Abstract
Objective
To evaluate and validate the proposed 8th edition AJCC system for T and N staging of pancreatic adenocarcinoma.
Summary Background Data
Investigators have questioned the clinical relevance and reproducibility of previous AJCC staging for pancreatic adenocarcinoma.
Methods
Prospective databases at Memorial Sloan Kettering (MSK), Massachusetts General Hospital (MGH), and Johns Hopkins Hospital (JHH) were queried for patients who had undergone resection for pancreatic adenocarcinoma. Patients who underwent a margin-negative (R0) resection, and who had previously undergone pathologic review, were included. Patients were staged according to 7th edition AJCC criteria, as well as the proposed 8th edition system that includes different definitions of tumor size (T) and nodal status (N). The dataset was randomly split into training and test sets.
Results
2,318 patients were identified who met inclusion criteria. Recursive partitioning on the training set (n=1,551) identified statistically appropriate cutoffs for tumor size (<2.2cm, ≥4.8cm,) and nodal status (no positive nodes, 1–3 positive nodes, ≥4 positive nodes) that supported the proposed 8th edition changes. Median survival in patients staged as T3, N0 by the 7th edition definitions was different between institutions (median Center 1, 24mo; Center 2, 37mo; Center 3, 29mo; p=0.054). This difference was not observed when patients were staged as T3, N0 by 8th edition criteria. Stage, and stage-specific outcome (7th edition), on the test set revealed a predominance of patients (68%) within the IIB subgroup, and a concordance probability estimate (CPE) of 0.57 for stage-specific survival. When assessed with 8th edition criteria, no stage subgroup had a majority of patients, and the CPE was 0.58.
Conclusions
The proposed 8th edition changes for T and N classification were statistically valid and may allow a more reproducible system of T staging. This system also stratifies patients more evenly across stages without sacrificing prognostic accuracy.
INTRODUCTION
Cancer staging is the process of determining how much cancer is present within a given patient, and where the cancer is located. Staging can be done clinically, radiographically, and/or pathologically. The American Joint Committee on Cancer (AJCC) has developed a common language for solid tumor staging that is generally based on three factors: tumor size and extent of tumors (T), whether or not the cancer has spread to adjacent lymph nodes (N), and whether or not the cancer has spread to distant areas of the body (M).1 For any given malignancy, the stage at presentation should be one of the strongest prognostic factors of disease-specific outcome. Reliable determination of stage at diagnosis is a critical factor in determining treatment strategy, and in the selection of patients with similar prognoses for therapeutic clinical trials.
There are several principles for creating a particular cancer staging system that are critical for the system to be clinically applicable. First, the individual stage should stratify patients into prognostic groups that are both statistically significant and clinically relevant. If the prognosis of patients with stage IV disease is not different from that of patients with stage III disease, or from patients with stage I or II disease, then the staging information will not be useful to either patients, or physicians. Second, the criteria utilized for determining stage should be reproducible across hospitals and providers. If the criteria for determining T, N, or M are not reproducible, then comparison of stage-related outcomes between individual treatments and between hospitals will be meaningless. Finally, there is a general acceptance that individual T, N, and M definitions should be easily incorporated into clinical practice. This latter principle is one that balances statistical significance with ease of practice, and has resulted in common definitions for T staging (tumor-size cutoffs) and N staging (number of positive nodes) between a variety of sites within the gastrointestinal tract.
Investigators have recently questioned the clinical relevance and reproducibility of previous AJCC staging for the T and N categories in pancreatic adenocarcinoma.2 Concerns regarding reproducibility have centered on the definitions for T-stage, particularly the term “extension beyond the pancreas.” Such extension is considered challenging to define histopathologically, and has been thought to be potentially inconsistent between pathologists (not reproducible). The N classification for pancreatic cancer has also been criticized because it has only included a node-negative (N0) and a node-positive (N1) category, without an additional N2 category for patients with multiple positive nodes. Recent studies have shown that not only does the presence of nodal involvement predict survival outcome, but also the given number of positive lymph nodes has been reported to be a predictor of decreased survival.3–5
There have been several proposed changes for the 8th edition of the AJCC staging system for pancreatic adenocarcinoma that have altered the definitions of T and N in the 8th edition.6 These changes have focused on improving the reproducibility of T-stage, decreasing the percentage of tumors designated as T3, and adding an N2 category similar to other gastrointestinal disease sites. The goal of the current study was to evaluate, and attempt to validate, the proposed 8th edition AJCC system for T and N staging of pancreatic adenocarcinoma on a large multi-institutional dataset.
