Abstract
BACKGROUND
The mechanisms of skin aging have not been completely elucidated. Anecdotal data suggests that EGFR inhibition accelerates aging-like skin changes.
OBJECTIVE
To evaluate the clinical characteristics and investigate the cellular and molecular mechanisms underlying skin changes associated with the use of EFGRIs.
PATIENTS AND METHODS
Patients during prolonged treatment with EGFRIs (>3 months) were analyzed for aging-like skin changes. Baseline EGFR expression was compared in young (< 25 years old) vs. old (> 65 years old) skin. In addition, the regulation of extracellular matrix, senescence-associated genes, and cell cycle status was measured in primary human keratinocytes treated with erlotinib in vitro.
RESULTS
Progressive signs of skin aging, including xerosis cutis, atrophy, rhytide formation and/or actinic purpura in 12 patients. Keratinocytes treated with erlotinib in vitro showed a significant down-modulation of hyaluronan synthases (HAS2 and HAS3), whereas senescence-associated genes (p21, p53, IL-6, maspin) were upregulated, along with a G1 cell cycle arrest and stronger SA β-Gal activity. There was significantly decreased baseline expression in EGFR-density in aged skin, when compared to young controls.
CONCLUSIONS
EGFR inhibition results in molecular alterations in keratinocytes that may contribute to the observed skin aging of patients treated with respective targeted agents.
Keywords: Aging, EGFR, Erlotinib, Senescence, Targeted therapy
INTRODUCTION
Epidermal growth factor (EGF) receptor (EGFR) inhibitors (EGFRIs) have proven to be remarkably successful in the targeted therapy of cancers of epithelial cell origin including colorectal, non-small-cell lung, pancreatic cancers, and squamous-cell carcinomas. Despite their clinical efficacy, treatment with these agents can lead to dermatologic adverse events (AEs) in more than 90% of the treated patients, and result in dose modifications, impairment of quality of life, and patient non-compliance [1, 2].
An inflammatory papulopustular eruption (“acneiform rash”), primarily localized to seborrheic areas (e.g. face and trunk) is one of the characteristic AEs. Additionally, patients may develop xerosis cutis, pruritus, painful paronychia, bacterial skin colonization and/or superinfections, alopecia, and hair changes [3–6]. This broad variety of AEs underscores the central physiologic role that EGF and its receptor play in cutaneous homeostasis (including adnexae). In fact, recent studies have demonstrated that the loss of epidermal EGFR leads to induction of pro-inflammatory cytokines and chemokines, which recruit an inflammatory infiltrate into the skin. This is accompanied by a concomitant down-regulation of antimicrobial peptide and barrier gene expression, leading to bacterial infections and progressive xerosis cutis [7].
In our clinical practice, we have observed aging-like skin changes in patients receiving prolonged EGFRI treatment. Since this AE has not been hitherto recognized, we investigated the potential cellular and molecular mechanisms underlying its development, and report our findings.
MATERIALS AND METHODS
Study design
We retrospectively reviewed the medical records of cancer patients at three different institutions (Department of Dermatology, University of Duesseldorf, Duesseldorf, Germany; Dermatology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, USA; Dermatology Service and Paris-Sud University, Gustave Roussy Cancer Campus, Paris, France) who had received prolonged treatment with an EGFRI (>3 months), and also developed aging-like skin changes. To validate our findings, we analyzed the regulation of established (skin-) aging markers in primary human keratinocytes treated in vitro with the EGFRI, erlotinib, using quantitative real-time PCR. Measured markers included the extracellular matrix (ECM)-associated genes hyaluronan synthase 2 (HAS2) and hyaluronan synthase 3 (HAS3). The progressive loss of dermal hyaluronan is a hallmark of skin aging. A major factor that contribute to this loss is the aging-associated downregulation of HAS2 and HAS3 [8, 9]. Moreover, we measured the expression of the senescence-associated genes p53 [10], p21 upregulation [11], maspin[12], and IL-6 [13]. Next, we performed immunohistochemical and flow cytometric analyses of cell cycle status in erlotinib-treated primary human keratinocytes. To determine the relative expression of EGFR in skin (young vs. aged), we obtained biopsies of young skin (<25 years old, n=4) and aged skin from healthy control volunteers (>65 years old, n=8). In the latter, the UV-exposed (extrinsically aged) and non-UV-exposed (intrinsically aged) areas were biopsied, and immunohistochemical analyses were conducted. The clinical data was sourced from all our centers, and the laboratory experiments were conducted at the University of Duesseldorf, Germany. The study was approved by the local ethics committee and informed consent was obtained.
Culture of human epidermal cells
Human primary epidermal keratinocytes were isolated and cultured in keratinocyte serum-free medium (SFM, Invitrogen) at 37°C, 5% CO2. Cells were treated with erlotinib (500 nM and 1000 nM; Roche Pharmaceuticals) or medium (vehicle control), either in the presence or absence of TNF-α (10 ng/ml; AbD Serotec) and IL-1β (5 ng/ml; R&D Systems) for 24 hours, following which the cells were harvested for RNA extraction.
