Abstract
This study investigated the effect of the serotonin selective reuptake inhibitors (SSRIs) fluoxetine, sertraline, fluvoxamine and the tricyclic antidepressant (TCA) impiramine on oxidative stress in brain and liver induced by lipopolysaccharide administration in mice. Each drug was administered subcutaneously at doses of 10 or 20 mg/kg, for two days prior to intraperitoneal (i.p.) administration of lipopolysaccharide E (LPS: 200 µg/kg). Mice were euthanized 4 h after administration of the lipopolysaccharide. Lipid peroxidation (malondialdehyde; MDA), reduced glutathione (GSH) and nitric oxide (nitrite/nitrate) concentrations were measured in brain and liver.
Results: The administration of lipopolysaccharide increased oxidative stress in brain and liver; it increased brain MDA by 36.1 and liver MDA by 159.8 %. GSH decreased by 34.1 % and 64.8 % and nitric oxide increased by 78.7 % and 103.8 % in brain and liver, respectively. In brain, MDA decreased after the administration of sertraline and by the lower dose of fluoxetine or fluvoxamine, but increased after the higher dose of imipramine. Reduced glutathione increased after sertraline, fluvoxamine and the lower dose of fluoxetine or imipramine. Nitric oxide decreased by sertraline, fluoxetine, fluvoxamine and by the lower dose of imipramine. In the liver, all drugs decreased MDA and increased GSH level. Nitric oxide is decreased by sertraline, fluvoxamine and by the lower dose of fluoxetine or imipramine. It is concluded that, during mild systemic inflammatory illness induced by peripheral bacterial endotoxin injection, the SSRIs fluoxetine, sertraline and fluvoxamine reduced, while the TCA impiramine increased oxidative stress induced in the brain. The SSRIs as well as imipramine reduced oxidative stress due to lipopolysaccharide in liver tissue.
Keywords: lipopolysaccharide, brain, liver, antidepressant drugs, oxidative stress, mice
Introduction
The last decade has witnessed the introduction of several new antidepressant drugs for the pharmacological management of depressive disorders. In particular, the serotonin reuptake inhibitors (SSRIs) fluoxetine, sertraline, fluvoxamine, paroxetine and citaopram are the most commonly prescribed antidepressant drugs nowadays. These agents have replaced the older classic tricyclic antidepressant (TCA) drugs since they are well tolerated and generally lack the anticholinergic and antihistaminic side effects of the tricyclic agents. The SSRIs are believed to selectively inhibit neuronal reuptake of serotonin, thereby increasing its concentration in the synapse (Fuller, 1994[19]). This effect results in alleviation of the symptoms of depression (Gibbons, 2000[21]). The reuptake of other neurotransmitters is inhibited as well by some members of this group of drugs. Sertraline for example also inhibits dopamine reuptake to a degree that possibly have clinical consequences (Goodnick and Goldstein, 1998[23]; Sánchez and Hyttel, 1999[62]; Kitaichi et al., 2010[33]). There is also an evidence suggesting inhibition of noradrenaline and dopamine reuptake by fluoxetine (Pozzi et al., 1999[57]; Bymaster et al., 2002[7]). The tricyclic drug imipramine, on the other hand, inhibits both noradrenaline and serotonin reuptake (Felton et al., 2003[18]).
Studies have suggested a neuroprotective and an antiapoptotic effect for a number of antidepressant drugs (Drzyzga et al., 2009[16]; Peng et al., 2008[56]). In culture, fluoxetine or the TCA amitriptyline protected rat pheochromocytoma (PC12) cells against the neurotoxicity of hydrogen peroxide (Kolla et al., 2005[34]). The SSRI escitalopram protected against ischemia-induced neuronal death in the gerbil hippocampal CA1 region after transient cerebral ischemia (Lee et al., 2011[39]). In vitro, imipramine as well as fluoxetine increased the survival rate of neural stem cells (Peng et al., 2008[56]; Chen et al., 2007[9]). In addition, the effect of antidepressant drugs on the liver integrity in the healthy and diseased liver is of particular importance. This is because (1) the liver is the site of metabolism for many of these drugs; (2) depression is common in patients with chronic liver disease and can complicate antiviral therapy with ribavirin-interferon (Lang et al., 2003[37]; Cai et al., 2005[8]; Cozzi et al., 2006[12]) and therefore antidepressant drugs are also prescribed in these patients (Gleason et al., 2002[22]; Schäfer et al., 2000[63]); (3) serotonin plays an important role in pathogenesis of liver injury as well as in liver regeneration (Lang et al., 2008[38]; Lesurtel et al., 2008[40]); (4) alterations in brain neurotransmitters have been implicated in the pathogenesis of hepatic encephalopathy (Lozeva-Thomas, 2004[43]), a serious neuropsychiatric complication of both acute and chronic liver disease.
The administration of lipopolysaccharide endotoxin is a widely used model to study the effect of systemic endotoxaemia and the resultant systemic inflammatory responses on tissue function. The administration of lipopolysaccharide endotoxin to experimental animals elicits increased oxidative stress. The model is also used to study the effect of peripheral inflammatory stimuli on brain function. Therefore, it looked pertinent to study the effect of the SSRIs compared with the TCA imipramine on oxidative stress in brain and liver after endotoxin treatment.
Materials and Methods
Animals
Swiss male albino mice 20-22 g of body weight (age: 5-6 weeks) were used. Mice were obtained from animal house colony of the National Research Centre (Cairo, Egypt). Standard laboratory food and water were provided ad libitum. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Equal groups of 6 mice each were used in all experiments.