METHODS
This study was approved by a waiver of authorization from each of the three participating organizations’ Institutional Review Boards. The prospectively maintained pancreatic cancer databases at Memorial Sloan Kettering (MSK), Massachusetts General Hospital (MGH), and Johns Hopkins Hospital (JHH) were queried for patients who had undergone resection for pancreatic ductal adenocarcinoma. Patients with the less common histologic subtypes of pancreatic cancer such as intraductal papillary mucinous neoplasms or mucinous cystic neoplasms with invasive cancer, neuroendocrine carcinomas, adenosquamous carcinomas, and acinar cell carcinomas were excluded. All patients who underwent a partial or total pancreatectomy without neoadjuvant therapy were included. The inclusive time period of study differed between institutions, depending on the availability of data that had previously undergone review by dedicated GI pathologists (Center 1, Jan 2004 – Jan 2015; Center 2, Jan 2004 – Jan 2015; Center 3, Jan 1985 – Jan 2012).
Clinicopathologic variables were retrieved from the respective databases, and included demographic data, pathologic features of the tumor, operative data, and follow-up data. Tumor diameter was defined as the maximum diameter recorded from the measurements of the gross lesion (+/− microscopic assessment). All three centers have utilized an axial slicing technique, and at the time of pathologic review, a margin was considered positive if there were tumor cells at the margin, or within 1 mm of the margin. The number of positive lymph nodes, as well as the number of nodes pathologically assessed, was also recorded. Additional patient follow-up was extracted from hospital electronic records, or the Social Security Death Index.
The T, N, and M definitions were utilized from the 7th edition of the AJCC staging manual (Table 1). Stage-related overall survival outcomes (7th edition) were then calculated for all patients who had undergone R0 resection. Patients were then staged according to the T and N definitions proposed for the AJCC 8th edition (Table 2). Proposed T-stage definitions are the following: T1 ≤2 cm maximal diameter, T2 >2 ≤4 cm maximal diameter, T3 >4 cm maximal diameter, T4 = locally unresectable. Extrapancreatic extension was not included in these T-stage definitions. Proposed N-stage definitions included the following: N0 = node negative, N1 = 1–3 nodes positive for metastatic disease, N2 ≥4 nodes positive for metastatic disease. Stage groupings were then determined according to general principles that have been applied to other GI disease sites.
Table 1.
AJCC staging system for pancreatic adenocarcinoma (7th edition)
| Primary Tumor (T) |
Regional Lymph Nodes (N) |
Distant Metastases (M) |
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Tumor limited to the pancreas, ≤2 cm in greatest dimension | N0 | No regional lymph node metastasis | M0 | No distant metastases | ||||||||||||||||||||||||||||
| T2 | Tumor limited to the pancreas, >2 cm in greatest dimension | N1 | Regional lymph node metastases | M1 | Distant metastases | ||||||||||||||||||||||||||||
| T3 | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
|
|||||||||||||||||||||||||||||||
| T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) | ||||||||||||||||||||||||||||||||
AJCC, American Joint Committee on Cancer
Table 2.
Proposed AJCC staging system for pancreatic adenocarcinoma (8th edition)
| Primary Tumor (T) |
Regional Lymph Nodes (N) |
Distant Metastases (M) |
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Maximum tumor diameter ≤2 cm | N0 | No regional lymph node metastasis | M0 | No distant metastases | ||||||||||||||||||||||||||||||||
| T2 | Maximum tumor diameter >2 ≤4 cm | N1 | 1–3 positive regional lymph node metastasis | M1 | Distant metastases | ||||||||||||||||||||||||||||||||
| T3 | Maximum tumor diameter >4 cm | N2 | ≥4 regional lymph node metastases | ||||||||||||||||||||||||||||||||||
| T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
|
|||||||||||||||||||||||||||||||||||
AJCC, American Joint Committee on Cancer
Patient and disease characteristics are presented as median and range for continuous variables, and frequency and percentage for categorical variables. Overall survival was calculated from date of surgery to date of death or last follow-up. To assess the validity of the proposed 8th edition staging system (T and N), the dataset was randomly split using a common strategy: two-thirds for a training dataset and the remaining one-third for a testing dataset.7 This strategy allows us to have a balance between accurate estimation of the relationship between overall survival and the staging variables in the training dataset and the ability to assess the strength of the new staging system in the testing dataset. Recursive partitioning was performed on the training dataset to determine any thresholds (cutoffs) for tumor diameter (T-stage) and number of positive nodes (N-stage) that were most predictive of risk for death. Recursive partitioning is an algorithm that searches through the predictor variables (in our case, T-stage and N-stage) to find a split that is most predictive of the response variable (death). Once a split is made, the algorithm continues to search for sub-splits that would further improve predictions on the response variable. For validation of the proposed staging system, patients in the test dataset were restaged using the proposed staging system. Concordance probability estimates (CPE) were calculated for the 7th AJCC staging system and the proposed 8th AJCC staging system. CPE is a measure of how well the staging system discriminates between those who are high and low risk of death. A CPE > 0.5 indicated good prediction ability, while a CPE of 0.5 implies no predictive ability. All analyses were performed using R version 3.2.2 (cran.r-project.org), including the rpart and CPE packages.
