Abstract
Background and Purpose:
Seborrheic dermatitis is a chronic and recurrent superficial dermatitis in which Malassezia species play an important role. There are different Malassezia species, which have been recently reported to be resistant to common antifungals. Natural sources can be useful alternatives to reduce the emergence of this resistance. Kombucha tea is believed to have potential antimicrobial properties. Regarding this, the present study aimed to investigate the antifungal activity of Kombucha tea ethyl acetate fraction (KEAF) against Malassezia species obtained from the patients with seborrheic dermatitis.
Materials and Methods:
A total of 23 clinical isolates were identified by direct microscopic examination and Tween assimilation, and then confirmed by DNA sequencing of ITS regions for Malassezia species. Kombucha tea was fractionated using ethyl acetate (1:2 v/v). The minimum inhibitory concentration (MIC) microdilution assay was used to evaluate the anti-Malssezia activity of KEAF at three concentrations of 10, 40, and 80 mg/mL.
Results:
The results of the DNA sequence analysis indicated that M. furfur (39.13%) was the predominant species, followed by M. globosa (30.43%), M. sloofie (13.04%), M. sympodialis (13.04%), and M. restricta (4.34%), respectively. Furthermore, KEAF showed inhibitory activity against Malassezia species. Accordingly, KEAF had the lowest and highest MIC value against M. sloofie and M. restricta, respectively. Moreover, the inhibitory effect of the extract was equivalent to that of ketoconazole at 4.8 µg/mL.
Conclusion:
The findings of the current study highlighted the antifungal properties of KEAF. Therefore, this extract can be promoted as complementary medicine for the treatment of the infections caused by Malassezia.
Key Words: Antifungal activity, Ethyle acetate fraction, Ketoconazole, Kombucha tea, Malassezia spp
Introduction
Malassezia genus is known to have 14 species of lipophilic yeasts. These species are members of skin microbiota in humans, mammals, and birds [1]. There are particular predisposing factors that may turn these species into opportunistic yeasts causing superficial and systemic infections in their hosts [2]. Seborrhoeic dermatitis is a common skin disease that can appear most often on the face, especially nasolabial folds, scalp, trunk, and sebaceous gland-rich areas. Malassezia species play an important role in this skin disease [3].
While seborrhoeic dermatitis caused by Malassezia species is generally treated with a combination of oral and topical medicines, treatments do not always lead to favorable outcomes due to the chronic and recurrent nature of the infection [4]. However, a limited range of drugs, particularly topical antifungal solutions with 2% ketoconazole, can be administered to manage the mentioned infection [5]. Long periods of treatment with such medicines are associated with high toxicity and recurrence [6].
The oral administration of this highly lipophilic drug will lead to its accumulation in the fatty tissues. This results in major side effects, such as toxic hepatitis, acquired cuta-neous adherence, nausea, vomiting, decreased appetite, rash, pruritus, irregular period, decreased libido, and breast enlargement in men [7]. Moreover, this medication is contraindicated for the pregnant or breastfeeding women [8].
Other topical antifungal agents, such as clotrimazole, miconazole, and terbinafine, are less commonly administered due to their association with higher recurrence risk [9]. Recently, much attention has been paid to replace the synthetic anti-microbial medications with natural alternatives, which have less side effects and lower cost [10, 11]. Kombucha tea is one of these natural alternatives, which is a popular traditional fermented drink in many countries.
This tea is prepared from the fermentation of sugared black tea with a symbiotic culture of acetic bacteria and kombucha fungi [12]. The beneficial compounds of this beverage include polyphenols (flavenoids), glucuronic acid, gluconic acid, lactic acid, antibiotics, folic acid, D-lactic acid, epigallocatechin gallates, amino acids, as well as vitamins B1, B2, B3, B6, and B12 [13, 14].
There are reports indicating the potential antimicrobial properties of Kombucha tea against such pathogenic bacteria as Escherichia, Klebsiella, Proteus, and Salmonella [15, 16]. According to the literature, Kombucha tea, especially its ethyl acetate fraction, has high amounts of flavonoid and phenolic compounds [17] with antimicrobial activity [14, 18]. How-ever, no study has determined the effective fraction of Kombucha tea against Malassezia species yet.
With this background in this mind, the current study was comducted with a two-fold aim: a) identification of Malassezia species obtained from the patients with seborrheic dermatitis and b) evaluation of the antifungal effect of Kombucha tea ethyl acetate fraction (KEAF) on inhibiting the growth of these isolates.