Drugs and chemicals
Lipopolysaccharide derived from Escherichia coli (Serotype 055: B5, Sigma, St Louis, MO, USA) was used and dissolved in sterile saline, aliquoted, and frozen at −20 °C. Fluoxetine hydrochloride (Amoun Pharmaceutical Co., Cairo, A.R.E.), sertraline hydrochloride (Pfizer Egypt, Cairo, A.R.E.), imipramine hydrochloride (Novartis Pharma, Cairo, A.R.E.) and fluvoxamine maleate (Pharco Pharmaceuticals, Alexandria, A.R.E.) were used and dissolved in isotonic (0.9 % NaCl) saline solution immediately before use. The doses of drugs employed were based upon the human dose after conversion to that of mice according to Paget and Barnes (1964[54]) conversion tables.
Study design
Mice were divided into 8 equal groups (6 mice each). Mice were treated with fluoxetine (10, 20 mg/kg) (groups 1, 2), sertraline (10, 20 mg/kg) (groups 3, 4), fluvoxamine (10, 20 mg/kg) (groups 5, 6), impiramine (10, 20 mg/kg) (groups 7, 8) or saline (group 9, control) once daily orally for 2 days before and just before lipopolysaccharide injection (200 mg/kg, i.p.). In addition, an eighth group (n=6) received only saline (-ve control). Mice were euthanized 4 h after lipopolysaccharide by decapitation under ether anaesthesia. Mice had free access to food and drinking water during the study. At the end of the experiments, brains and livers were removed, washed with ice-cold saline solution (0.9 % NaCl) and stored at -80 ºC for further determination of biochemical parameters. They were homogenised with 0.1 M phosphate buffer saline at pH 7.4, to give a final concentration of 10 % w/v for the biochemical assays.
Biochemical assessment
Determination of lipid peroxidation
Lipid peroxidation was assayed by measuring the level of malondialdehyde (MDA). Malondialdehyde forms a 1:2 adduct with thiobarbituric acid which can be measured by spectrophotometry. Malondialdehyde was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al. (1994[61]), in which the thiobarbituric acid reactive substances react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm.
Determination of reduced glutathione
Reduced glutathione (GSH) was determined by Ellman's method (1959[17]). The procedure is based on the reduction of Ellman´s reagent by -SH groups of GSH to form 2-nitro-s-mercaptobenzoic acid. The nitromercaptobenzoic acid anion has an intense yellow color which can be determined spectrophotometrically.
Determination of nitric oxide
Nitric oxide measured as nitrite was determined by using Griess reagent, according to the method of Moshage et al. (1995[49]) where nitrite, stable end product of nitric oxide radical, is mostly used as indicator for the production of nitric oxide.
Statistical analysis
Data are expressed as mean ± SEM. The data were analyzed by one way ANOVA followed by Duncan's multiple range test, using SPSS software (SAS Institute Inc., Cary, NC). A probability value of less than 0.05 was considered statistically significant.
Results
Effect of antidepressant drugs on brain oxidative stress
Lipid peroxidation
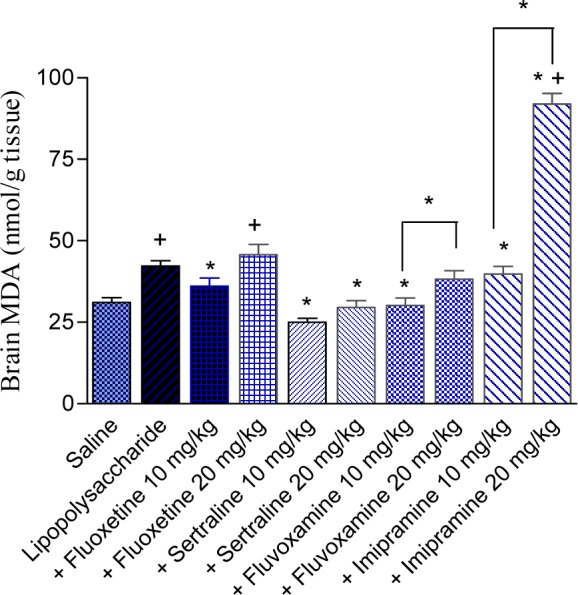
The administration of lipopolysaccharide significantly increased brain MDA by 36.1 % (42.18 ± 1.67 vs 31.0 ± 1.54 nmol/g, p<0.05). Fluoxetine or fluvoxamine given at 10 mg/kg, decreased brain MDA by 14.6 and 28.6 %, respectively (36.03 ± 2.48 and 30.1 ± 2.37 vs 42.18 ± 1.67 nmol/g, p<0.05). The higher dose of either drug, however, did not significantly alter MDA level. Sertraline administered at 10 or 20 mg/kg decreased MDA by 40.7 and 30.0 %, respectively (24.99 ± 1.23 and 29.52 ± 2.12 vs 42.18 ± 1.67 nmol/g, p<0.05). Brain MDA was not altered by imipramine at 10 mg/kg. The higher dose of the drug, however, significantly increased the level of MDA by 118.1 % (91.98 ± 3.19 vs 42.18 ± 1.67 nmol/g, p<0.05) (Figure 1(Fig. 1)).