Total RNA isolation and RT-PCR analysis of human primary keratinocytes cells
Total RNA from cultured epidermal keratinocytes was isolated with TRIzol® Reagent (Invitrogen). cDNA synthesis was performed with SuperScript First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Primers were obtained from Eurofins MWG, Ebersberg, Germany (HAS2 forward 5′-GTG GAT TAT GTA CAG GTT TGT GA-3′, reverse 5′-TCC AAC CAT GGG ATC TTC TT-3′; HAS3 forward 5′-GAG ATG TCC AGA TCC TCA ACA A-3′, reverse 5′-CCC ACT AAT ACA CTG CAC AC-3′; p21 forward 5′-CTG GAG ACT CTC AGG GTC GAA-3′, reverse 5′-CCA GGA CTG CAG GCT TCC T-3′; p53 forward 5′-AAG AAA CCA CTG GAT GGA GAA-3′, reverse 5′-CAG CTC TCG GAA CAT CTC GAA-3′; IL-6 forward 5′-TCT CCA CAA GCG CCT TCG-3′, reverse 5′-CTC AGG GCT GAG ATG CCG-3′; Maspin forward 5′-CAG ACA CGG TCG CCT CCA CA-3′, reverse 5′-TTG CAG GGC ATC CAT TGC GG-3′). Gene-specific PCR products were measured by means of an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA). Target gene expression was normalized to the expression of 18S rRNA.
SA β-Gal enzyme activity
Cellular senescence was examined by measuring senescence-associated beta-galactosidase (SA β-Gal) activity (BioVision, Milpitas, CA). Human primary epidermal keratinocytes were grown for 24 hours in 6-well plates either with erlotinib (1000 nM; Roche Pharmaceuticals) or medium (vehicle control). The culture media was then decanted and the cells were stained for SA β-Gal activity as directed by the supplier (BioVision). Histologic images were acquired with a Zeiss microscope (Axiovert 200 M) (Zeiss, Jena, Germany) using Axiovision 4.7 software (Zeiss).
Flow cytometric cell cycle analysis
Cell cycle analysis of keratinocytes was performed via flow cytometry. In short, the cells were harvested by trypsinization and washed two times with PBS. Subsequently, the nuclei were isolated via incubation in a hypotonic buffer containing propidium iodide (0.1% sodium citrate, 0.1% Triton X-100, 50 mg/µL propidium iodide). The stained DNA content of the nuclei was analyzed in a FACS-Calibur (BD Bioscience) and the measured peaks corresponding to certain cell cycle phases (G1: 2n; G2 or M: 4n; S-Phase in between) were quantified with the CellQuest software (BD Bioscience).
Immunofluorescence
Cryosections from young (age <25 years) and aged (age >65 years) human skin were routinely fixed and stained with an antibody directed against human EGFR (mouse monoclonal; DAKO, Hamburg, Germany) or IgG1 isotype control antibody (R&D Systems, Minneapolis, MN). Primary antibody binding was detected using the secondary antibody anti-mouse IgG Alexa Fluor 555 (Life Technologies, Carlsbad, CA). Sections were fixed with Fluoromount G and immunoreactions were detected by use of the microscope Axiovert 200 M (Zeiss, Jena, Germany) using Axiovision 4.7 software (Zeiss). For quantification of EGFR staining, computer-assisted image analyses were performed using NIS Elements AR software (NIKON, Duesseldorf, Germany).
Statistical analysis
Data are represented as means ± SEM, and were evaluated with a two-tailed, unpaired Student’s t test with 95% confidence intervals. The p value of ≤ 0.05 was taken to be statistically significant, and the values are represented with asterisks in the figures (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001).
