Abstract
The ability of an aqueous extract of the rhizomes of Sansevieria liberica to protect against carbon tetrachloride induced liver injury was investigated in Wistar albino rats. The carbon tetrachloride was prepared 1:5 (v:v) in olive oil, and administered subcutaneously at 1 mL/kg body weight. The extract was administered to both normal and carbon tetrachloride treated rats at 100, 200 and 300 mg/kg. On gas chromatographic analysis of the extract, twenty nine known flavonoids were detected, consisting mainly of 31.94 % apigenin, 20.66 % quercetin, 11.28 % kaempferol, 5.99 % naringenin, 5.83 % (-)-epicatechin, 3.69 % biochanin, 3.58 % (+)-catechin, 2.72 % diadzein, 2.20 % ellagic acid, 2.04 % butein. Compared to test control, the treatment dose dependently produced significantly (P<0.05) lower alkaline phosphatase, aspartate transaminase and alanine transaminase activities. The plasma total bilirubin and total protein levels of the test animals were lower though not significantly. The hepatic histopathological studies showed that carbon tetrachloride caused fatty degeneration of hepatocytes, which was inhibited by pre-treatment with the extract; thus, confirming the results of the biochemical studies. The results of this study indicated that treatment with the plant extracts protects the liver against carbon tetrachloride induced hepatotoxicity. This supports the use of Sansevieria liberica in traditional health care for managing liver problems.
Keywords: apigenin, carbon tetrachloride, flavonoids, hepatospecific markers, histopathology, Sansevieria liberica Gérôme and Labroy
Introduction
Liver dysfunction as a result of infection, toxic chemicals, certain drugs and environmental pollutants has been largely increased in the last few decades (Atta et al., 2006[5]). Herbs play a major role in the management of various liver disorders. Sansevieria liberica is one of such plants used in African traditional health care for the treatment of liver disorders.
Sansevieria liberica (Family Agavaceae or Ruscaceae or Dracaenaceae), is one of the bowstring hemp species (Evans, 2005[10]), with concave, short petioled leaves that are in part transversely banded with light and dark green, or may be linearly striated with whitish to light green and dark green striations (Reed, 1978[29]). The leaves are very rich in fibers (Ikewuchi et al., 2010[12]; Osabohien and Egboh, 2008[25]), protein (Ikewuchi et al., 2010[12]), potassium, calcium, magnesium, vitamin C, biotin, and riboflavin (Ikewuchi and Ikewuchi, 2009[13]). This plant has long rhizomes with long fibrous roots and a rapid rate of growth, and produces red or orange berry fruits. It is a rather stout herb with several stiff red-margined leaves about 2 feet high arising from the creeping plant, 50-80 cm long inflorescence longer than leaves with abundant white flowers. They are grown as ornamental plants (United States Development Agency, 2008[34]), and are widely distributed throughout the tropics. In Nigeria, the leaves and roots of Sansevieria liberica are used in traditional medicine for the treatment of abdominal pain, asthma, colic, diarrhea, eczema, gonorrhea, hemorrhoid, hypertension, monorrhagia, piles, sexual weakness, wounds, and alleviating the effect snake bite (Adeyemi et al., 2009[1]; Amida et al., 2007[4]; Gill, 1992[11]; Osabohien and Egboh, 2008[25]; Osabohien, 2009[24]). The roots are used for the treatment of convulsion, epilepsy, paralysis, malnutrition, pulmonary troubles, vermifuges, cough and debility. The anti-anaemic (Ikewuchi et al., 2010[14]) and sedative and anticonvulsant (Adeyemi et al., 2007[2]) activities of the leaves and roots have been reported. In this study, the ability of an aqueous extract of the rhizomes of Sansevieria liberica, to protect against carbon tetrachloride induced liver damage was investigated in Wistar albino rats.
