Abstract
Peganum harmala L. (Zygophyllaceae) is one of the most famous medicinal plants used in traditional medicine of Iran. The aim of this study was to consider antibacterial effects of the methanolic extract of different parts of P. harmala including root, stem, leaf, flower and seed against some important human pathogenic bacteria. Antibacterial properties of methanolic extract of mentioned parts were assessed by disc diffusion method. Active extract was fractioned using Thin Layer Chromatography; also their synergism activity in combination with synthetic antibiotic was evaluated. Among the evaluated parts of P. harmala, the root and seed extracts presented antibacterial activity against all of tested bacteria even at the lowest concentration. Antibacterial effect of leaf part was moderate while stem and flower extracts showed relatively poor activity. Antibacterial activity of root extract against most of the tested Gram positive bacteria was better than seed extract. Tested against Gram negative bacteria the obtained results were inconsistent. MIC (Minimal Inhibitory Concentration) and MBC (Minimal Bactericidal Concentration) values for both extracts against MRSA (Methicillin Resistant Staphylococcus aureus) and for seed extract against E. coli and S. typhi were equal (0.625 mg/ml). TLC (Thin Layer Chromatography) results revealed that seed and root extracts were different in terms of nature and content of their constituents. Furthermore, these two extracts showed an excellent stability to temperature and pH treatment. Also, the seed and root extracts showed synergism in combination with novobiocin, colistin and carbenicillin. In conclusion, P. harmala can be assigned as a source of antibacterial compounds for treatment of infections caused by multi-drug resistant (MDR) bacterial pathogens.
Keywords: antibiotic resistance, medicinal plants, Peganum harmala, E. coli, S. aureus, synergism
Introduction
Nowadays, bacterial infections especially those caused by multi-drug resistant (MDR) bacteria have become one of the great challenges for modern healthcare. Therefore, discovering new antibacterial compounds with improved activity is necessary. Majority of scientists define multi-drug resistance (MDR) as ˝resistance to at least 3 classes of antimicrobial agents˝ (Falagas et al., 2006[16]). Since the ancient time, the idea of finding healing powers in plants has been noteworthy. Plants produce a vast array of secondary metabolites that in many cases, these substances act as defense mechanisms against predation by herbivores, microorganisms and insects; also similar substances can be produced by plants as a part of their normal growth and development or in response to stress (Mirjana et al., 2004[22]; Cowan, 1999[12]). Peganum harmala L. (Zygophyllaceae), that is also called Harmal, Suryin Rue, is a perennial, bushy, and wild-growing flowering plant with short creeping root which may grow to 30-100 cm high (Shamsa et al., 2007[30]; Goel et al., 2009[19]; Mahmoodian et al., 2002[20]) is known as “Espand'' in Iran and Harmal in North Africa and African Rue, Mexican Rue, Syrian Rue or Turkish Rue in United States (Mahmoodian et al., 2002[20]). It's native to semi-arid rangeland, steppe area and sandy soils (Mahmoodian et al., 2002[20]). This plant is widely distributed in North Africa, Mediterranean, the Middle East, Pakistan, India and Iran and has been introduced in America and Australia (Asghari and Lockwood, 2002[6]; Ehsanpour and Saadat, 2002[15]; Yousefi et al., 2009[35]). P. harmala traditionally has been used in Iran as an antiseptic and disinfectant agent by burning its seeds (Fathiazada et al., 2006[17]; Arshad et al., 2008[5]). This plant has been considered for the treatment of a variety of human ailments, such as lumbago, asthma, colic, jaundice and as a stimulant emmenagogue (Bukhari et al., 2008[8]). The most pharmacological active compounds of P. harmala are several alkaloids which are found in the seeds and roots (Mirzaie et al., 2007[23]). There are various reports that P. harmala had different pharmacological activities including spontaneous effect (Fathiazada et al., 2006[17]), anti-tumor effect (Goel et al., 2009[19]), insecticidal effect (Goel et al., 2009[19]), caving malaria (Goel et al., 2009[19]), anti-leishmanial (Mirzaie et al., 2007[23]), anti-spasmodic (Asghari and Lockwood, 2002[6]), anti-histaminic (Asghari and Lockwood, 2002[6]), vasorelaxant effect (Asghari and Lockwood, 2002[6]), wound healing (Derakhshanfar et al., 2009[14]), anti-oxidant activity (Astulla et al., 2008[7]), leukemic healing (Zaker et al., 2007[36]), hypoglycemic effect (Singh et al., 2008[32]), immuno-modulator properties (Astulla et al. 2008[7]), analgesic and anti-inflammatory properties (Muhi-eldeen et al., 2008[25]). Also, it has been reported that this plant had antibacterial, antifungal and antiviral effects (Shonoudam et al., 2008[31]); this study focused on the seed extract of P. harmala. However, a study with focusing on antibacterial activity of different parts of P. harmala has not yet been documented. The objective of the present study was to assess the antibacterial property of the methanol extract from different parts of P. harmala including flower, seed, leave, stem and root against some most important human pathogenic bacteria.
