Abstract
The Ixodidae family of hard ticks has cement-producing and non-cement-producing species. Involved skins of four patients bitten by cement-producing ticks and two by non-cement-producing ticks were histopathologically examined. Those of the latter two patients were also studied immunohistochemically to characterize the infiltrating inflammatory cells. In patients with cement-producing ticks, the cement substance was observed as external cement or outer zone of internal cement, respectively. Coagulative necrosis was present in the epidermis in one patient and from the epidermis to the dermis in another patient. Epidermal cells were damaged in the remaining two patients. Despite these severe tissue damages, the cutaneous inflammatory reaction in all four patients was very mild. In contrast, the patients bitten by non-cement-producing ticks had severe cutaneous inflammatory reaction. In addition to caseous necrosis-like change in the entrance site of the inserted mouthparts, extensive interstitial lymphohistiocytic infiltrate was present diffusely from the dermis to the subcutaneous tissue. In one of the patients coagulative necrosis was present from the dermis to the subcutaneous tissue. Immunohistochemically, the infiltrating lymphocytes were T-cell dominant and mixed moderately with B-cells. Pathogenetically, the cutaneous inflammatory reaction is only mild in the skins involved by the cement-producing ticks, perhaps because inflammatory reaction in the host skin is suppressed by antiinflammatory and immunosuppressive substances contained in tick’s saliva in order to prevent position of their mouthparts fixed to the host skin from rejection of the host until finishing their engorgement. In contrast, the cutaneous inflammatory reaction induced by the non-cement-producing ticks is severe, possibly because these ticks have no antiinflammatory and immunosuppressive substances in their saliva, and because their saliva is much more injurious than that of the cement-producing ticks.
Keywords: cement substance, cutaneous inflammatory reaction, histopathology, tick bite, tick saliva
Immediately after a tick attaches to the host skin, its mouthparts are inserted into the skin for feeding, followed by the secretion of saliva. One of the physiologically active substances contained in the saliva is “cement.” According to a recent paper,1 tick bites on the human skin in Japan are caused by the Ixodidae family only. However not all species in the Ixodidae family produce the cement substance.2 Although there are several studies concerning the histopathology of the tick bite,3–9 to my knowledge, there are no reports, to date, that have critically evaluated the relationship between the cement substance and the tissue reaction of the host skin.
This paper describes two patterns of inflammatory tissue reaction in the human skin in the early stage after tick bite depending on whether or not the cement substance is produced.
PATIENT REPORT
Six patients with a single tick bite were examined. Skin of five of the patients was assessed by dermoscopy. The lesions were surgically excised and processed for histopathology. Infiltrating cells of two patients (cases 5 and 6) were immunostained with antibodies to cluster of differentiation antigens (CD).
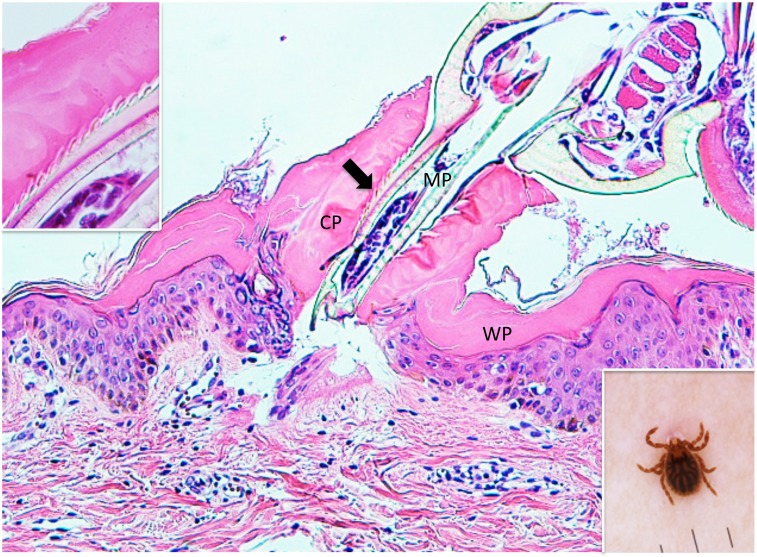
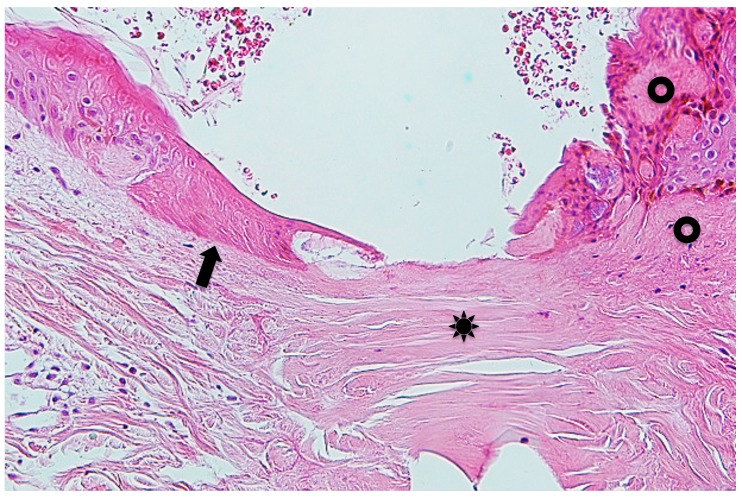
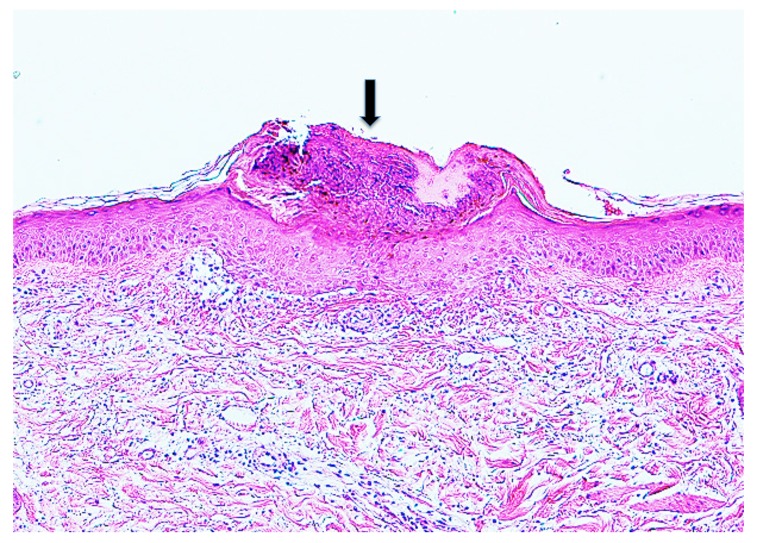
Case 1. A 1-year-old girl presented with a tick to the left abdomen, which the mother had noticed that morning. Clinically and dermoscopically, a tick was confirmed on the skin (Insert in the right lower corner of Fig. 1), with a narrow erythematous halo surrounding it. Histopathologically, the mouthparts of the tick were seen, penetrating the epidermis (Fig. 1). The mouthparts were located within highly eosinophilic, amorphous, mucoid substance which formed a tube with thick wall. The mucoid substance spread in a thick homogeneous layer over the epidermis. Based on a description by Chinery,10 the author considered this mucoid substance to be typical external cement, as discussed later. The cement substance made up of the wall of the tube was in a shade of eosinophilic color (Fig. 1). The inner surface of the tube, which had serrated structures, was much more eosinophilic (Fig. 1). The serrated structures were associated with spicula of the tick’s hypostomal teeth, interlocking closely with each other (Insert in the left upper corner of Fig. 1). A circumscribed part of the epidermis just beneath the cement substance was much more eosinophilic than the intact parts of the epidermis (Fig. 2), and some of the epidermal cells exhibited coagulative necrosis. These findings suggested that the saliva of the cement-producing ticks contained certain toxic agents permeating the surrounding tissues. In the subepidermis just beneath the tip of the mouthparts, a cleavage was artificially formed, under which were band-like accumulation of fibrin nets (Fig. 2). A slight perivascular lymphohistiocytic infiltrate mixed with a few neutrophils was present in the upper dermis (Fig. 1). The cement substance, the area involved by the permeable toxic agents, and the mouthparts could not be evaluated histochemically because they were lost in the remaining specimen.
