Abstract
Aim of the study
To evaluate an association between food products consumption, dietary intake and the incidence of selected gastrointestinal symptoms (nausea, vomiting, diarrhea and constipation) in cancer patients undergoing chemotherapy.
Material and methods
Fifty six women receiving chemotherapy for ovarian cancer were eligible for the study. Anthropometrical measurements were assessed. The dietary intake was evaluated by 24-hours food records. The association between the consumption of selected food products and gastrointestinal symptoms incidences was assessed by modified semi-quantitative food frequency questionnaire including 77-different food items that was developed and applied in cancer patients undergoing chemotherapy.
Results
BMI values indicated 9%, 45%, 30% and 16% of patients as underweight, normal weight, overweight and obese respectively. Only 23% and 32% of patients never experienced nausea and constipation when 43% and 45% never experienced vomiting and diarrhea. Nausea was promoted by oils, constipation by chocolate and chocolate products and diarrhea by dairy products, stone fruit and apple. Significant inverse correlations were found between vomiting and the intake of energy, fat, protein, carbohydrates, B groups vitamins, vitamin D, phosphorus and zinc. The difference in energy intake between marginal values of vomiting incidence exceeded 400 kcal.
Conclusions
Dietary intake as well as specific food products influence on gastrointestinal side effect of chemotherapy in cancer patients. The dietary approach based on either exclusion or limited intake of selected food products and improvement of diet could reduce and prevent chemotherapy induced gastrointestinal symptoms therefore should be taken under consideration in clinical practice.
Keywords: Chemotherapy induced nausea and vomiting (CINV), diarrhea, constipation, chemotherapy, cancer, diet
Introduction
The incidences of cancer rise up in recent decades. Despite the new drugs and techniques in medicine are developing, cytotoxic chemotherapy is the only treatment option for many patients. While the use of chemotherapy has significantly improved survival rates, the symptoms associated with chemotherapy remain a major burden for patients [1].
The most common chemotherapy side effects associated with gastrointestinal tract are taste changes, chemotherapy induced nausea and vomiting (CINV), constipation and diarrhea [2, 3]. The prevention of CINV has been revolutionized over the past years. Guideline-based treatment means that vomiting can be prevented in the majority, but not in all patients [4]. The incidences of CINV depend mainly on chemotherapy regimen, where high emetogenic agents (> 90% of patients) include i.e. cisplatin, cyclophosphamide and dacarbazin [5]. Constipation occurs in an average of 41% of patients undergoing chemotherapy, most common when treated with the vinca alkaloid group [6]. However, other factors such as advanced age, decreased mobility, dietary errors, psychological alterations and cancer related complications (tumor growth, plexus invasion, adhesions, hernias, radiotherapy, peritoneal carcinomatosis, and opioids) may increase its occurrence [6]. The incidences of chemotherapy-induced diarrhea (CID) vary depending on the regimen used. It has been reported to be as high as 80% when treated with 5-fluorouracil and irinotecan [7]. Other chemotherapeutic agents associated with diarrhea include: cisplatin, cyclophosphamide, doxorubicin, paclitaxel, topotecan, etc. [8]. However, also in the case of CID other factors such as diet (deficiency of vitamin A and zinc, excess of vitamin C, high fiber diet, high osmolar dietary supplements, milk or milk products), infection, inflammatory factors, malabsorption, medications, neuroendocrine factors and psychological factors appear to be associated with an increased incidence of CID [7, 8].
The knowledge concerning dietary modification depending on cancer side effects based mostly on clinical experience and there is a lack of the scientific data in this area. Still little is known also about the self-management dietary behaviors in patients suffering from cancer. In clinical practice the implementation of non-pharmacologic strategies play an important role as adjuncts to pharmacological agents in alleviating chemotherapy induced gastrointestinal symptoms. Therefore we aimed this study to find association between dietary intake, consumption of specific food products and chemotherapy related gastrointestinal side effects (nausea, vomiting, diarrhea and constipation) in cancer patients undergoing chemotherapy.