Materials and Methods
Preparation of plant extract
Samples of fresh Sansevieria liberica plants (Figure 1(Fig. 1)) were procured from horticulturists by Air Force Gate, Aba Road, and at the University of Port Harcourt's Abuja campus, and from behind the Ofrima complex of University of Port Harcourt, all in Port Harcourt, Nigeria. After due identification at the University of Port Harcourt Herbarium, Port Harcourt, the identity was confirmed/authenticated by Dr. Michael C. Dike of Taxonomy Unit, Department of Forestry and Environmental Management, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria; and Mr. John Ibe, the Herbarium Manager of the Forestry Department, National Root Crops Research Institute (NRCRI), Umuahia, Nigeria. The rhizomes were removed, rid of dirt, oven dried at 55 °C and ground into powder. The resultant powder was soaked in hot distilled water for 12 h, after which the resultant mixture was filtered and the filtrate (herein referred to as the aqueous extract) was stored in the refrigerator for subsequent use. A known volume of this extract was evaporated to dryness, and the weight of the residue was used to determine the concentration of the filtrate, which was in turn used to determine the dose of administration of the extract. The percentage recovery of the crude extract was 29 %. The resultant residue from the aqueous extract was used for the phytochemical study, in order to determine its flavonoid composition.
Figure 1. Sansevieria liberica Gérôme and Labroy.

Determination of the flavonoid content of the leaf extract
Calibration, identification and quantification
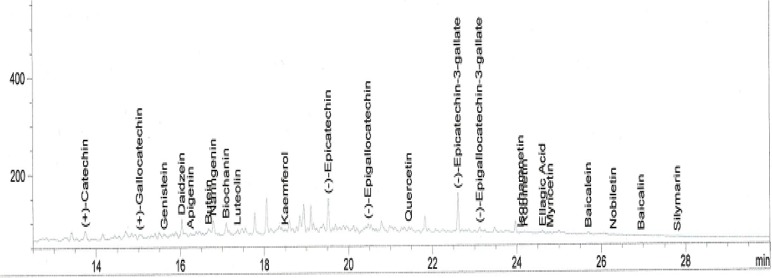
Standard solutions were prepared in methanol. The linearity of the dependence of response on concentration was verified by regression analysis. Identification was based on comparison of retention times and spectral data with standards. Quantification was performed by establishing calibration curves for each compound determined, using the standards. The chromatogram of the extract is shown in Figure 2(Fig. 2).
Figure 2. Chromatogram of the flavonoid composition of an aqueous extract of the rhizomes of Sansevieria liberica.
Determination of flavonoid composition
The extraction was carried out according to the method of Millogo-Kone et al. (2009[21]). The residue from the aqueous extract above was extracted with methanol and the resultant extract was subjected to gas chromatographic analysis. Chromatographic analyses were carried out on an HP 6890 (Hewlett Packard, Wilmington, DE, USA), GC apparatus, fitted with a flame ionization detector (FID), and powered with HP Chemstation Rev. A 09.01 [1206] software, to quantify and identify compounds. The column was a capillary HP INNOWax Column (30 m × 0.25 mm × 0.25 μm film thickness). The inlet and detection temperatures were 250 and 320 °C. Split injection was adopted with a split ratio of 20:1. Nitrogen was used as the carrier gas. The hydrogen and compressed air pressures were 22 psi and 35 psi. The oven was programmed as follows: initial temperature at 50 °C, first ramping at 8 °C/min for 20 min, maintained for 4 min, followed by a second ramping at 12 °C/min for 4 min, maintained for 4 min.
Experimental design for the hepatoprotective study
Male Wistar albino rats (190-210 g) were collected from the animal house of the Department of Physiology, University of Nigeria, Enugu Campus. Studies were conducted in compliance with applicable laws and regulations for handling experimental animals. The rats were weighed and sorted into eight groups (Table 1(Tab. 1)) of five animals each, so that their average weights were approximately equal.
Table 1. Experimental design for the hepatoprotective screening.