RESULTS
‘Aging-skin’ phenotype in long-term EGFRI-treated patients
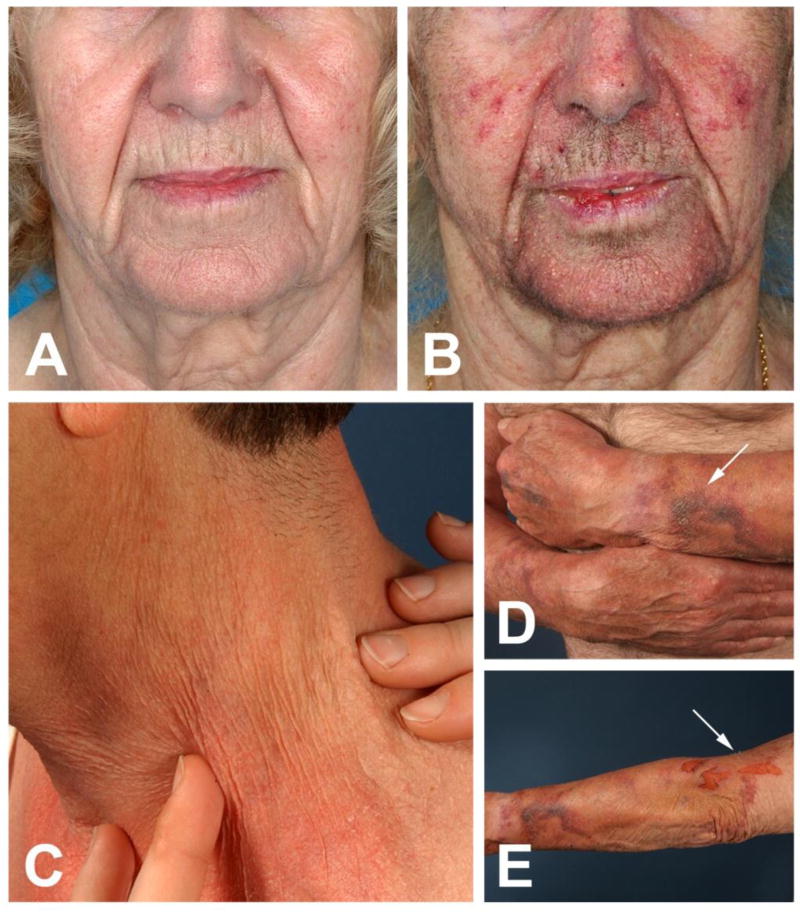
We identified a total of 12 patients with a mean age of 65.4 years (range, 62–74 years), who had all developed characteristic signs of skin aging during long-term therapy with EGFRIs. As signs of (skin-) aging in tumor patients are often associated with significant weight loss or general wasting, concomitant anti-tumor therapies or systemic steroids, we did not included patients in our analysis that fulfilled any of these criteria. Four drugs (erlotinib, gefitinib, dacomitinib, cetuximab) were represented in our patient group, however, dermatologic AEs were associated with all drugs, suggestive of a “class effect”. Patients presented with progressive generalized xerosis cutis, rhytides (wrinkles), skin atrophy, skin fragility (purpura, erosions and lacerations upon minimal trauma), atrophy of the subcutaneous tissue, and/or actinic purpura favoring UV-exposed areas (Figure 1A–E). All patients acknowledged that their skin-appearance was age-appropriate prior to EGFRI treatment, and that the changes developed within months after initiation of the EGFRI.
Figure 1. A–E. “Aging-skin”-phenotype in patients treated with EGFRIs for >3 months.
A 64-year-old female (A) before and (B) after long-term (>3 months) treatment with erlotinib 75 mg orally once daily for NSCLC. The patient complained about progressive aging of her facial skin. Rhytides and skin atrophy could be appreciated upon clinical examination. (C) Fine wrinkles of the neck of a 62-year-old male treated with gefitinib 250 mg for 4 months. (D, E) Xerosis cutis, excessive skin atrophy, disseminated fine wrinkles and (D; arrow) extensive purpura of the forearms of a 74-year-old male treated with erlotinib 150 mg for 12 months. (E; arrow) The patient reported that the erosions on his left arm had been caused by simple removal of band-aids.
Comparative analysis of EGFR-density in aged vs. young skin
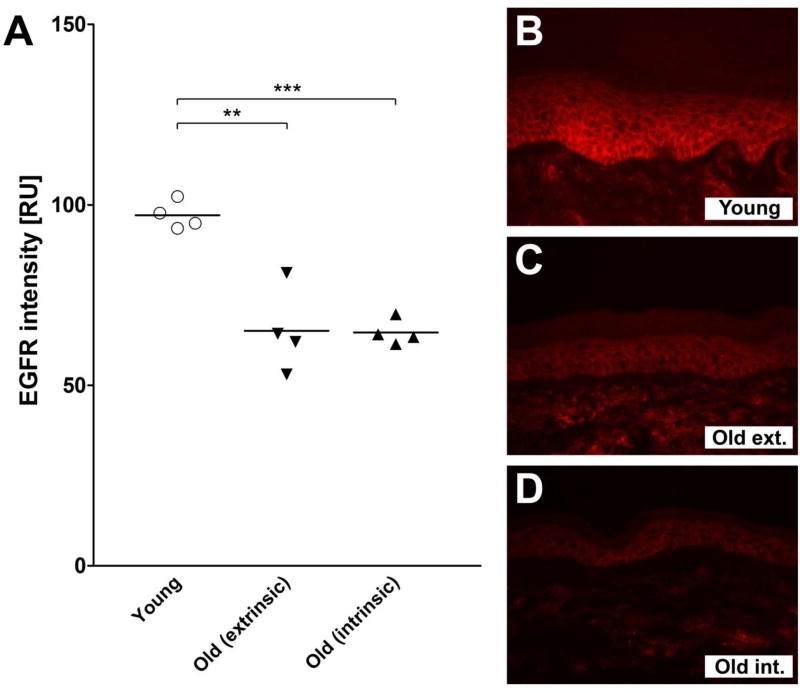
Immunofluorescence analyses of skin sections revealed a significant decline in EGFR density in aged skin, as compared to young skin. This was true for samples obtained from the UV-exposed (p ≤ 0.01) as well as non-UV-exposed (p ≤ 0.001) areas. In the latter however, the EGFR density in both the areas was comparable (Figure 2A–D).
Figure 2. A–D. EGFR expression in young vs. aged skin.