Material and methods
Participants
From 56 women who were eligible for the study 44 completed and provided all questionnaires. The study was performed in patients receiving chemotherapy for ovarian cancer at the Department of Oncology at Poznan University of Medical Sciences (Poznan, Poland) from May to December 2014. Patients were eligible if they had been receiving at least 2 cycles of chemotherapy (radical or palliative) with histological confirmed cancer. Exclusion criteria were as follow: non-malignant diseases or other that mentioned above cancer, bad performance status (≥ 3) according to Eastern Cooperative Oncology Group (ECOG) [9]. All study women in our clinic received emetogenic prophylaxis based on actual guidelines [10]. The study was performed in accordance with the Helsinki Declaration. The subjects gave their written consent for the study.
Nutritional status and energy intake
Body weight and height were measured with accuracy to 0.10 kg and 0.5 cm respectively (Radwag, Radom, Poland). Body mass index (BMI) was calculated in all patients. The energy intake was evaluated by 24-hours food records with a dietician checking of data completion (Dietetyk, National Institute of Food and Nutrition, Warsaw, Poland). Patients filled out the questionnaire 3 day prior the admission to the clinic for the next chemotherapy cycle.
Gastrointestinal symptoms and food consumption
Previously validated a 77-item questionnaire [11, 12] was used to evaluate the incidence of chemotherapy induced gastrointestinal symptoms and describe an association between them and selected food products. Patients were asked to answer question “how often have you had”: nausea, vomiting, constipation and diarrhea on a 6-point scale ranging from 0 (“Never”) to 5 (“very often”). Next they marked food products that they recognized as increasing gastrointestinal symptoms, separately for: nausea, vomiting, constipation and diarrhea. Food products that were indicated by 5–10% of patients were classified as moderately connected with specific symptoms and those highlighted by more than 10% of women as significant. Additionally patients were asked about the dietary supplements intake that could potentially influence the occurrence of nausea and vomiting or constipation and diarrhea.
Statistical analysis
Categorical data were presented as raw numbers and percentages. Continuous data were presented as means and standard error of mean (SEM). Data were compared using Kruskal-Wallis test with Dunn post-hoc test. The Spearman correlation coefficient (r) was calculated to measure the strength and direction of a relationship. The following interpretation of correlation coefficient was used: 0 indicates no relationship, values between 0.7 and 1.0 (–0.7 and –1.0) indicate a strong positive (negative) relationship, between 0.3 and 0.7 (0.3 and –0.7) moderate, between 0 and 0.3 (0 and –0.3) a weak positive (negative), –1 a perfect negative and +1 a perfect positive relationship. Statistical analysis was performed with the use of Statistica 12 Software (StatSoft, Tulsa, US).
Results
The characteristics of studied population are presented in Table 1. The most of the woman were characterized by advanced stages of cancer and undergone subsequent line of chemotherapy. Mean value of BMI (25.2 kg/m2) indicated overweight, which refer to 30% of patients; however only 9% were underweight, 16% obese and 45% normal weight.
Table 1.
Characteristic of study population (n = 44)
| Race / ethnicity | n (%) |
|---|---|
| Caucasian | 44 (100) |
| Stage at diagnosis | n (%) |
| Early | 11 (25) |
| Advanced | 33 (75) |
| Line of chemotherapy | n (%) |
| 1 | 15 (34) |
| 2 + | 29 (66) |
| Chemotherapy regimen | n (%) |
| Platinum based | 35 (79.5) |
| Antracyclines | 5 (11.5) |
| Topotecan | 4 (9) |
| Anthropometry | Mean (SEM) |
| Age (years) | 58.6 (1.5) |
| Body weight (kg) | 65.1 (2.2) |
| Body height (cm) | 160.4 (0.89) |
| BMI (kg/m2) | 25.2 (0.74) |
SEM – standard error of mean
The incidence of chemotherapy induced gastrointestinal symptoms and intake of dietary supplements were presented in Table 2. Constipation and nausea were much more frequently observed than diarrhea and vomiting (20%, 18%, 7% and 7% respectively for often and very often answer). From all patients 66% used anti CINV supplements and 57% anti-constipation or diarrhea supplements. Only 23% and 32% of patients never experienced nausea and constipation respectively when 43% and 45% never experienced vomiting and diarrhea. However, the use of anti CINV supplements was reported in 8 of 10 patients who have never experienced nausea and in all who have never experienced vomiting. Similarly, 11 of 14 patients with no constipation and 14 of 20 with no diarrhea symptoms used anti-constipation or diarrhea supplements respectively.
Table 2.