Fig. 1.
(case 1). Histopathology and dermoscopy. Mouthparts (MP) penetrate the epidermis. They are located within conical part (CP) of the external cement which forms a tube. Wing-like part (WP) of it spreads over the epidermis. An arrow indicates serrated structures. Hematoxylin and eosin stain. Original magnification: x100.
Insert in the left upper corner. Magnified serrated structures. Hematoxylin and eosin stain. Original magnification: x200.
Insert in the right lower corner. Dermoscopic feature of an attached tick.
Fig. 2.
Magnified Fig. 1. A circumscribed part of the epidermis surrounded by thin arrows is much more eosinophilic than the intact parts of the epidermis. A thick arrow indicates fibrin nets. CP: conical part of the external cement. MP: mouthparts. WP: wing-like part of the external cement. Hematoxylin and eosin stain. Original magnification: x200.
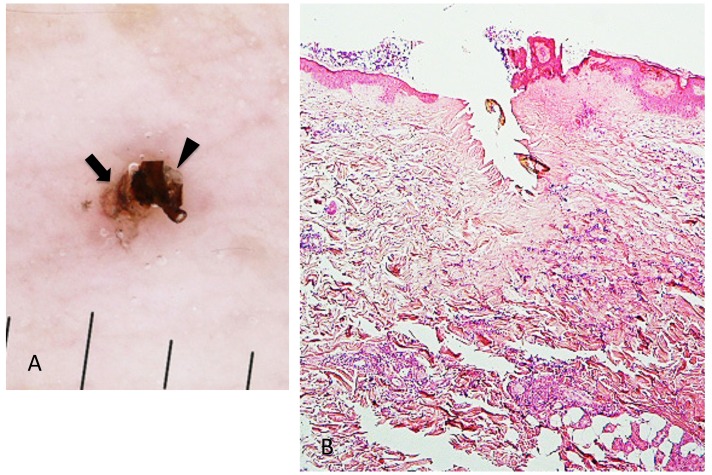
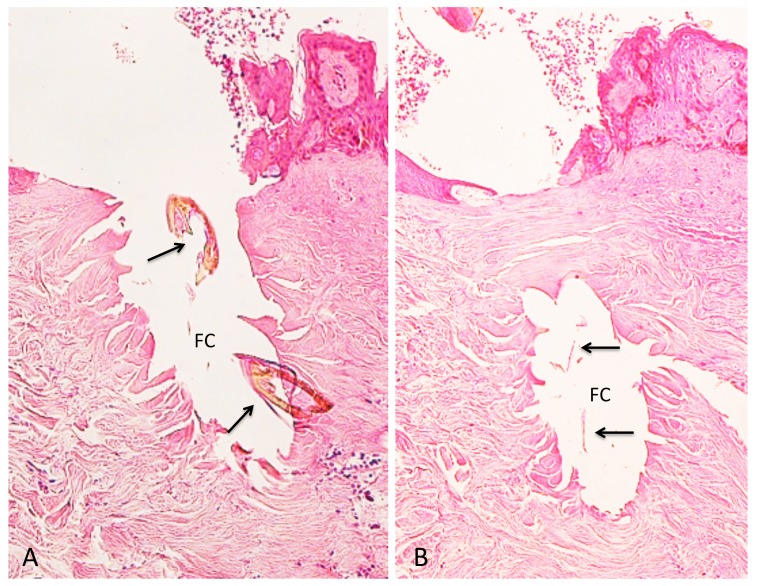
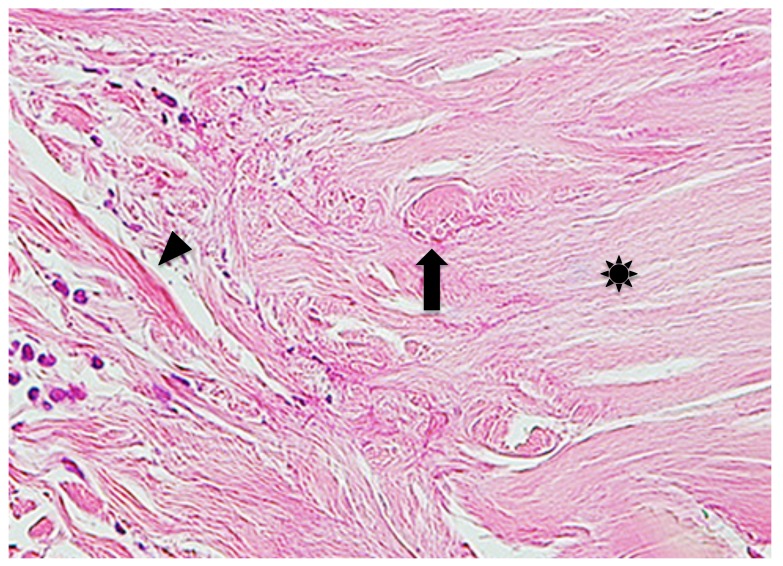
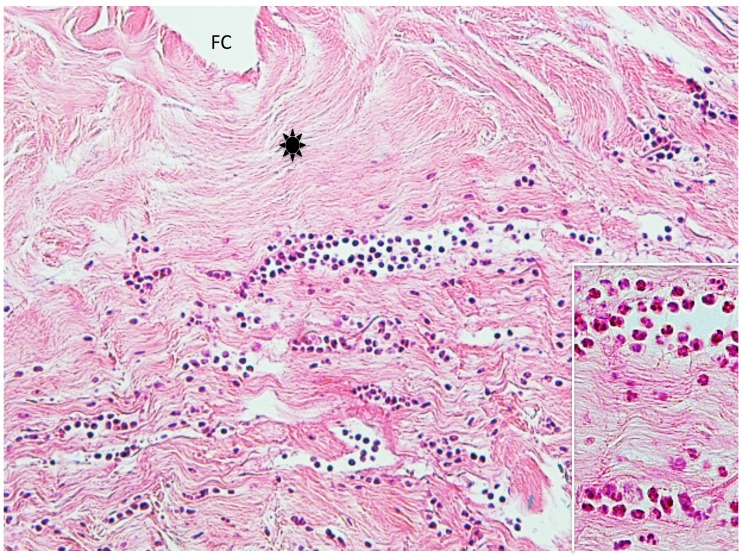
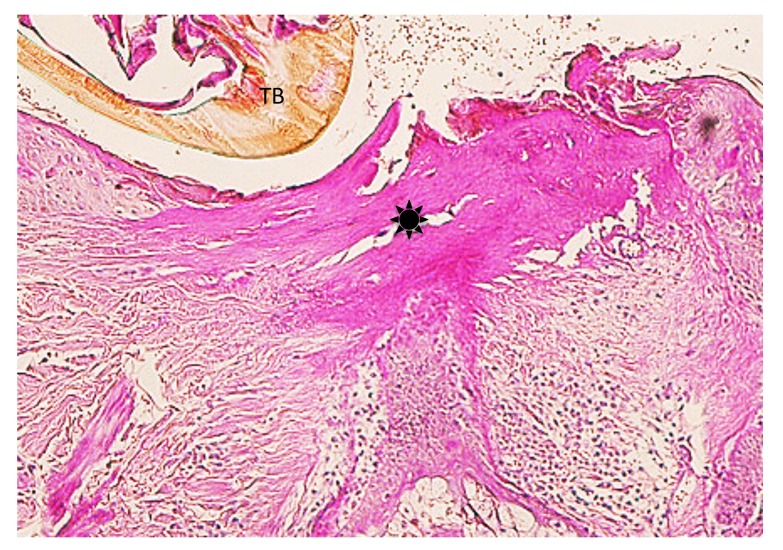
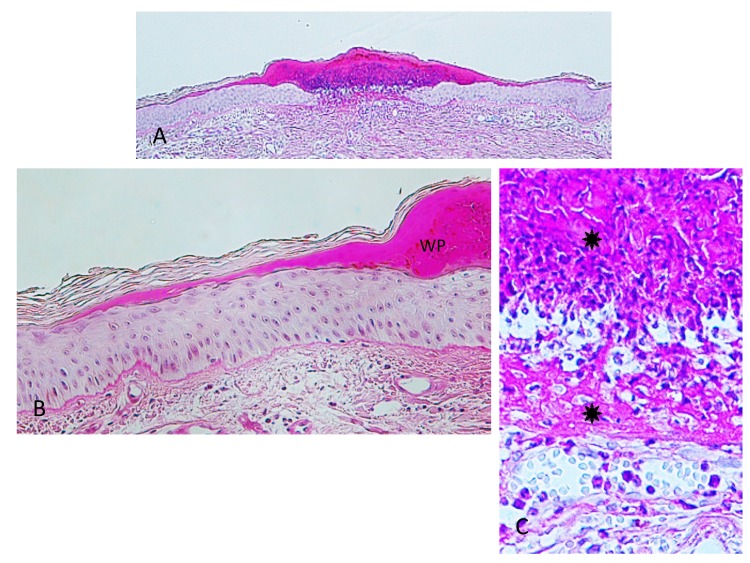
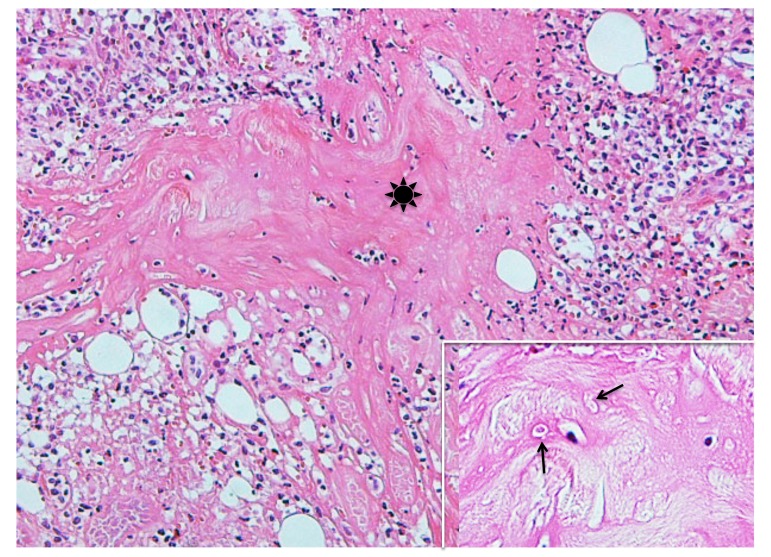
Case 2. A 53-year-old woman presented with a part of a tick body attached to the right clavicular region. Two days previously she had tried to remove an embedded tick with her fingers, but a part of it remained in the skin. On examination, there was a round erythematous area measuring about 10 x 10 mm, with a barely visible brown punctate structure. Dermoscopy revealed that the punctate structure consisted of a part of the tick mouthparts measuring about 0.5 mm in maximum width, with external cement enveloping it (Fig. 3A). Histopathologically, the central portion of the lesion was ulcerated and had a V-shaped deep cleavage containing two sectioned mouthparts (Figs. 3B and 4A). In the step section the cleavage was seen as a spindle-shaped empty space in the dermis containing several small remnants of the mouthparts (Fig. 4B). This kind of cleavage or empty space has been referred to as “the cavity in the cement tube” or “feeding cavity” which was formed by collagen destruction.10–11 The inner surfaces of the feeding cavity showed irregularly shaped thick projections or smooth appearance (Fig. 4), both