Figure 1. Effect of antidepressant drugs on the brain tissue level of malondialdehyde (MDA; nmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Reduced glutathione
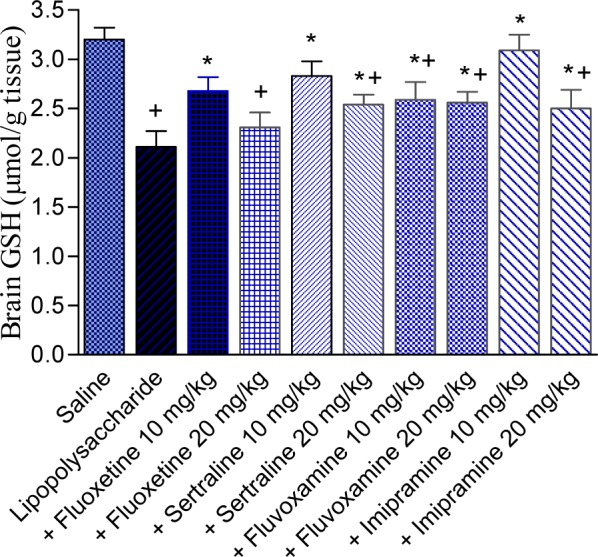
The administration of lipopolysaccharide significantly decreased brain GSH by 34.1 % (2.11 ± 0.16 vs 3.20 ± 0.12 µmol/g, p<0.05) compared with the saline control group. Significant increase in the level of GSH was observed after fluoxetine treatment at 10 mg/kg (27 % increase; 2.68 ± 0.14 vs 2.11 ± 0.16 µmol/g, p<0.05). The higher dose of the drug had no significant effect on GSH level. Reduced glutathione significantly increased by 34.6 and 20.4 % by sertraline at 10 and 20 mg/kg (2.83 ± 0.15 and 2.54 ± 0.10 vs control value of 2.11 ± 0.16 µmol/g, p<0.05) and by 22.7 and 21.3 % by fluvoxamine at 10 and 20 mg/kg (2.59 ± 0.18 and 2.56 ± 0.11 vs control value of 2.11 ± 0.16 µmol/g, p<0.05). Imipramine administered at 10 mg/kg increased GSH by 46.4 %. The higher dose of imipramine, however, resulted in 18.5 % increase in GSH %, compared with the LPS control group. Values of GSH were: 3.09 ± 0.16 and 2.5 ± 0.19 for imipramine doses of 10 and 20 mg/kg, respectively vs LPS control value of 2.11 ± 0.16 µmol/g, p<0.05 (Figure 2(Fig. 2)).
Figure 2. Effect of antidepressant drugs on the brain tissue level of reduced glutathione (GSH: µmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Nitric oxide
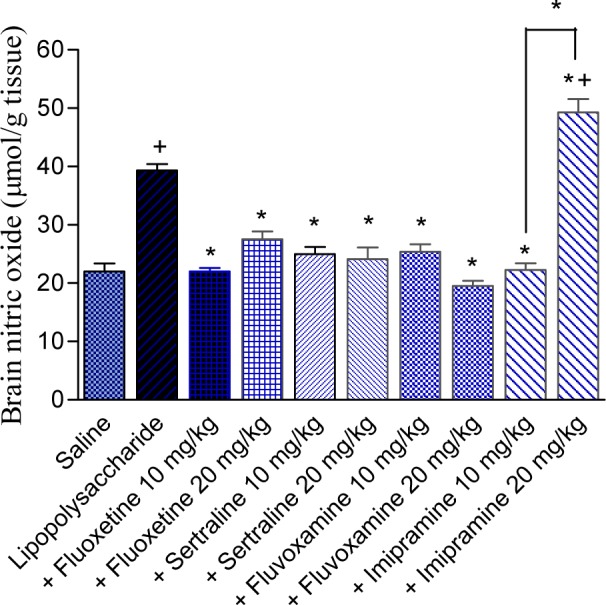
The level of nitric oxide increased by 78.7 % after lipopolysaccharide injection compared with the saline control group (39.32 ± 1.08 vs 22.0 ± 1.40 µmol/g, p<0.05). In lipopolysaccharide-treated rats, nitric oxide level was markedly and significantly decreased by fluoxetine, sertraline or fluvoxamine. Nitric oxide decreased by 44 and 30.1 % by fluoxetine at 10 and 20 mg/kg (22.0 ± 0.6 and 27.5 ± 1.38 vs control value of 39.32 ± 1.08 µmol/g, p<0.05). It decreased by 36.7 and 38.7 % by sertraline at 10 and 20 mg/kg (24.9 ± 1.24 and 24.1 ± 2.00 vs control value of 39.32 ± 1.08 µmol/g, p<0.05) and by 35.4 and 50.4 % by fluvoxamine at 10 and 20 mg/kg (25.4 ± 1.30 and 19.5 ± 0.94 vs control value of 39.32 ± 1.08 µmol/g, p<0.05). The level of nitric oxide was significantly decreased by 43.3 after the administration of imipramine at 10 mg/kg. The higher dose of imipramine, however, increased nitric oxide by 25.4 %, compared with the LPS control group. Values of nitric oxide were: 22.3 ± 1.14 and 49.3 ± 2.27 for imipramine doses of 10 and 20 mg/kg, respectively vs LPS control value of 39.32 ± 1.08 µmol/g, p<0.05 (Figure 3(Fig. 3)).