Incidence of chemotherapy related gastrointestinal symptoms and use of dietary supplements
| Symptom | Incidence of chemotherapy related gastrointestinal symptoms no (%) | |||||
|---|---|---|---|---|---|---|
| never | very rare | rare | sometimes | often | very often | |
| Nausea | 10 (23) | 11 (25) | 9 (20) | 6 (14) | 8 (18) | 0 (0) |
| Vomiting | 19 (43) | 10 (23) | 6 (14) | 6 (14) | 3 (7) | 0 (0) |
| Constipation | 14 (32) | 10 (23) | 6 (14) | 5 (11) | 5 (11) | 4 (9) |
| Diarrhea | 20 (45) | 13 (30) | 6 (14) | 2 (5) | 1 (2) | 2 (5) |
| Use of dietary supplements no (%) | ||||||
| Nausea and vomiting | Yes | 29 (66) | No | 15 (34) | ||
| Constipation and diarrhea | Yes | 25 (57) | No | 19 (43) | ||
The list of food products increasing the incidences of chemotherapy induced gastrointestinal symptoms was presented in Table 3. From those, oils were the only that significantly increased the incidence of nausea (moderately also the incidence of vomiting). The consumption of chocolate and chocolate products was significantly related to increased incidence of constipation. Products like: dairy products, stone fruit and apple were significantly related to the incidence of diarrhea.
Table 3.
Food products increasing the incidence of chemotherapy related gastrointestinal symptoms
| Symptoms occurrence | Nausea | Vomiting | Constipation | Diarrhea |
|---|---|---|---|---|
| Significant | Oils | None | Chocolate and chocolate products | Dairy products Stone fruit Apple |
| Moderately | Milk and dairy products Eggs Candies Cookies Ice cream Citrus fruits Tropical fruits Berries Pear Apple Banana Cruciferous vegetables Bulb vegetables Tomato Paprika Legumes Vegetable juice Processed meat Fat-rich fish |
Oils Bulb vegetables Processed meat |
Cheeses Salty snacks White bread Animal fats |
Ice cream Citrus fruits Tropical fruits Berries Pear Banana Cruciferous vegetables Tomato Paprika |
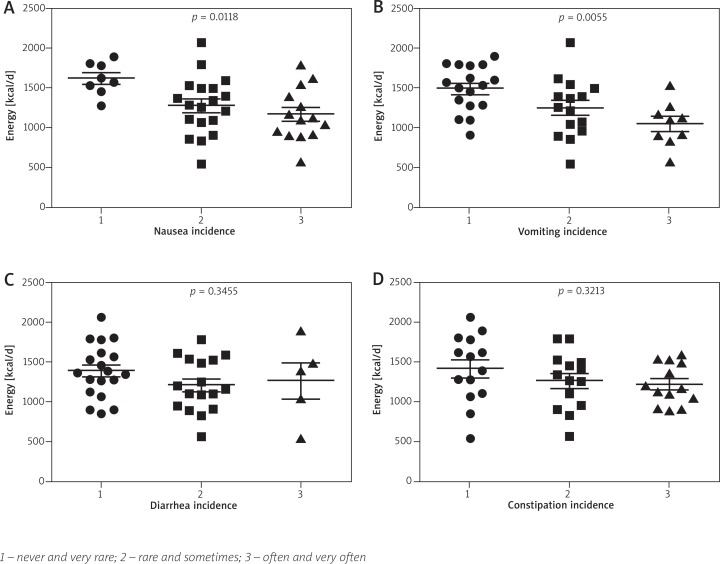
The energy intake depending on gastrointestinal symptoms incidence was presented on Fig. 1. A significantly reduced energy intake was related to the occurrence of nausea (r = –0.38; 95% CI: from –0.62 to –0.08, p = 0.0141) and vomiting (r = –0.56; 95% CI: from –0.74 to –0.30, p = 0.0002). Mean energy intake was 452 kcal lower comparing marginal values by nausea incidence (1619 to 1167 kcal) and 443 kcal lower (1493 to 1050 kcal) by vomiting respectively. No relationship between diarrhea (r = –0.05; 95% CI: from –0.35 to 0.26, p = 0.7513), constipation (r = –0.21; 95% CI: from –0.49 to 0.10, p = 0.180) and energy intake was observed. Nausea was additionally inversely correlated to the intake of fat, saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA), when vomiting inversely correlated to the intake of protein, carbohydrates, fat, SFA, MUFA, polyunsaturated fatty acids (PUFA), phosphor, zinc, and B group vitamins (B1, B2, B6, B12, PP). MUFA intake was also inversely correlated with the incidence of constipation when no significant correlations in diarrhea were found (Table 4).