Materials and Methods
Preparation of Kombucha tea
In order to prepare Kombucha tea, black tea (Golestan, Tehran, Iran) was added to boiling water (1.2% w/v), which was then mixed and left to brew for 5 min. Subsequently, the tea was passed through a sterile sieve. After the dissolution of sucrose (10%) in the hot tea, it was left to cool. In the next step, 3% (w/v) freshly grown tea fungus (previously cultured in the same medium for 14 days) and 10% (v/v) fermented liquid tea broth were added to the jar under sterile condition. The jar was covered with clean clothes and fastened tightly. The solutuion was allowed to ferment in the dark at 24°C for 14 days. On the 14th day, the fermented tea was centrifuged at 4,000 rpm for 20 min [19].
Ethyl acetate fraction of Kombucha tea
Kombucha tea was fractionated using ethyl acetate (1:2 v/v). A vacuum rotary evaporator (R-200, Büchi, Germany) was used to concentrate the Kombucha tea ethyl acetate solution. The obtained sticky mass in distilled water was passed through a 0.22 μm Millipore membrane. Finally, the filtered solution was left to degas [20].
Culture and identification of Malassezia species
This study was conducted on 19 patients referring to the Skin Department at Bahonar Hospital, Karaj, Iran. Lesions with yellowish scale, pityriasiform scaling, as well as greasy, seborrhea, and erythematous plaques were clinically diagnosed as seborrheic dermatitis. The patients who had used antifungal drugs during the previous six weeks were excluded from the study. The samples were collected from the skin lesions of the patients and inoculated onto modified Dixon’s agar as recommended by Gueho et al. (3.6% malt extract agar, 0.6% peptone, 1% agar, 2% oxbile, 1% Tween-40 [all from Merck, Germany], 0.2% glycerol, and 0.2% oleic acid [Sigma, Germany]).
The medium was supplemented with cyclo-hexamide (0.5%) and chloramphenicol (0.05%) (Sigma, Germany) and incubated at 32°C for 10 days. The growing colonies were identified based on the morphology of the colonies, attendance of catalase, tolerance of 37°C [21], and Tween assimilation test [22]. This study was approved by the Ethics Committee of Alborz University of Medical Sciences, Karaj, Iran (Abzums.Rec. 1395.51).
Tween assimilation technique
In order to identify the species of Malassezia, these yeasts were subjected to utilize individual Tween compounds (i.e., Tween 20, 40, 60, and 80). The growth and precipitation of the lipophilic yeasts around individual wells revealed the utilization of Tween. Briefly, a suspension about 105 CFU/mL was prepared from Malassezia yeasts on the mDixon agar. These suspensions were mixed with 16 ml of melted sterile Sabouraud agar (Merck, Germany) that allowed them to cool to approximately 50°C. Afterwards, the mixtures were plated, and four holes with 2-mm diameter punch were made and filled with5 µl of Tween 20, 40, 60, and 80 (Merck, Germany). The plates were incubated at 32°C for one week [22].
Amplification of internal transcribed spacer regions
One colony of yeast pure culture was subcultured on 10 ml of Dixon broth for DNA extraction by phenol chloroform method as described by Yamada et al. [23]. The polymerase chain reaction (PCR) technique was performed by the primers internal transcribed spacer (ITS) 4 and ITS5 to amplify ITS-5.8S rDNA region (5΄-TCCTCCGCTTATTGATATGC-3΄) and ITS5 (5΄-GGAAGTAAAAGTCGTAACAAGG-3΄). For each PCR, we used 25 µL Taq 2x PCR Master Mix (SinaClon BioScience Co., Karaj, Iran), 0.5 µl of each primer, and 2 µl DNA template in a 25 µl volume.
The amplification of the gene fragment was performed in a PCR thermal cycler (Peqlab, Belgium) by the denaturation of 5 min at 94°C, followed by 35 cycles of annealing at 56°C for 40 sec and elongation at 72°C for 2 min. The second denaturation occurred at 94°C for 45 sec. The PCR was completed by a final elongation step at 72°C for 10 min. The molecular masses of the amplified DNA were estimated by comparison with a 100-bp DNA ladder (Bio-Rad Laboratories S.A., Barcelona, Spain).