Figure 3. Effect of antidepressant drugs on the brain tissue level of nitric oxide (nitrite/nitrate; µmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Effect of antidepressant drugs on liver oxidative stress
Lipid peroxidation
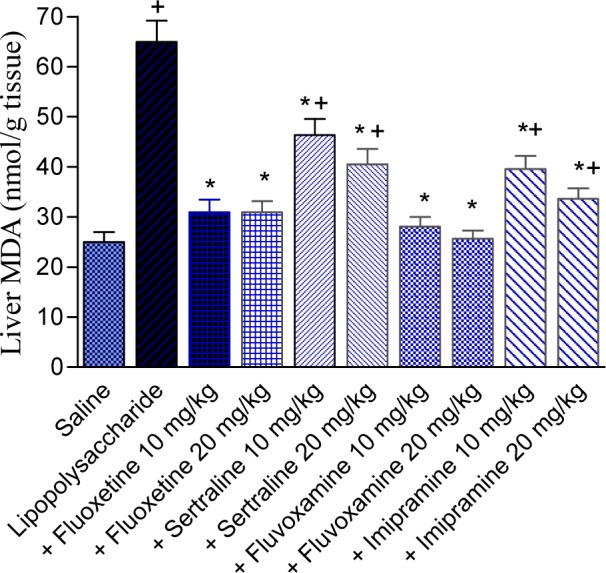
The level of MDA was significantly increased by 159.8 % by the administration of LPS (64.94 ± 4.3 vs 25.0 ± 2.0 nmol/g tissue; p<0.05). In LPS-treated mice, MDA significantly decreased by fluoxetine (-52.3 and -52.4 %), sertraline (-28.6 and -37.6 %), fluvoxamine (-56.8 and -60.5 %), or imipramine (-39.1 and -48.2 %) given at doses of 10 and 20 mg/kg, compared with the LPS control group. Values of MDA were: 30.96 ± 2.47 and 30.93 ± 2.23 for fluoxetine doses of 10 and 20 mg/kg; 46.36 ± 3.17 and 40.5 ± 3.11 for sertraline doses of 10 and 20 mg/kg; 28.07 ± 1.92 and 25.63 ± 1.67 for fluvoxamine doses of 10 and 20 mg/kg; 39.58 ± 2.62 and 33.62 ± 2.13 for imipramine doses of 10 and 20 mg/kg, respectively vs LPS control value of 64.94 ± 4.3 nmol/g, p<0.05 (Figure 4(Fig. 4)).
Figure 4. Effect of antidepressant drugs on the liver tissue level of malondialdehyde (MDA; nmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Reduced glutathione
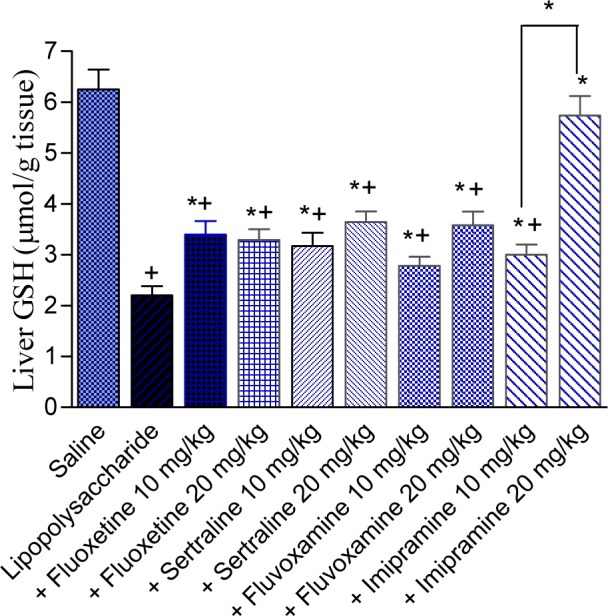
The level of GSH was reduced by 64.8 % after LPS treatment (2.2 ± 0.18 vs 6.25 ± 0.39 µmol/g, p<0.05). In LPS-treated mice, Reduced glutathione was significantly increased by fluoxetine (54.5 and 49.5 %), sertraline (44.1 and 65.4 %), fluvoxamine (26.4 and 62.7 %), or imipramine (36.4 and 160.9 %) given at doses of 10 and 20 mg/kg, compared with the LPS control group. Values of GSH were: 3.4 ± 0.26 and 2.29 ± 0.21 for fluoxetine doses of 10 and 20 mg/kg; 3.17 ± 0.26 and 3.64 ± 0.21 for sertraline doses of 10 and 20 mg/kg; 2.78 ± 0.18 and 3.58 ± 0.27 for fluvoxamine doses of 10 and 20 mg/kg; 3.00 ± 0.20 and 5.74 ± 0.38 for imipramine doses of 10 and 20 mg/kg, respectively vs LPS control value of 2.2 ± 0.18 µmol/g, p<0.05 (Figure 5(Fig. 5)).
Figure 5. Effect of antidepressant drugs on the liver tissue level of reduced glutathione (GSH: µmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Nitric oxide
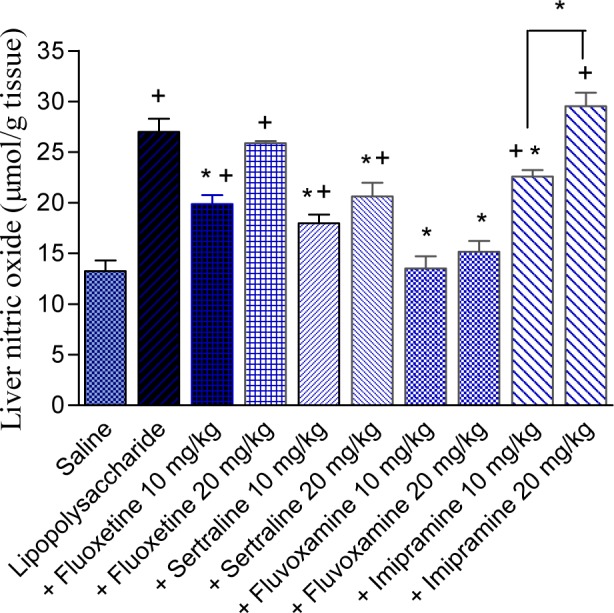
Nitric oxide increased by 103.8 % after LPS administration (27.0 ± 1.3 vs 13.25 ± 1.05 µmol/g, p<0.05) compared with the saline control group. The lower dose of fluoxetine decreased nitric oxide by 26.3 % compared with the LPS control group (19.89 ± 0.85 vs 27.0 ± 1.3 µmol/g, p<0.05). However, no significant effect on the level of nitric oxide was observed in mice treated with fluoxetine at 20 mg/kg. Nitric oxide decreased by 33.4 and 23.5 % by sertraline at doses of 10 and 20 mg/kg, respectively (17.98 ± 0.86 and 20.65 ± 1.32 vs 27.0 ± 1.3 µmol/g, p<0.05) and was restored to near normal value by fluvoxamine treatment. The level of nitric oxide decreased by 16.3 % by the lower dose of imipramine (22.6 ± 0.62 vs 27.0 ± 1.3 µmol/g, p<0.05). The effect of imipramine at the highest dose of 20 mg/kg was not significant (Figure 6(Fig. 6)).