of which were amorphous and distinctly eosinophilic. According to a description by Chinery,10 these histopathologic features were compatible with those of the outer zone of internal cement, as discussed later. The outer zone merged gradually into the dermal collagen fibers. Bundles of the collagen fibers increased in thickness and the interspaces between them were narrow or absent (Fig. 5). The infiltrating cells or the fibroblasts adjacent to those collagen bundles showed coagulative necrosis (Fig. 5); this finding suggested that the above-mentioned changes of the collagen fibers had been caused by certain permeable toxic agents in the tick saliva which induced coagulative necrosis. In the epidermis, the massive epidermal cells had undergone coagulative necrosis (Fig. 6). As a result, the feeding cavity was surrounded by coagulative necrosis from the epidermis to the dermis. Aside from the necrotic portion of the epidermis, the epidermal cells were unaltered. The papillary and subpapillary dermis had a homogeneous, prominently eosinophilic appearance (Fig. 6) and only a few infiltrating cells. This appearance suggested that these areas had undergone a peculiar type of degenerative alteration, which probably belonged to coagulative necrosis and that it was possibly induced by permeable toxic agents of the cement-producing tick saliva. Numerous neutrophils infiltrated in the mid-dermis which was interposed by a narrow coagulative necrosis area just beneath the feeding cavity (Fig. 7). In the entire dermis outside this area, there was slight perivascular lymphohistiocytic infiltrate with a few neutrophils (Fig. 3B). In the boundary area between the dermis and the subcutaneous tissue, considerable perivascular lymphohistiocytic infiltrate was present (Fig. 3B). Histochemically, the degenerative areas of the papillary and subpapillary dermis were stained strongly positive with periodic acid-Schiff stain (PAS) before and after diastase digestion (Fig. 8); negative with alcian blue (pH 2.5) stain (AB); deep brown with phosphotungstic acid hematoxylin stain; and red with elastica van Gieson stain (EVG). The dermal connective tissue around the feeding cavity could not be evaluated histochemically because the remaining specimen did not have such portions.