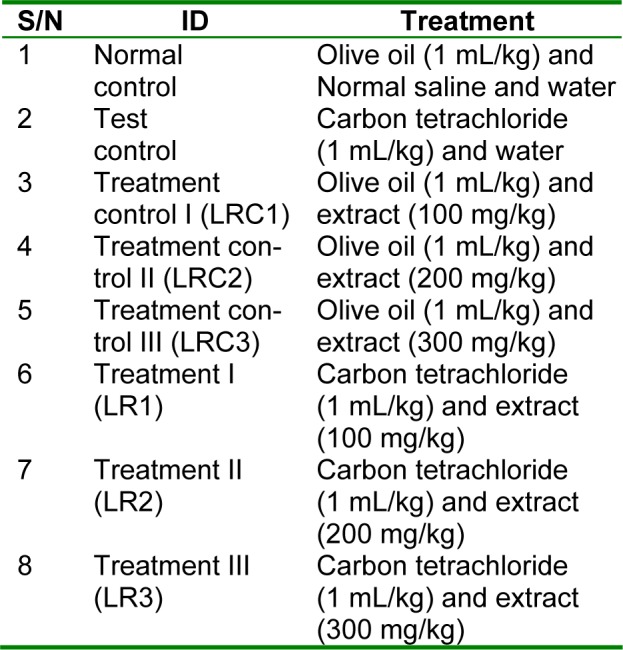
The animals were housed in plastic cages in the animal house of the Department of Biochemistry, University of Port Harcourt. After a one-week acclimatization period on guinea growers mash (Port Harcourt Flour Mills, Port Harcourt, Nigeria), the treatment commenced. The extracts were administered orally on daily basis for eight days. The dosages of administration of the extract was adopted and modified from Adeyemi et al. (2007[2]) and Ikewuchi et al. (2010[14]). The carbon tetrachloride was prepared 1:5 (v:v) in olive oil, and administered subcutaneously at 1 mL/kg body weight of carbon tetrachloride, on days 4 and 8. The dosage and method of administration of carbon tetrachloride was adapted from Obi and Uneh (2003[22]), with modification. Twenty four hours after the last administration of carbon tetrachloride, the rats were weighed and anaesthetized by exposure to chloroform. While under anesthesia, they were painlessly sacrificed and blood was collected from each rat into heparin sample bottles, after which the livers were collected and preserved in 10 % formalin, for histological studies. The heparin anti-coagulated blood samples were centrifuged at 1000 g for 10 min, after which their plasma was collected and stored for subsequent analysis.
Determination of plasma hepatospecific markers
The plasma activities of alanine transaminase, aspartate transaminase, and alkaline phosphatase, were determined using Randox test kits (Randox Laboratories Ltd., Crumlin, England, UK). The activities of alanine and aspartate transaminases were respectively measured by monitoring at 546 nm the concentrations of pyruvate and oxaloacetate hydrazones formed with 2,4-dinitrophenylhydrazine. The activity of alkaline phosphatase was determined by monitoring the degradation of p-nitrophenylphosphate to p-nitrophenol, at 405 nm.
Plasma total bilirubin and protein concentrations were determined using Randox test kits (Randox Laboratories Ltd., Crumlin, England, UK). The wavelength for the determination of total bilirubin was 578 nm, while that of total protein was 560 nm.
Determination of percentage protection (% protection)
The percentage protection provided by the extract against carbon tetrachloride induced liver damage was calculated using the formula below adapted from Al-Qarawi et al. (2004[3]).
Histopathological study
The histopathology study was carried out by Professor S.O. Nwosu, of the Department of Anatomical Pathology, University of Port Harcourt Teaching Hospital. Small pieces of liver tissues were collected in 10 % formalin for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5-6 μm in thickness were cut, mounted on slide and stained with hematoxylin and eosin. The sections were then examined via light microscopy (Opticphot-2; Nikon, Tokyo, Japan) at 100× magnification.
Statistical analysis of data
All values are reported as the mean ± s.e.m. (standard error in the mean). The values of the various parameters were analyzed for statistical significant differences between the groups, using the Student's t-test, with the help of SPSS Statistics 17.0 package (SPSS Inc., Chicago Ill). P<0.05 was assumed to be significant. Graphs were drawn using Microsoft Office Excel, 2010 software.