RESULTS
Patients and outcome
The query identified 3,085 patients who had undergone pancreatectomy for pancreatic adenocarcinoma at Center 1 (n=1394), Center 2 (n=970), and Center 3 (n=766). A negative microscopic margin (R0) was achieved in 2,358 patients (76%), a microscopically positive margin (R1) was identified in 693 patients (23%), and 34 patients (1%) underwent a macroscopically incomplete (R2) resection. Within the group of 2,358 patients who underwent an R0 resection, there were 31 patients who had an indeterminate nodal status and nine patients in whom the tumor diameter could not be determined. These 40 patients were excluded, and thus the study cohort comprised 2,318 patients who had undergone an R0 resection at one of the three institutions and had previously undergone pathologic review performed by dedicated GI pathologists (Center 1, n=1050; Center 2, n=731; Center 3, n=537).
The demographic, operative, and pathologic variables are presented in Table 3. The median age was 68 years (range 30 – 93 years), and the most common operation was pancreaticoduodenectomy (77%). The median tumor diameter was 3 cm (range 0.1 – 30 cm) and the majority of patients (76%) underwent resection for T3 tumors as defined by the AJCC 7th edition system. The majority of patients (67%) had node-positive disease, and the median number of positive lymph nodes was two (range 0 – 35 positive nodes). Thus, the vast majority (88%) of patients underwent resection for stage II disease, and 66% of all patients were stage IIB. By definition, no patient underwent resection for stage III disease (locally unresectable), and 32 patients (1%) were found to have pathologically confirmed distant metastatic disease (omentum, bowel implant) on the final pathology results after resection had been performed.
Table 3.
Patient and tumor characteristics of the 2318 patients who underwent R0 resection.
| All Patients (n = 2318) |
Training Set (n = 1551) |
Test Set (n = 767) |
|
|---|---|---|---|
|
| |||
| FACTOR | n (%) | ||
| Gender | |||
|
| |||
| Female | 1197 (52) | 804 (52) | 393 (51) |
|
| |||
| Male | 1121 (48) | 747 (48) | 374 (49) |
|
| |||
| Age, median | 68 years | 68 years | 68 years |
|
| |||
| Operation | |||
| Pancreaticoduodenectomy | 1792 (77) | 1194 (77) | 598 (78) |
| Distal pancreatectomy | 435 (19) | 293 (19) | 142 (19) |
| Total pancreatectomy | 48 (2) | 37 (2) | 11 (1) |
| Other | 43 (2) | 27 (2) | 16 (2) |
|
| |||
| Tumor Size, median | 3 cm (0.1 – 30) | 3 cm (0.1 – 18) | 3 cm (0.1 – 30) |
|
| |||
| T Stage (7th edition AJCC) | |||
|
| |||
| T1 | 189 (8) | 128 (8) | 61 (8) |
|
| |||
| T2 | 375 (16) | 247 (16) | 128 (17) |
|
| |||
| T3 | 1754 (76) | 1176 (76) | 578 (75) |
|
| |||
| Total nodes assessed, median | 18 (1 – 100) | 18 (1 – 100) | 18 (1 – 58) |
|
| |||
| # positive nodes, median | 2 (0 – 35) | 1 (0 – 35) | 2 (0 – 19) |
|
| |||
| N Stage | |||
|
| |||
| N0 | 767(33) | 525 (34) | 242 (32) |
|
| |||
| N1 | 1551 (67) | 1026 (66) | 525 (68) |
|
| |||
| AJCC Stage | |||
|
| |||
| IA | 107 (5) | 70 (5) | 37 (5) |
|
| |||
| IB | 157 (7) | 102 (7) | 55(7) |
|
| |||
| IIA | 501 (22) | 352 (23) | 149 (19) |
|
| |||
| IIB | 1521 (66) | 1001 (65) | 520 (68) |
|
| |||
| III | 0 (0) | 0 (0) | 0 (0) |
|
| |||
| IV | 32 (1) | 26 (2) | 6 (1) |
|
| |||
| Center | |||
|
| |||
| Center 1 | 1050 (45) | 703 (45) | 347 (45) |
|
| |||
| Center 2 | 731 (32) | 489 (32) | 242 (32) |
|
| |||
| Center 3 | 537 (23) | 359 (23) | 178 (23) |
R0, negative margin; AJCC, American Joint Committee on Cancer
The median follow-up time for all patients was 16 months (range 1 – 195 months). At the time of last follow-up, 894 patients were alive (39%), and the median follow-up in this group of patients was 14 months (range 1 – 195 months). The median overall survival was 23 months and the estimated five-year survival rate was 20%.