Materials and Methods
Plant material collection and identification
Different parts of P. harmala were collected from hills around Behbahan (south east of Khuzestan province, Iran) in 2009. Sample collection was done in April for leave and stem, in May for flower, in June for seed and in September for root. The plant used in this study was taxonomically identified by the Herbarium of Agricultural Science Faculty, Shahid Chamran University, Ahvaz, Iran. Voucher specimens were deposited at the Department of Biology, Shahid Chamran University, Iran.
Preparation of extract
The leaf, flower, seed and stem were shade dried at room temperature for ten days while the root was shade dried for twenty days. These mentioned parts were ground to a fine powder. The powder (1 g) was extracted using 10 ml of methanol-distilled water solution (8:2 v/v), centrifuged at 3000 rpm for 15 min and collecting the supernatant. This process was repeated for three times. Then, solvent removed by evaporation (Darabpour et al., 2010[13]).
Tested microorganisms
A total of thirteen bacteria were selected for this study. Gram positive bacterial species were Bacillus anthracis, Bacillus cereus, Bacillus pumilus, Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes and Streptococcus pyogenes. Gram negative bacterial species were Pseudomonas aeruginosa, Brucella melitensis, Proteus mirabilis, Salmonella typhi, Escherichia coli and Klebsiella pneumoniae. These species were originally isolated from clinical specimens and identified by standard biochemical reactions based on morphological and biochemical characters according to the methods described in Bergey's manual for systematic bacteriology (Garrity et al., 2006[18]). Different characteristics were Gram stain, pigmentation, motility and utilization of different carbon and nitrogen sources and so on.
Inoculums preparation for antibacterial assay
All of the tested bacteria were cultured over night at 37 °C in the Muller-Hinton Broth (MHB, Merck) and used as inoculums. The turbidimetry of the suspension was adjusted to the McFarland 0.5 turbidity standard (108 cfu/ml) (Owen and Palombo, 2007[27]; Chomnawang et al., 2005[10]).
Antibacterial assay procedure
At first, a total of 0.1 ml of bacterial suspension was poured on each plate containing Muller-Hinton Agar (MHA, Merck). The lawn culture was prepared by sterile cotton swab and allowed to remain in contact for 1 min. Four concentrations of methanolic extracts (50, 100, 200, and 400 mg/ml) from each of the used parts of P. harmala were prepared. The sterile filter paper discs (6 mm diameter) (Cermelli et al., 2008[9]) were saturated by 35 μl of different concentrations of each extract and then were placed on lawn cultures. The Petri dishes were subsequently incubated at 37 °C for 24 h and the inhibition zone around each disc was measured in mm. This experiment was carried out in duplicate. As positive controls, discs containing novobiocin 30 μg, nalidixic acid 30 μg, carbenicillin 100 μg, colistin 10 μg, streptomycin 10 μg and methicillin 5 μg were used. All of these synthetic antibiotic discs were prepared from Difco. Discs impregnated with 80 % and methanol was also included to test if they had an inhibitory effect on the test bacteria.
MIC and MBC determination
The MIC (Minimal Inhibitory Concentration) and MBC (Minimal Bactericidal Concentration) of methanolic extracts from the most effective parts of P. harmala were determined against somewhat important and more sensitive bacteria. MIC was determined by the macro broth dilution assay method (NCCLS, 2008[26]; Motamedi et al., 2010[24]). In the tube dilution assay, standard bacterial suspension and different concentrations of extract (0.312, 0.625, 1.25, 2.5, 5, 10, 20, 40, 80, 160, and 200 mg/ml) were added to tubes containing 1 ml Muller-Hinton Broth (MHB, Merck). These tubes were incubated at 37 °C for 24 h. The first tube in the above series without sign of visible growth was considered as the MIC. MBC was determined by culturing one standard loop of the tubes with no apparent growth on MHA and subsequent incubation at 37 °C for 24 h. The least concentration that inhibited colony formation on agar was considered as MBC for the extract.
Study of the synergistic effect
To determine the synergistic effect of the most effective parts of P. harmala with synthetic antibiotics, 400 mg/ml concentration of their extract was added to the discs containing these antibiotics (novobiocin, colistin and carbenicillin) and their effect was evaluated by disc diffusion method on the most important bacterial pathogens among tested bacteria (Motamedi et al., 2010[24]).
Physicochemical treatment of the active extracts
To find out thermostability of antibacterial compound, 1 ml aliquots of the most effective parts extract at 100 mg/ml concentration were added to the sterile screw capped ampoules and treated by temperature at 4 °C in refrigerator for 24 h and 25, 37, 56, 70 and 90 °C in a water bath for 30 min (Salama and Marraiki, 2009[29]; Amin et al., 2010[4]). To determine the effect of pH, the methanolic extract of the most effective parts at 100 mg/ml concentration were treated at pH ranges from 2 to 10 using either 1N HCl or 1N NaOH solutions for 30 min at 4 °C (Salama and Marraiki, 2009[29]). Finally, the result of these treatments was found by measurement of the inhibition zone against one of the most important human pathogenic bacterium.