Fig. 3.
(case 2). A (left). Dermoscopic feature. A part of the mouthparts (arrowhead) gets into the external cement (arrow). B (right). Histopathology. There is a V-shaped deep cleavage which is referred to as feeding cavity. There is slight perivascular lymphohistiocytic infiltrate in the entire dermis. Hematoxylin and eosin stain. Original magnification: x20.
Fig. 4.
Magnified feeding cavity.
A (left). Magnified V-shaped cleavage in Fig. 3B. There are two sectioned mouthparts (arrows) in the feeding cavity (FC). They are disorderly scattered. Hematoxylin and eosin stain. Original magnification: x50.
B (right). Step section of Fig. 3B. A feeding cavity which is spindle-shaped, has several remnants (arrows) of the mouthparts. Hematoxylin and eosin stain. Original magnification: x50.
Fig. 5.
Step section of Fig. 3B. Arrow indicates fibroblasts or infiltrating cells which undergo coagulative necrosis. Asterisk indicates collagen bundles in the coagulative necrosis area. Arrowhead indicates intact collagen fiber. Hematoxylin and eosin stain. Original magnification: x200.
Fig. 6.
Manified Fig. 4B. Arrow indicates massive epidermal cells which undergo coagulative necrosis. Asterisk indicates collagen bundles in the coagulative necrosis area. Two circles indicate papillary and subpapillary areas which show homogeneous, prominent eosinophilic appearance, indicating existence of the permeable toxic agents in the tick saliva. Hematoxylin and eosin stain. Original magnification: x100.
Fig. 7.
Step section of Fig. 3B. This picture shows neutrophilic infiltrate in the dermis. Asterisk: collagen bundles in the coagulative necrosis area. FC; feeding cavity. Hematoxylin and eosin stain. Original magnification: x100.
Insert: Magnified a part of the infiltrating area of neutrophils in Fig. 7. Hematoxylin and eosin stain. Original magnification: x200.
Fig. 8.
(case 2). Histochemistry. Diastase-resistant, strongly PAS-positive area (asterisk). TB: tick body. Original magnification: x50.
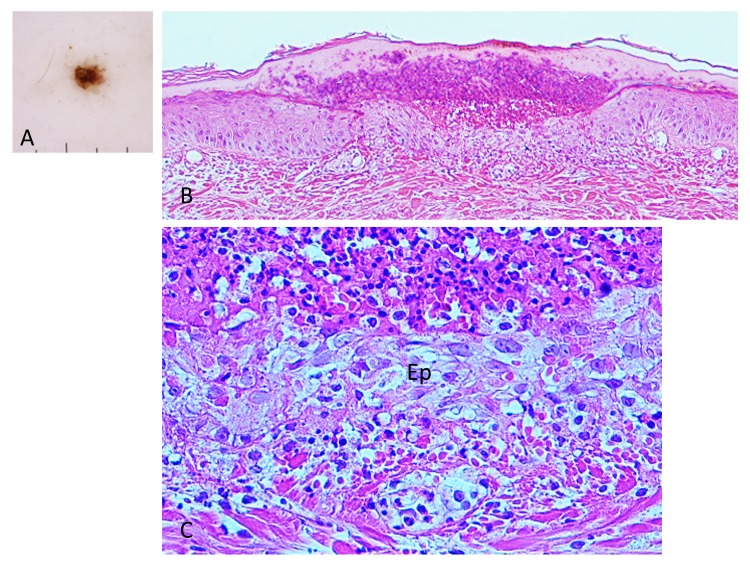
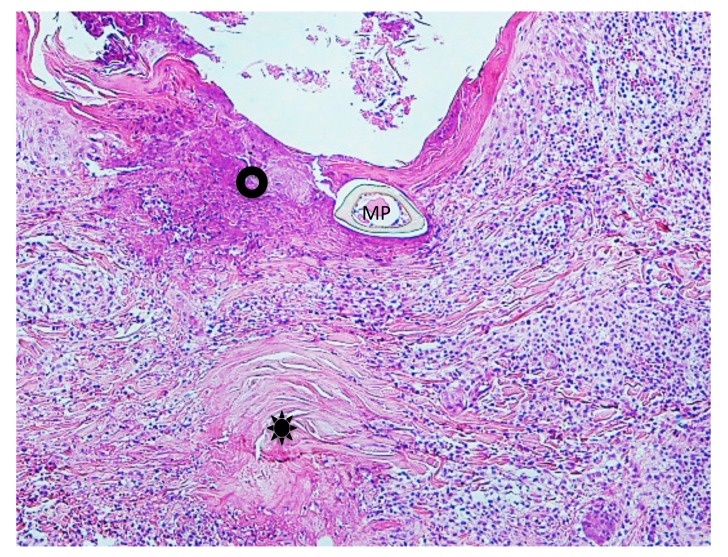
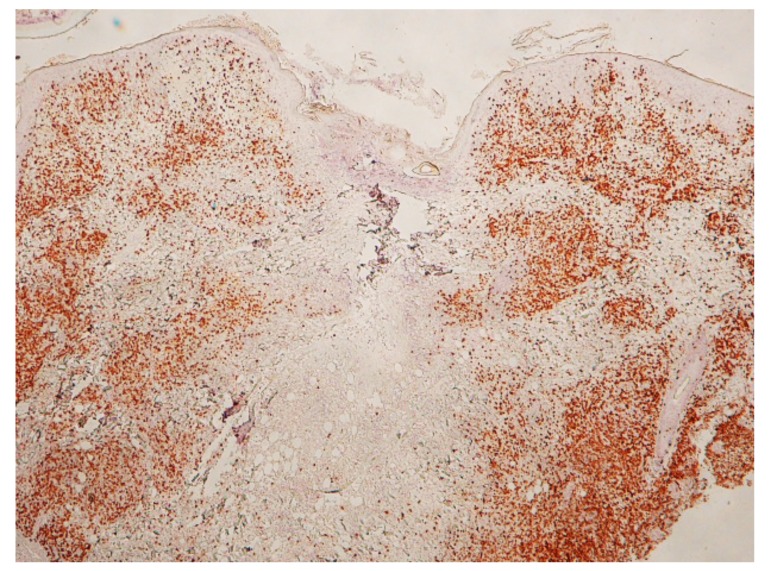
Case 3. A 54-year-old woman presented with a black dotted lesion on the right lower leg. She had noticed an attached tick just one week before and removed it with her fingers, but the cutaneous lesion had remained. On examination, a brown-black dotted lesion with a surrounding narrow erythematous halo was present. Dermoscopically, a brown-black, irregularly square structure measuring about 1 x 1 mm was present (Fig. 9A). Histopathologically, there was a crust consisting of the mucoid substance and the underlying aggregated infiltrating inflammatory cells (Fig. 9B). The mucoid substance spread widely over the epidermis, becoming less thick towards its distal end, like a gentle slope below a mountain. (Figs. 10A and B). According to a description by Chinery,10 the author regarded the mucoid substance as a wing-like part of the external cement. The aggregated infilitrating inflammatory cells consisted of neutrophils, lymphocytes and histiocytes. The lower portion of the crust contained numerous erythrocytes and infiltrating inflammatory cells (Fig. 9B and C). Just beneath the crust, the epidermis was damaged with spongiosis and numerous erythrocytes. The dermoepidermal junction was indistinct due to the extravasated erythrocytes