(A) EGFR density in young (<25 years old; n =4), aged UV-exposed (extrinsically aged, >65 years old; n = 4) and aged non-UV-exposed (intrinsically aged, >65 years old; n = 4) skin. Skin sections were analyzed by NIKON NIS Elements AR software. Values are expressed as relative units (RU) and represent the mean ± SEM (Student’s t-test; **p ≤ 0.01, ***p ≤ 0.001). (B–D) Exemplary immunofluorescence slides of each of the datasets presented in Fig. 2A: (B) Young skin, (C) Aged UV-exposed (Old ext.), and (D) Aged non-UV-exposed skin (Old int.).
Effect of erlotinib on primary human keratinocytes in vitro
Expression of Hyaluronan synthase genes
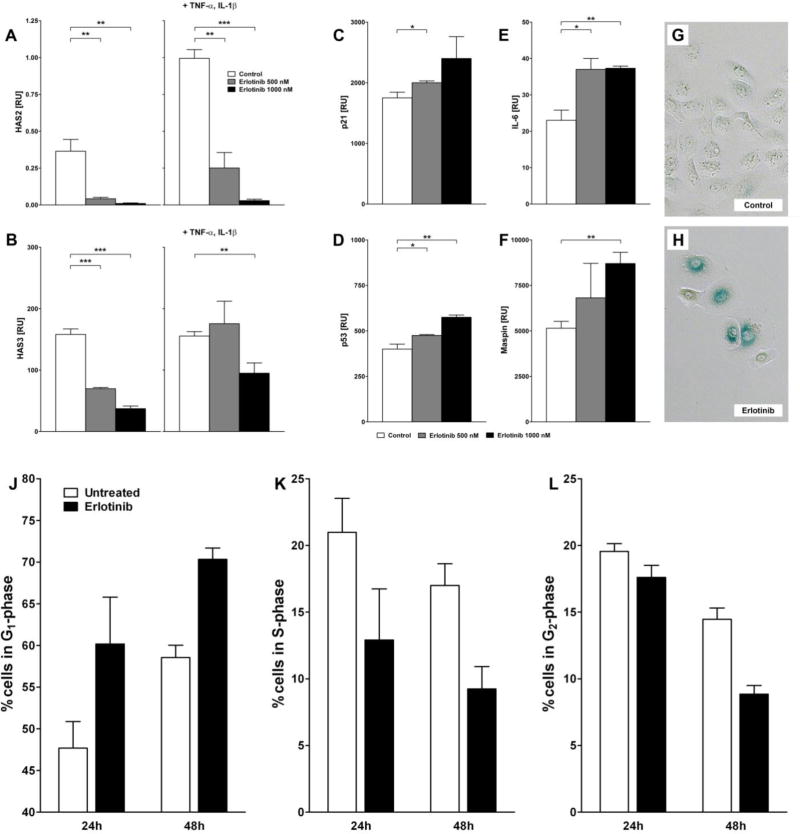
The expression of the genes, HAS2 and HAS3, was significantly impaired in a dose-dependent manner following incubation with erlotinib for 24h, both in the presence and absence of the pro-inflammatory cytokines TNF-α and IL-1β (Figure 3A, B).
Figure 3. A–L. Erlotinib impairs the expression of hyaluronan synthase genes, induces senescence-associated genes and SA β-galactosidase activation as well as a time-dependent G1 cell cycle arrest in primary human keratinocytes in vitro.
(A–F) mRNA expression of (A) HAS2, (B) HAS3, (C) p21, (D) p53, (E) IL-6 and (F) maspin in primary human keratinocytes treated with erlotinib (0 nM, 500 nM, 1000 nM) for 24 hours, in case of (A) and (B) in presence or absence of the pro-inflammatory cytokines TNF-α (10 ng/ml) and IL1β (5 ng/ml). (G–H) Cells were incubated overnight with (G) medium (vehicle control) or (H) erlotinib (1000 nM), fixed and incubated with X-gal and analyzed by microscopy. (J–L) For flow cytometric cell cycle analysis cells were treated with erlotinib (1000 nM) for up to 48 hours. Displayed are percentages of cells in the (J) G1-, (K) S-, and (L) G2-phase. Data shown represent the mean ± SEM of three independent experiments (Student’s t-test; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). RU = relative units.
Expression of Senescence-associated genes
There was a statistically significant increase in the expression of senescence-associated genes p21, p53, IL-6 and maspin, as compared to controls (Figure 3C–F). Moreover, primary human keratinocytes treated with erlotinib (1000 nM) showed a stronger SA β-Gal activity as compared to medium-treated controls (Figure 3G, H).
Effect on cell cycle
In order to further investigate the senescence-inducing effect of erlotinib, we determined the cell cycle phase distribution in primary human keratinocytes after treatment with erlotinib for up to 48 hours. Interestingly, and in concordance with its senescence-inducing effect (Figure 3C–H), treatment with erlotinib leads to strong time-dependent increase in cells in the G1-phase (Figure 3J). On the other hand, populations of cells residing in the S- and G2-phase were markedly decreased (Figure 3K, L). This indicates the formation of a G1-arrest induced by erlotinib, finally leading to cellular senescence.