Figure 6. Effect of antidepressant drugs on the liver tissue level of nitric oxide (nitrite/nitrate; µmol/g tissue) in lipopolysaccharide-treated mice. The plus sign indicates significant change from the saline group. Asterisks indicate significant change from saline group and between different groups as indicated.
Discussion
In the present study, the induction of mild systemic inflammation by peripheral administration of a small dose of LPS endotoxin increased oxidative stress in both the brain and liver. Malondialdehyde a marker of lipid peroxidation activity (Gutteridge, 1995[24]) is increased in both organs. This was associated with marked and significant decrease in the level of reduced glutathione, an important antioxidant defense mechanism. LPS administration resulted also in markedly increased nitric oxide levels in brain and liver. The significance of these findings implies that mild systemic inflammation by itself impairs antioxidant defense mechanisms and consequently make the brain (or liver) more susceptible to further oxidative insults. Studies have shown decreased brain reduced glutathione and glutathione reductase activity in rats after the administration of lipopolysaccharide (1 mg/kg, i.p.) (Jacewicz et al., 2009[31]). Similarly, a single intraperitoneal dose of lipopolysaccharide (250 µg/mouse) was associated with GSH depletion, cyclooxygenase-2 expression, lipid peroxidation and impairment in mitochondrial redox activity (Noble et al., 2007[52]). Thus endotoxin-induced peripheral systemic inflammation can affect brain functions. In this context, studies have also shown that induction of systemic inflammation by intravenous or intraperitoneal lipopolysaccharide injection causes brain inflammation. Thus, Jeong et al. (2010[32]) have shown that acute induction of systemic inflammation with intravenous lipopolysaccharide at 100 or 500 µg/kg causes brain inflammation although this was not sufficient to induce neuronal injury. Other researchers reported neuronal damage after higher dose of the endotoxin (Qin et al., 2007[58]). It has also been shown that LPS (50 µg/kg, i.p.)-induced peripheral inflammation exacerbated ischaemic hippocampal cell loss after global brain ischaemia, independently of hippocampal pro-inflammatory cytokine production, hippocampal microglial activation or temperature changes (Spencer et al., 2007[66]). These observations are important in view of the evidence linking both increased oxidative stress and inflammation in the pathogenesis of several neurological, neuropsychiatric and neurodegenerative disorders such as Parkinson's disease and Alzheimer's disease, schizophrenia as well as depression (Maes, 2008;[44] Wood et al., 2009[70]).
The present study also provided an evidence for a beneficial effect for the SSRIs fluoxetine, fluovoxamine or sertraline in decreasing brain oxidative stress induced by endotoxin administration. In contrast, increased lipid peroxidation was observed after the TCA impiramine. These findings point to a likely neuroprotective properties for some of the SSRIs. In this context, studies have shown that the SSRIs fluoxetine and paroxetine lessened the loss of nigral dopaminergic neurons caused by lipopolyscaccharide or by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Chung et al., 2010[10][11]), while escitalopram protected against ischemia-induced neuronal death in the gerbil hippocampal CA1 region after transient cerebral ischemia (Lee et al., 2011[39]). These effects were mediated through decreased microglia activation, reactive oxygen species generation and oxidative stress. Studies also have shown reduced lipid and protein oxidation, in rat brain, increased superoxide dismutase and catalse activities by imipramine (Réus et al., 2010[60]). In culture, fluoxetine and amitriptyline, a tricyclic agent protected rat pheochromocytoma (PC12) cells from neurotoxicity of hydrogen peroxide and increased superoxide dismutase activity (Kolla et al. 2005[34]). In vitro also, imipramine increased the survival rate of neural stem cells and prevented the endotoxin-induced apoptosis (Peng et al., 2008[56]).
In the current study, GSH which is decreased by endotoxin administration, is increased in the brain by both the SSRIs and imipramine. Glutathione, an intracellular tripeptide (glycyl-glutamic acid-cysteine) is the most abundant sulfhydryl-containing compound in the cells and the brain's most important cellular free radical scavenger (Wang and Ballatori, 1998[69]; Wu et al., 2004[71]). The increments in reduced glutathione by antidepressant drugs seem to correlate with their effect on lipid peroxidation. For example, the increase in brain level of MDA by LPS endotoxin was decreased by the lower dose of fluoxetine, which also increased glutathione, suggesting an antioxidant effect. In contrast, MDA was not altered by the higher dose of fluoxetine which also had no significant effect on reduced glutathione, suggesting an increased free radical production with the higher dose of the antidepressant drug. The TCA imipramine elicited marked increase in reduced glutathione at a dose which did not alter brain lipid peroxidation. Meanwhile, a lesser increase in reduced glutathione was observed at the higher dose of imipramine which markedly enhanced lipid peroxidation in brain. Nevertheless, an increase in reduced glutathione by antidepressant drugs is important, since decreased glutathione content has been reported in brain of patients suffering from a number of neurological diseases (Schulz et al., 2000[64]; Dean et al., 2009[15]; Gawryluk et al., 2011[20]), thereby implicating GSH consumption by free radicals in the pathogenesis of these disorders.