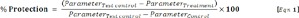
Results and Discussion
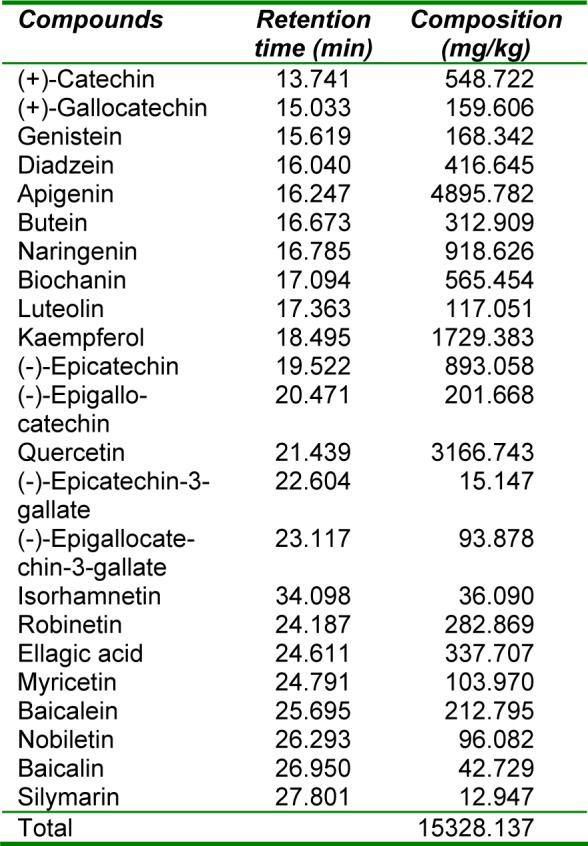
Table 2(Tab. 2) shows the flavonoid composition of an aqueous extract of the rhizomes of Sansevieria liberica. Twenty nine known flavonoids were detected, consisting mainly of 31.94 % apigenin, 20.66 % quercetin, 11.28 % kaempferol, 5.99 % naringenin, 5.83 % (-)-epicatechin, 3.69 % biochanin, 3.58 % (+)-catechin, 2.72 % diadzein, 2.20 % ellagic acid, 2.04 % butein, 1.85 % robinetin, 1.39 % baicalein, 1.32 % (-)-epigallocatechin, 1.10 % genistein and 1.04 % (+)-gallocatechin. Most of these compounds are bioactive, with antineoplasmic and anticarcinogenic properties (Dillard and German, 2000[8]; Evans, 2005[10]).
Table 2. Composition of flavonoid fraction of an aqueous extract of the rhizomes of Sansevieria liberica.
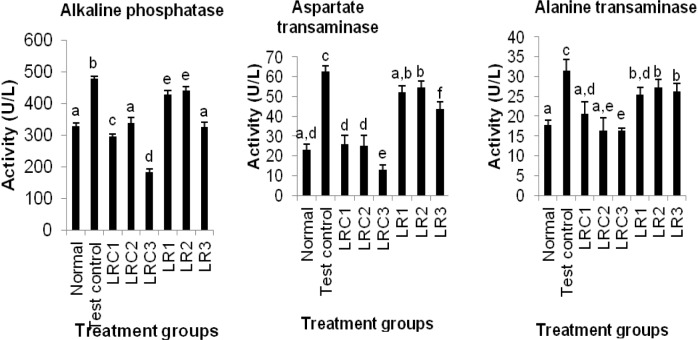
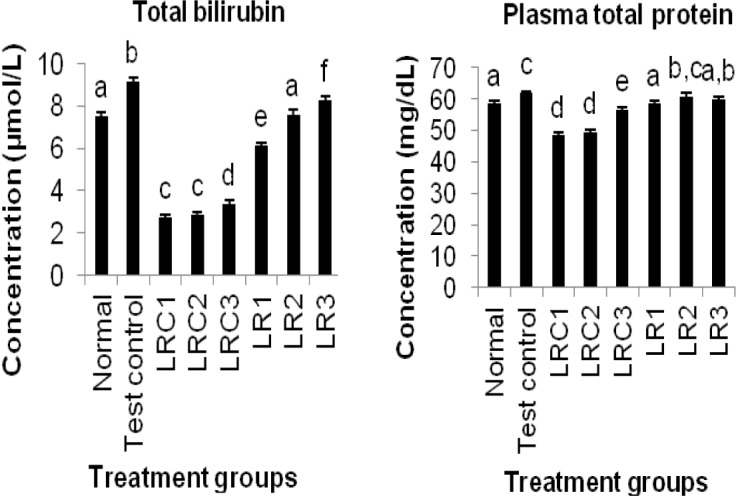
Figures 3-4(Fig. 3)(Fig. 4) show the effects of an aqueous extract of the rhizomes of Sansevieria liberica on plasma markers of liver function. The carbon tetrachloride treatment produced significantly higher (P<0.05) plasma alkaline phosphatase, alanine and aspartate transaminases activities, and total bilirubin and total protein levels. Plasma alkaline phosphatase, alanine and aspartate transaminases activities, and total bilirubin level of treated animals were significantly lower (P<0.05) than test control. The plasma total protein concentrations of the treated animals (except LR2) were significantly lower (P<0.05) than test control.