Fractionation of the most effective extract by Thin Layer Chromatography (TLC)
The methanolic extracts of the most effective parts of P. harmala were analyzed by TLC; the presence of different constituent types in screened extracts was established by adding 15 µl of extracts at 100 mg/ml concentration on silica gel plate (Merck 60 F254 10-20 cm), the plate was developed in a chamber saturated with solvent system n-butanol: acetic acid: distilled water (v/v 4:4:1) as mobile phases. The separated components were visualized under visible and ultraviolet light (254 and 366 nm). Also, the retardation factor (Rf) values of the spots were determined.
Results
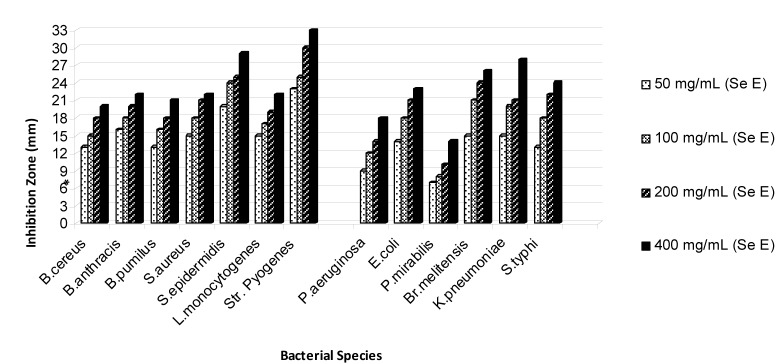
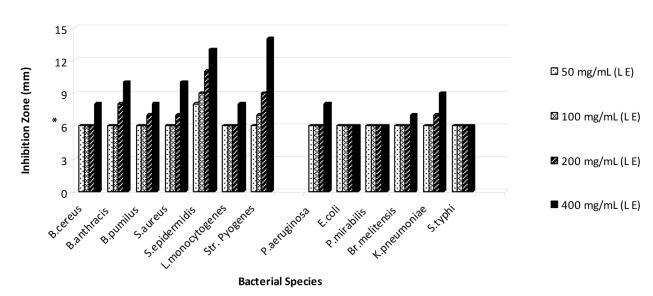
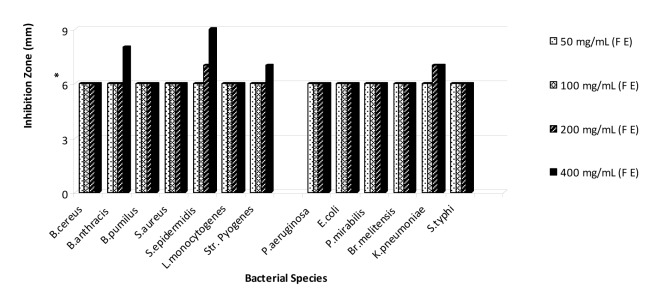
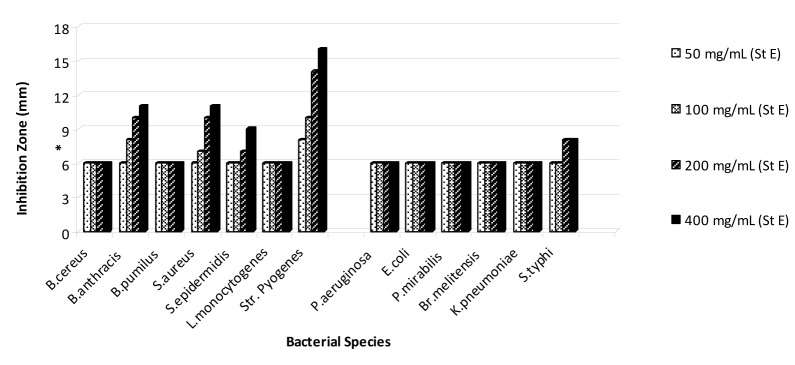
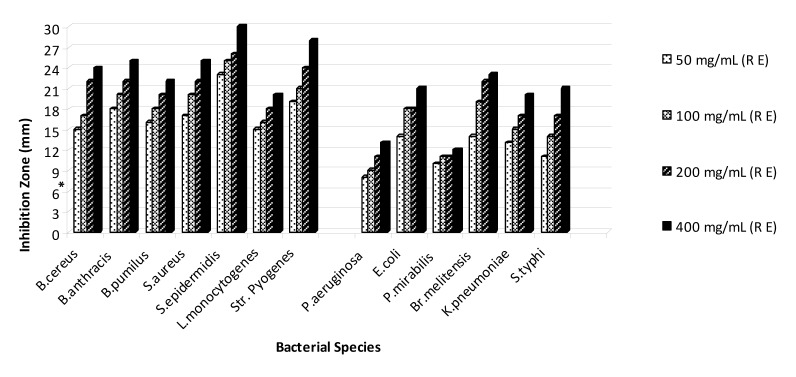
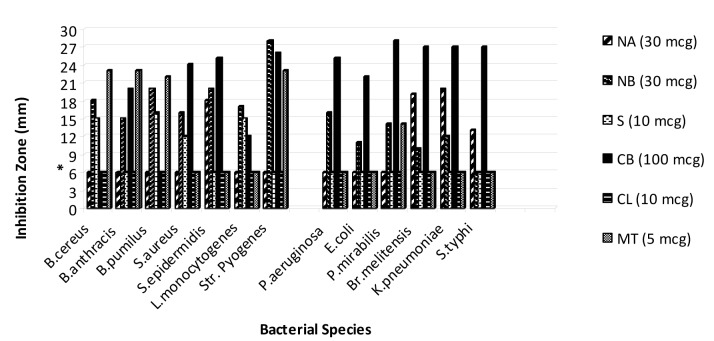
On the basis of the obtained results that are shown in Figures 1-5(Fig. 1)(Fig. 2)(Fig. 3)(Fig. 4)(Fig. 5), the seed and root extracts of P. harmala have a broad antibacterial activity so that all of the tested clinical bacteria were sensitive to these two extracts while flower, leaf and stem were presented a relatively poor antibacterial activity. Increasing the antibacterial activity of the different parts of P. harmala was observed at higher concentrations. Among Gram positive bacteria, S. epidermidis and Str. pyogenes were the most sensitive strains to the seed and root extracts while among Gram negative bacteria, K. pneumoniae and Br. melitensis had the most sensitivity to the seed extract and K. pneumoniae and E. coli to the root extract too. P. mirabilis and P. aeruginosa were the most resistant strains to these two extracts. Antibacterial activity of the root extract against tested Bacillus species and S. aureus was better than the seed extract. Moreover, the seed extract of P. harmala was more effective than the root extract against Gram negative bacteria. The flower, leaf and stem extracts of P. harmala showed inhibitory effect against S. epidermidis, Str. pyogenes and B. anthracis at the highest concentration. Among the used parts of P. harmala, the flower extract showed the least antibacterial activity. All of the tested clinical bacteria were MDR strains. E. coli, P. aeruginosa, S. typhi and L. monocytogenes were the most resistant strains to the synthetic antibiotics (Figure 6(Fig. 6)). Tested S. aureus exhibited resistance to methicillin. Also, all of the tested bacteria were resistant to colistin. Considering antibacterial activity of the different parts of P. harmala, seed and root extracts were chosen for further study.
