Abstract
Background and Purpose:
Formation of pseudohyphae is considered a virulence factor in Candida species. Generally, Candida glabrata grows as budding yeast cells; however, reports illustrated that C. glabrata could form pseudohyphal cells in response to some stimuli. In this study, we provided insight into the ability of C. glabrata in forming pseudohyphal cells under different levels of carbon dioxide (CO2).
Materials and Methods:
Candida glabrata reference strain (ATCC 90030) was used in this study. Yeast samples were cultured on Sabouraud dextrose broth (SDB) medium and incubated under 3%, 5%, and 10% CO2 levels for 24, 48 and 72 h. Control cultures were prepared without CO2 pressure for three days. The possibility of pseudohyphae and mycelium formation in C. glabrata was investigated.
Results:
The results of this study revealed that the most branching filament-like cells were obtained at high CO2 pressure (10%) after 72 h. After three days of low CO2 pressure (3%), only yeast and budding cells were observed without any pseudohyphae formation.
Conclusion:
CO2 could act as a stimulus and induced formation of pseudohyphae in Candida glabrata yeast cells.
Key Words: Candida glabrata, CO2 pressure, Pseudohyphae
Introduction
The members of the genus Candida were reported as the most common fungal agents causing nosocomial infections [1, 2]. Over the past decade, the incidence rate of infections caused by non-albicans Candida species has been globally on the rise [2]. Among Candida species, Candida glabrata (C. glabrata) is known as the second most common yeast isolated from blood stream infections, particularly in immunocompromised patients [3].C. glabrata is also among the most frequent causes of candidemia with high mortality rate, especially due to natural resistance of this species to antifungal agents [4, 5].
Filamentous growth is deemed as a virulence factor in Candida species; filamentation is indicated to play an important role in pathogenesis through evading host defenses [6, 7]. In addition, formation of pseudohyphae could be involved in biofilm formation resulting in drug resistance [7].
Switching to pseudohyphal growth usually occurs in response to some stimuli such as nitrogen starvation [8-10]. Carbon dioxide (CO2) is a gaseous molecule with important signaling and stimulating roles in some fungal pathogens [11-13]. Besides, CO2 can act as a stimulus inducing filamentation in C. albicans [14].
Filamentous growth of C. glabrata in response to diverse physiological conditions and the molecular mechanisms involved in switching still remain inconspicuous. In this study, we sought to evaluate the ability of C. glabrata in forming pseudohyphae under various CO2 pressures.
Materials and Methods
Culture condition
C. glabrata reference strain (ATCC 90030) was employed in this study. Yeast cells were cultured on Sabouraud dextrose agar (SDA; Merck, Germany) and incubated at 37°C for 48 h. The suspension of yeast cells was then prepared using distilled water at final concentration of 1×105 CFU/ml [15].C. albicans reference strain was also utilized as positive control for pseudohyphae formation.
Inducing filamentous growth
For examination of filamentous growth, 200 µl of the final suspension was added to three plates containing SDB (Merck, Germany).The plates were then incubated at 37°C in the presence of 3%, 5%, and 10% CO2 separately. All the samples under each CO2 level were then incubated for 24, 48, and 72 h. Control cultures were prepared under the same conditions without any CO2.
Microscopic examination
Microscopic characteristics of all the samples were investigated to assess pseudohyphae formation, and then they were compared to yeast cells as negative control. In addition, scanning electron microscopy (SEM) examination was conducted based on the standard protocol [16, 17]. Briefly, the C. glabrata cells exposed to 3%, 5% and 10% CO2 were collected separately and mounted on 12 mm slides. The samples were then pre-fixed with 2.5% glutaraldehyde (GA) in 0.1 M cacodylate buffer for 50 min. After washing three times in 0.1 M sodium cacodylate (CAC) buffer, the samples were fixed with 10% osmium tetroxide (OsO4) in 0.1 M cacodylate buffer followed by washing in cacodylate buffer. Post-fixing of C. glabrata cells was carried out with 1% OsO4. The cells were then washed twice in dH2O and dehydrated and dried with ethanol [17, 18]. Thereafter, the obtained samples were mounted, coated with gold/palladium, and viewed by DSM-960A scanning electron microscope.
In order to investigate the reversibility of the formed pseudohyphae, C. glabrata cells exposed to 10% CO2 pressure were inoculated in tryptic soy broth (TSB) medium (Merck, Germany) and stored at -20°C for two weeks. The sample was sub-cultured without CO2 pressure in SDB medium plate and incubated at 37°C. Further formation of pseudohyphae was then evaluated.