T staging
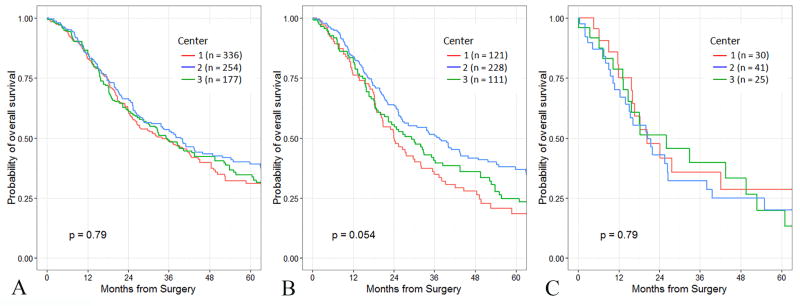
The initial assessment of the association between T-stage and survival was performed on the subgroup of patients with node-negative disease (n=767). Maximum tumor diameter could be determined in 764 of the 767 node-negative patients, and all of the patients in this group had T-stage defined according to the AJCC 7th edition. There were 141 patients (18%) with T1 tumors, 166 patients (22%) with T2 tumors, and 460 patients (60%) with T3 tumors. Survival by T-stage in node-negative patients according to the AJCC 7th edition is presented in Figure 1. Within the entire group of R0 and node-negative patients, survival was similar between institutions (Figure 2A). Because of the concern regarding the reproducibility of T-stage between institutions (extrapancreatic extension), we compared T3 survival outcomes between patients from the three different institutions (Figure 2B). Median survival rates in resected AJCC 7th edition T3, N0 patients were different between institutions and approached statistical significance (median Center 1, 24 mo.; Center 2, 37 mo.; Center 3, 29 mo.; p=0.054).
Figure 1.
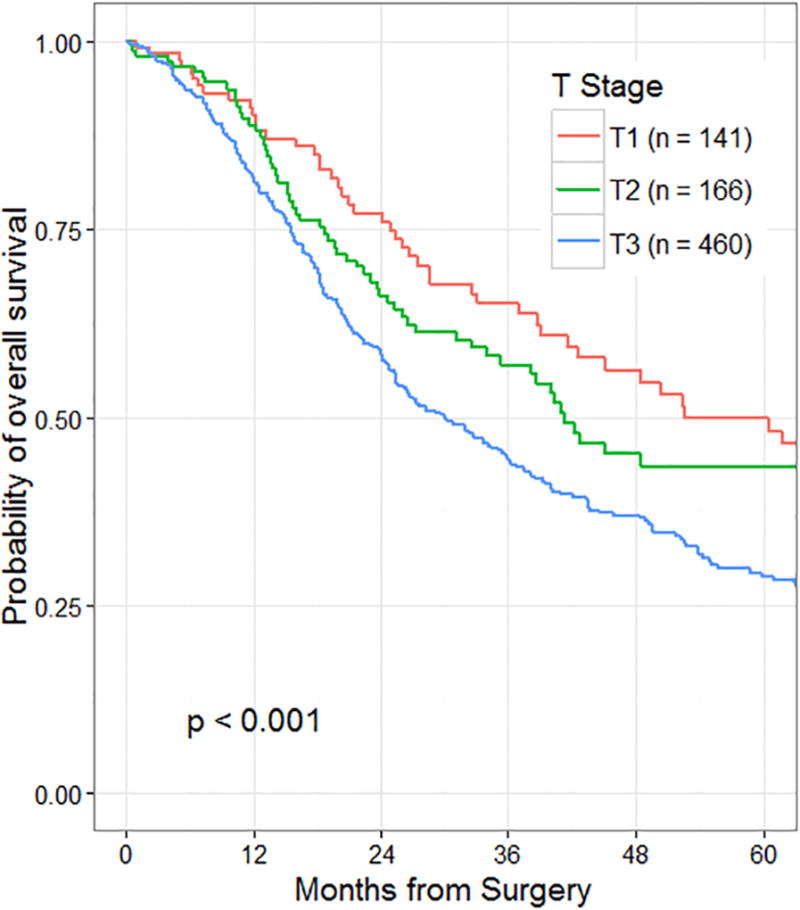
Overall survival by T-stage of 767 patients who underwent resection for node-negative pancreatic cancer. T-stage defined by AJCC 7th edition criteria.