and the infiltrating inflammatory cells (Fig. 9C). There was a slight perivascular lymphohistiocytic infiltrate in the upper dermis together with dilatation of small blood vessels (Fig. 9B). Histochemically, the wing-like part of the external cement was stained strongly positive with PAS before and after diastase digestion (Figs. 10A and B); negative with AB; negative with mucicarmine stain; negative with colloidal iron stain. Furthermore the diastase-resistant, strongly PAS-positive substance was also seen both in the area of the aggregated infiltrating inflammatory cells in the crust and around the epidermal cells (Fig. 10C).
Fig. 9.
(case 3). Dermoscopy and histopathology. A (left upper corner). Dermoscopic feature. There is a brown-black, irregularly shaped square structure. B (top). There is a crust over the epidermis. The crust consists of the wing-like part of the external cement and the underlying aggregated infiltrating inflammatory cells. Hematoxylin and eosin stain. Original magnification: x50. C (bottom). Magnified Fig. 9B. There are numerous erythrocytes and the infiltrating inflammatory cells over the epidermis (Ep). Dermoepidermal junction is indistinct due to the extravasated erythrocytes and the infiltrating inflammatory cells. Hematoxylin and eosin stain. Original magnification: x200.
Fig. 10.
(case 3). Histochemistry. A (top). Diastase-resistant. strongly PAS-positive substance is seen over the epidermis and in the crust. Original magnification: x20. B (left bottom). Magnified Fig. 10A. Wing-like part of the external cement which is diastase-resistant, strongly PAS-positive, spreads widely over the epidermis and becomes less thick toward the distal end, like a gentle slope below a mountain. Original magnification: x100. C (right bottom). Diastase-resistant, strongly PAS-positive substance (asterisks) in the crust and around the epidermal cells. This substance is regarded as the permeable toxic agents in the tick saliva. Original magnification: x200.
Case 4. A 63-year-old woman presented with an erythema on the left forearm, which developed after she had attempted to remove an attached tick with her fingers the previous afternoon. On examination, a coin-sized, round erythematous area was seen, but residual tick body parts were not identified. Dermoscopiccally, an irregularly shaped dark brown structure measuring about 0.8 x 0.2 mm was seen. Histopathologically, there was a small crust over the epidermis consisting of the mucoid substance and an aggregated infiltrating inflammatory cells, mostly neutrophils (Fig. 11). The staining property of the mucoid substance suggested that it was a small part of the wing-like part of a tick’s external cement. Just beneath the crust, the upper spinous cells were slightly flattened. Their cytoplasm was eosinophilic and their nucleus was pyknotic. In this area, liquefaction degeneration of basal cells with the cleavage was present (Fig. 11). From the subepidermal area to the upper dermis, slight perivascular lymphohistiocytic infiltrate with dilated small blood vessels and lymphatic vessels was present (Fig. 11).
Fig. 11.
(case 4). Histopathology. There is a crust (arrow) over the epidermis. The colloid substance in the crust is regarded as a part of the wing-like part of the external cement. Hematoxylin and eosin stain. Original magnification: x50.