Figure 3. Effects of an aqueous extract of the rhizomes of Sansevieria liberica on the activities of plasma hepatospecific marker enzyme in normal and carbon tetrachloride treated rats. Values are mean ± S.D., n=5, per group. a,b,cValues in the same group with different superscripts are significantly different at P<0.05.
Figure 4. Effects of an aqueous extract of the rhizomes of Sansevieria liberica on the concentrations of plasma hepatospecific marker molecules in normal and carbon tetrachloride treated rats. I: total bilirubin, II: conjugated bilirubin. Values are mean ± S.D., n=5, per group. a,b,cValues in the same column with different superscripts are significantly different at P<0.05.
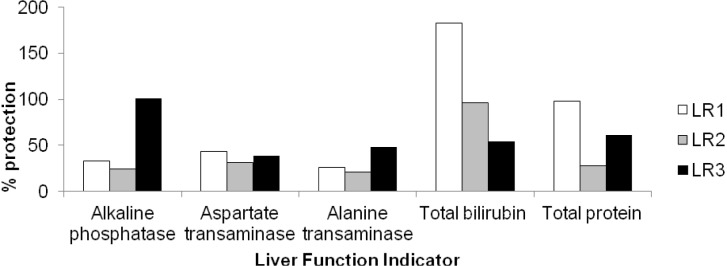
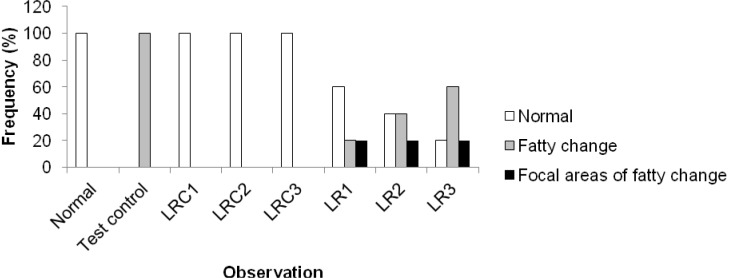
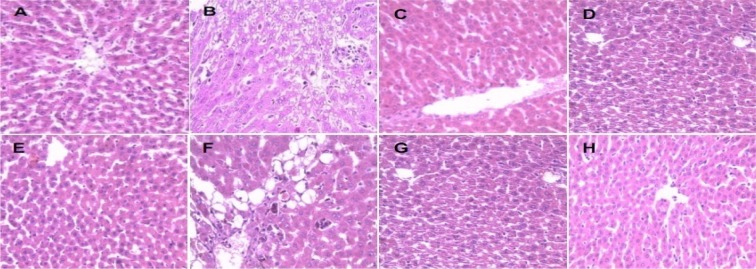
The hepatoprotective activity of an aqueous extract of the rhizomes of Sansevieria liberica on carbon tetrachloride-induced hepatotoxicity in Wistar rats is shown in Figure 5(Fig. 5). The protection seemed to be dose dependent. The frequency distribution of the effects of an aqueous extract of the rhizomes of Sansevieria liberica on the liver histology of normal and carbon tetrachloride treated rats is presented in Figure 6(Fig. 6). Sections of the liver samples are shown in Figure 7(Fig. 7). Histopathological studies of liver sections, showed that carbon tetrachloride caused fatty degeneration, necrosis and ballooning degeneration of hepatocytes; while pre-treatment with aqueous extract of the rhizomes of Sansevieria liberica exhibited protection, which confirmed the results of the biochemical studies. The results of our study indicated that treatment with the plant extract protects the liver against carbon tetrachloride-induced hepatotoxicity.
Figure 5. Hepatoprotective activity of an aqueous extract of the rhizomes of Sansevieria liberica on carbon tetrachloride-induced hepatotoxicity in Wistar rats.