DNA sequencing of internal transcribed spacer regions
Sequencing analysis was performed on purified products using the ITS4 forward primer by Biosystems 3730 XL Bioneer Corporation (made in Korea). The sequences were compared with the those present in the gene bank database (http://www.ncbi.nlm.nih.gov) by using the Blast system.
Assessment of the antifungal properties of Kombucha ethyl acetate fraction
The minimum inhibitory concentrations (MICs) of KEAF were determined by the modified broth microdilution method based on the Clinical and Laboratory Standards Institute M27-A3 protocol [24]. Malassezia isolates were obtained in 5 ml of sterile distilled water using 5-day-old colonies grown on mDixon agar at 32°C. The cell density was adjusted with spectrophotometer at 530 nm to obtain the transmittance of 0.5 McFarland, corresponding to 2-4×104 cells/mL [25].
Subsequently, 80 μl KEAF with three concentrations of 10, 40, and 80 mg/mL diluted in RPMI 1640 (Sigma Aldrich, Germany) was transferred into a 96-well microplate (Jetbiofil, China) and mixed with 80 μl of yeast suspension and 80 μl of Dixon broth. For the purpose of control and comparison, we also prepared the serial dilutions of ketoconazole (Sigma Aldrich, Germany) ranged within 16-0.016 µg/mL and assessed their antifungal properties in a procedure similar to that described for KEAF.
All tests were incubated at 32°C for five days. The MIC was regarded as the lowest concentration of drug or fraction inducing growth inhibition, compared to the amount in the wells with no drug or fraction agent (negative control). After incubation, 50 µl of each concentration was transferred to the surface of modified Dixon agar. The minimum fungicidal concentration (MFC) of drug or fraction was obtained by the absence of colonies in plate. All bioassays were performed twice, and the mean values were calculated.
Statistical analysis
The data were analyzed using the ANOVA and paired t-test through the SPSS version 19.0. P-value less than 0.05 was considered statistically significant.
Results
During the study period, 89.5% of the samples were positive for the presence of Malassezia species. These isolates were identified according to their microscopic characteristics and their ability to assimilate Tweens 20, 40, 60, and 80. However, a total of 23 Malassezia strains were isolated from the patients with seborrheic dermatitis (Table 1). The ITS5.8S rDNA region was amplified using ITS4 and ITS5 primers. Figure 1 presents the gel electrophoresis of amplification products.
Table 1.
Isolation of Malassezia clinical strains and demographic characteristics of patients with seborrhoeic dermatitis
| Malassezia species | Number tested (%) | Gender | Age |
|---|---|---|---|
| M. furfur | 9 (39.13) | Male=7 Female=9 |
Range=17-48 Mean=30.4 |
| M. globosa | 7 (30.43) | ||
| M. sloofie | 3 (13.04) | ||
| M. sympodialis | 3 (13.04) | ||
| M. restricta | 1 (4.34) |
Figure 1.
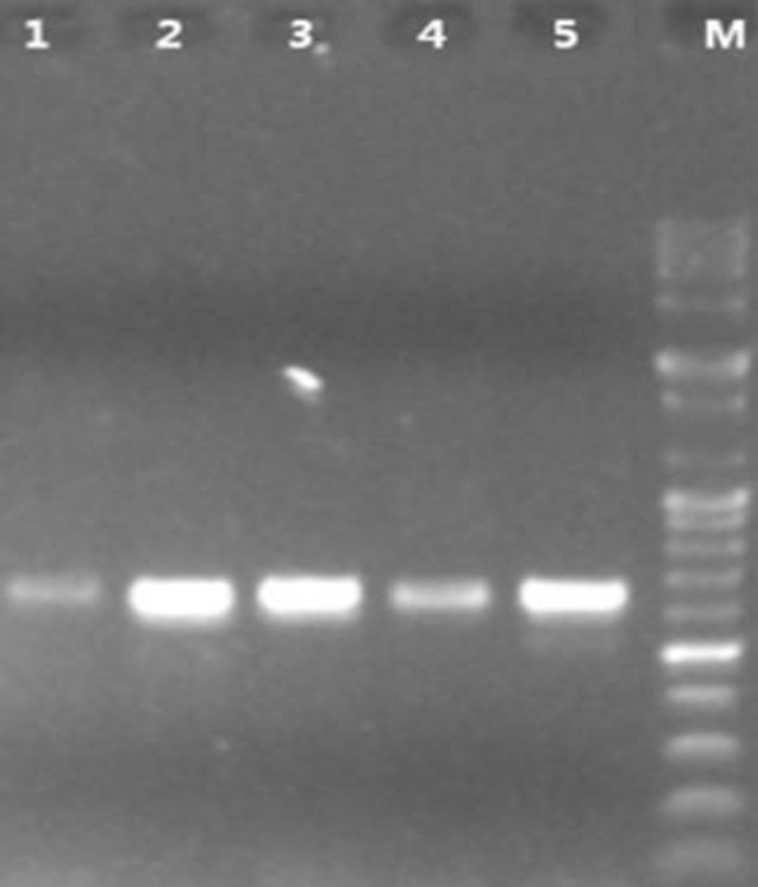