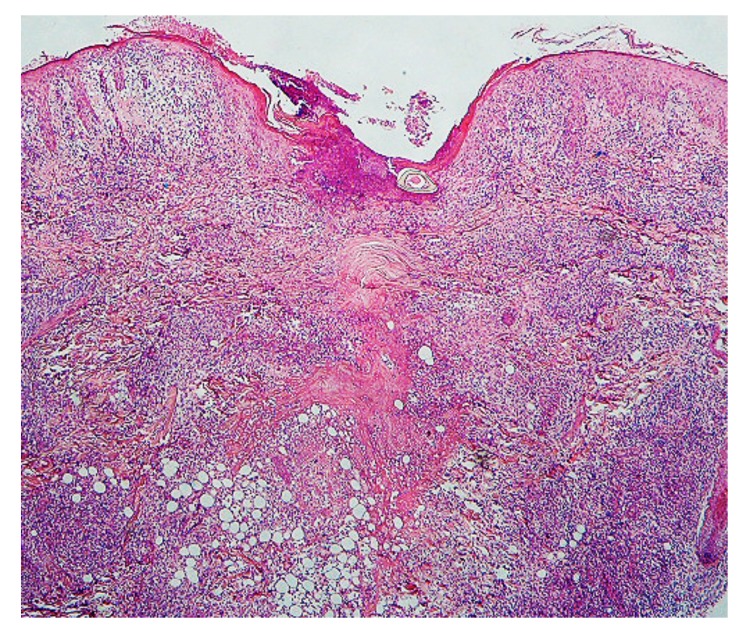
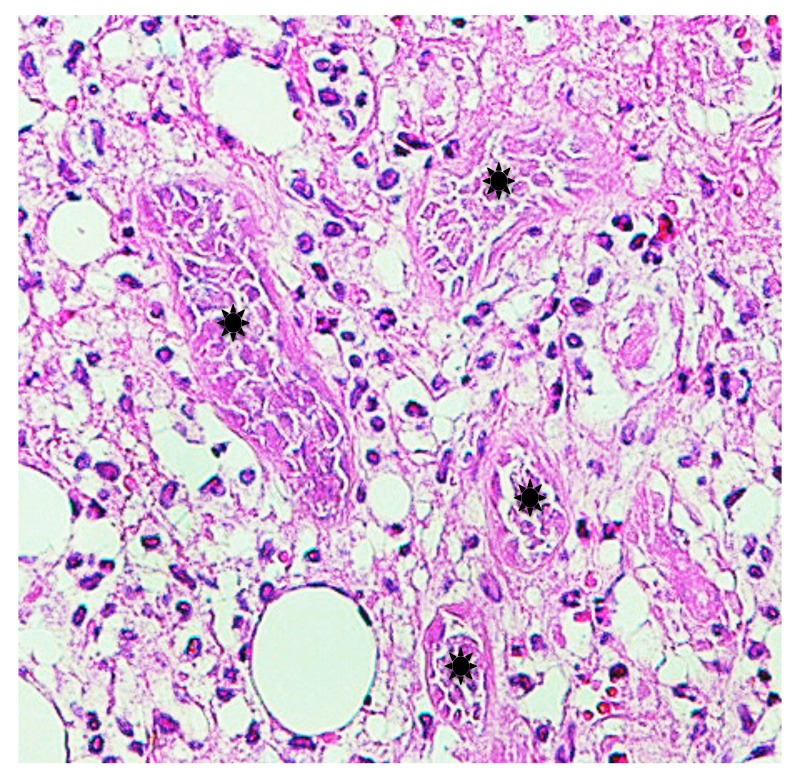
Case 5. A 3-year-old boy presented with a tick attached to the left retroauricular region, which his mother had noticed after he awoke that morning. On examination, the tick was surrounded by a slightly edematous, moderately erythematous halo. Dermoscopically, an engorged tick measuring about 3 x 4 mm was observed. Histopathologically, the lesion had severe acute inflammatory reaction with necrotic change from the epidermis to the subcutaneous tissue (Fig. 12). Penetrated mouthparts in the area hollowed from the skin surface and ulceration were present. Around the mouthparts, necrosis with marked destruction of the normal structures from the epidermis to the superficial dermis was seen (Fig. 13). The normal structures were replaced with amorphous granular basophilic material, so that the epidermis and the dermis could not be differentiated. These histopathologic findings closely resembled those in caseous necrosis except for having basophilic, rather than eosinophilic staining. The epidermis adjacent to the caseous necrosis-like change had severe exocytosis of the lymphocytes and the histiocytes. Below the caseous necrosis-like change, coagulative necrosis of the dermal tissue was seen which ran vertically from the skin surface toward the deep dermis and extended into the subcutaneous tissue (Figs. 12–14). Near the area of coagulative necrosis in the subcutaneous tissue, groups of completely necrotic small veins were present (Fig. 15); their entire walls as well as intravascular blood cells were necrotic. From the entire dermis to the subcutaneous tissue there was extensive interstitial lymphohistiocytic infiltrate (Fig. 12), and the subcutaneous tissue had lobular panniculitis (Fig. 14). No cement substance was found. Histochemically, the area of caseous necrosis-like change was stained strongly positive with PAS; stained weakly positive with PAS after diastase digestion; and not stained with AB. The collagen fibers in the coagulative necrosis area were stained red with EVG and considerably increased in thickness. They were moderately positive with PAS; weakly positive with PAS after diastase digestion; and negative with AB. Immunohistochemically, the patterns of distribution of CD+cells were these: numerous cells stained with anti-CD3 and anti-CD4 antibodies were present in the entire dermis (Fig. 16); moderate numbers of cells stained with positive with anti-CD8 antibody were present in the entire dermis; small numbers of cells stained with anti-CD20 antibody from the upper dermis to the mid-dermis, and moderate numbers were present in the deep dermis; small numbers of cells stained with anti-CD56 antibody were in the entire dermis; and moderate numbers of cells stained with anti-CD68 antibody were present in the entire dermis. For immunostaining with anti-CD1a antibody, many positive cells were present in the epidermis and the follicular epithelium. In the areas of caseous necrosis-like change and coagulative necrosis, the immunostained cells were rare (Fig. 16).
Fig. 12.
Histopathology. Extensive interstitial lymphohistiocytic infiltrate which means severe acute inflammatory reaction of the skin, is seen from the epidermis to the subcutaneous tissue. Hematoxylin and eosin stain. Original magnification: x20.
Fig. 13.
Magnified Fig. 12. Caseous necrosis-like change (circle) is seen adjacent to the mouthparts (MP). Asterisk indicates collagen bundles in the coagulative necrosis area. Hematoxylin and eosin stain. Original magnification: x50.
Fig. 14.
Magnified Fig. 12. Coagulative necrosis area (asterisk) is seen in the subcutaneous tissue which shows lobular panniculitis. Hematoxylin and eosin stain. Original magnification: x100. Insert; Magnified a part of the coagulative necrosis area in Fig. 14. Arrows indicate coagulative necrosis of the infiltrating inflammatory cells. Hematoxylin and eosin stain. Original magnification: x200.
Fig. 15.
Magnified a part of the subcutaneous tissue in Fig. 12. Coagulative necrosis of grouped small veins (asterisks) near the coagulative necrosis area. Hematoxylin and eosin stain. Original magnification: x100.
Fig. 16.
(case 5). Immunohistochemistry. There are numerous CD3+T cells from the dermis to the subcutaneous tissue. Notice that they are very few in the areas of the caseous necrosis-like change and the coagulative necrosis. Original magnification: x20.
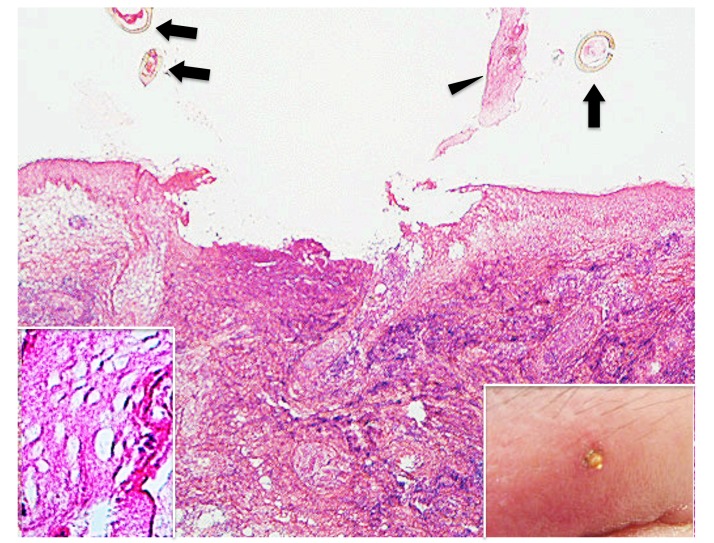
Case 6. A 58-year-old woman presented with an attached residual part of a tick body on the left upper eyelid. She had noticed an embedded tick just after waking up the day before presentation. She had tried to remove it with her fingers, but a small part of the tick body had remained in the skin. On examination, a part of the tick body was observed as a small, pear-shaped, yellow-brown projection which was surrounded by erythema and marked edema (Insert in the right lower corner of Fig. 17). Histopathologically, the central portion of the lesion was ulcerated with a caseous necrosis-like change similar to that in case 5 (Fig. 17), and adjacent epidermis was much destroyed (Fig. 17 and insert in the left lower corner of Fig. 17). In the entire dermis, extensive interstitial lymphohistiocytic infiltrate was diffusely present. Unlike the findings in case 5, there was no coagulative necrosis in the dermal tissue. The cement substance was not observed. The histochemical findings of caseous necrosis-like change and the immunohistochemical staining of the infiltrating inflammatory cells were similar to those in case 5.