Figure 6. Frequency distribution of the effects of an aqueous extract of the rhizomes of Sansevieria liberica on the histology of normal and carbon tetrachloride treated rats.
Figure 7. Sections of the liver samples of showing effect of an aqueous extract of the rhizomes of Sansevieria liberica on the liver histology of normal and carbon tetrachloride treated rats. A: Section of the liver of rats administered olive oil (1 mL/kg) and treated with water, showing normal cells. B: Section of the liver tissue of rats administered carbon tetrachloride (1 mL/kg) and treated with water, showing fatty change. C: Section of the liver of rats administered olive oil (1 mL/kg) and treated with 100 mg/kg extract, showing normal cells. D: Section of the liver of rats administered olive oil (1 mL/kg) and treated with 200 mg/kg extract, showing normal cells. E: Section of the liver of rats administered olive oil (1 mL/kg) and treated with 300 mg/kg extract, showing normal cells. F: Section of the liver of rats administered carbon tetrachloride (1 mL/kg) and treated with 100 mg/kg extract, showing normal cells. G: Section of the liver of rats administered carbon tetrachloride (1 mL/kg) and treated with 200 mg/kg extract, showing normal cells. H: Section of the liver of rats administered carbon tetrachloride (1 mL/kg) and treated with 300 mg/kg extract, showing focal areas of fatty change.
From Figures 3(Fig. 3) and 4(Fig. 4) it is evident that the extract was able to reduce all the elevated biochemical parameters due to the hepatotoxin intoxication. The extract showed very significant hepatoprotection against carbon tetrachloride-induced hepatotoxicity in the rats, by reducing plasma total bilirubin and protein, plasma alkaline phosphatase, alanine and aspartate transaminases levels. The reduction towards the normal value, of the levels of these plasma indices of liver integrity/function is an indication of the ability of the extract to protect normal structural/functional integrity of the poisoned liver, and also to protect against subsequent carbon tetrachloride hepatotoxicity, enabling regeneration process. A fact that was confirmed by histopathological studies on liver sections: that revealed that the treated animals had normal hepatic cells. This hepatoprotective activity may have been produced via any of the following mechanisms.
Reduced metabolic activation of carbon tetrachloride by cytochrome P450 depresses the initial formation of trichloromethyl free radical, resulting in the diminished initiation of lipid peroxidation (Middleton et al., 2000[20]), and the consequent toxicity of carbon tetrachloride. So, any hepatoprotective drug should be able to inhibit the aromatase activity of cytochrome P450, and thereby, favor liver regeneration. Therefore, it can be suggested that flavonoids in rhizomes of Sansevieria liberica (Table 2(Tab. 2)), could be responsible for its hepatoprotective ability. Flavonoids have been reported to inhibit lipid peroxidation by exerting a membrane-stabilizing action (Middleton et al., 2000[20]) or inhibiting cytochrome P450 aromatase (Kowalska et al., 1990[15]; Middleton et al., 2000[20]).
Many hepatoprotective flavonoids were detected in the aqueous extract of the rhizomes of Sansevieria liberica. They include apigenin, quercetin, kaempferol, naringenin and silymarin. The hepatoprotective activities of apigenin (El Alfy et al., 2010[9]; Zheng et al., 2005[36]), quercetin (Chen, 2010[7]; Lee et al., 2003[16]; Mandal and Das, 2005[18]; Mandal et al., 2007[19]; Pavanato et al., 2003[27]; Peres et al., 2000[28]), kaempferol (Oh et al., 2004[23]; Song et al., 2003[32]; Xiong et al., 2000[35]), naringenin (Lee et al., 2004[17]; Pari and Gnanasoundari, 2006[26]) and silymarin (Suja et al., 2004[33]) have all been documented.
Another component of Sansevieria liberica that may also have contributed to its hepatoprotective activity is vitamin C, a compound that has been reported to be abundant in Sansevieria liberica (Ikewuchi and Ikewuchi, 2009[13]). In vivo studies have indicated that hepatic microsomal drug metabolism decreases in ascorbic acid deficiency and is augmented when high supplements of the vitamin are given to guinea pigs (Burtis and Ashwood, 2001[6]; Sato and Zannoni, 1976[31]). Rikans et al. (1978[30]) had also reported that liver cytochrome P450 is significantly reduced in ascorbic acid-deficiency.