Fig. 1.
The energy intake depending on chemotherapy induced gastrointestinal symptoms incidence. Scatter plots show means with standard errors
Table 4.
Associations between dietary intake and chemotherapy related side effect
| Parameter | Nausea | Vomiting | Constipation | Diarrhea |
|---|---|---|---|---|
| Energy (kcal) | –0.38* | –0.56*** | –0.21 | –0.05 |
| Protein (g) | –0.24 | –0.49** | –0.22 | –0.04 |
| Carbohydrates (g) | –0.19 | –0.36* | –0.03 | –0.14 |
| Fat (g) | –0.43** | –0.47** | –0.28 | 0.10 |
| SFA (g) | –0.37* | –0.39* | –0.28 | 0.14 |
| MUFA (g) | –0.43** | –0.45** | –0.33* | 0.12 |
| PUFA (g) | –0.26 | –0.32* | –0.03 | –0.06 |
| Cholesterol (mg) | –0.28 | –0.28 | –0.20 | 0.06 |
| Saccharose (g) | –0.12 | –0.10 | 0.12 | –0.04 |
| Fibre (g) | 0.04 | –0.13 | 0.08 | –0.03 |
| Na (mg) | –0.23 | –0.29 | –0.14 | –0.06 |
| K (mg) | –0.11 | –0.26 | 0.02 | –0.17 |
| Ca (mg) | –0.17 | –0.20 | –0.08 | –0.12 |
| P (mg) | –0.22 | –0.42** | –0.09 | –0.16 |
| Mg (mg) | –0.13 | –0.25 | 0.04 | –0.17 |
| Fe (mg) | –0.19 | –0.25 | 0.01 | –0.11 |
| Zn (mg) | –0.27 | –0.34* | –0.14 | –0.25 |
| Vit. A (μg) | –0.24 | –0.17 | –0.08 | –0.21 |
| Vit. D (μg) | –0.19 | –0.33* | 0.14 | 0.19 |
| Vit. E (mg) | –0.14 | –0.14 | 0.006 | –0.17 |
| Vit. B1 (mg) | –0.25 | –0.36* | –0.03 | –0.13 |
| Vit. B2 (mg) | –0.23 | –0.30* | –0.12 | –0.11 |
| Vit. PP (mg) | –0.07 | –0.39* | –0.03 | –0.10 |
| Vit. B6 (mg) | –0.17 | –0.37* | 0.02 | –0.19 |
| Folate (μg) | 0.01 | –0.11 | 0.09 | –0.02 |
| Vit. B12 (μg) | –0.30 | –0.41** | –0.06 | –0.18 |
| Vit. C (mg) | 0.15 | 0.002418 | 0.11 | 0.05 |
p < 0.05;
p < 0.01;
p < 0.001
SFA – saturated fatty acids; MUFA – monounsaturated fatty acids; PUFA – polyunsaturated fatty acids
Discussion
The presented study provides consistent evidence that almost 80% of cancer patients experienced ever nausea, almost 70% constipation and over 50% vomiting and diarrhea during the chemotherapy. It has been shown that that significant link exists between chemotherapy induced gastrointestinal symptoms and dietary intake or specific food product intake.
Guidelines for CINV treatment and prevention describe specific pharmacological interventions depending on chemotherapeutic agent potential, although there is lack of information regarding nutritional modifications [4, 5, 13, 14]. The current study aimed to fill this gap in the literature. In one of the already published systematic review, focusing on dietary management in gastrointestinal complications from chemotherapy, it has been suggested rather specific dietary behaviors than avoiding specific food (except overly fatty and sweet foods) [6]. Several self-care strategies were described by Williams et al. [15] in the following categories using complementary medicine as framework: diet/nutrition/lifestyle change (eg: use of nutritional supplements; modifications of food and of eating habits; naps, sleep, and rest), mind/body control (eg, relaxation methods, prayer, music, attending granddaughter’s sports events) biologic treatments (vitamins), herbal treatments (green mint tea), and ethnomedicine (lime juice and garlic). There are also eating habits that usually apply cancer patients as for example: eating slowly, with small and frequent meals; avoiding intake of liquids during meals, using well-tolerated foods with neutral odors, preferring dry foods, staying away from the kitchen during food preparation, eating in a pleasant, cool environment with fresh air [6]. We have decided to take an advantage of our patients and use directly their experience with diet and consumption of different food products during the chemotherapy. In the current study, more than 10% of patients indicated oils as products significantly increasing incidence of nausea. Further, next 5 to 10% of patients indicated much more potential candidates within food products that may increase CINV. Of course for proper assessment of the relationship between food and the incidence of gastrointestinal symptoms we should take under consideration possible dietary errors that could be related to subjective evaluation of patients and indication of only several food products associated with an increased incidence of mentioned above chemotherapy side effect. However, the strong support of medical staff during the dietary examination seems to exclude major confusions or misunderstanding of dietary questions by analyzed patients.
