Abstract
Background
Regular physical exercises may help people to be more resistant to everyday problems; however, how acute and intense exercises affect the heart tissues functioning with maximum capacity and how melatonin changes the effect of acute and intense exercises are still not obvious. We aimed to comprehend whether melatonin intravenous injection supports the oxidative/antioxidative conditions and energy charge in heart tissues of rats exposed to acute swimming exercise.
Methods
Thirty Wistar-albino male rats were categorized into 3 groups with equal number of subjects. Control group performed no application, and acute intensive swimming exercise group were subjected to acute intensive swimming exercise for 30 minutes, and melatonin group were applied 25 mg/kg single dose melatonin administration prior to 30 minutes acute intensive swimming exercise. The levels of malondialdehyde (MDA), and superoxide dismutase, catalase and glutathione peroxidase activities were measured by spectrophotometric method; and the levels of 3-nitrotyrosine (3-NT) and energy charge were determined by a high performance liquid chromatography.
Results
Tissue MDA and 3-NT levels of the acute intensive exercise group were found to be higher than the control group. It was also found that the melatonin administration increased the energy charge and antioxidant activities, while decreased tissue MDA and 3-NT levels in heart tissues. Our results provide evidence for melatonin that can exert potent protective effects on oxidative stress and energy charge for heart tissues in acute swimming exercise.
Conclusions
These findings suggest that the direct beneficial effects of melatonin could be potentially applied on prevention of oxidative stress and energy deficit.
Keywords: Aerobic capacity, Cardiac pharmacology, Cardiovascular risk
INTRODUCTION
Regular physical exercise may help people to be more resistant to problems such as the development of cardiovascular events and organisms due to changes in body composition and blood pressure.1 Likewise, strenuous sports such as swimming and running, when conducted reasonably, may protect the heart against diseases by raising resistance to oxidative stress. However, when they are performed intensively, they can lower the stability of the heart, increase oxidative stress, induce apoptosis, and lead to heart injuries.2,3 Nonetheless, even mild exercise can raise oxygen consumption by 8-10 times, and oxygen flow through the muscles can increase by 90-100 times.4 It has been reported that very arduous dynamic exercise approaching an anaerobic situation can result in more intense oxidative stress.5 When organs such as the heart become ischemic, the production of reactive oxygen species (ROS) increases after stopping the exercise and the resumption of tissue blood flow.6
Melatonin is a largely neurotransmitter-like compound derived principally from the pineal gland. The diverse range of actions and biological functions of melatonin suggest the potential for a number of clinical and health improving uses.7 Therefore, melatonin has attracted increasing attention for the therapeutic management of various diseases. Many scientific studies have demonstrated that melatonin can reduce lipid peroxidation through a free radical scavenging effect and also by directly raising antioxidant activity.8,9 An early investigation also suggested that pharmacological and physiological concentrations of melatonin can preserve deoxyribonucleic acid (DNA) from damage by free radicals.10
The overproduction of ROS causes DNA fragmentation that maybe detrimental to heart tissue through peroxidative harm to the mitochondria and plasma membrane.11 Since cell growth and protein regeneration are low after the embryonic phase in the heart, antioxidant shielding capacity is restricted in this organ.2,3 Accordingly, factors increasing heart metabolism may jeopardize heart muscles.12 The mechanism by which exercise protects against heart injuries is unclear. One hypothesis is an increase in mitochondrial superoxide dismutase (SOD) activity and adenosine triphosphate (ATP)-sensitive potassium channels on the sarcolemma land mitochondrial inner membranes.13 However, how acute and intense exercise affects working heart tissues with a maximum capacity and how melatonin changes the effect of acute and intense exercises are still unclear. The combined effects of melatonin and acute intensive swimming training on heart tissues have not been investigated simultaneously. Therefore, we conducted this study to evaluate the effects of exercise and injections of melatonin on oxidative/antioxidative parameters and energy charge in rat hearts after acute exercise.
MATERIALS AND METHODS
Animal procedure
The experiments were performed on 30 healthy male Wistar rats weighing 260-300 g (3 months old) which were randomly selected from the Erciyes University, Experimental Research and Application Centre. Six rats were placed per cage under standard laboratory conditions, with a 12/12-hour light-dark cycle (lights on at 7:00 a.m.), an ambient temperature of 23 °C-25 °C, and 55 ± 5% humidity. The animals were fed with standard laboratory food and water ad libitum. Before the experiment was commenced, the animals were given a one-week acclimation period. The animal protocols used in this work were evaluated and approved by the Experimental Animals Ethics Committee of Erciyes University (Protocol: 5-08/25-09.04.2008). The experimental procedures were performed in the central laboratory of Erciyes University in accordance with the national guidelines and protocols for care and use of laboratory animals, approved by the Institutional Animal Ethical Committee (Law no: 2911/2007).