Conclusion
This study clearly demonstrates that aqueous extract of the rhizomes of Sansevieria liberica is an effective agent in the treatment and prevention of carbon tetrachloride-induced hepatic cytotoxicity. The data suggest that the daily oral consumption of the extract was prophylactic to carbon tetrachloride poisoning. This confirms the use of Sansevieria liberica in traditional health care for the treatment of liver problems.
References
- 1.Adeyemi OO, Akindele AJ, Ogunleye EA. Evaluation of the antidiarrhoeal effect of Sanseviera liberica Gerome and Labroy (Agavaceae) root extract. J Ethnopharmacol. 2009;123:459–463. doi: 10.1016/j.jep.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Adeyemi OO, Yemitan OK, Adebisi OO. Sedative and anticonvulsivant activities of the aqueous root extract Sanseviera liberica Gerome and Labroy (Agavaceae) J Ethnopharmacol. 2007;113:111–114. doi: 10.1016/j.jep.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Al-Qarawi AA, Mousa HM, Ali BE-DH, Abdel-Rahman H, El-Mougy SA. Protective effect of extracts from Dates (Phoenix dactylifera L.) on carbon tetrachloride–induced hepatotoxicity in rats. Internat J Appl Res Vet Med. 2004;2:176–80. [Google Scholar]
- 4.Amida MB, Yemitan OK, Adeyemi OO. Toxicological assessment of the aqueous root extract of Sanseviera liberica Gerome and Labroy (Agavaceae) J Ethnopharmacol. 2007;113:171–175. doi: 10.1016/j.jep.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Atta AH, Nasr SM, Mouneir SM. Potential protective effect of some plant extracts against carbon tetrachloride–induced hepatotoxicity. Afr J Trad Compl Alt Med. 2006;3(3):1–9. [Google Scholar]
- 6.Burtis CA, Ashwood ER. Tietz’s fundamentals of clinical chemistry, Vol 2. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 7.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6(22):135–41. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–56. [Google Scholar]
- 9.El Alfy TS, El Sawi SA, Sleem A, Moawad DM. Investigation of flavonoidal content and biological activities of Chorisia Insignis Hbk. Leaves. Aust J Basic Appl Sci. 2010;4:1334–1348. [Google Scholar]
- 10.Evans WC. A taxonomic approach to the study of medicinal plants and animal-derived drugs. In: Evans WC, editor. Trease and Evans pharmacognosy. 15th ed. India: Elsevier; 2005. pp. 15–40. [Google Scholar]
- 11.Gill LS. Ethnomedical uses of plants in Nigeria. Benin City, Nigeria: Uniben Press; 1992. [Google Scholar]
- 12.Ikewuchi CC, Ikewuchi CJ, Ayalogu OE, Onyeike NE. Proximate and phytochemical profile of Sansevieria liberica Gérôme and Labroy. J Appl Sci Environ Mgt. 2010;14:103–6. [Google Scholar]
- 13.Ikewuchi CC, Ikewuchi JC. Amino acid, mineral, and vitamin composition of Sansevieria liberica Gérôme and Labroy. Pac J Sci Technol. 2009;10:477–482. [Google Scholar]
- 14.Ikewuchi CC, Ikewuchi JC, Onyeike EN, Ayalogu EO. Effect of Sansevieria liberica Gérôme and Labroy on plasma chemistry and hematological indices of salt-loaded rats. Res J Sci Technol. 2010;2(5):110–114. [Google Scholar]
- 15.Kowalska MT, Brandt ME, Puett D. Inhibition of cytochrome P-450 aromatase activity by plant extracts. Planta Med. 1990;56:675–7. [Google Scholar]
- 16.Lee E-S, Lee H-E, Shin JY, Yoon S, Moon J-O. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J Pharmacy Pharmacol. 2003;55:1169–74. doi: 10.1211/0022357021396. [DOI] [PubMed] [Google Scholar]
- 17.Lee M-H, Yoon S, Moon J-O. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27(1):72–76. doi: 10.1248/bpb.27.72. [DOI] [PubMed] [Google Scholar]
- 18.Mandal AK, Das N. Sugar coated liposomal flavonoid: a unique formulation in combating carbon tetrachloride induced hepatic oxidative damage. J Drug Target. 2005;13:305–15. doi: 10.1080/10611860500230278. [DOI] [PubMed] [Google Scholar]