Figure 1. Antibacterial activity of seed extract of P. harmala against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
(Se E): Seed extract
Figure 2. Antibacterial activity of leaf extract of P. harmala against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
(L E): Leaf extract
Figure 3. Antibacterial activity of flower extract of P. harmala against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
Figure 4. Antibacterial activity of stem extract of P. harmala against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
(St E): Stem extract
Figure 5. Antibacterial activity of root extract of P. harmala against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
(R E): Root extract
Figure 6. Antibacterial activity of synthetic antibiotics against some clinical bacterial pathogens.
*: The diameter of disc is 6 mm.
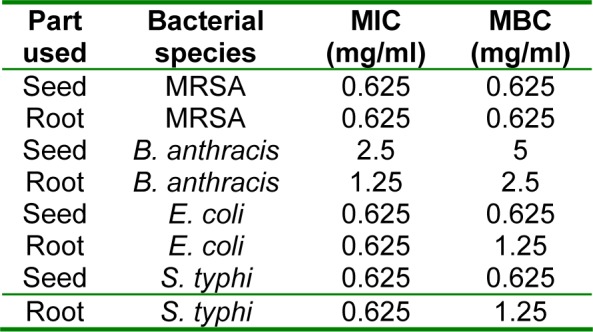
Results of MIC and MBC determination (Table 1(Tab. 1)) showed that MIC and MBC values for the seed and root extract of P. harmala against MRSA are equal (0.625 mg/ ml). Also, these values for seed extract against E. coli and S. typhi were the same (0.625 mg/ml) while for the root extract were different. Moreover, MIC values for seed and root extracts against B. anthracis were 2.5 and 1.25 mg/ml, respectively while MBC values were 5 and 2.5 mg/ml, respectively.
Table 1. The MIC and MBC values of seed and root extracts of P. harmala against some important bacterial pathogens.
Based on the results from pH treatment (Figure 7(Fig. 7)), the best activity of the seed extract was observed at the pH ranges from 4 to 7 while for root extract was in pH 6 and 7. The observed zone of inhibition after treatment of the seed extract with the different temperature was equal to untreated extract (20 mm) (Figure 8(Fig. 8)) but, antibacterial activity of the root extract partially was affected when treated at 70 and 90 °C. In general, antibacterial activity of these two extracts was not considerably affected after temperature and pH treatment.
Figure 7. Results of pH stability treatment of P. harmala seed and root extracts.
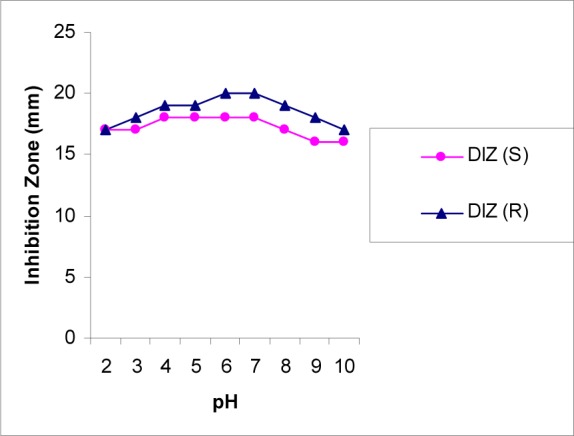
DIZ (S): Diameter of Inhibition Zone for Seed Extract against MRSA
DIZ (R): Diameter of Inhibition Zone for Root Extract against MRSA
Figure 8. Results of the temperature treatment on P. harmala seed and root extracts.