Study groups
The control group was fed a normal diet and was not subjected to any procedures. Stress caused by injections in the other groups may have led to changes in biochemical parameters; and therefore physiological saline was injected into the control group to induce the same level of stress. The rats were sacrificed under ether anesthesia, and heart tissue samples were taken and frozen in liquid nitrogen at -80 ° C until analysis. Total tissue homogenate was used for biochemical determinations.
The acute exercise group were made to perform swimming exercises in a container (length 100 cm, width 50 cm, depth 50 cm) containing water kept at a constant 37 °C. The swimming exercises were conducted as a one-time (30 minutes) acute intensive exercise session.14 The rats were sacrificed under ether anesthesia, and heart tissue samples were taken and frozen in liquid nitrogen at -80 °C until analysis. Total tissue homogenate was used for biochemical determinations.
The melatonin group were made to do swimming exercises as a one-time (30 minutes) acute intensive exercise session. After 20 minutes, the animals in this group were given intravenous injections of 25 mg/kg melatonin as an intraperitoneal single dose.15 The rats were sacrificed under ether anesthesia, and heart tissue samples were taken and frozen in liquid nitrogen at -80 °C until analysis. Total tissue homogenate was used for biochemical determinations.
The release of melatonin has a special circadian rhythm. Melatonin level starts to rise in the evening from 21:00-22:00, and reaches the highest level (50-200 pg/dl) at 02:00-04:00. It then starts to decrease from 05:00-07:00 in the morning, and falls to basal levels (0-20 pg/dl) after 07:00 a.m.16 Accordingly, the melatonin injections, acute swimming exercise and sacrifice of the rats for extraction of the heart tissues were conducted in all groups at same time between 11:00-12:00 in the daytime.
Tissue preparation and biochemical analysis
Measurement of 3-nitrotyrosine
Heart tissues were homogenized to 0.5 g of tissue/ 1.5 ml potassium phosphate buffer (50 mM, pH 7.4). The nitrotyrosine levels of the serum samples were measured using the high-performance liquid chromatography (HPLC) method described by Cimen et al.15 The acid hydrolysis process for the serum samples was performed as follows: 0.3 ml of serum was used to precipitate protein, with the addition of an equal amount of 10% trichloroacetic acid (TCA) to serum and vortexed. After centrifugation at 3000 rpm for 10 minutes, the supernatants were separated and the protein precipitates were hydrolyzed in 6 N HCl at 100 °C for 18-24 hours. Sonication was performed in order to increase the acid penetration for hydrolysis to take place in whole protein content and in order for hydrolysis to take place in whole protein content. The samples were placed in specific hydrolysis tubes after sonication, and analyzed with a Hewlett Packard 1050 diode array detector HPLC system (Hewlett Packard, Waldbron, Germany). The analytical column was a 5-μm pore size Spherisorb ODS-2 C18 reverse-phase column (4.6 × 250 mm; Alltech, Dearfield, IL, USA). The guard column was a C18 cartridge (Alltech), and the mobile phase was 50 mmol/l sodium acetate/50 mmol/l citrate/8% (v/v) methanol pH 3.1. HPLC analysis was conducted under isocratic conditions at a flow range of 1 ml/dk, with the ultraviolet detector adjusted to 274 nm. nTyr peaks were found in accordance with its retention time, and approved by spiking with added exogenous 3 nTyr. Concentrations of nTyr were computed from a nTyr standard curve and stated as nmol/g tissue.17
Determination of malondialdehyde levels
The heart tissue samples were homogenized in 50 mM Tris-HCl (pH 7.4), and levels of heart tissue malondialdehyde (MDA) were measured using the thiobarbituric acid method,18 in which 1.5 ml of 0.8% thiobarbituric acid was added to 1 ml of the heart tissue homogenate sample followed by the addition of 0.4 ml of 8.1% sodium dodecyl sulfate and 1.5 ml of acetic acid. The mixture was then made upto 5 ml with distilled water and put in a water bath at 95 °C for 1 hour. After being allowed to cool, 1.0 ml of distilled water and 5 ml of the mixture of n-butanol and pyridine (15:1, v/v) were added. The mixture was vortexed and after centrifugation at 4000 rpm for 10 minutes, absorbance of the upper layer was measured using a spectrophotometer at 532 nm against a blank utilizing distilled water. The outcomes were reported as nmol/g tissue.
Determination of SOD activity
The heart tissues were homogenized with distilled water at a ratio of 1/10 (v/v). The supernatants were mixed with a chloroform/methanol mixture ratio at 1/1 (v/v), then the homogenates were centrifuged at 5000 xg for 2 hours at 4 °C. SOD activity was determined in the supernatant obtained after centrifugation according to the method developed by Sun et al.19 The principle of the method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine-xanthine oxidase system as a superoxide generator. NBT was used to form blue formazan which has an absorbance at 560 nm. SOD activity was also expressed as units per milliliter for heart tissue measurements and units per milligram heart tissue protein for tissue measurements. The results were expressed as U/mg protein.