Figure 2.
Overall survival of 767 patients who underwent resection for node-negative pancreatic cancer.
2A: Overall survival stratified by institution.
2B: Overall survival of T3, N0 patients (AJCC 7th edition) stratified by institution.
2C: Overall survival of T3, N0 patients (AJCC 8th edition) stratified by institution.
In an effort to evaluate the statistical validity of the proposed AJCC 8th edition tumor size cutoff points, recursive partitioning was then performed on a training set (n=1551) that comprised roughly two-thirds of patients who had undergone a R0 resection. In this analysis, two separate cutoff points were identified for tumor size: the first was for tumors <2.2 cm in diameter, and the second for tumors ≥4.8 cm in diameter. These findings supported the use of the following proposed T staging definitions: T1 ≤2 cm maximal diameter, T2 >2 ≤4 cm maximal diameter, T3 >4 cm maximal diameter, T4 = locally unresectable. Survival by proposed T-stage in node-negative patients according to the AJCC 8th edition is presented in Figure 3. Comparison of T3, N0 survival outcomes between the three different institutions by this proposed T-staging system showed no difference in survival between institutions (Figure 2C, p=0.79).
Figure 3.
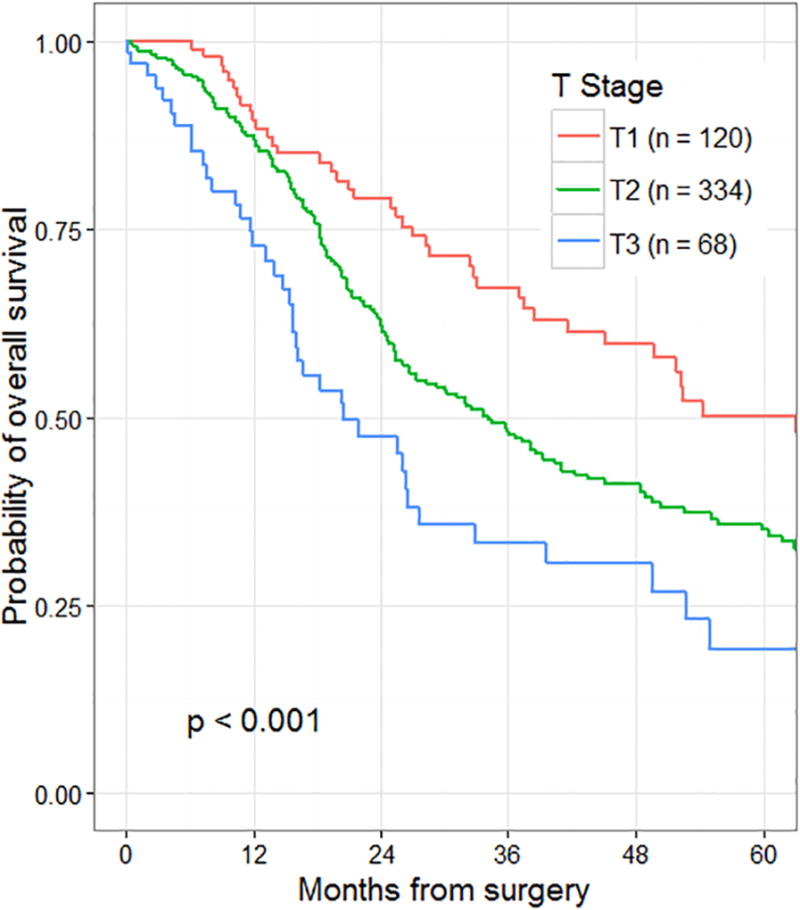
Overall survival by T-stage of 525 patients who underwent resection for node-negative pancreatic cancer stratified by proposed AJCC 8th edition criteria (training set only).
N staging
Recursive partitioning was also performed on the training set to assess for cutoff points for nodal status. In this analysis, two separate cutoffs were identified: the first was for patients with fewer than 0.5 positive nodes, and the second for patients with ≥ 3.5 positive nodes. These findings supported the use of the following proposed AJCC 8th edition N-staging definitions: N0 = node negative, N1 = 1–3 nodes positive for metastatic disease, N2 ≥4 nodes positive for metastatic disease. Survival by the proposed N-stage for all patients who underwent a R0 resection is presented in Figure 4 (p<0.001).