Electrophoresis of polymerase chain reaction products from Malassezia clinical isolates; lane M: DNA size marker 100 bp (5 μg/μL), lane 1-5: isolates including M. globosa, M. furfur, M. restricta, M. sloofie, and M. sympodialis (~650bp), sample: 3 ul, 45 min running on 1% agarose gel
According to the results of DNA sequence analysis, M. furfur (39.13%) was the most common Malassezia species isolated from the seborrheic dermatitis lesions, followed by M. globosa (30.43%), M. sloofie (13.04%), M. sympodialis (13.04%), and M. restricta (4.34 %), respectively. (Table 1). The in-vitro inhibitory effects of KEAF on 23 clinical isolates of Malassezia species obtained in this study are illustrated in Table 2.
Table 2.
Average percentage of minimum inhibitory and fungicidal concentrations of Kombucha tea ethyl acetate fraction and ketoconazol against Malassezia species (n=23)
| MIC | MFC | |
|---|---|---|
| KEAF (10 mg/mL) | 33% | - |
| KEAF (40 mg/mL) | 59% | - |
| KEAF (80 mg/mL) | 95.7% | 78.7% |
| KTC (4.5-16 µg/mL) | 100% | 57% |
KEAF: Kombucha tea ethyl acetate fraction, KTC: ketoconazol, MIC: minimum inhibitory concentration, MFC: minimum fungicidal concentration (The values are the mean of two experiments)
The antifungal property of KEAF was evident when the fraction was assayed at 80 mg/mL (P<0.05). Furthermore, KEAF in concentration of 80 mg/mL demonstrated an inhibiting growth of 95.7% in the evaluated Malassezia strains. The fungicidal effect was observed in 78.7% of the samples. Although KEAF inhibited 33% and 59% of Malassezia strains at concentrations of 10 and 40 mg/mL, respectively, it showed no fungicidal effects in these concentrations.
Regarding ketoconazole (16-4.5 µg/mL), it inhibited 100% of the Malassezia strains; nevertheless, the fungicidal effect was observed in only 57% of the samples (Table 2). M. furfur had a higher MIC value, compared to other species, indicating a low susceptibility to KEAF and ketoconazole (Table 3). The comparison of the fraction and ketoconazole in terms of the anti-Malassezia effects revealed that the inhibitory effect of the extract was equivalent to that of ketoconazole at 4.8 µg/mL (P<0.05; Table 2).
Table 3.
In vitro antifungal of Kombucha tea ethyl acetate fraction (80 mg/mL) and ketoconazol against 23 clinical isolates of Malassezia
|
M.furfur
(n=9) |
M. globosa
(n=7) |
M.sloofie
(n=3) |
M. sympodialis
(n=3) |
M.restricta
(n=1) |
|
|---|---|---|---|---|---|
| MIC (KEAF ) | 1.5±0.3 | 1.2±0.2 | 1.08±0.2 | 1.1±0.05 | 0.9±0.08 |
| MIC (KTC ) | 0.6±0.02 | 0.2±0.01 | 0.1±0.04 | 0.1±0.01 | 0.3±0.02 |
MIC: minimum inhibitory concentration, KEAF: Kombucha tea ethyl acetate fraction, KTC: ketoconazol (The values are the mean of two experiments)
Discussion
In this study, DNA sequencing method was used to identify Malassezia species obtained from seborrheic dermatitis patients. Five Malassezia species were identified in the Iranian patients inflicted with seborrheic dermatitis. In this regard, M. furfur (39.13 %) was the predominant species, followed by M. globosa (30.43%), M sloofie, M. sympodialis (13.04%), and M. restricta (4.34 %), respectively (Figure 1, Table 2).