Within the central nervous system, nitric oxide is an important physiological signaling molecule involved in neurotransmission and control of vascular tone, but when generated in excess, neurotoxicity might ensue (Brown, 2010[6]). Nitric oxide is generated by inflammatory cytokines due to the action of inducible nitric oxide (iNOS) (Moncada and Bolaños, 2006[47]). In the present study, the administration of LPS endotoxin resulted in increased nitric oxide in brain. Other studies implicated nitric oxide in the induction of fever (Kozak and Kozak, 2003[35]) and in activation of apoptotic pathways in the brain during systemic inflammation induced by lipopolysaccharide (Czapski et al., 2007[13]). Furthermore, LPS-activated microglia were shown to mediate oligodendrocyte progenitor cell death involving nitric oxide-dependent oxidative pathway (Pang et al., 2010[55]). The results of the present study demonstrate that the rise in nitric oxide in brain due to LPS is markedly reduced by treatment with the SSRIs fluoxetine, sertraline and fluvoxamine. One notable observation was the reduction of nitric oxide by the lower dose of imipramine which did not alter brain lipid peroxidation. This finding contrasted with the increase in nitric oxide by the higher dose of the drug which also elicited marked increase in lipid peroxidation, suggesting a role for nitric oxide in this latter process. Several antidepressant drugs have been shown to modulate nitric oxide levels in brain. For example, imipramine has been reported to decrease nitrite/nitrate concentration in brain (Krass et al., 2011[36]) and nitric oxide production in INF-γ stimulated microglia cells (Hashioka et al., 2007[27]). In vivo, imipramine decreased nitric oxide synthase activity and nitrite in rat brain (Harvey et al., 2006[26]; Liu et al., 2011[42]). Fluoxetine has been reported to have no effect on brain nitrite/nitrate (Krass et al., 2011[36]), to increase mRNA expression of iNOS (NOS2) (Ha et al., 2006[25]) or to inhibit endotoxin-induced release of nitric oxide, iNOS mRNA (Liu et al., 2011[42]). Sertraline inhibited the generation of nitric oxide from INF-γ activated microglia (Horikawa et al., 2010[28]).
The current study also investigated the effect of antidepressant drugs on oxidative status of the liver after treatment with LPS endotoxin. Similar to the findings that were observed in the brain, systemic endotoxaemia increased liver oxidative stress, increased lipid peroxidation, decreased reduced glutathione and increased nitric oxide generation in the liver. These biochemical alterations were ameliorated to a large extent by antidepressant drugs. The present findings are in accordance with other studies suggesting a beneficial effect of a number of antidepressant drugs in reducing experimental hepatic injury (Abdel-Salam et al., 2006[3], 2010[1][2], 2011[4]). Several mechanisms are likely to contribute to the effect of antidepressant drugs and in particular the SSRIs on the development of hepatic injury. These agents decrease oxidative stress in the liver as seen in the present study. Reduced glutathione is increased and nitric oxide levels decreased following the administration of antidepressant drugs. In the liver, nitric oxide is involved in regulation of hepatic haemodynamics (Mittal et al., 1994[46]; Lhuillier et al., 2006[41]) and in hepatic regeneration (Tuncyurek et al., 2006[67]). Studies indicated that inhibition of nitric oxide synthase increased hepatic lipid peroxidation, serum liver enzymes and bilirubin in CCl4-intoxicated rats (Muriel, 1998[51]), while increasing nitric oxide availability with L-arginine improved hepatic arterial and portal blood flow and sinusoidal oxygenation in experimental hepatic steatosis (Ijaz et al., 2005[30]). The increase in nitric oxide and consequent vasodilatation can benefit tissue function against toxic insults, but another consequence of the elevated levels of nitric oxide is its cellular toxicity resulting in increased lipid peroxidation. This is because of the ability of nitric oxide to react with biomolecules or with other free radicals e.g., superoxide anion, yielding the highly reactive peroxynitrite radical capable of evoking the oxidation of important cellular biomolecules (Moncada et al., 1991[48]; Dawson and Dawson,1996[14]).
It is to be noted also that antidepressant drugs possess anti-inflammatory properties (Bianchi et al., 1995[5]) and have been shown to inhibit pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF-α) (Hwang et al., 2008[29]; Horikawa et al., 2010[28]; Liu et al., 2011[42]); these effect are likely to benefit the liver against toxic injury. Moreover, the effect of antidepressant drugs and in particular the SSRIs on serotonin metabolism is likely to contribute to their hepatic effects. Since these agents inhibit platelet aggregation and dense granule release (Serebruany et al., 2001[65]; Maurer-Spurej et al., 2004[45]), they might act to reduce the vasoconstrictor effect of platelet-derived serotonin on the hepatic microcirculation (Reilly et al., 1984[59]; Murata et al., 2003[50]; Nocito et al., 2007[53]) which would benefit hepatic function. In contrast to other antidepressant drugs such as the SSRIs, the tertiary amine tricyclics, possess anticholinergic and antihistaminic properties and TCA have been shown to possess arterial vaso-relaxant properties (Vila et al., 1999[68]).
In conclusion, the present study suggested a beneficial effect for the SSRIs fluoxetine, sertraline and fluvoxamine in reducing oxidative stress in brain and liver during mild systemic inflammatory illness induced by peripheral bacterial endotoxin injection.