Determination of glutathione peroxidase activity
The heart tissues were homogenized in 50 mM phosphate buffer containing 0.5 mM EDTA (pH 7.5). The homogenates were then centrifuged at 12000 xg for 15 minutes at 4 °C. Glutathione peroxidase (GPx) activity was determined in the supernatant obtained after centrifugation. Absorbance at 340 nm was recorded for 5 minutes, and the activity was calculated from the slope of these lines as μmoles of nicotinamide adenine dinucleotide phosphate (NADPH) oxidized per minute taking into account that the millimolar absorption coefficient for NADPH is (6.22 × 103 M-1.cm-1). One unit of GPx was defined as the amount of enzyme required to oxidize 1 μmol of NADPH/min. The results were expressed as U/g protein.20
Determination of catalase activity
The heart tissues were homogenized in 50 mM phosphate buffer (pH 7.4) ratio of 1/10 (v/v). The homogenates were then centrifuged at 15000 xg for 15 minutes at 4 °C. Catalase (CAT) activity was determined in the supernatant obtained after centrifugation. CAT activity was measured using a method which takes advantage of the peroxidatic activity of catalase and allows a semiautomated method to be performed. In phosphate buffered medium, the destruction of hydrogen peroxide by the effect of catalase of the sample caused a decrease in absorbance as 240 nm. The results were expressed as k/g-protein.21 Protein concentrations in the heart tissues were measured spectrophotometrically using the Lowry method.22
Measurements of adenosine monophosphate (AMP), adenosine diphosphate (ADP) and ATP levels
Heart tissue (0.15 g) in 1.5 ml ice-cold 0.6 N perchloric acid was homogenized and placed on ice for 1 hour, followed by neutralization with 1 mol I-1 of K2HPO4, centrifugation for 15 minutes at 10000 g at 4 °C, and then filtration through a 0.2-μ m syringe filter. The supernatant was stored at 70 °C until analysis. AMP, ADP and ATP were measured using an HPLC diode array detector at a wavelength of 254 nm. The analytical column was 4.6 × 250 mm, (Allosphere ODS-2, C18 5 mm reverse-phase column). The mobile phase was 160 mM KH2PO4 with 100 mM KCl at pH 6.5. AMP, ADP and ATP peaks were identified according to the corresponding retention times and confirmed by ‘spiking’ with added exogenous AMP, ADP and ATP. Concentrations of AMP, ADP and ATP were calculated from standard curves and expressed as mmolg/1 tissue. The cellular energy charge was calculated as ([ATP] + 0.5[ADP])/([AMP] + [ADP] + [ATP]).15
Statistical analysis
Statistical analyses were performed using SPSS software version 21 and 3.5 sigmastat (IBM Corp., Armonk, New York, USA). The mean values for the groups were compared using the Student’s t test. Non-parametric analysis of the data did not fit normal distribution performed with the Mann-Whitney U test. Group mean differences were examined using unpaired (for group comparisons) or paired t-tests (for within group comparisons before and after treatment). The normally distributed data were presentedas mean ± standard deviation, and median (min-max) for non-normally distributed data. The Shapiro-Wilk test was used to assess compliance with normal distribution. Groups were assessed using Kruskal-Wallis analysis. The Student-Newman-Keuls test was used for non-parametric multiple comparisons. Statistical significance was set at 0.05.
RESULTS
The energy charge levels are shown in Table 1. Acute exercise resulted in depletion of energy charge of the rat heart tissues compared to the controls (p < 0.001; Figure 1). The results indicated that the mean heart tissue energy charge was significantly (p < 0.001) higher in the melatonin group compared with the control and acute exercise groups (Table 1). AMP levels were not measurable in the study groups. ADP and ATP levels decreased by 45-50% in the acute exercise group compared to the controls. The mean heart tissue ADP and ATP levels were significantly (p < 0.001) higher in the melatonin group compared with the acute exercise group (Table 1). The mean heart tissue MDA levels were significantly higher in the acute exercise group compared with the control and melatonin groups (Figure 2). 3-nitrotyrosine (3-NT) levels were not measurable in the control group (Figure 3). The results indicated that the mean heart tissue 3-NT levels were significantly (p < 0.001) lower in the melatonin group compared with the acute exercise group (0.032 ± 0.01 vs. 0.090 ± 0.01 ng/ml, respectively). On the other hand, CAT, GPx and SOD activities were significantly lower in the acute exercise group compared with the control and melatonin groups (p < 0.001). Although there were significant differences between the melatonin group and control group in terms of CAT, GPx and SOD activities, the values of the control and melatonin groups were close to each other (Table 2; Figure 4; Figure 5).