However, our findings were slightly different with those obtained by the previous studies in the sense that M. sloofie was isolated from the Iranian seborrheic dermatitis patients for the first time. Furthermore, in contrast to other studies, M. furfur had the highest frequency, and M. pachydermatitis and M. obtuse were not isolated in this study [3, 26, 27]. The results of the previous studies may lead to the false identification of species due to not employing molecular methods or investigating different geographic regions.
Findings regarding M. furfur were mostly compatible to those of other studies carried out in Japan [28], Korea [29], China [30], Sweden [31], and Canada [32]. DNA sequence analysis is a rapid, stable, and effective method for the identification and strain typing of Malassezia species [30, 33]. In the present study, the use of universal primers for the amplification of ITS regions led to the separation of non-Malassezia species and Malassezia species. Therefore, it is recommended to use more specific primers for the identification of Malassezia species.
The present study also investigated the inhibitory effects of KEAF against these Malassezia clinical isolates. According to the results, the inhibitory effect of KEAF was notable at the concentration of 80 mg/mL (Table 2). The antifungal property of KEAF was not evident when the fraction was assayed at 40 mg/mL, and specially 10 mg/mL (Table 2), which was indicative of the dose-dependent inhibitory activity of fraction.
Furthermore, the comparisons between the anti-Malassezia effects of the extract and ketoconazole revealed that the inhibitory effect of this fraction was equivalent to that of ketoconazole at 4.8 µg/mL. Seborrheic dermatitis is a growing disease [10, 34], especially in Iran, for unknown reasons, which might be genetic factors or climatic conditions (based on our unpublished clinical observations).
Azole derivatives, especially ketoconazole, are the most commonly antifungal agents used forthe treatment of this disease [5]. Nevertheless,since several Malassezia species have developed resistance to existing antifungal drugs, the prevalence of infections caused by these micro-organisms is on a growing trend [6, 10, 34]. Furthermore, the toxicity and low efficacy of the available antifungal agents along with the scarcity of novel medicines are barriers to the effective use of infection management modalities [6, 9].
Kombucha tea is known to inhibit the growth of Candida albicans and also a wide range of gram-positive and gram-negative bacteria, including Staphylococcus aureus, Vibrio cholerae, Campylobacter jejuni, Escherichia coli, and Helicobacter pylori [15, 16]. However, no report has determined the effective fraction of Kombucha tea against Malassezia species obtained from the patients with seborrheic dermatitis.
Overall, the comparison of KEAF with ketoconazol demonstrated that KEAF could be a good option for the topical management of seborrheic dermatitis, mainly in the recurrent forms. It seems that the anti-Malassezia activity was due to the presence of some compounds in the fraction. Nonetheless, the mechanism behind the anti-Malassezia activity of Kombucha tea has not been fully understood yet.
Jayaban et al. showed that ethyl acetate fraction of Kombucha tea contains two compounds, namely dimethyl malonate and vitexin with antimicrobial properties [17]. Moreover, these extracts have been found to have high amounts of flavonoidand phenolic compounds. These compounds have strong DPPH radical scavenging and antimicrobial activities [17, 18]. The presence of such compounds in the extract might have been responsible for their antifungal properties.
Nevertheless, further studies are required to confirm these findings. Finally, the topical use of KEAF at concentrations of ≥ 80 mg/mL can be offered to prevent and treat the diseases caused by Malassezia species, such as pityriasis versicolor, dandruff, folliculitis, and atopic dermatitis. However, the in vitro efficacy of this extract needs to be further evaluated to find the correlation between the in vitro MIC and clinical outcomes.
Conclusion
In summary, the obtained results demonstrated that ethyl acetate fraction of Kombucha tea has a marked antifungal activity against Malassezia species and that it may be used to treat and prevent Malassezial infections. We believe that the observed anti-malassezia activity is due to some compounds present in the fraction. However, future studies are required to analyze the extract.
Acknowledgments
The authors appreciate the technical assistance of the Institute of Medicinal Plants and the Academic Center for Education, Culture, and Research, Karaj, Iran.
Author’s contribution
E. M., M. S., and M. M. designed the experiments and analyzed the data. They were also involved in writing, drafting, and revising the manuscript. Furthermore, A. M., V. M., and M. Z cooperated in sample collection and laboratory examinations. All the authors submitted their opinions during all study stages.