References
- 1.Abdel Salam OME, Sleem AA, Shafee N. Effect of trazodone and nefazodone on hepatic injury induced by carbon tetrachloride. Drug Discov Ther. 2010;4:285–297. [PubMed] [Google Scholar]
- 2.Abdel Salam OME, Sleem AA, Shafee N. The effect of serotonin reuptake inhibitors on hepatic injury induced by crbon tetrachloride. J Comp Pathol. 2010 Available from: http://dx.doi.org/10.1007/s00580-010-1067-5. [Google Scholar]
- 3.Abdel Salam OME, Sleem AA, Shaffie NM. Effect of the selective serotonin reuptake inhibitor fluoxetine on carbon tetrachloride induced hepatic damage in rats. J Pharmacol Toxicol. 2006;1:395–406. [Google Scholar]
- 4.Abdel-Salam OME, Sleem AA, Shaffie N. Hypericum perforatum protects against hepatic injury induced by carbon tetrachloride. Comp Clin Pathol. 2011 Available from: http://dx.doi.org/10.1007/s00580-011-1252-1. [Google Scholar]
- 5.Bianchi M, Rossoni G, Sacerdote P, Panerai AE, Berti F. Effects of chlomipramine and fluoxetine on subcutaneous carrageenin-induced inflammation in the rat. Inflamm Res. 1995;44:466–469. doi: 10.1007/BF01837911. [DOI] [PubMed] [Google Scholar]
- 6.Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, et al. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Chen SJ, Kao CL, Chang YL, Yen CJ, Shui JW, Chien CS, et al. Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr Neurovasc Res. 2007;4:19–29. doi: 10.2174/156720207779940707. [DOI] [PubMed] [Google Scholar]
- 10.Chung ES, Chung YC, Bok E, Baik HH, Park ES, Park JY, et al. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res. 2010;1363:143–150. doi: 10.1016/j.brainres.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson's disease. J Immunol. 2010;185:1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- 12.Cozzi A, Zignego AL, Carpendo R, Biagiotti T, Aldinucci A, Monti M, et al. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13:402–408. doi: 10.1111/j.1365-2893.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 13.Czapski GA, Cakala M, Chalimoniuk M, Gajkowska B, Strosznajder JB. Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation. J Neurosci Res. 2007;85:1694–1703. doi: 10.1002/jnr.21294. [DOI] [PubMed] [Google Scholar]
- 14.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 15.Dean OM, van den Buuse M, Bush AI, Copolov DL, Ng F, Dodd S, et al. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem. 2009;16:2965–2976. doi: 10.2174/092986709788803060. [DOI] [PubMed] [Google Scholar]
- 16.Drzyzga LR, Marcinowska A, Obuchowicz E. Antiapoptotic and neurotrophic effects of antidepressants: A review of clinical and experimental studies. Brain Res Bull. 2009;79:248–57. doi: 10.1016/j.brainresbull.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL. Tissue sulfhydryl groups. Arch Biochem. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Felton TM, Kang TB, Hjorth S, Auerbach SB. Effects of selective serotonin and serotonin/noradrenaline reuptake inhibitors on extracellular serotonin in rat diencephalon and frontal cortex. Naunyn-Schmiedebergs Arch Pharmacol. 2003;367:297–305. doi: 10.1007/s00210-002-0688-x. [DOI] [PubMed] [Google Scholar]
- 19.Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55:163–7. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- 20.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons SS. Antidepressants. In: Edmunds MW, Mayhew MS, editors. Pharmacology for the primary care provider. St. Louis, MI: Mosby; 2000. pp. 602–620. [Google Scholar]
- 22.Gleason OC, Yates WR, Isbell MD, Philipsen MA. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63:194–198. doi: 10.4088/jcp.v63n0304. [DOI] [PubMed] [Google Scholar]
- 23.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders. I. Basic pharmacology. J Psychopharmacol. 1998;12(3 Suppl B):S5–20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 24.Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 25.Ha E, Jung KH, Choe BK, Bae JH, Shin DH, Yim SV, et al. Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neurosci Lett. 2006;397:185–189. doi: 10.1016/j.neulet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Harvey BH, Retief R, Korff A, Wegener G. Increased hippocampal nitric oxide synthase activity and stress responsiveness after imipramine discontinuation: role of 5HT 2A/C-receptors. Metab Brain Dis. 2006;21:211–220. doi: 10.1007/s11011-006-9018-1. [DOI] [PubMed] [Google Scholar]
- 27.Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, et al. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Horikawa H, Kato TA, Mizoguchi Y, Monji A, Seki Y, Ohkuri T, et al. Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1306–1316. doi: 10.1016/j.pnpbp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Hwang J, Zheng LT, Ock J, Lee MG, Kim SH, Lee HW, et al. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55:826–834. doi: 10.1016/j.neuropharm.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 30.Ijaz S, Yang W, Winslet MC, Seifalian AM. The role of nitric oxide in the modulation of hepatic microcirculation and tissue oxygenation in an experimental model of hepatic steatosis. Microvasc Res. 2005;70:129–136. doi: 10.1016/j.mvr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Jacewicz M, Czapski GA, Katkowska I, Strosznajder RP. Systemic administration of lipopolysaccharide impairs glutathione redox state and object recognition in male mice. The effect of PARP-1 inhibitor. Folia Neuropathol. 2009;47:321–328. [PubMed] [Google Scholar]
- 32.Jeong H-K, Jou I, Joe E-h. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp Mol Med. 2010;42:823–832. doi: 10.3858/emm.2010.42.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharmacol. 2010;647:90–96. doi: 10.1016/j.ejphar.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Kolla N, Wei Z, Richardson JS, Li XM. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. Psychiatry Neurosci. 2005;30:196–201. [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak W, Kozak A. Selected contribution: Differential role of nitric oxide synthase isoforms in fever of different etiologies: studies using NOS gene-deficient mice. J Appl Physiol. 2003;94:2534–44. doi: 10.1152/japplphysiol.01042.2002. [DOI] [PubMed] [Google Scholar]