Table 1. The results of the categorical comparison between study groups in terms of heart tissue levels of energy charge, AMP, ADP and ATP.
| Parameters | Study groups | Comparisons | ||||
| Control (n = 10) | Acute exercise (n = 10) | Melatonin (n = 10) | Control/acute exercise | Control/melatonin | Acute exercise/melatonin | |
| AMP (μmol/g) | - | - | - | - | - | - |
| ADP (μmol/g) | 13.41 ± 8.3 | 7.61 ± 30 | 13.78 ± 4.5 | p > 0.05 | p > 0.05 | p < 0.05 |
| ATP (μmol/g) | 0.52 ± 0.30 | 0.32 ± 0.20 | 0.62 ± 0.23 | p > 0.05 | p > 0.05 | p < 0.05 |
| Energy charge | 0.86 ± 0.42 | 0.62 ± 0.35 | 1.55 ± 0.59 | p > 0.05 | p < 0.05 | p < 0.001 |
AMP, adenosine monophosphate; ADP, adenosine diphosphate; ATP, adenosine triphosphate; SD, standard deviation.
Data are expressed as mean ± SD for continuous variables.
Figure 1.
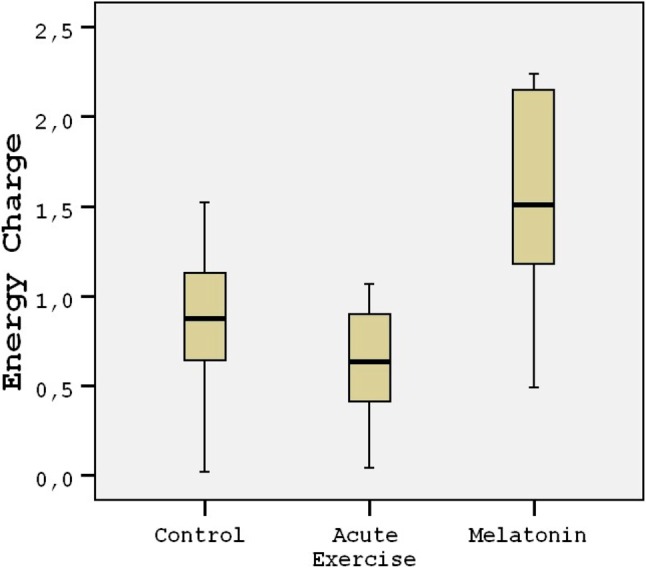
Tukey box plots and scatter plots illustrating the results of the categorical comparisons between study groups in terms of energy charge values of heart tissues (p < 0.001).
Figure 2.
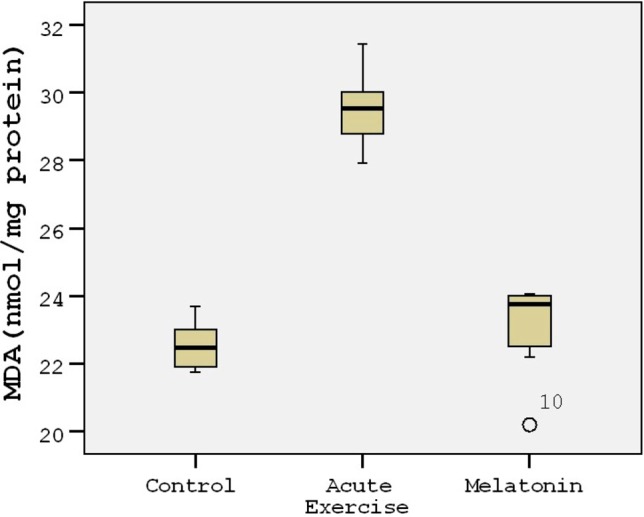
Tukey box plots and scatter plots illustrating the results of the categorical comparisons between study groups in terms of MDA levels (nmol/mg) of heart tissues (p < 0.001).
Figure 3.
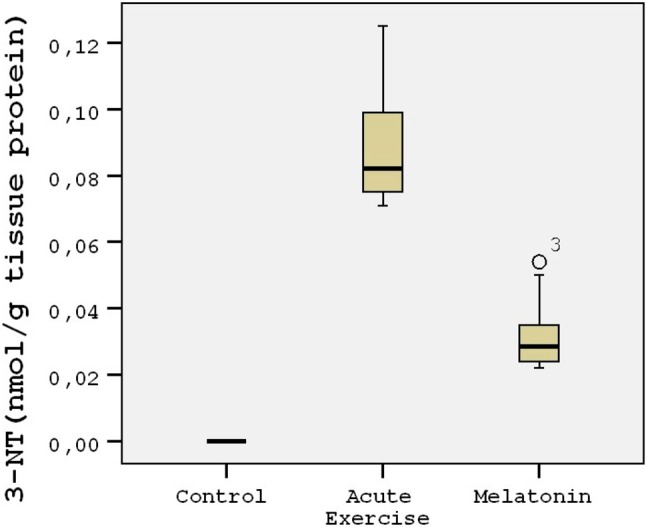
Tukey box plots and scatter plots illustrating the results of the categorical comparisons between study groups in terms of 3-NT levels (nmol/g) of heart tissues (p < 0.001).