Conflict of interest
The authors have declared no conflicts of interest.
Financial disclosure
This study was financially supported by the Deputy of Research of Alborz University of Medical Sciences under the reference number of 2757591.
References
- 1.Thayikkannu AB, Kindo AJ, Veeraraghavan M. Malassezia-Can it be Ignored? Indian J Dermatol. 2015;60(4):332–9. doi: 10.4103/0019-5154.160475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25(1):106–41. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarei-Mahmoudabadi A, Zarrin M, Mehdinezhad F. Seborrheic dermatitis due to Malassezia species in Ahvaz, Iran. Iran J Microbiol. 2013;5(3):268–71. [PMC free article] [PubMed] [Google Scholar]
- 4.Carrillo‐Munoz AJ, Rojas F, Tur‐Tur C, de Lose Ángeles Sosa M, Diez GO, Espada CM, et al. In vitro antifungal activity of topical and systemic antifungal drugs against Malassezia species. Mycoses. 2013;56(5):571–5. doi: 10.1111/myc.12076. [DOI] [PubMed] [Google Scholar]
- 5.Di Fonzo E, Martini P, Mazzatenta C, Lotti L, Alvino S. Comparative efficacy and tolerability of Ketomousse (ketoconazole foam 1%) and ketoconazole cream 2% in the treatment of pityriasis versicolor: results of a prospective, multicentre, randomised study. Mycoses. 2008;51(6):532–5. doi: 10.1111/j.1439-0507.2008.01508.x. [DOI] [PubMed] [Google Scholar]
- 6.Como JA, Dismukes WE. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330(4):263–72. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 7.Filip R, Davicino R, Anesini C. Antifungal activity of the aqueous extract of Ilex paraguariensis against Malasseziafurfur. Phytother Res. 2010;24(5):715–9. doi: 10.1002/ptr.3004. [DOI] [PubMed] [Google Scholar]
- 8.Zani MB, Soares RC, Arruda AC, Arruda LH, Paulino LC. Ketoconazole does not decrease fungal amount in patients with Seborrhoeic dermatitis. Br J Dematol. 2016;175(2):417–21. doi: 10.1111/bjd.14501. [DOI] [PubMed] [Google Scholar]
- 9.Robert ME, Kalia YN. New developments in topical antifungal therapy. Am J Drug Deliv. 2006;4(4):231–47. [Google Scholar]
- 10.Kim YR, Kim JH, Shin HJ, Choe YB, Ahn KJ, Lee YW. Clinical evaluation of a new-formula shampoo for scalp seborrheic dermatitis containing extract of Rosa centifolia petals and epigallocatechin gallate: a randomized, double-blind, controlled study. Ann Dermatol. 2014;26(6):733–8. doi: 10.5021/ad.2014.26.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oro D, Heissler A, Rossi EM, Scapin D, da Silva Malheiros P, Boff E. Antifungal activity of natural compounds against Candida species isolated from HIV-positive patients. Asian Pacific J Trop Biomed. 2015;5(9):781–4. [Google Scholar]
- 12.Dufresne C, Farnworth E. Tea, Kombucha, and health: a review. Food Res Int. 2000;33(6):409–21. [Google Scholar]
- 13.Jayabalan R, Malbasa RV, Loncar ES, Vitas JS, Sathishkumar M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Safety. 2014;13(4):538–50. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Ji B, Wu W, Wang R, Yang Z, Zhang D, et al. Hepatoprotective effects of kombucha tea: identification of functional strains and quantification of functional components. J Sci Food Agric. 2014;94(2):265–72. doi: 10.1002/jsfa.6245. [DOI] [PubMed] [Google Scholar]
- 15.Santos Jr RJ, Batista RA, Rodrigues Filho SA. Antimicrobial activity of broth fermented with kombucha colonies. J Microbial Biochem Technol. 2009;1(1):72–8. [Google Scholar]
- 16.Cetojevic-Simin DD, Bogdanovic GM, Cvetkovic DD, Velicanski AS. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L Kombucha. J BUON. 2008;13(3):395–401. [PubMed] [Google Scholar]
- 17.Jayabalan R, Chen PN, Hsieh YS, Prabhakaran K, Pitchai P, Marimuthu S, et al. Effect of solvent fractions of kombucha tea on viability and invasiveness of cancer cells-Characterization of dimethyl 2-(2-hydroxy-2-methoxypropylidine) malonate and vitexin. Indian J Biotechnol Pharm Res. 2011;10(1):75–82. [Google Scholar]