Fig. 17.
(case 6). Histopathology and clinical feature. The epidermis and the underlying dermis are lost, resulting in ulcer formation. Arrows indicate sectioned mouthparts. Arrowhead indicates the epidermis peeled off. Hematoxylin and eosin stain. Original magnification: x20. Insert in the left lower corner. Magnified the epidermis peeled off in Fig. 17. Hematoxylin and eosin stain. Original magnification: x200. Insert in the right lower corner. Clinical feature. There is a part of the tick body which is surrounded by erythema and marked edema on the left upper eyelid.
DISCUSSION
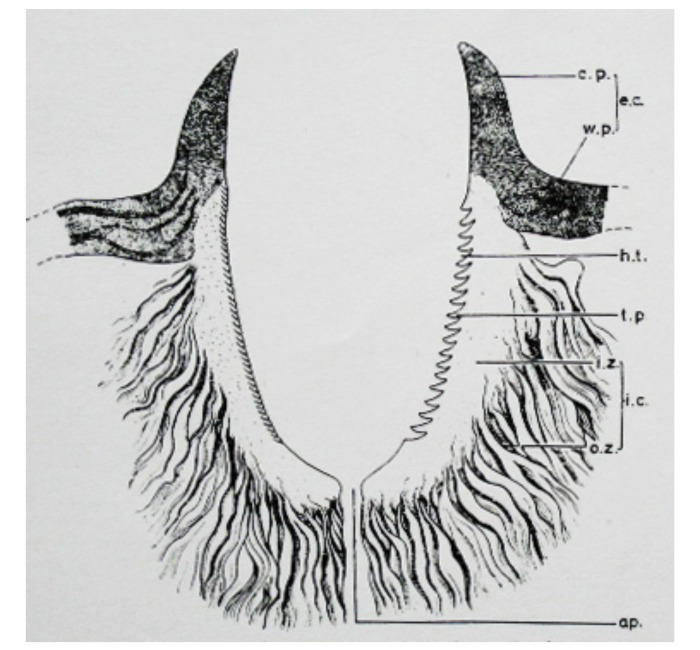
The cement substance of tick saliva is crucial for prolonged attachment of the tick to the host skin during feeding.12 According to a previous histopathologic study of the cement substance using rabbits by Chinery,10 the cement substance is bright pink with hematoxylin and eosin stain (HE) and is PAS-negative because it is protein rich in tyrosine and tryptophan. The cement substance is divided roughly into external and internal cement. The external cement is located in and over the epidermis which is named conical part (or cone) and wing-like part, respectively. The internal cement is present in the dermis which consists of inner zone (or tube of internal cement) and outer zone (or lateral strands). Schematic illustration of the cement substance is presented in Fig. 18.10 As described above, the mucoid substance in case 1 was typical external cement. In case 2, the external cement was identified by dermoscopy (Fig. 3A). However, histopathologically, neither the external cement nor the inner zone of the internal cement was found; the outer zone only was seen. The author had expected in this case that the mouthparts and the inner zone of the internal cement would be locked firmly to each other. However, two sectioned mouthparts were scattered in the feeding cavity (Fig. 4A). Such a histopathologic feature strongly suggests that both the external cement and the inner zone of the internal cement were artificially lost by an unknown factor(s) during the staining of the sections. Probably, the outer zone was preserved because it is formed from meshing with the fibrils of the surrounding dermal tissue.10 In cases 3 and 4, only the wing-like part of the external cement was present as a part of the crust. Unlike a description by Chinery,10 in case 3 the cement substance was strongly PAS-positive diastase-resistant, which indicates that it contained various glycoproteins.
Fig. 18.
Schematic illustration of the cement substance in the sagittal section by Chinery.10 (a.p.: aperture; c.p.: conical part, or cone; e.c.: external cement; h.t.: imprints of hypostomal teeth; i.c.: internal cement; i.z.: inner zone, or tube of internal cement; o.z.: outer zone, or lateral strands; t.p.: tooth-like process alternating with the imprints of hypostomal teeth; and w.p.: wing-like part.)
All six cases in this study presented in early stage after the tick bite, and the lesions in four of the patients (cases 1–4) had the cement substance. All four of them had severe epidermal and dermal damages including, in patients 1 and 2, coagulative necrosis in the epidermal and dermal tissues around the inserted mouthparts. Coagulative necrosis is often observed in the involved skins of tick bite.5, 10 Although there have been no descriptions, to my knowledge, about the pathogenesis of coagulative necrosis for tick bite, tissue damages or coagulative necrosis of the present cases are probably caused by trauma due to physical and chemical agents such as penetration of the mouthparts and permeation of the saliva which contains various biologically active substances such as esterase, aminopeptidase, hyaluronidase, metalloproteinase, cytolysin.7, 13, 14 Generally speaking, severe tissue damage or tissue necrosis is usually a potent triggers that induces acute inflammation.18 Contrary to expectation, however, the all involved lesions of the present four cases had very mild lymphohistiocytic infiltrate, i.e., mild inflammatory reaction. Also the neutrophilic infiltrate in the dermis of patient 2 was seen. Neutrophilic infilitrate is a well-known histopathologic feature in the early stage of a tick bite.2, 5, 19 The neutrophilic infiltrate in this patient implies that the lesion was in the stage of early neutrophilic infiltrate, not in the stage of later mononuclear cellular infiltrate on acute inflammation.18 From a viewpoint of normal immune responses, these findings are considered the early innate immune responses of the host,20 and it is not likely that cell-mediated immune mechanism may be operated in the involved host skin. In fact, it is already described that tick saliva has antiinflammatory and immunomodulatory or immunosuppressive substances, which suppress inflammatory reaction of the host skin,7, 15–17 although it is not known whether the cement substance itself has these effects. Thus it is possible that these bioactive substances of the tick saliva may make the cement-producing ticks prevent interruption of the fixation of their inserted mouthparts to the host skin by rejection of the host until their engorgement is finished.