Table 2. The results of the categorical comparison between study groups in terms of heart tissue levels of MDA, 3-NT, CAT, GPx and SOD.
| Parameters | Study groups | Comparisons | ||||
| Control (n = 10) | Acute exercise (n = 10) | Melatonin (n=10) | Control/acute exercise | Control/melatonin | Acute exercise/melatonin | |
| MDA (nmol/mg protein) | 22.4 (21.8-23.0) | 29.5 (28.7-30.1) | 23.7 (22.4-24.0) | p < 0.001 | p > 0.05 | p < 0.001 |
| 3-NT (nmol/g tissue protein) | - | 0.090 ± 0.01 | 0.032 ± 0.01 | - | - | p < 0.001 |
| CAT (μmol/g tissue protein) | 4.10 (4.08-4.12) | 3.09 (3.06-3.10) | 3.91 (3.90-3.93) | p < 0.001 | p < 0.001 | p < 0.001 |
| GPx (U/mg tissue protein) | 1.6 (1.59-1.62) | 0.80 (0.79-0.82) | 1.44 (1.42-1.46) | p < 0.001 | p < 0.001 | p < 0.001 |
| SOD (U/mg tissue protein) | 1.56 (1.54-1.58) | 1.10 (1.2-1.14) | 1.52 (1.50-1.53) | p < 0.001 | p < 0.001 | p < 0.001 |
CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; 3-NT, 3-nitrotyrosine.
Data are expressed as mean ± SD or median (25th-75th percentile) for continuous variables.
Figure 4.
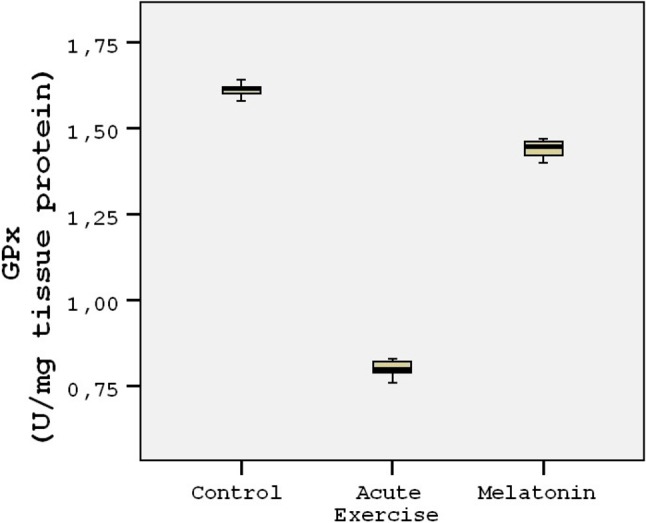
Tukey box plots and scatter plots illustrating the results of the categorical comparisons between study groups in terms of GPx levels (U/mg) of heart tissues (p < 0.001).
Figure 5.
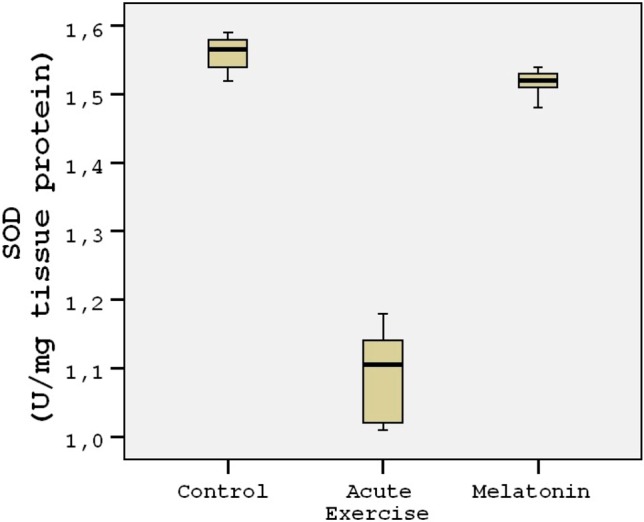
Tukey box plots and scatter plots illustrating the results of the categorical comparisons between study groups in terms of SOD levels (U/mg) of heart tissues (p < 0.001).
DISCUSSION
To the best of our knowledge, the present study is the first to demonstrate the protective effects of melatonin against acute exercise-induced oxidative stress and antioxidant changes in a rat model. We found that levels of MDA and 3-NT increased, while the activities of CAT, GPx and SOD in the heart tissues in the acute intensive exercise group decreased compared to the control and melatonin groups. We also found that the decrease in intracellular ATP was followed by an increase in ADP levels in the acute intensive exercise group compared to the control and melatonin groups.