Figure 4.
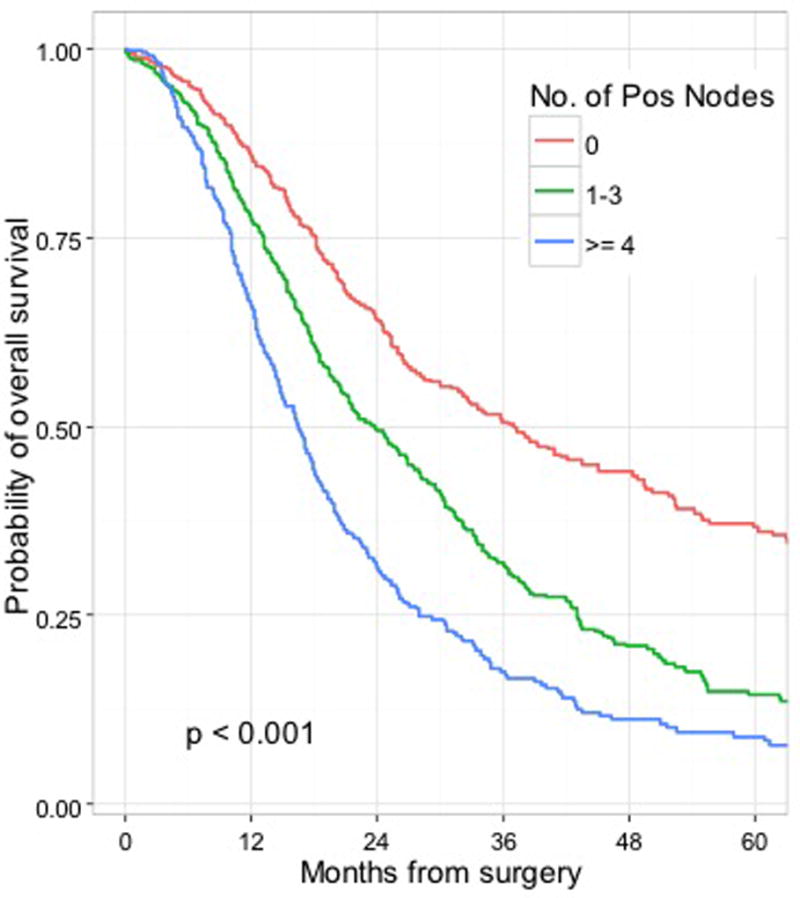
Overall survival by number of positive nodes for all patients who underwent a R0 resection (training set, n=1551) stratified by proposed AJCC 8th edition criteria.
AJCC stage groupings
The test set was then used to evaluate the performance of the 7th edition and the proposed 8th edition staging systems. The 7th edition stage-specific survival outcomes are presented in Supplementary Figure 1A. The CPE was 0.57. We then evaluated stage-specific survival with the system proposed for the AJCC 8th edition. The 8th edition stage-specific survival outcomes are presented in Supplementary Figure 1B. The CPE was 0.58. The comparison between the 7th and 8th edition systems with respect to the number and percentage of patients within each stage grouping is presented in Supplementary Table 1. Within the proposed 8th edition system, there was no longer a majority of patients within stage IIB, nor is there a majority in any single stage strata. The median survival of N2 patients, now defined as stage 3 disease (along with locally unresectable), was 16.3 months.
DISCUSSION
There have been several criticisms of the AJCC definitions for the T and N staging of patients with pancreatic adenocarcinoma. A primary concern regarding the T-stage criteria has been the inclusion of the criterion “extension beyond the pancreas” as a defining characteristic of a T3 tumor.2,8,9 Because the pancreas is relatively thin, most carcinomas will have some component that extends to a surface and thus the vast majority of resected patients have been defined as having T3 tumors - regardless of size – because of this criterion. In addition, the pancreas does not have a capsule, and the soft tissue surface of the pancreas often makes deep invaginations between the lobules throughout the pancreas. Furthermore, chronic pancreatitis associated with invasive carcinoma may obliterate the border between the pancreatic parenchyma and the extra-pancreatic soft tissue. These can make the interpretation of “extension beyond the pancreas” a pathologist-dependent factor and one that may not be reproducible between pathologists.