- 19.Mandal AK, Das S, Basu MK, Chakrabarti RN, Das N. Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis. J Pharmacol Exp Therap. 2007;320:994–1001. doi: 10.1124/jpet.106.114215. [DOI] [PubMed] [Google Scholar]
- 20.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 21.Millogo-Kone H, Lompo M, Kini F, Asimi S, Guissou IP, Nacoulma O. Evaluation of flavonoids and total phenolic contents of stem bark and leaves of Parkia biglobosa (Jacq.) Benth. (Mimosaceae)-free radical scavenging and antimicrobial activities. Res J Med Sci. 2009;3(2):70–74. [Google Scholar]
- 22.Obi FO, Uneh E. pH dependent prevention of carbon tetrachloride–induced lipoperoxidation in rats by ethanolic extract of Hibiscus rosasinensis petal. Biokemistri. 2003;13:42–50. [Google Scholar]
- 23.Oh H, Kim D-H, Chob J-H, Kim Y-C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol. 2004;95:421–424. doi: 10.1016/j.jep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Osabohien E. Effects of epoxidation on the thermal conductivity and equilibrium swelling properties of bowstring hemp fibre natural rubber composites. Adv Nat Appl Sci Res. 2009;7:169–76. [Google Scholar]
- 25.Osabohien E, Egboh SHO. Utilization of bowstring hemp fiber as a filler in natural rubber compounds. J Appl Polymer Sci. 2008;107:210–4. [Google Scholar]
- 26.Pari L, Gnanasoundari M. Influence of naringenin on oxytetracycline mediated oxidative damage in rat liver. Basic Clin Pharmacol Toxicol. 2006;98:456–61. doi: 10.1111/j.1742-7843.2006.pto_351.x. [DOI] [PubMed] [Google Scholar]
- 27.Pavanato A, Tuñón MJ, Sonia SC, Marroni CA, Llesuy S, González-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Digest Dis Sci. 2003;48:824–829. doi: 10.1023/a:1022869716643. [DOI] [PubMed] [Google Scholar]
- 28.Peres W, Tunon, MJ, Collado PS, Herrmann S, Marroni N, Gonzalez-Gallego J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol. 2000;33:742–50. doi: 10.1016/s0168-8278(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 29.Reed DW. Sansevieria - Identification and culture. Louisiana Soc Hort Res. 1978;14(2):30–77. [Google Scholar]
- 30.Rikans LE, Smith CR, Zannoni VG. Ascorbic acid and cytochrome P-450. J Pharmacol Exp Ther. 1978;204:702–5. [PubMed] [Google Scholar]
- 31.Sato PH, Zannoni VG. Ascorbic acid and hepatic drug metabolism. J Pharmacol Exp Ther. 1976;198:295–307. [PubMed] [Google Scholar]
- 32.Song E-K, Kim J-H, Kim J-S, Cho H, Nan J-X, Sohn D-H, et al. Hepatoprotective phenolic constituents of Rhodiola sachalinensis on tacrine-induced cytotoxicity in Hep G2 cells. Phytotherapy Res. 2003;17:563–5. doi: 10.1002/ptr.1166. [DOI] [PubMed] [Google Scholar]
- 33.Suja SR, Latha PG, Pushpangandan P, Rajasekharan S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L) Hook against carbon tetrachloride induced liver damage in Wistar rats. J Ethnopharmacol. 2004;92:61–66. doi: 10.1016/j.jep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 34.United States Department of Agriculture, Agricultural Research Service. Germplasm Resources Information Network (GRIN). GRIN taxonomy for plants [database on the Internet] 2008. Available from: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?33057.
- 35.Xiong Q, Fan W, Tezuka Y, Adnyana IK, Stampoulis P, Hattori M, et al. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med. 2000;66(2):127–33. doi: 10.1055/s-2000-11135. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Q-S, Sun X-L, Xu B, Li G, Song M. Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed Environ Sci. 2005;18:65–70. [PubMed] [Google Scholar]