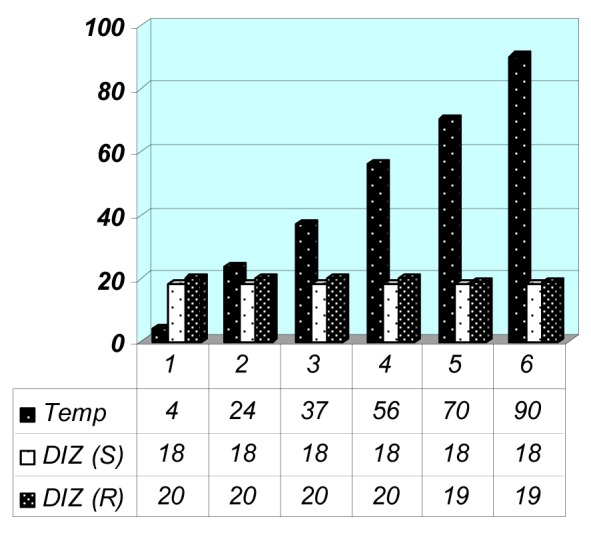
DIZ (S): Diameter of Inhibition Zone for Seed Extract against MRSA
DIZ (R): Diameter of Inhibition Zone for Root Extract against MRSA
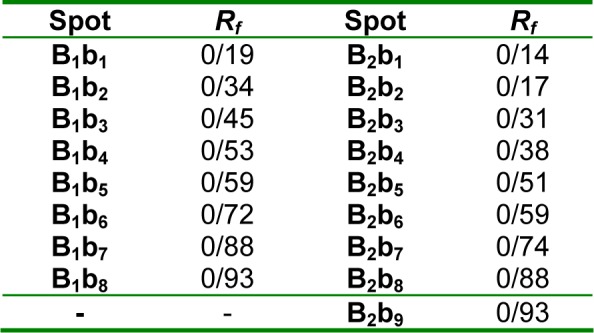
As we can see in Figures 9A and B(Fig. 9) and Table 2(Tab. 2), there are spots (at 254 and 336 nm) with different Rf values or molecular weight in seed and root extracts of P. harmala. Some spots are more intensive in one extract comparing with other extract. In general, the obtained results from TLC revealed that these two parts of P. harmala have several different constituents.
Figure 9. TLC analysis for P. harmala seed and root extracts.
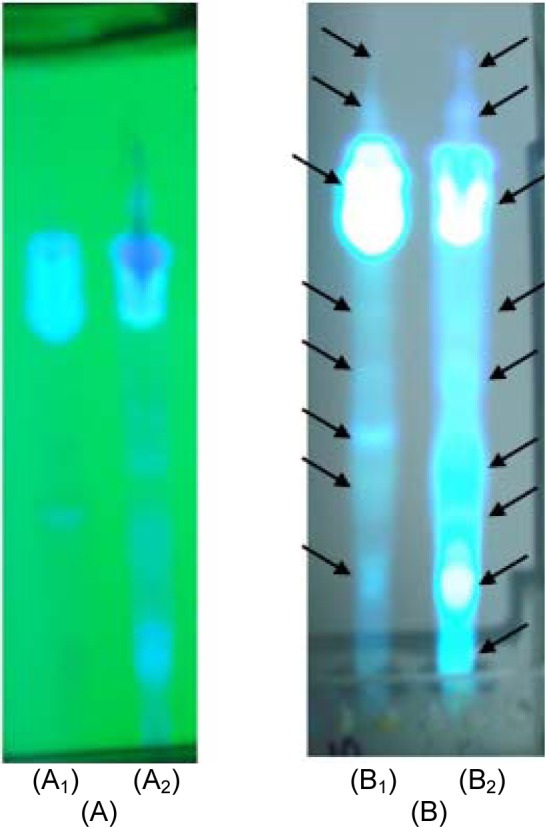
A: TLC plate visualized at 254 nm
B: TLC plate visualized at 366 nm
A1 and B1: Seed extract
A2 and B2: Root extract
Table 2. Rf values of the obtained spots (at 336 nm): From high molecular weight to low molecular weight.