The diameter of the tumor has been shown to be a strong predictor of survival in a variety of malignancies, including pancreatic adenocarcinoma, and has been proposed to be the sole factor governing T-stage in pancreatic cancer.9,10 Although the current cutoff points of ≤2 cm, >2 ≤4 cm, and >4 cm were chosen to be consistent with other gastrointestinal and pancreatic tumors (pancreatic neuroendocrine), the results from the current study suggest that they are statistically sound. In the current study, recursive partitioning was performed to evaluate for tumor diameter cutoff points on 1,551 patients (training set) who had undergone a R0 resection. In this analysis, two separate cutoffs were identified that were significantly associated with survival, and matched the proposed changes. The first cutoff point was for tumors <2.2 cm in diameter, and the second for tumors ≥4.8 cm in diameter. These findings support the use of the proposed diameter cutoffs: T1 ≤2 cm maximal diameter, T2 >2 ≤4 cm maximal diameter, T3 >4 cm maximal diameter.
The proposed shift of T-stage criteria to size alone also appears to result in a system that is reproducible between pathologists and institutions. In the current study, survival comparisons between patients with AJCC 7th edition T3, N0 tumors revealed differences between institutions with respect to median survival. The median survival difference between AJCC 7th edition T3, N0 patients resected at Center 1 and at Center 2 was 13 months. When T3, N0 was defined according to the proposed AJCC 8th edition criteria, or when survival was assessed within the entire group of node-negative patients (all T-stages), the survival differences between institutions were not identified. This suggests that the threshold for defining a patient as T3 within the 7th edition criteria is variable, at least between our three institutions. This is a critical finding, as having definitions that can be accurately and consistently reproduced between institutions is essential for appropriate stage-specific enrollment into clinical trials, and for comparison of stage-related treatments and outcomes between organizations.
A variety of thresholds have been evaluated for nodal status and survival for patients with pancreatic cancer. Several studies have evaluated 0 vs. 1 vs. 2 or more; as well as 0 vs. 1–2 vs. 3 or more.3,4,11 Within the gastrointestinal tract, the current convention in colorectal and other cancer sites has become 0 (N0) vs. 1–3 (N1) vs. 4 or more (N2). In the current study, recursive partitioning on the training set supported the use of this latter system. In our analysis, two separate cutoff points were identified: the first was for patients with >0.5 positive nodes, and the second for patients with ≥3.5 positive nodes. Survival estimates between patients with N0 (node negative), N1 (1–3 positive nodes), and N2 (≥4 positive nodes) disease were statistically significant (p<0.001). We believe that this structure for the definitions of N-stage is appropriate given the findings of the current study and the adoption of this system within other areas of the gastrointestinal tract.
The stage groupings for the proposed 8th edition criteria have been created by the AJCC with consideration given to the conventions of the previous 7th edition system as well as those of other disease sites. When patients within the test set were staged according to this proposed system, 8%, 22%, 4%, 40% and 26% had stage IA, IB, IIA, IIB, and III disease, respectively. When the test set patients were staged according to the AJCC 7th edition, 68% had stage IIB disease. It is difficult to compare the accuracy or stratification of these two systems, however, the CPEs suggest that the systems are similar in this respect. The new classification of resected patients with ≥ 4 positive nodes as stage III appears to be statistically sound. The median survival of N2 patients was 16.3 months in this study, and the reported survival of patients with locally unresectable tumors (sole classification for AJCC 7 stage III) has been typically between 13 – 17 months.12,13
There are several weaknesses of this study that are inherent to the multi-institutional dataset. First, our goal was to include all patients at each of three institutions that had previously undergone pathologic review by dedicated gastrointestinal pathologists for determination of tumor size, and nodal status. Although this allowed us to generate a large multi-institutional dataset with well-annotated specimens, it resulted in a dataset where the majority of patients had undergone resection between 2004–2012, with a more limited number resected between 1985–2004 and also between 2012–2014. An argument could be made that it would be more appropriate to only pick a more limited time period, and thus have a group of patients in which the preoperative radiographic assessment, and postoperative adjuvant systemic therapies are more uniform. We chose to include all patients as a method to increase the power of the study, and unfortunately, because previous studies evaluating survival outcome following resection have not shown significant improvement in overall survival outcome over time.14 We were also unable to evaluate the use of adjuvant therapies over time, and recognize that this is a limitation of this study. Adjuvant therapies however have a somewhat limited benefit, and these benefits have been noted in both node negative and node positive patients.15 In addition, we recognize that the median follow-up was relatively short (16 months) and that with longer follow-up there may be meaningful differences in outcome between the T and N groupings. We note however, that even at the 12 month time point the differences in survival between stage can be observed, and that these do not appear to diminish over time.