Regular physical activity is known to increase resistance against oxidation protein and ROS-induced lipid peroxidation.23 However, acute-intense exercise does not elicit the same response as long-term exercise training. It has been shown by many researchers that acute physical exercise causes oxidative stress.24 Consistent with previous studies, the mean heart tissue MDA and 3-NT levels were significantly higher in the acute exercise group compared with the control and acute exercise groups. However, with the administration of melatonin, we were able to reduce MDA and 3-NT levels in the acute exercise group-induced rat hearts. The results indicated that the mean heart tissue 3-NT levels were significantly lower in the melatonin group compared with the acute exercise group.
Elevated levels of free radicals during acute swimming exercise interact with lipids, proteins and nucleic acids to cause loss of membrane integrity, structural and functional changes in proteins, and genetic mutations.25 Elevated MDA levels in various tissues of rats subjected to swimming exercise have also been reported to be inhibited by melatonin administration,16 which also supports our results in the acute swimming exercise group that was given melatonin. It is known that lipid peroxidation significantly increases in heart tissue with exercise.26 On the other hand, melatonin levels have been found to be correlated with the total antioxidant capacity of rat and human serum and in all studies in which mammals are used, and where melatonin characteristically lowers the products of lipid peroxidation.27 NO-and O2- undergo a radical addition reaction to yield ONOO- that can react with DNA, proteins and lipids. The direct measurement of ONOO-mediated tissue damage can be observed by measuring 3-NT due to the fact that ONOO has a very short lifespan.15 It has also been shown that melatonin inhibits NO production and iNOS expression in the liver and lungs, leading to the occurrence of multiple organ failure in a model of lipopolysaccharides (LPS)-induced endotoxemia in rats.28 Melatonin has also been shown to have beneficial effects on iNOS inhibition in swine during (ischemia reperfusion).29
Taken together with the findings of previous studies, our results may provide evidence that melatonin can exert potent protective effects on oxidative stress status in heart tissue after acute swimming exercise as shown by the decreased concentrations of MDA and 3-NT. Our findings provide evidence of an increase antioxidant activity in acute exercise-induced rat hearts with the administration of melatonin. CAT, GPx and SOD activities were significantly lower in the acute exercise group compared with the control and melatonin groups. Moreover, although there were significant differences between the melatonin and control group in terms of CAT, GPx and SOD activities, the values of the control and melatonin groups were found to be close to each other.
Just as with other organs, heart muscles also possess a system of conservation against the detrimental action of free oxygen radicals.29,30 Nevertheless, the activity of GPx in cardiomyocytes attains merely 1-2% that in the liver, and SOD activity in the heart amounts to just 25-30% of the activity of enzymes in the liver.30 Accordingly, the heart is particularly susceptible to the action of substances with high amounts of free oxygen radicals at the time of their metabolism.31 It has been demonstrated that the administration of melatonin can result in a substantial rise in the activity of GPx in homogenates of rat brains.32 It has also been shown that melatonin can protect Harder’s gland cells from the damaging free radical effect of porphyrins and enhanced expression of mRNA liable for the production of SOD.33 In other works, increased activity of SOD has been demonstrated after melatonin administration in the small intestine and stomach exposed to ischemia/reperfusion so as to induce oxidative stress.34,35
In the present study, we detected a significant increase in cellular SOD, CAT and GPx activities in the heart tissue of the melatonin group compared with acute intensive swimming exercise group. This may be because melatonin decreased the oxidative stress in the heart tissue resulting in increased antioxidant enzyme activity in the heart tissue, possibly suggesting that melatonin plays an important role as an antioxidant and respiratory chain protector.
In the present study, acute intensive exercise resulted in a depletion of energy charge of the rat heart tissues compared to the controls. The functional consequence of this is that melatonin may also have the ability to maintain an adequate cellular ATP reserve. Our data show that ATP and energy charge levels in the heart tissues were significantly higher in the melatonin group compared with the acute intensive exercise groups. This finding is consistent with our previous studies in which melatonin administration prevented 3-nitrotyrosine formation while failing to prevent or restore changes in the energy charge ratio of the kidney.15
Previous studies suggest that ONOO- is a toxic oxidant species that may decrease cellular ATP by inhibiting mitochondrial respiratory enzymes and lowering cellular oxygen depletion in macrophages and in rat aortic smooth muscle cells.15 El-Sokkary et al. concluded that melatonin removes and inactivates O2- thereby decreasing the formation of ONOO-.36 This subsequently hinders the activation of poly-ADP-ribose synthase, an increase in the activity of which can cause final extreme energy consumption of the cells in inflammation and ischemia-reperfusion injury.15 It has also been demonstrated that exogenous melatonin can restore the energetic and functional status of cells during liver ischemia-reperfusion by inhibiting NO production and iNOS expression.37 Dugo et al. proved that the inhibition of ONOO- production is associated with the antioxidant effect of melatonin.38 In vivo therapy of animals with melatonin has been shown to substantially inhibit NO production in a dose-dependent manner and establish the depletion of intracellular NAD and ATP levels in pleural macrophage cultures.