In contrast to the four cases mentioned above, patients 5 and 6 did not have the cement substance and had a severe acute cutaneous inflammatory reaction. In both patients there was extensive interstitial lymphohistiocytic infiltrate from the entire dermis to the subcutaneous tissue, and almost all the infiltrating cells were lymphocytes and histiocytes. Particularly, the infiltrating lymphocytes were T-cell dominant in the entire dermis and mixed moderately with B-cells in the deep dermis, and also the histiocytes were diffusely present in the entire dermis. Such an infiltrating pattern of lymphocytes and histiocytes indicates mononuclear stage of acute inflammation.18, 20 Possibly this is an acute inflammatory reaction that is an adaptive immune response to the damaged tissues caused by both the mechanical stimulus of mouthpart insertion and biochemical stimulus of the salivary components. Unlike the cement-producing ticks, therefore, the non-cement-producing ticks may have no antiinflammatory and immunosuppressive substances in their saliva.
Patients 5 and 6 exhibited caseous necrosis-like change in the entrance site of the inserted mouthparts. Caseous necrosis results from the progression of coagulative necrosis.21 Thus the caseous necrosis-like change may develop due to much more injurious agents in the tick saliva than those in coagulative necrosis induced by the cement-producing ticks. In patient 5, grouped small veins had coagulative necrosis in the subcutaneous tissue near the area of coagulative necrosis. This finding may indicate that the soluble, highly toxic substances of the saliva of the non-cement-producing ticks were absorbed and accumulated in those veins in high concentration for a short time. Since such vascular changes were not observed in the lesions produced by the cement-producing ticks, probably toxic components of the saliva of the non-cement-producing ticks is very different in quality from those of the cement-producing ticks.
In summary, the cement-producing ticks induced mild cutaneous acute inflammatory reaction in the involved host skin in the early stage after bite, while the non-cement-producing ticks gave rise to severe cutaneous acute inflammatory reaction. This distinct difference of the host skin reaction may be caused by antiinflammatory and immunosuppressive agents contained in the saliva of the cement-producing ticks in order to prevent the position of their inserted mouthparts from rejection of the host. In contrast, possibly the non-cement-producing ticks may have no antiinflammatory and immunosuppressive agents in their saliva, but they have much more toxic agents than those in the cement-producing ticks.
This paper was read in part at the 261st Okayama Regional Meeting of Japanese Dermatological Association held in Okayama on January 18, 2014 and the Joint Meeting of the 127th San-in and the 23rd Schimane Regional Meetings of Japanese Dermatological Association in Izumo on March 2, 2014.
The authors declare no conflict of interest.
REFERENCES
- 1. Natsuaki M. The present state and countermeasure of tick bite. Nishinihon Hifuka. 2017;79:5-11. Japanese. [Google Scholar]
- 2. Wheeler CM, Coleman JL, Habicht GS, Benach JL. Adult Ixodes dammini on rabbits: Development of acute inflammation in the skin and immune responses to salivary glands, midgut, and spirochetal components. J Infect Dis. 1989;159:265-73. [DOI] [PubMed] [Google Scholar]
- 3. Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arhtropoda: Acari). Int J Dermatol. 1983;22:75-91. [DOI] [PubMed] [Google Scholar]
- 4. Bowman AS, Coons LB, Needham GR, Sauer JR. Tick saliva: recent advance and implications for vector competence. Medical and Veterinary Entomology. 1997;11:277-85. [DOI] [PubMed] [Google Scholar]
- 5. Stefanato CM, Phelps RG, Goldberg LJ, Perry AE, Bhawan J. Type-1 cryoglobulinemia-like histopathogic changes in tick bites: a useful clue for tissue diagnosis in the absence of tick parts. J Cutan Pathol. 2002;29:101-6. [DOI] [PubMed] [Google Scholar]
- 6. Galaria NA, Chaudhary O, Margo CM. Tick mouth parts occlusive vasculopathy; a localized cryoglobulinemic vasculitic response. J Cutan Pathol. 2003;30:303-6. [DOI] [PubMed] [Google Scholar]
- 7. Castelli E, Caputo V, Mollero V, Tomasino RM. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-8. [DOI] [PubMed] [Google Scholar]
- 8. Resnik KS. Intravascular eosinophilic deposits-when common knowledge is insufficient to render a diagnosis. Am J Dermatopathol. 2009;31:211-7. [DOI] [PubMed] [Google Scholar]
- 9. Krahl D, Sellheyer K. A scanning microscopic clue to the diagnosis of arthropod assault reaction: alteration of interstitial tissue is more common than a wedge-shaped inflammatory infiltrate. J Cutan Pathol. 2009;36:308-13. [DOI] [PubMed] [Google Scholar]
- 10. Chinery WA. The nature and origin of the “cement” substance at the site of attachment and feeding of adult Haemaphysalis spinigera (Ixodidae). J Med Ent. 1973;10:355-62. [DOI] [PubMed] [Google Scholar]
- 11. Arthur DR. Tick feeding and its implications. Adv Parasitol. 1970;8:275-92. [DOI] [PubMed] [Google Scholar]
- 12. Hovius JWR, Levi M, Fikrig E. Salivating for knowledge: potential pharmacological agents in tick saliva. Plos Med. 2008;5:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saeki E. Madani no Seibutsugaku [Biology of tick]. Dou Yaku Kenkyu. 1998;5:13-21. Japanese. [Google Scholar]
- 14. Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-42. [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro JMC. Role of saliva in blood-feeding by arthropods. Ann Rev Entomol. 1987;32:463-78. [DOI] [PubMed] [Google Scholar]
- 16. Ribeiro JMC, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of atick, Ixodes dammini. J Exp Med. 1985;161:332-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribeiro JMC, Weis JJ, Telford SR III. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382-8. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell RN. Inflammation and repair. In: Robins Basic Pathology, 9th ed Chapter 2. Kumar V, Abbas AK, Aster JC, editors. Philadelphia (PA): Elsevier; 2013. p.29-73. [Google Scholar]
- 19. Brown SJ. Antibody- and cell-mediated immune resistance by gunea pigs to adult Amblyomma americanum ticks. Am J Trop Med Hyg. 1982;31:1285-90. [DOI] [PubMed] [Google Scholar]
- 20. Maitra A. Disease of the immune system. In: Robins Basic Pathology, 9th ed Chapter 2. Kumar V, Abbas AK, Aster JC, editors. Philadelphia (PA): Elsevier; 2013. p.99-151. [Google Scholar]
- 21. Ogata T. Byori Gaku Nyumon [A Primer of Pathology]. 9th ed Tokyo: Nanzando; 1966. p.192. Japanese. [Google Scholar]