- 18.Vaquero MR, Alberto MR, de Nadra MM. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18(2):93–101. [Google Scholar]
- 19.Jayabalan R, Subathradevi P, Marimuthu S, Sathishkumar M, Swaminathan K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008;109(1):227–34. doi: 10.1016/j.foodchem.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Jayabalan R, Marimuthu S, Swaminathan K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007;102(1):392–8. [Google Scholar]
- 21.Gueho E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Leeuwenhoek. 1996;69(4):337–55. doi: 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 22.Gupta AK, Kohli Y, Faergemann J, Summerbell RC. Epidemiology of Malassezia yeasts associated with pityriasis versicolor in Ontario, Canada. Med Mycol. 2001;39(2):199–206. doi: 10.1080/mmy.39.2.199.206. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Makimura K, Merhendi H, Ueda K, Nishiyama Y, Yamaguchi H, et al. Comparison of different methods for extraction of mitochondrial DNA from human pathogenic yeasts. Jpn J Infect Dis. 2002;55(4):122–5. [PubMed] [Google Scholar]
- 24.Pakshir K, Zomorodian k, Zakaei A, Motamedi M, Rahimi Ghiasi M, Karamitalab M. Molecular identification and in-vitro antifungal susceptibility testing of Candida species isolated from patients with onychomycosis. Curr Med Mycol. 2015;1(4):26–32. doi: 10.18869/acadpub.cmm.1.4.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badiee P, Badali H, Diba K, Ghadimi Moghadam A, Hosseininasab A, Jafarian H, et al. Susceptibility pattern of Candida albicans isolated from Iranian patients to antifungal agents. Curr Med Mycol. 2016;2(1):24–9. doi: 10.18869/acadpub.cmm.2.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedayati M, Hajheydari Z, Hajjar F, Ehsani A, Shokohi T, Mohammadpour R. Identification of Malassezia species isolated from Iranian seborrhoeic dermatitis patients. Eur Rev Med Pharmacol Sci. 2010;14(1):63–8. [PubMed] [Google Scholar]
- 27.Saghazadeh M, Farshi S, Hashemi J, Mansouri P, Khosravi AR. Identification of Malassezia species isolated from patients with seborrheic dermatitis, atopic dermatitis, and normal subjects. J Med Mycol. 2010;20(4):279–82. [Google Scholar]
- 28.Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38(5):337–41. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 29.Lee YW, Byun HJ, Kim BJ, Kim DH, Lim YY, Lee JW, et al. Distribution of Malassezia species on the scalp in Korean seborrheic dermatitis patients. Ann Dermatol. 2011;23(2):156–61. doi: 10.5021/ad.2011.23.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lian CH, Shen LL, Gao QY, Jiang M, Zhao ZJ, Zhao JJ. Identification of Malassezia species in the facial lesions of Chinese seborrhoeic dermatitis patients based on DNA sequencing. Mycoses. 2014;57(12):759–64. doi: 10.1111/myc.12229. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Kohli Y, Summerbell RC, Faergemann J. Quantitative culture of Malassezia species from different body sites of individuals with or without dermatoses. Med Mycol. 2001;39(3):243–51. doi: 10.1080/mmy.39.3.243.251. [DOI] [PubMed] [Google Scholar]
- 32.Sandstrom Falk MH, Tengvall Linder M, Johansson C, Bartosik J, Back O, Sarnhult T, et al. The prevalence of Malassezia yeasts in patients with atopic dermatitis, seborrhoeic dermatitis and healthy controls. Acta Derm Venereol. 2005;85(1):17–23. doi: 10.1080/00015550410022276. [DOI] [PubMed] [Google Scholar]
- 33.Makimura K, Tamura Y, Kudo M, Uchida K, Saito H, Yamaguchi H. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Med Microbiol. 2000;49(1):29–35. doi: 10.1099/0022-1317-49-1-29. [DOI] [PubMed] [Google Scholar]
- 34.James WD, Berger T, Elston D. Andrews' diseases of the skin E-Book: clinical dermatology. New York: Elsevier Health Sciences; 2015. [Google Scholar]