It is clear that contracting skeletal muscles produce free radicals, and that prolonged and intense exercise can lead to oxidative damage to cellular constituents.5,6 Previous studies have also shown that melatonin administration can hinder the increase in free radical generation in connection with acute exercise.39 Indeed, melatonin has been demonstrated to substantially decrease ONOO-induced protein nitration in homogenates of cardiac tissues, and to significantly hinder the oxidative-induced tissue damage due to acute exercise.36,37 Consistent with previous studies, our results also demonstrated that melatonin protected the heart against the formation of MDA and 3-NT once it was administrated prior to acute swimming exercise. On the other hand, acute swimming exercise resulted in the reduction of energy generation of the rat heart tissues compared with the controls. These findings demonstrated that ATP and energy charge levels in the heart tissues were dramatically higher in the melatonin group compared to the acute swimming exercise groups. Therefore, injections of melatonin may be useful not just for oxidative stress but also in improving energy load in rat heart tissues for acute swimming exercise.
Our novel findings strengthen those of our former study in which there was a substantial rise in the formation of 3-nitrotyrosine and reduction in energy charge in the endotoxin-induced group. In the same study, we demonstrated that melatonin administration hampered 3-nitrotyrosine production while being unable to preventor restore changes in the energy charge ratio of the kidney.15
Physical exercise is known to have extensive impacts on myocardial metabolic, physiological, and anatomical functions.40 Heart mitochondrial respiratory features and antioxidant defense and oxidative capacity have been shown to increase after endurance exercise, leading to the development of resistance to oxidative stress and contractile performance under a number of experimental conditions, such as ischemia/reperfusion and anoxia/hypoxia.12 However, we suggest that this situation may not be possible for acute swimming exercise. In the present study, our results provide evidence of increased oxidative stress with acute swimming exercise as shown by increased concentrations of MDA and 3-NT, and decreased levels of CAT, GPx and SOD activities.
CONCLUSIONS
Our results provide evidence that melatonin can exert potent protective effects on oxidative stress status and energy charge in heart tissues after acute swimming exercise as shown by decreased concentrations of MDA and 3-NT with increased energy charge and activity of antioxidant enzymes in rats subjected to acute swimming exercise. These findings suggest that the direct beneficial effects of melatonin can be potentially applied to prevent oxidative stress and energy deficits.
Acknowledgments
The authors are grateful to Research Foundation of Erciyes University for supporting this study (TSY-10-3083).
REFERENCES
- 1.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadiasl N, Najafipour H, Soufi FG, Jafari A. Effect of short- and long-term strength exercise on cardiac oxidative stress and performance in rat. J Physiol Biochem. 2012;68:121–128. doi: 10.1007/s13105-011-0125-z. [DOI] [PubMed] [Google Scholar]
- 3.Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;18:1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- 4.Atalay M, Laaksonen DE. Diabetes, oxidative stress and physical exercise. J Sports Sci Med. 2002;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 5.Michailidis Y, Jamurtas AZ, Kickolaidis MG, et al. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med Sci Sports Exer. 2007;39:1107–1113. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]
- 6.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra S, Sawhney G, Pandhi P. The therapeutic potential of melatonin: a review of the science. Med Gen Med. 2004;6:46. [PMC free article] [PubMed] [Google Scholar]
- 8.Rapozzi V, Comelli M, Mavelli I, et al. Melatonin and oxidative damage in mice liver induced by the prooxidant antitumor drug, adriamycin. In Vivo 1999. 1999;13:45–50. [PubMed] [Google Scholar]
- 9.Reiter RJ, Tan DX, Mayo JC, et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 10.Upchurch GR, Jr., Welch GN, Fabian AJ, et al. Homocyst (e) ine decreases bioavailable nitric oxide by a mechanism involving glu¬tathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 11.Sahna E, Türk G, Atessahin A, et al. Remote organ injury induced by myocardial ischemia and reperfusion on reproductive organs, and protective effect of melatonin in male rats. Fertil Steril. 2007;88:188–192. doi: 10.1016/j.fertnstert.2006.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardio protection: biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol. 2007;117:16–30. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 13.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardio protection against myocardial ischemia-reperfusion injury. Free Radic Biol Med. 2008;44:193–201. doi: 10.1016/j.freeradbiomed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Baltaci AK, Ozyurek K, Mogulkoc R, et al. Effects of zinc deficiency and supplementation on some hematologic parameters of rats performing acute swimming exercise. Acta Physiol Hung. 2003;90:125–132. doi: 10.1556/APhysiol.90.2003.2.5. [DOI] [PubMed] [Google Scholar]
