a significant reduction in RV volumes at the first month after ASD closure, and significantly improved RV MPI.
Study limitations
There were some limitations to our study. Several studies have cited significant hemodynamic changes occurring in the first months after ASD closure.16,19,23 The present study was designed based on this evidence. However, patient follow-up of more than 1 month is needed for a complete evaluation of hemodynamic changes. Our study was conducted on a limited number of patients, which made it difficult to arrive at a definitive statement. In theory, myocardial strain can be measured in all dimensions (longitudinal, radial, and circumferential). Assessment of the RV function is very difficult due to complex RV morphology. We quantified the longitudinal strain, assuming that the contraction of the RV is predominantly longitudinal. In this study, the 2D strain analysis software that was originally designed for the left ventricle was applied to the RV, assuming that the algorithm can be transferred. Further studies about reproducibility and feasibility are necessary to validate this method.
CONCLUSIONS
2D-STE measurement is a novel approach used to quantitatively assess myocardial wall motion, which can also be applied to the RV. This method allows exact and objective assessment of global and regional myocardial function. The long-term volume overload is associated with decreased strain values and RV function, which return to normal after abolishment of the volume overload.
REFERENCES
- 1.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645–1653. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt AB, Landzberg MJ, Wu FM. Atrial Septal Defect. In. Crawford MH, DiMarco JP, Paulus WJ, editors: Cardiology. 3rd Edition. Philadelphia: Elsevier; 2010. pp. 1441–1446. [Google Scholar]
- 4.Walker RE, Moran AM, Gauvreau K, Colan SD. Evidence of adverse ventricular interdependence in patients with atrial septal defects. Am J Cardiol. 2004;93:1374–1377. doi: 10.1016/j.amjcard.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Jategaonkar SR, Scholtz W, Butz T, et al. Two-dimensional strain and strain rate imaging of the right ventricle in adult patients before and after percutaneous closure of atrial septal defects. Eur J Echocardiogr. 2009;10:499–502. doi: 10.1093/ejechocard/jen315. [DOI] [PubMed] [Google Scholar]
- 6.Sakata K, Uesugi Y, Isaka A, et al. Evaluation of right atrial function using right atrial speckle tracking analysis in patients with pulmonary artery hypertension. J Echocardiogr. 2016;14:30–38. doi: 10.1007/s12574-015-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marwick T. Measurement of strain and strain rate by echocardiography. Ready for prime time? J Am Coll Cardiol. 2006;47:113–127. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Vitarelli A, Sardella G, Roma AD, et al. Assessment of right ventricular function by three-dimensional echocardiography and myocardial strain imaging in adult atrial septal defect before and after percutaneous closure. Int J Cardiovasc Imaging. 2012;28:1905–1916. doi: 10.1007/s10554-012-0022-8. [DOI] [PubMed] [Google Scholar]
- 10.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Feigenbaum H, Mastouri R, Sawada S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circulation. 2012;76:1550–1555. doi: 10.1253/circj.cj-12-0665. [DOI] [PubMed] [Google Scholar]
- 12.Ozturk O, Ulgen MS, Tekes S, et al. Influence of the angiotensin converting enzyme I/D gene polymorphisms on right ventricular myocardial performance index in patients with a first acute anterior myocardial infarction. Circ J. 2005;69:211–215. doi: 10.1253/circj.69.211. [DOI] [PubMed] [Google Scholar]
- 13.Aslan M, Erturk M, Turen S, et al. Effects of percutaneous closure of atrial septal defect on left atrial mechanical and conduction functions. Eur Heart J Cardiovasc Imaging. 2014;15:1117–1124. doi: 10.1093/ehjci/jeu089. [DOI] [PubMed] [Google Scholar]
- 14.Favot M, Courage C, Ehrman R, et al. Strain echocardiography in acute cardiovascular diseases. West J Emerg Med. 2016;17:54–60. doi: 10.5811/westjem.2015.12.28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Veldtman GR, Razack V, Siu S, et al. Right ventricular form and function after percutaneous atrial septal defect device closure. J Am Coll Cardiol. 2001;37:2108–2113. doi: 10.1016/s0735-1097(01)01305-5. [DOI] [PubMed] [Google Scholar]
- 17.Akula VS, Durgaprasad R, Velam V, et al. Right ventricle before and after atrial septal defect device closure. Echocardiography. 2016;33:1381–1388. doi: 10.1111/echo.13250. [DOI] [PubMed] [Google Scholar]
- 18.Atashband A, Lakkis N. First comprehensive analysis of outcomes in adult patients after percutaneous closure of isolated secundum atrial septal defects. Cardiovasc Hematol Agents Med Chem. 2015;13:63–69. doi: 10.2174/187152571301150730115936. [DOI] [PubMed] [Google Scholar]
- 19.Thilén U, Persson S. Closure of atrial septal defect in the adult. Cardiac remodeling is an early event. Int J Cardiol. 2006;108:370–375. doi: 10.1016/j.ijcard.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Elsheikh RG, Hegab M, Szatmari A. NT-proBNP correlated with strain and strain rate imaging of the right ventricle before and after transcatheter closure of atrial septal defects. J Saudi Heart Assoc. 2013;25:3–8. doi: 10.1016/j.jsha.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo KS, Dundon BK, Molaee P, et al. Percutaneous closure of atrial septal defects leads to normalisation of atrial and ventricular volumes. J Cardiovasc Magn Reson. 2008;1;10:55. doi: 10.1186/1532-429X-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Ma G, Wang C, et al. Acute effect of transcatheter closure on right ventricular function in patients with atrial septal defect assessed by tissue Doppler imaging. Acta Cardiol. 2009;64:303–309. doi: 10.2143/AC.64.3.2038014. [DOI] [PubMed] [Google Scholar]
- 23.Pascotto M, Santoro G, Cerrato F, et al. Time-course of cardiac remodeling following transcatheter closure of atrial septal defect. Int J Cardiol. 2006;112:348–352. doi: 10.1016/j.ijcard.2005.10.008. [DOI] [PubMed] [Google Scholar]