DISCUSSION
The present study is the first to describe the clinical characteristics and molecular basis for an ‘aging-skin’ phenotype occurring in patients receiving long-term EGFRI treatment. The most frequent manifestations of this phenotype include xerosis cutis and rhytides, besides atrophy of the skin and subcutaneous tissue, skin fragility, and/or actinic purpura (Figure 1A–E). Our in vitro studies have demonstrated the significant down-modulation of HAS genes, induction of senescence-associated genes, and a cell cycle arrest in the G1 phase, all of which further corroborate our clinical observations. Moreover, there is now accumulating evidence that skin aging may manifest as a chronic cutaneous insufficiency/fragility syndrome, referred to as dermatoporosis. Clinical characteristics of this advanced stage of skin aging include: (a) morphological markers of fragility, such as purpura (Figure 1D) or skin atrophy (Figure 1C), and (b) a functional expression of skin fragility, including skin lacerations resulting from minor trauma (Figure 1E) [14].
In general, skin aging is a consequence of genetic programming (intrinsic aging) and/or exposure to environmental factors (extrinsic aging; e.g. UV rays, drugs). Yet, even under extreme conditions, the development of an advanced “skin-aging” phenotype similar to that seen in our patients would require years to develop. It is likely that chronic UV-exposure predisposes to EGFRI-induced skin aging, as the skin alterations in our patients were observed almost exclusively in UV-exposed areas (face, neck, arms), and it has been shown that the EGFRI-induced rash also can be aggravated or even provoked by UV-exposure [15]. On the other hand, it is rather unlikely that acute UV-exposure could have played a major role, as our patients were advised intensive sun-protection measures at the initiation of EGFRI-therapy. Furthermore, none of our patients had received extensive doses of systemic or topical glucocorticosteroids, ruling out this potential cause of skin atrophy. Taken together, our clinical observations and in particular, the short time-period for the development of the AEs presented here, suggest that impaired EGFR signaling may promote human (skin) aging. This hypothesis is supported by the fact that intrinsically aged skin, and the skin of patients treated with an EGFRI share common histopathologic characteristics such as atrophic epidermis, with a thin, compact stratum corneum [16, 17].
An age-related reduction in EGF/EGFR levels has been observed in a number of settings. Recent studies in Caenothabditis elegans longevity models demonstrate that reducing the activity of EGF signaling is associated with system-wide evidence of aging-related changes [18–20]. Similar effects were noted by Shurin and coworkers in humans. Of the 30 serum biomarkers tested, they identified EGF and EGFR as the two candidates with the most significant down-modulation with age, in contrast to the interferon-induced chemokines, CXCL9 (MIG), CXCL10 (IP-10), and CCL11 (eotaxin), and IL-6 which were all increased by aging [21]. While in skin, Green et al. demonstrated a downregulation of EGFR on basal epidermal keratinocytes and dermal fibroblasts in the dorsal skin of young (neonatal, day 1) vs. old (days 23, or 51) rats [22]. In addition, Shiraha et al demonstrated decreased levels of EGFR on old vs. young human dermal fibroblasts [23]. This argues well that EGFR decline may be associated with impaired regenerative capacities of aging skin. In this context, and to the best of our knowledge, we here for the first time have demonstrated a decline in (epidermal) EGFR levels in `young` (<25 years) vs. `aged` (UV-exposed: `old extrinsic`; non-UV-exposed: `old intrinsic`) human skin (>65 years) (Figure 2).
But what is known about a link between reduced EGFR and skin aging? In general, two major hypotheses for the mechanisms of intrinsic skin aging have been proposed: (a) the oxidative stress theory of skin aging suggests that aging is heavily influenced by external stressors which influence the genetic program through the modulation of redox sensitive genes, and, (b) the cellular senescence theory that invokes a combination of factors including (i) decreased proliferation of skin cells, (ii) decreased matrix synthesis, and (iii) increased expression of matrix-degrading enzymes in skin aging [24]. In this hypothesis, few senescent cells may exert significant deleterious effects on the tissue microenvironment and promote aging and/or tumor progression (“Senescent cells are good citizens but bad neighbours!”) [25, 26].