- 15.Cimen B, Türközkan N, Ünlü A. Effects of melatonin on 3-nitrotyrosine formation and energy charge in rat kidney in LPS-induced stress. Cell Biochem Function. 2004;23:273–277. doi: 10.1002/cbf.1151. [DOI] [PubMed] [Google Scholar]
- 16.Claustrat B, Brun J, Chazot G. The basic physiology and pathophisology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Unlu A, Türközkan N, Cimen B, et al. The effect of E. coli-derived lipopolysaccharides on plasma levels of malondialdehyde and 3-nitrotyrosine. Clin Chem Lab Med. 2001;39:491–493. doi: 10.1515/CCLM.2001.081. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Yi-Sun S, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 20.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 21.Yasmineh WG, Theologides A. Catalase as a roving scavenger of hydrogen peroxide: a hypothesis. J Lab Clin Med. 1993;122:110–114. [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Biochem. 1951;157:145–157. [PubMed] [Google Scholar]
- 23.Radak Z, Sasvari M, Nyakas C, et al. Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int. 2001;39:33–38. doi: 10.1016/s0197-0186(01)00003-1. [DOI] [PubMed] [Google Scholar]
- 24.Gul M, Demircan B, Taysi S, et al. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:239–245. doi: 10.1016/j.cbpa.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Cayir A, Ugan RA, Albayrak A, et al. The lung endothelin system: a potent therapeutic target with bosentan for the amelioration of lung alterations in a rat model of diabetes mellitus. J Endocrinol Invest. 2015;38:987–998. doi: 10.1007/s40618-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 26.Naderi R, Mohaddes G, Mohammadi M, et al. Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv Pharm Bull. 2015;5:231–236. doi: 10.15171/apb.2015.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benot S, Goberna R, Reiter RJ, et al. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J Pineal Res. 1999;27:59–64. doi: 10.1111/j.1600-079x.1999.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 28.Crespo E, Macías M, Pozo D, et al. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999;13:1537–1546. [PubMed] [Google Scholar]
- 29.Isobe M, Katsuramak T, Hirata K, et al. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803–813. doi: 10.1097/00007890-199909270-00013. [DOI] [PubMed] [Google Scholar]
- 30.Seifert CF, Nesser ME, Thompson DF. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother. 1994;28:1063–1072. doi: 10.1177/106002809402800912. [DOI] [PubMed] [Google Scholar]
- 31.Singal PK, Iliskovic N, Li T, et al. Heart failure due to doxorubicin. Kuwait Med J. 2001;33:111–115. [Google Scholar]
- 32.Barlow-Walden LR, Reiter RJ, Abe M, et al. Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int. 1995;26:497–502. doi: 10.1016/0197-0186(94)00154-m. [DOI] [PubMed] [Google Scholar]
- 33.Antolin I, Rodriguez C, Sainz RM, et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10:882–890. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- 34.Ustundag B, Kazez A, Demirbag M, et al. Protective effect of melatonin on antioxidative system in experimental ischemia reperfusion of rat small intestine. Cell Physiol Biochem. 2000;10:229–236. doi: 10.1159/000016354. [DOI] [PubMed] [Google Scholar]
- 35.Cabeza J, Motilva V, Martin MJ, et al. Mechanisms involved in gastric protection of melatonin against oxidant stress by ischemia-reperfusion in rats. Life Sci. 2001;68:1405–1415. doi: 10.1016/s0024-3205(01)00935-3. [DOI] [PubMed] [Google Scholar]
- 36.El-Sokkary GH, Omar HM, Hassanein AF, et al. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic Biol Med. 2002;32:319–332. doi: 10.1016/s0891-5849(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez Reynoso S, Leal C, Portilla E, et al. Effect of exogenous melatonin on hepatic energetic status during ischemia-reperfusion. J Surg Res. 2001;100:141–149. doi: 10.1006/jsre.2001.6185. [DOI] [PubMed] [Google Scholar]
- 38.Dugo L, Serraino I, Fulia F, et al. Effects of melatonin on cellular energy depletion mediated by peroxynitrite and poly(ADP-ribose) synthetase activation in an acute model of inflammation. J Pineal Res. 2001;31:76–84. doi: 10.1034/j.1600-079x.2001.310111.x. [DOI] [PubMed] [Google Scholar]
- 39.Borges Lda S, Dermargos A, da Silva Junior EP, et al. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J Pineal Res. 2015;58:166–172. doi: 10.1111/jpi.12202. [DOI] [PubMed] [Google Scholar]
- 40.Andrew F, Glenn H, Dan D. Cardiac adaptation to endurance exercise in rats. Mol Cell Biochem. 2003;251:51–59. doi: 10.1007/978-1-4419-9238-3_8. [DOI] [PubMed] [Google Scholar]