Chemotherapy induced diarrhea recommendations include, beside pharmacological treatment also diet modifications [7, 8]. These consider avoiding spicy foods, caffeine, alcohol and fruit juices, high osmolar dietary supplements, vegetables, especially cruciferous as well as lactose containing products, high fiber and high fat food [8]. However, due to the lack of information regarding nutritional modification, recommendations based rather on clinical practice than scientific evidences. In the previously published systematic review it has been indicated even more food products that should be avoid by cancer patients [6]. Among them dairy products, apples and stone fruits (apricot, cherry, nectarine, peach, and plum) are listed and indicated by majority of our patients as increasing the incidence of diarrhea.
Constipation is also recognized as one of the main symptoms reported by cancer patients. Nevertheless, behind the chemotherapy there are also biologically active substances that may cause the constipation [16, 17]. Müller-Lissner et al. [18] pointed out that these patients with constipation report altered stool form after food and beverage consumption more often than healthy subjects. Additionally, among these chocolate was the most frequently mentioned and perceived as caused constipation. Therefore, psychological factors could also influence our patients during the selection of these products.
Currently, on the market, there are many ready to use either products or beverages containing energy by cancer patients to increase calories intake. However, none from the examined patients consumed such products, which could not influence the obtained results of energy intake. We have already reported in previously published study that patients suffering from ovarian cancer did not reach even 25 kcal per kg of body weight during the day [11]. The changes in energy and nutrients intake are especially visible when we take into account separated cycles of cytotoxic chemotherapy [12]. Usually, the changes in energy intake are directly related to body weight changes [19]. Especially, as pointed out Grosvenor et al. [20] gastrointestinal symptoms (nausea indicated in 39%, vomiting in 27% and constipation in 41% of patients) potentially influencing weight loss are prevalent early in the course of cancer patients with regard to lower caloric intake, nutritional status and prior therapy experience. However, in current study only small percentage of patients was underweight, which stay in line with previously published data [11, 21]. In early 90’s has been already indicated that a significant percentage of patients who even received exclusive oral feeding did not cover a minimum acceptable quantity of their energy requirements [22].
Limitations
Despite an increasing number of dietary intervention studies applying pharmacologic and non-pharmacologic strategies in alleviating chemotherapy induced nausea and vomiting, the body of evidence remains limited by small sample size. Only future clinical trials with long-term follow-up periods can address this limitation. The heterogeneity of study population could influence results of this study. We believe that by change in dietary behaviors patients could increase energy intake which will result, in most cases, in a higher quality of life.