The EGFR has been shown to play a central role for oxidative stress driven skin aging. Under physiological conditions the EGFR is activated by the binding of specific ligands such as EGF or transforming growth factor-α (TGF-α) [27]. Yet, exogenous stimuli, such as UV- and gamma-irradiation, H2O2 or polycyclic aromatic hydrocarbons, can also activate the EGFR via the generation of reactive oxygen species (ROS) in a ligand-independent manner [28, 29]. These effects become evident when the generation of ROS exceeds cellular scavenger capacities (stress tolerance), resulting in oxidative stress. Stress-induced activation of EGFR-signaling leads to the activation of the transcription factor activator protein (AP-1), and finally to an induction of matrix metallo-proteinases, eventually resulting in extrinsic skin aging [30]. In this context, pharmacological inhibition of EGFR and/or EGFR-dependent signaling pathways has been proposed as a strategy for the prevention of photoaging [31–33]. However, it has also been shown that pharmacological EGFR-inhibition results in an increase of UVB-induced H2O2-generation and hence, increased oxidative stress in primary human keratinocytes [34]. Furthermore, hepatocytes obtained from old rats (24–26 months) were more sensitive to H2O2 as compared to those obtained from young rats (4–6 months). This effect could be mimicked in young hepatocytes by pharmacologic inhibition of the EGFR down-stream kinases ERK and Akt [35]. Hence, it can be proposed that the activation status of the EGFR may influence skin aging in multifarious ways and that other variables that determine pro- and anti-aging effects still need to be identified (e.g. acute UV-induced EGFR-activation vs. chronic pharmacologic EGFR-inhibition).
Concerning the cellular senescence theory of skin aging, decreased EGFR-expression in aging skin has been associated with decreased proliferative capacities that may clinically correlate with impaired wound healing in the elderly [23]. We have recently shown that pharmacologically or genetically impaired EGFR-signaling induces the expression of pro-inflammatory cytokines and chemokines, such as IL-6, IL-1α, IL-1β, CCL2, CXCL1 and CXCL2, that define a senescence-associated secretory phenotype [7, 36]. Furthermore, we have demonstrated that the EGFR is critically involved in TGF-β1-induced hyaluronan (HA) synthesis and HA signaling. Strikingly, pharmacologic EGFR-inhibition blocked the paracrine effect of UVB- plus estrogen (E2)-treated epidermal keratinocytes on dermal fibroblasts and suppressed the expression of HAS3 and versican (V2) and hence ECM synthesis [37]. Our current data are consistent with these findings and demonstrate that the EGFRI, erlotinib, significantly and dose-dependently impairs the expression of HAS2 and HAS3 in primary human keratinocytes in vitro (Figure 3A, B). Furthermore, pharmacological inhibition of the EGFR and its downstream kinase BRAF have been shown to induce premature senescence in a variety of cell types, including non-small cell lung cancer or melanoma cells [38, 39]. A recent study by Schad and coworkers outlined similarities in dermatologic AEs in patients treated with MEK inhibitors, and patients treated with EGFRIs. In this context, the authors proposed that long-term inhibition of the EGFR downstream kinase MEK may result in a phenotype that resembles senescence-driven skin aging [40]. Again, these findings are in line with our data that demonstrate that erlotinib significantly and dose-dependently induces the expression of senescence-associated genes (p21, p53, IL-6, maspin) (Figure 3C–F), as well as SA β-gal activity (Figure 3G, H).
Finally, we demonstrate that erlotinib is capable of inducing G1 cell cycle arrest of primary human keratinocytes (Figure 3J–L). This is consistent with the findings of El-Abaseri et al that show that activation of EGFR, in this case using UV irradiation, leads to cell proliferation, but in absence of the receptor (EGFR −/− mouse skin) cell cycle arrest and apoptosis occurs with an increase in p21 and p53 levels. Thus, absence or reduction of EGFR signaling, leads to an increase in senescence, associated with cell cycle arrest [41]. Hence, there is accumulating evidence demonstrating that EGFR-inhibition promotes processes that are critical to skin aging. These pro-aging effects may affect both the oxidative stress and senescence-driven aging, and are likely to exert multiple alterations in signaling pathways.
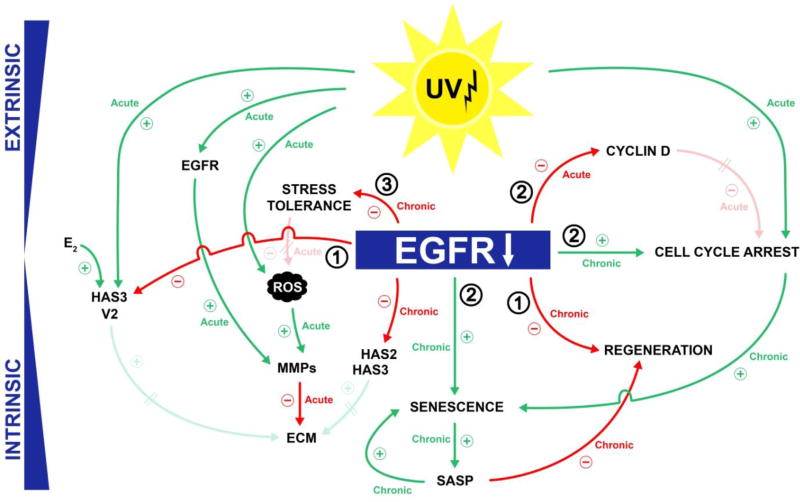
To conclude, targeted inhibition of signaling pathways involved in the survival and growth of tumor cells has extended the therapeutic armamentarium for the management of cancer. Paradoxically, the associated AEs of these drugs have also been valuable and given medical research new insights into the physiological functions of these pathways. Inhibition of the EGFR has uncovered a critical role for EGF and its receptor in the homeostasis of normal human skin and skin aging. The changes that lead to premature skin aging are summarized in a hypothetical model (Figure 4). With this concept in hand, future studies on the mechanisms of aging can be focused on confirming the relationships proposed and should also focus to discriminate acute effects, such as the activation of EGFR signaling by UV, from chronic effects such as a long-term pharmacologic inhibition of the EGFR and its downstream kinases by targeted cancer agents.