- 36.Krass M, Wegener G, Vasar E, Volke V. The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behav Brain Res. 2011;218:57–63. doi: 10.1016/j.bbr.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Lang JP, Meyer N, Doffoel M. Benefits of a preventive psychiatric accompaniment in patients Hepatitis C Virus seropositive (HCV): prospective study concerning 39 patients (in French) Encephale. 2003;29:362–365. [PubMed] [Google Scholar]
- 38.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 39.Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, et al. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011;229:450–459. doi: 10.1016/j.expneurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastrointestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–952. doi: 10.1007/s00018-007-7377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lhuillier F, Robert MO, Crova P, Goudable J, Arnal F, Cespuglio R, et al. Nitric oxide and liver microcirculation during autoregulation and haemorrhagic shock in rabbit model. Br J Anaesth. 2006;97:137–46. doi: 10.1093/bja/ael097. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide (LPS)-stimulated microglial cells. Neuropharmacology. 2011;61:592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Lozeva-Thomas V. Serotonin brain circuits with a focus on hepatic encephalopathy. Metab Brain Dis. 2004;19:413–420. doi: 10.1023/b:mebr.0000043985.25055.b3. [DOI] [PubMed] [Google Scholar]
- 44.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinol Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- 45.Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91:119–128. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- 46.Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416–22. doi: 10.1152/ajpgi.1994.267.3.G416. [DOI] [PubMed] [Google Scholar]
- 47.Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 48.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 49.Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determination in plasma: A critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 50.Murata R, Hamada N, Nakamura N, Kobayashi A, Fukueda M, Taira A, et al. Serotonin activity and liver dysfunction following hepatic ischemia and reperfusion. In Vivo. 2003;17:567–572. [PubMed] [Google Scholar]
- 51.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998;56:773–779. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 52.Noble F, Rubira E, Boulanouar M, Palmier B, Plotkine M, Warnet JM, et al. Acute systemic inflammation induces central mitochondrial damage and mnesic deficit in adult Swiss mice. Neurosci Lett. 2007;424:106–110. doi: 10.1016/j.neulet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, et al. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 54.Paget GE, Barnes JM. Toxicity tests. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities. Pharmacometrics. London: Academic Press; 1964. p. 161. [Google Scholar]
- 55.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 56.Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, et al. Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Pozzi L, Invernizzi R, Garavaglia C, Samanin R. Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: a comparison with citalopram. J Neurochem. 1999;73:1051–1057. doi: 10.1046/j.1471-4159.1999.0731051.x. [DOI] [PubMed] [Google Scholar]
- 58.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reilly FD, Cilento EV, McCuskey RS. Hepatic microvascular regulatory mechanisms. V. Effects of lodoxamide tromethamine or phentolamine-HCl on vascular responses elicited by serotonin. Microcirc Endothelium Lymphatics. 1984;1:671–689. [PubMed] [Google Scholar]
- 60.Reus GZ, Stringari RB, de Souza B, Petronilho F, Dal-Pizzol F, Hallak JE, et al. Harmine and imipramine promote antioxidant activities in prefrontal cortex and hippocampus. Oxid Med Cell Longev. 2010;3:325–331. doi: 10.4161/oxim.3.5.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/a:1006986824213. [DOI] [PubMed] [Google Scholar]
- 63.Schäfer M, Schmidt F, Amann B, Schlösser S, Loeschke K, Grunze H. Adding low-dose antidepressants to interferon alpha treatment for chronic hepatitis C improved psychiatric tolerability in a patient with schizoaffective psychosis. Neuropsychobiology. 2000;42(Suppl 1):43–45. doi: 10.1159/000054852. [DOI] [PubMed] [Google Scholar]
- 64.Schulz JB, Lindenau J, Seyfried J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 65.Serebruany VL, Gurbel PA, O'Connor CM. Platelet inhibition by sertraline and N-desmethylsertraline: a possible missing link between depression, coronary events, and mortality benefits of selective serotonin reuptake inhibitors. Pharmacol Res. 2001;43:453–462. doi: 10.1006/phrs.2001.0817. [DOI] [PubMed] [Google Scholar]
- 66.Spencer SJ, Mouihate A, Pittman QJ. Peripheral inflammation exacerbates damage after global ischemia independently of temperature and acute brain inflammation. Stroke. 2007;38:1570–1577. doi: 10.1161/STROKEAHA.106.476507. [DOI] [PubMed] [Google Scholar]
- 67.Tuncyurek P, Yenisey C, Doger FK, Soyder A, Bicakci T, Cevikel MH. Nitric oxide as an independent regulatory factor in regenerating rat liver. Acta Chir Belg. 2006;106:581–587. doi: 10.1080/00015458.2006.11679956. [DOI] [PubMed] [Google Scholar]
- 68.Vila JM, Medina P, Segarra G, Lluch P, Pallardo F, Flor B, et al. Relaxant effects of antidepressants on human isolated mesenteric arteries. Br J Clin Pharmacol. 1999;48:223–229. doi: 10.1046/j.1365-2125.1999.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 70.Wood SJ, Yücel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38:396–401. [PubMed] [Google Scholar]
- 71.Wu G, Fang Y, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]