In conclusions, dietary intake as well as specific food products influence on gastrointestinal side effect of chemotherapy in cancer patients. The dietary approach based on either exclusion or limited intake of selected food products and improvement in dietary intake could reduce and prevent chemotherapy induced gastrointestinal symptoms and should be taken under consideration in clinical practice. The arguments for such an approach come from practical experience showing that the total energy intake is the driving force for better nutritional status and leads to better outcomes in the treatment of cancer patients.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kearney N, Miller M, Maguire R, et al. WISECARE+: Results of a European study of a nursing intervention for the management of chemotherapy-related symptoms. Eur J Oncol Nurs. 2008;12:443–8. doi: 10.1016/j.ejon.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME. Cancer: disease and nutrition are key determinants of patients’ quality of life. Support Care Cancer. 2004;12:246–52. doi: 10.1007/s00520-003-0568-z. [DOI] [PubMed] [Google Scholar]
- 3.Grant M, Kravits K. Symptoms and their impact on nutrition. Semin Oncol Nurs. 2000;16:113–21. doi: 10.1053/on.2000.5738. [DOI] [PubMed] [Google Scholar]
- 4.Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol. 2015;26:1081–90. doi: 10.1093/annonc/mdv138. [DOI] [PubMed] [Google Scholar]
- 5.Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202. doi: 10.1016/j.ejphar.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Calixto-Lima L, Martins de Andrade E, Gomes AP, Geller M, Siqueira-Batista R. Dietetic management in gastrointestinal complications from antimalignant chemotherapy. Nutr Hosp. 2012;27:65–75. doi: 10.1590/S0212-16112012000100008. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, 3rd, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918–26. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 8.Richardson G, Dobish R. Chemotherapy induced diarrhea. J Oncol Pharm Pract. 2007;13:181–98. doi: 10.1177/1078155207077335. [DOI] [PubMed] [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 10.Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202. doi: 10.1016/j.ejphar.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 11.Mardas M, Jamka M, Mądry R, Walkowiak J, Krótkopad M, Stelmach-Mardas M. Dietary habits changes and quality of life in patients undergoing chemotherapy for epithelial ovarian cancer. Support Care Cancer. 2015;23:1015–23. doi: 10.1007/s00520-014-2462-2. [DOI] [PubMed] [Google Scholar]
- 12.Mardas M, Mądry R, Stelmach-Mardas M. Dietary intake variability in the cycle of cytotoxic chemotherapy. Support Care Cancer. 2016;24:2619–25. doi: 10.1007/s00520-015-3072-3. [DOI] [PubMed] [Google Scholar]
- 13.Gralla RJ, Roila F, Tonato M, Herrstedt J. MASCC/ESMO Antiemetic Guideline Multinational Association of Supportive Care in Cancer. [Online, 03 January 2016] http://www.mascc.org/antiemetic-guidelines. [Google Scholar]
- 14.NCCN Clinical Practice Guideline in Oncology . Antiemesis. . Version 2.2015 [Online, 03 January 2016]. Available at: http://www.nccn.org. [Google Scholar]
- 15.Williams PD, Piamjariyakul U, Ducey K, Badura J, Boltz KD, Olberding K, Wingate A, Williams AR. Cancer treatment, symptom monitoring, and self-care in adults: pilot study. Cancer Nurs. 2006;29:347–55. doi: 10.1097/00002820-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Motoyama T, Katayama Y, Watanabe H, Okazaki E, Shibuya H. Functioning ovarian carcinoids induce severe constipation. Cancer. 1992;70:513–8. doi: 10.1002/1097-0142(19920715)70:2<513::aid-cncr2820700223>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Huang L, Cai W, Cao S, Yuan Y, Lu S, Zhao Y, Lu P. Relationship between serotonin transporter gene polymorphism and constipation in cancer patients. Contemp Oncol (Pozn) 2015;19:17–21. doi: 10.5114/wo.2014.41391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller-Lissner SA, Kaatz V, Brandt W, Keller J, Layer P. The perceived effect of various foods and beverages on stool consistency. Eur J Gastroenterol Hepatol. 2005;17:109–12. doi: 10.1097/00042737-200501000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Zabłocka-Słowińska K, Porębska I, Gołecki M, Prescha A, Pieczyńska J, Kosacka M, Ilow R, Grajeta H, Jankowska R, Biernat J. Dietary habits of lung cancer patients from the Lower Silesia region of Poland. Contemp Oncol (Pozn) 2015;19:391–395. doi: 10.5114/wo.2015.54084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosvenor M, Bulcavage L, Chlebowski RT. Symptoms potentially influencing weight loss in a cancer population. Correlations with primary site, nutritional status, and chemotherapy administration. Cancer. 1989;63:330–4. doi: 10.1002/1097-0142(19890115)63:2<330::aid-cncr2820630221>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira D, Guimarães TG, Marcadenti A. Acceptance of hospital diets and nutritional status among inpatients with cancer. Einstein (Sao Paulo) 2013;11:41–6. doi: 10.1590/S1679-45082013000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabal J, Leyes P, Forga MT, Hervás S. Quality of life, dietary intake and nutritional status assessment in hospital admitted cancer patients. Nutr Hosp. 2006;21:505–10. [PubMed] [Google Scholar]