Figure 4. Impaired EGFR-signaling promotes skin aging by alteration of miscellaneous mechanisms.
(1) The deprivation of an essential growth factor results in impairment of regenerative capacities and impaired ECM synthesis, (2) EGFR-inhibition causes premature cellular senescence and induces a SASP that affects neighboring cells, and (3) EGFR-inhibition reduces cellular stress-tolerance and promotes a UV-induced cell cycle arrest.
Acknowledgments
Funding sources: This work was supported by a grant of the Duesseldorf Science Commission and a grant by Roche Pharmaceuticals.
PAG has a speaking, consultant or advisory role with Amgen, Galderma, and Roche; MEL has a speaking, consultant or advisory role with Advancell, Amgen, AstraZeneca, Augmentium, Aveo, Bayer, Berg Pharma, Biopharm Communications, Boehringer Ingelheim, Brickell Biotech, Bristol-Myers Squibb, Clinical Assistance Programs, Clinical Care Options, EMD Serono, Envision Communications, Foamix, Galderma, Genentech, GlaxoSmithKline, Helsinn, Institute for Medical Education and Research, Integro-MC, Lindi Skin, Medscape, Medtrend International, Merck, Nerre Therapeutics, Novartis, Novocure, Oncology Specialty Group, OSI Pharmaceuticals, Permanyer, Physicians Education Resource, Pierre Fabre, Pfizer, Reata Pharmaceuticals, Roche, Sandoz, Sanofi Aventis, and Threshold Pharmaceuticals.
ABBREVIATIONS/ ACRONYMS USED
- AE
adverse event
- ECM
extracellular matrix
- EGFRI
epidermal growth factor (EGF) receptor (EGFR) inhibitor
- HAS
hyaluronan (HA) synthase
- IL
interleukin
- MSKCC
Memorial Sloan Kettering Cancer Center
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- SA β-Gal
senescence-associated β-galactosidase
- TGF
transforming growth factor
- TNF
tumor necrosis factor
Footnotes
Submission Declaration: The contents of this manuscript have not been presented, and are not under consideration for publication elsewhere.
CONFLICT OF INTEREST
BAB, HS, PH, EB, DS, VRB, and CR have no conflicts of interest to declare.
References
- 1.Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, Rosenbaum SE, Lacouture ME. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116:3916–3923. doi: 10.1002/cncr.25090. [DOI] [PubMed] [Google Scholar]
- 2.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nature reviews Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 3.Gerber PA, Buhren BA, Cevikbas F, Bolke E, Steinhoff M, Homey B. Preliminary evidence for a role of mast cells in epidermal growth factor receptor inhibitor-induced pruritus. Journal of the American Academy of Dermatology. 2010;63:163–165. doi: 10.1016/j.jaad.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. The New England journal of medicine. 2011;364:486–487. doi: 10.1056/NEJMc1013027. [DOI] [PubMed] [Google Scholar]
- 5.Gerber PA, Homey B. Images in clinical medicine. Erlotinib-induced hair alterations. The New England journal of medicine. 2008;358:1175. doi: 10.1056/NEJMicm073144. [DOI] [PubMed] [Google Scholar]
- 6.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:1425–1433. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P, Mackenzie C, Wollenberg A, Kislat A, Fischer JW, Rock K, Harder J, Schroder JM, Homey B, Sibilia M. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Science translational medicine. 2013;5:199ra111. doi: 10.1126/scitranslmed.3005886. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, Kurkciyan A, Liu N, Jiang D, Noble PW. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix biology : journal of the International Society for Matrix Biology. 2016 doi: 10.1016/j.matbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzellos TG, Klagas I, Vahtsevanos K, Triaridis S, Printza A, Kyrgidis A, Karakiulakis G, Zouboulis CC, Papakonstantinou E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Experimental dermatology. 2009;18:1028–1035. doi: 10.1111/j.1600-0625.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 10.Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 11.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature medicine. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickoloff BJ, Lingen MW, Chang BD, Shen M, Swift M, Curry J, Bacon P, Bodner B, Roninson IB. Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer research. 2004;64:2956–2961. doi: 10.1158/0008-5472.can-03-2388. [DOI] [PubMed] [Google Scholar]
- 13.Garbers C, Kuck F, Aparicio-Siegmund S, Konzak K, Kessenbrock M, Sommerfeld A, Haussinger D, Lang PA, Brenner D, Mak TW, Rose-John S, Essmann F, Schulze-Osthoff K, Piekorz RP, Scheller J. Cellular senescence or EGFR signaling induces Interleukin 6 (IL-6) receptor expression controlled by mammalian target of rapamycin (mTOR) Cell cycle. 2013;12:3421–3432. doi: 10.4161/cc.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaya G, Saurat JH. Dermatoporosis: a chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology. 2007;215:284–294. doi: 10.1159/000107621. [DOI] [PubMed] [Google Scholar]