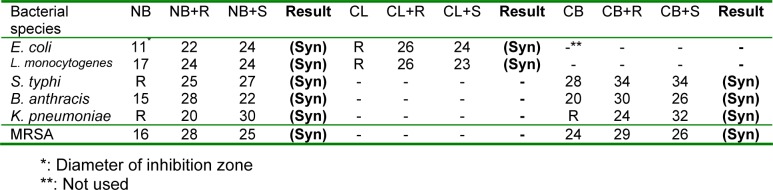
The results of the combination of seed and root extracts of P. harmala with novobiocin, colistin and carbenicillin showed that these combinations have synergistic effect against tested bacteria (Table 3(Tab. 3)). Combination of seed and root extract with novobiocin against E. coli, B. anthracis, K. pneumoniae and MRSA showed a remarkable synergistic effect. Although E. coli and L. monocytogenes were resistant to colistin, the combination of this synthetic antibiotic with seed and root extract showed excellent antibacterial activity against these two bacteria. Synergistic effect of seed extract in combination with novobiocin against E. coli, S. typhi and K. pneumoniae was better than root extract while about of B. anthracis and S. aureus, efficiency of the root extract was better than seed extract. Furthermore, the observed synergism of root extract with colistin against E. coli and L. monocytogenes was better than the seed extract. Combination of the root extract of P. harmala with carbenicillin presented a better antibacterial activity against B. anthracis and MRSA compared with seed extract. Meanwhile, the seed and root extract in combination with carbenicillin showed a relatively weak synergistic activity against MRSA.
Table 3. Results of the synergistic effects of P. harmala seed and root extracts with antibiotic discs.
Discussion
In recent years, an explosive spread of MDR bacterial pathogens has become a serious concern worldwide in terms of public health and economic effects. Medicinal plants have been shown to be a potential source of pharmaceutical natural products. The comparison between pharmaceutical effect of different parts of a medicinal plant can give a good vision for accomplishing further study with more efficiency. In this study, for the first time we have attempted to compare antibacterial activity of the different parts of P. harmala. Each of the used parts of P. harmala was collected at the best month of collection in terms of their secondary metabolite content. Among the evaluated different parts of P. harmala, the seed and root extract showed the best antibacterial activity against tested clinical bacterial pathogens (Figures 1-5(Fig. 1)(Fig. 2)(Fig. 3)(Fig. 4)(Fig. 5)). The seed and root are known as reservoir parts in plants, so, it is very likely that these parts served as arsenal of the secondary metabolites with antibacterial activity in P. harmala. Based on the obtained results, the root extract has a better antibacterial activity against Gram positive bacteria except L. monocytogenes compared with seed extract while about of Gram negative bacteria the observed result is inverse. Thin Layer Chromatography can justify this difference, because the TLC results for seed and root extract showed that these extracts contain several bands with different density or even some bands exist in one extract that we have not been able to find in another one. So, the content of each of the constituents and nature of constituents for these two extracts can be the determinant factors in their antibacterial mechanisms.
Unlike other Gram positive bacteria, L. monocytogenes was more resistant to the seed extract than the root extract, this result may be caused by genetic factors which change the affinity of the active phytochemical of root extract to this bacterium or also it can be related to the nature and number of active phytochemicals in the seed and root extract of P. harmala. Tested E. coli was sensitive to the seed and root extract, this result is in agreement with the document of antibacterial activity of seed extract of P. hamala against E. coli serotype O1:K1 which has been reported previously (Arshad et al., 2008[5]). Antibacterial effect of active extracts against K. pneumoniae especially seed extract was remarkable compared with synthetic antibiotic (Figure 6(Fig. 6)); this is an encapsulated bacterium that is considered as one of the most important causative agents of mortality in patients with bloodstream infections (Tenover, 2006[34]). So, these extract can be evaluated against other encapsulated clinical bacteria such as Streptococcus pneumoniae, which in this case forms a rather large capsule as its virulence factor and is involved in 60 % to 70 % of bacterial pneumonias which primarily affect immunocompromised patients (Talaro, 2009[33]). Figures 8(Fig. 8) and 9(Fig. 9) revealed that the seed and root extracts remained stable over a wide range of pH and temperature, so these extract can also be useful in some particular cases. For instance, pH stability is important in the treatment of brucellosis, one of the most important factors for the treatment of brucellosis is that the antibiotic should be able to act under acidic condition (pH 5) in phagolysosomes of macrophages because brucellae grow and replicate in this pH value (Akova et al., 1999[3]). Thus, considering antibacterial activity of the seed and root extracts of P. harmala against Br. melitensis and their pH resistance ability, this plant can be suggested for the treatment of brucellosis but in vivo efficacy remains to be confirmed. MIC and MBC values (Table 1(Tab. 1)) for both seed and root extract against MRSA and seed extract against E. coli and S. typhi were the same, it is generally held that for bactericidal agents, the MIC and MBC are often near or equal values; so, it can be concluded that these extracts of P. harmala have a bactericidal effect on the mentioned bacteria (Reuben et al., 2008[28]). Nowadays, development of bactericidal agents against MRSA as the most important causative agent of nosocomial infections is valuable so antibacterial activity of seed and root extract of P. harmala must be tested against MRSA with more details. Our results indicate that crude extract of P. harmala seed and root exhibited synergistic effect in combination with novobiocin, colistin and carbenicillin against most important clinical pathogens. In general, combination therapy such as one resulted from synergism between antibiotic and plant extract can be used to as new choice for treatment of infections caused by MDR pathogens, to expand antibacterial spectrum, to prevent emergence of MDR pathogens and to minimize the possible toxic effects (Aiyegoro et al., 2009[1][2]). Also, synergism between plant extract and synthetic antibiotics can develop standardization of herbal medicine for treatment and prevention of infectious diseases. Unlike the results that are shown in Figures 1(Fig. 1) and 5(Fig. 5) (without combination of extract with antibiotic), antibacterial activity of root extract associated with colistin against E. coli and L. monocytogenes was better than combination of the seed extract with this antibiotic (Table 3(Tab. 3)). Colistin is an antibiotic that affects the cell membrane of bacteria. So, it is likely that the active phytochemicals, which exist in root extract, interfere with cell-wall petidoglycan synthesis in bacteria more than seed extract and finally resulted in increasing of the access of colistin to the cell membrane, the outcome of this event is increasing the synergistic effect. On the other hand, the active efflux pump in E. coli might be responsible for the high levels of intrinsic resistance of this bacterium to a wide range of antibiotics. So, may these extracts interfere with this pump and assumed as resistance modifying agents (McKeegan et al., 2002[21]; Coutinho et al., 2009[11]). So far, several alkaloids with pharmaceutical activity including harmine, harmane, harmalol, harmaline, vasicine, vasicinon and peganine have been obtained from the various parts of this plant (Fathiazada et al., 2006[17]; Goel et al., 2009[19]). It has been reported that harmane as a highly aromatic planar alkaloid exerts its antibacterial activity through interchalate with DNA (Cowan, 1999[12]), thus, this antibacterial mechanism must be considered for active extract of P. harmala.