A staging system that perfectly discriminates between stage has not been developed, and there are multiple weaknesses in this study that suggest further improvements in T and N staging are possible. First, with regard to T, we included in the primary analysis only patients who had undergone an R0 resection. An argument could be made that the analysis should have been performed on all patients regardless of margin status. We chose to limit the analysis to R0 patients as it was impossible to discriminate between R1 and R2 resections pathologically, and we did not want include in the T analysis any R2 patients in which we did not know the actual gross tumor size. This may decrease the “real-word” applicability of the current T-definition, however when we evaluated the R1 patients the CPE in the 7th edition staging system was 0.53 while the CPE in the proposed 8th edition was 0.54, suggesting that this system appropriately stratifies even in the setting of a margin positive resection. Second, with regard to N, we did include in the primary analysis anyone who had at least a single node assessed in the pathologic specimen. A variety of techniques for lymphadenectomy, and cut-offs for nodal assessment have been proposed for patients undergoing resection of pancreatic cancer, and there was debate as to whether or not we should include only patients with a certain number of nodes assessed.16,17 We decided to include all patients who had nodal assessment as not only would this be “real world”, but previous work from the MSK group found that the number of pathologically assessed LN did not correlate with survival for N0 patients undergoing either pancreaticoduodenectomy or distal pancreatectomy.18 We do however recognize the importance of nodal involvement on prognosis, and recognize that as larger datasets are developed there may be an ability to better discriminate and identify specific cut-offs for pathologic nodal assessment.
In conclusion, this multi-institutional study of 2,318 patients who underwent an R0 resection of pancreatic adenocarcinoma validates independently the changes proposed for the AJCC 8th edition staging system. The proposed cutoff points for T-stage and N-stage have been found to be statistically valid. Furthermore, the use of tumor diameter alone was shown to be a variable that is more reproducible between institutions and pathologists, and allows for a more even distribution of patients within stage groupings without the sacrifice of prognostic accuracy. Although AJCC stage remains one of the strongest predictors of outcome, the CPEs in this study demonstrate a continued need for improved prognostic tools.
Supplementary Material
Supplementary Figure 1. Overall survival stratified by AJCC stage for patients within the test set (n=767).
S1A: 7th edition stage-specific survival outcomes (CPE = 0.57).
S1B: 8th edition stage-specific survival outcomes (CPE = 0.58).
Acknowledgments
Funding: This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant).
References
- 1.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 2.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–141. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261:961–969. doi: 10.1097/SLA.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 4.Murakami Y, Uemura K, Sudo T, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211:196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Basturk O, Saka B, Balci S, et al. Substaging of Lymph Node Status in Resected Pancreatic Ductal Adenocarcinoma Has Strong Prognostic Correlations: Proposal for a Revised N Classification for TNM Staging. Ann Surg Oncol. 2015;22(Suppl 3):1187–1195. doi: 10.1245/s10434-015-4861-0. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, editor. AJCC Cancer Staging Manual. Eighth. New York: Springer-Verlag; 2016. Pancreatic Adenocarcinoma. [Google Scholar]
- 7.Harrell FE, Jr, Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 8.Oliva I, Bandyopadhyay S, Coban I. Peripancreatic soft tissue involvement by pancreatic ductal adenocarcinomas: incidence, patterns and significance. Modern path. 2009;22(1):318–319. Ref Type: Abstract. [Google Scholar]
- 9.Saka B, Balci S, Basturk O, et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1:</=2, pT2: >2-</=4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter JM, Jiang W, Basturk O, et al. Recurrence and Survival After Resection of Small Intraductal Papillary Mucinous Neoplasm-associated Carcinomas (</=20-mm Invasive Component): A Multi-institutional Analysis. Ann Surg. 2016;263:793–801. doi: 10.1097/SLA.0000000000001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 12.Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol. 2012;3:326–334. doi: 10.3978/j.issn.2078-6891.2012.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillmore R, Laurence V, Raouf S, et al. Chemoradiotherapy with or without induction chemotherapy for locally advanced pancreatic cancer: a UK multi-institutional experience. Clin Oncol (R Coll Radiol) 2010;22:564–569. doi: 10.1016/j.clon.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 15.Berger AC, Winter K, Hoffman JP, et al. Five year results of US intergroup/RTOG 9704 with postoperative CA 19-9 </=90 U/mL and comparison to the CONKO-001 trial. Int J Radiat Oncol Biol Phys. 2012;84:e291–e297. doi: 10.1016/j.ijrobp.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 18.House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Overall survival stratified by AJCC stage for patients within the test set (n=767).
S1A: 7th edition stage-specific survival outcomes (CPE = 0.57).
S1B: 8th edition stage-specific survival outcomes (CPE = 0.58).