- 15.Gerber PA, Enderlein E, Homey B. The Koebner-phenomenon in epidermal growth factor receptor inhibitor-induced cutaneous adverse effects. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2790–2792. doi: 10.1200/JCO.2007.16.0077. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J. Targeting the epidermal growth factor receptor with tyrosine kinase inhibitors: small molecules, big hopes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2217–2219. doi: 10.1200/JCO.2002.20.9.2217. [DOI] [PubMed] [Google Scholar]
- 17.Stern RS. Clinical practice. Treatment of photoaging. The New England journal of medicine. 2004;350:1526–1534. doi: 10.1056/NEJMcp023168. [DOI] [PubMed] [Google Scholar]
- 18.Iwasa H, Yu S, Xue J, Driscoll M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging cell. 2010;9:490–505. doi: 10.1111/j.1474-9726.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rongo C. Epidermal growth factor and aging: a signaling molecule reveals a new eye opening function. Aging. 2011;3:896–905. doi: 10.18632/aging.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S, Driscoll M. EGF signaling comes of age: promotion of healthy aging in C. elegans. Experimental gerontology. 2011;46:129–134. doi: 10.1016/j.exger.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–129. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Green MR, Basketter DA, Couchman JR, Rees DA. Distribution and number of epidermal growth factor receptors in skin is related to epithelial cell growth. Developmental biology. 1983;100:506–512. doi: 10.1016/0012-1606(83)90243-9. [DOI] [PubMed] [Google Scholar]
- 23.Shiraha H, Gupta K, Drabik K, Wells A. Aging fibroblasts present reduced epidermal growth factor (EGF) responsiveness due to preferential loss of EGF receptors. The Journal of biological chemistry. 2000;275:19343–19351. doi: 10.1074/jbc.M000008200. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins G. Molecular mechanisms of skin ageing. Mechanisms of ageing and development. 2002;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 25.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews Molecular cell biology. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Fragoso L, Melendez K, Hudson LG, Lauer FT, Burchiel SW. EGF-receptor phosphorylation and downstream signaling are activated by benzo[a]pyrene 3,6-quinone and benzo[a]pyrene 1,6-quinone in human mammary epithelial cells. Toxicology and applied pharmacology. 2009;235:321–328. doi: 10.1016/j.taap.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. The Journal of biological chemistry. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S, Chung JH, Lee JH, Fisher GJ, Wan YS, Duell EA, Voorhees JJ. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. The Journal of investigative dermatology. 2003;120:835–841. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 32.Wan YS, Wang ZQ, Shao Y, Voorhees JJ, Fisher GJ. Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. International journal of oncology. 2001;18:461–466. doi: 10.3892/ijo.18.3.461. [DOI] [PubMed] [Google Scholar]
- 33.Xu YR, Fisher GJ. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J Dermatol Sci. 2005:S1–S8. [Google Scholar]
- 34.Peus D, Vasa RA, Meves A, Beyerle A, Pittelkow MR. UVB-induced epidermal growth factor receptor phosphorylation is critical for downstream signaling and keratinocyte survival. Photochemistry and photobiology. 2000;72:135–140. doi: 10.1562/0031-8655(2000)072<0135:uiegfr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Ikeyama S, Kusumoto K, Ogata S, Miyake H, Teshima S, Nikawa T, Rokutan K, Tashiro S. A nontoxic heat shock protein 70 inducer, geranylgeranylacetone, prevents apoptosis of primary cultures of rat hepatocyted induced by hydrogen peroxide or ethanol. Gastroenterology. 1999;116:A1222–A1222. [Google Scholar]
- 36.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer metastasis reviews. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock K, Meusch M, Fuchs N, Tigges J, Zipper P, Fritsche E, Krutmann J, Homey B, Reifenberger J, Fischer JW. Estradiol protects dermal hyaluronan/versican matrix during photoaging by release of epidermal growth factor from keratinocytes. The Journal of biological chemistry. 2012;287:20056–20069. doi: 10.1074/jbc.M112.353151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haferkamp S, Borst A, Adam C, Becker TM, Motschenbacher S, Windhovel S, Hufnagel AL, Houben R, Meierjohann S. Vemurafenib induces senescence features in melanoma cells. The Journal of investigative dermatology. 2013;133:1601–1609. doi: 10.1038/jid.2013.6. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Morsbach F, Sander D, Gheorghiu L, Nanda A, Benes C, Kriegs M, Krause M, Dikomey E, Baumann M, Dahm-Daphi J, Settleman J, Willers H. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer research. 2011;71:6261–6269. doi: 10.1158/0008-5472.CAN-11-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schad K, Baumann Conzett K, Zipser MC, Enderlin V, Kamarashev J, French LE, Dummer R. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1058–1064. doi: 10.1158/1078-0432.CCR-09-1766. [DOI] [PubMed] [Google Scholar]
- 41.El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27:225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]