Conclusion
In conclusion, among evaluated parts of P. harmala, seed and root extract were possessed the most active phytochemicals and showed a broad spectrum and strong antibacterial activity against clinical bacterial pathogens. The reason of the difference between antibacterial effect of these two extracts against Gram positive and negative bacteria was justified using TLC, because of the difference in nature and content of active phytochemicals existing in these extract. The low values of MIC and MBC for these two active extracts against the most important of clinical bacterial pathogens especially MRSA was valuable. Also, pH and temperature stability of seed and root extract is important especially for treatment of brucellosis. On the other hand, these active extracts showed synergism in combination with novobiocin, carbenicillin and colisin against the most important clinical pathogens. More studies concerning about the molecular basis of this interaction must be performed in future.
Acknowledgements
The authors wish to thank the vice chancellor for research of Shahid Chamran University, Ahvaz, Iran, for the research grant and financial support.
References
- 1.Aiyegoro OA, Afolayan AJ, Okoh AI. In vitro antibacterial activities of crude extracts of the leaves of Helichrysum longifolium in combination with selected antibiotics. Afr J Pharm Pharmacol. 2009;3:293–300. [Google Scholar]
- 2.Aiyegoro OA, Afolayan AJ, Okoh AI. Synergistic interaction of Helichrysum pedunculatum leaf extracts with antibiotics against wound infection associated bacteria. Biol Res. 2009;42:327–338. [PubMed] [Google Scholar]
- 3.Akova M, Gur D, Livermore DM, Kocagoz T, Akalin HE. In vitro activities of antibiotics alone and in combination against Brucella melitensis at neutral and acidic pHs. Antimicrob Agents Chemother. 1999;43:1298–1300. doi: 10.1128/aac.43.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin M, Kalantar E, Mohammad-Saeid N, Ahsan B. Antibacterial effect and physicochemical properties of essential oil of Zataria multiflora Boiss. Asian Pac J Trop Med. 2010;3:439–442. [Google Scholar]
- 5.Arshad N, Neubauer C, Hasnain S, Hess M. Peganum harmala can minimize Escherichia coli infection in poultry, but long-term feeding may induce side effects. Poult Sci. 2008;87:240–249. doi: 10.3382/ps.2007-00341. [DOI] [PubMed] [Google Scholar]
- 6.Asghari G, Lockwood GB. Stereospecific biotransformation of (±) phenylethyl propionate by cell cultures of Peganum harmala L. Iran Biomed J. 2002;6:43–46. [Google Scholar]
- 7.Astulla A, Zaima K, Matsuno Y, Hirasawa Y, Ekasari W, Widyawaruyanti A, et al. Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med. 2008;62:470–472. doi: 10.1007/s11418-008-0259-7. [DOI] [PubMed] [Google Scholar]
- 8.Bukhari N, Choi JH, Jeon CW, Park HW, Kim WH, Khan MA, et al. Phytochemical studies of the alkaloids from Peganum harmala. Appl Chem. 2008;12:101–104. [Google Scholar]
- 9.Cermelli C, Fabio A, Fabio G, Quaglio P. Effect of Eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 10.Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol. 2005;101:330–333. doi: 10.1016/j.jep.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Coutinho HDM, Costa JGM, Lima EO, Falcao-Silva VS, Junior JPS. Herbal therapy associated with antibiotic therapy: potentiation of the antibiotic activity against methicillin-resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complement Altern Med. 2009;9:1–4. doi: 10.1186/1472-6882-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darabpour E, Motamedi H, Seyyed Nejad SM. Antimicrobial properties of Teucrium polium against some clinical pathogens. Asian Pac J Trop Med. 2010;3:124–127. [Google Scholar]
- 14.Derakhshanfar A, Oloumi MM, Mirzaie M. Study on the effect of Peganum harmala extract on experimental skin wound healing in rat: pathological and biomechanical findings. Comp Clin Pathol. 2009;19:169–172. [Google Scholar]
- 15.Ehsanpour AA, Saadat E. Plant regeneration from hypocotyl culture of Peganum harmala. Pak J Bot. 2002;34:253–256. [Google Scholar]
- 16.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55:1619–1629. doi: 10.1099/jmm.0.46747-0. [DOI] [PubMed] [Google Scholar]
- 17.Fathiazada F, Azarmi Y, Khodaie L. Pharmacological effects of Peganum harmala seeds extract on isolated rat uterus. Iranian J Pharmaceut Sci. 2006;2:81–86. [Google Scholar]
- 18.Garrity GM, Bell JA, Lilburn TI. Bergey's manual of systematic bacteriology. 2nd. New York: Springer; 2006. [Google Scholar]
- 19.Goel N, Singh N, Saini R. Efficient in vitro multiplication of Syrian Rue (Peganum harmala L.) using 6-benzylaminopurine pre-conditioned seedling explants. Nat Sci. 2009;7:129–134. [Google Scholar]
- 20.Mahmoodian M, Jalilpour H, Salehian P. Toxicity of Peganum harmala: Review and a case report. Iran J Pharmacol Ther. 2002;1:1–4. [Google Scholar]
- 21.McKeegan KS, Borges-Walmsley MI, Walmsley AR. Microbial and viral drug resistance mechanisms. Trends Microbiol. 2002;10:103–109. doi: 10.1016/s0966-842x(02)02429-0. [DOI] [PubMed] [Google Scholar]
- 22.Mirjana S, Nada B, Valerija D. Variability of Satureja cuneifolia : ten essential oils and their antimicrobial activity depending on the stage of development. Eur Food Technol. 2004;218:367–371. [Google Scholar]
- 23.Mirzaie M, Nosratabadi SJ, Derakhshanfar A, Sharifi I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Vet Arhiv. 2007;77:365–375. [Google Scholar]
- 24.Motamedi H, Darabpour E, Gholipour M, Seyyed Nejad SM. In vitro assay for the anti-brucella activity of medicinal plants against tetracycline-resistant Br. melitensis. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11:506–511. doi: 10.1631/jzus.B0900365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhi-eldeen Z, Al-Shamma K, Al-Hussainy T, Al-Kaissi E, Al-Daraji AM, Ibrahim H. Acute toxicological studies on the extract of Iraqi Peganum harmala in rats. Eur J Scientific Res. 2008;22:494–500. [Google Scholar]
- 26.NCCLS. Performance standards for antimicrobial susceptibility testing; 9th informational supplement. Wayne, PA: National Committee for Clinical Laboratory Standards; 2008. pp. 120–126. (NCCLS document M100-S9). [Google Scholar]
- 27.Owen RJ, Palombo EA. Anti-listerial activity of ethanolic extracts of medicinal plants, Eremophila alternifolia and Eremophila duttonii, in food homogenates and milk. Food Cont. 2007;18:387–390. [Google Scholar]
- 28.Reuben KD, Abdulrahman FI, Akan JC, Usman H, Sodipo OA, Egwu GO. Phytochemical screening and in vitro antimicrobial investigation of the methanolic extract of Croton zambesicus Muell ARG stem bark. Eur J Scientific Res. 2008;23:134–140. [Google Scholar]
- 29.Salama HMH, Marraiki N. Antimicrobial activity and phytochemical analysis of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Aust J Basic Appl Sci. 2009;3:2008–2015. doi: 10.1016/j.sjbs.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamsa F, Monsef HR, Ghamooghi R, Verdian Rizi MR. Spectrophotometric determination of total alkaloids in Peganum harmala L. using bromocresol green. Res J Phytochem. 2007;1:79–82. [Google Scholar]
- 31.Shonoudam M, Osman S, Salama O, Ayoub A. Toxical effect of Peganum harmala L. leaves on the cotton leaf worm, Spodotera littoralis Boised and its parasitoids Microlitis refiventris Kok. Pak J Biol Sci. 2008;11:546–552. doi: 10.3923/pjbs.2008.546.552. [DOI] [PubMed] [Google Scholar]
- 32.Singh AB, Chaturvedi JP, Narender T, Srivastava AK. Preliminary studies on the hypoglycemic effect of Peganum harmala L. seeds ethanol extract on normal and streptozotocin induced diabetic rats. Ind J Clin Biochem. 2008;23:391–393. doi: 10.1007/s12291-008-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaro KP. Foundations in microbiology. 7th. New York: McGraw Hill; 2009. [Google Scholar]
- 34.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi R, Ghaffarifar F, Dalimi A. The effect of Alkanna tincturia and Peganum harmala extracts on Leishmania major (MRHO/IR/75/ER) in vitro. Iran J Parasitol. 2009;4:40–47. [Google Scholar]
- 36.Zaker F, Oody A, Arjmand A. A study on the antitumoral and differentiation effects of Peganum harmala derivatives in combination with ATRA on Leukaemic cells. Arch Pharm Res. 2007;30:844–849. doi: 10.1007/BF02978835. [DOI] [PubMed] [Google Scholar]