Results and Discussion
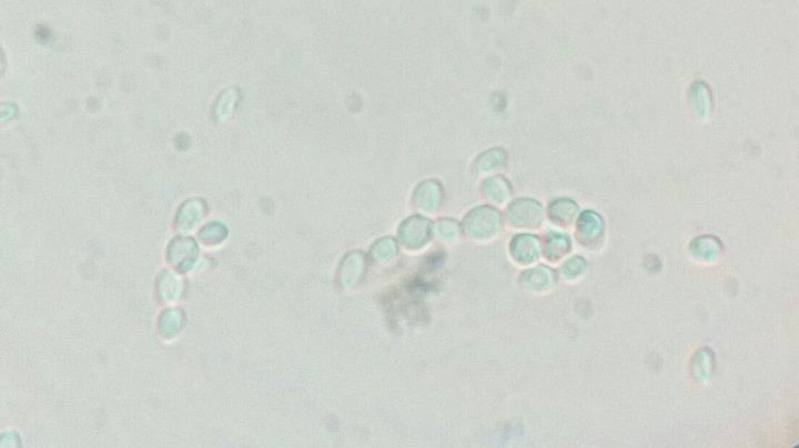
Observing cell aggregation and chains of more than three branching budding cells of C. glabrata were considered as positive result for formation pseudohyphae. Our outcomes indicated no pseudo-hyphal body in the presence of low CO2 pressure (3%) during 24, 48, and 72 h of incubation. However, few filament-like cells were noted at 5% and 10% CO2 pressure after 24 h. In addition, the most branching filament-like cells with larger sizes were detected in cultures exposed to 10% CO2 pressure after 72 h. Generally, high CO2 pressure diminished bud cells, while it propagated filament-like cells (Figure 1).
Figure 1.
Microscopic examination of filamentous growth in C. glabrata (ATCC 90030); the most cell aggregation and branching pseudohyphal cells at 10% CO2 pressure after 72 h
According to these results, C. glabrata dem-onstrated the ability to undergo morphological changes in response to CO2 pressure as a stimulus. Figure 2 illustrates SEM results of pseudohyphal growth in C. glabrata.
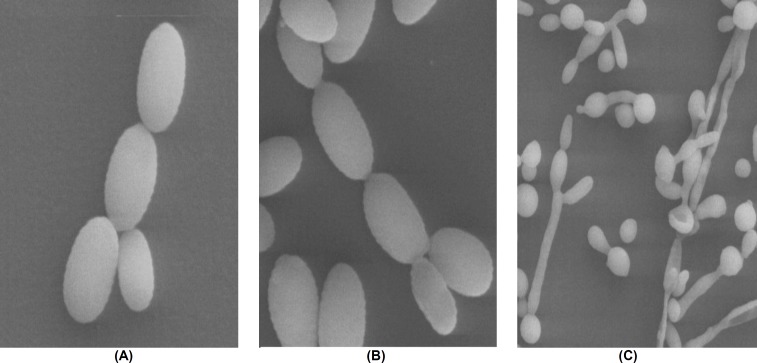
Figure 2.
Scanning electron microscopy examination of C. glabrata ATCC 90030 filamentous growth; (A) few branching budding cells were observed at 5% CO2 pressure after 72 h; (B) the most branching pseudohyphal cells were observed in cultures exposed to 10% CO2 pressure after 72 h; (C) pseudohyphae formation in Candida albicans standard strain
In addition, we investigated reversibility of filamentous growth through sub-culture of the samples after a week in the absence of CO2 pressure. The obtained results suggested that C. glabrata could form pseudohyphal bodies in a similar manner to the prior filamentation.
Candida species are considered as important fungal pathogens causing a wide spectrum of diseases ranging from superficial to life-threatening infections [19]. Infections due to non-C. albicans species such as C. glabrata increased over the past decade. Generally, various virulence factors are postulated for pathogenicity of Candida species including morphological switching, biofilm formation, and production of hydrolytic enzymes [6, 7].
Although C. glabrata usually grows as budding yeast cells, reports indicated that this species could form pseudohyphal cells during nitrogen starvation [10]. In addition, changes in oxygenand carbon dioxide levels influence cell activity of Saccharomyces cerevisiae, and high-pressure oxygen, as well as CO2 lead to cell inactivation [20].
In the present study, we evaluated the effect of CO2 on formation of pseudohyphae in C. glabrata. The obtained results indicated that high-pressure CO2 could induce pseudohyphal switching of theC. glabrata cells. As went before, a great number of filament-like cells were observed under 10% CO2 pressure after 72 h. Interestingly, the morphology of the yeast cells seems to be linked with physiological conditions since the most branching filament-like cells were obtained at higher CO2 level.
CO2 is applied for antimicrobial activity, especially for the preservation of foodstuffs, as it can affect microorganisms by inactivation of the cells [21]. Shimoda et al. reported that temperature and CO2 pressure independently contributed to inactivation of Saccharomyces cerevisiae cells [22].
Assessment of C. glabrata morphological alterations under different CO2 pressures described in this study revealed that the highest rate of pseudohyphae formation was attained upon prolonging the incubation time and enhancing CO2 pressure. High-pressure CO2 was associated with further switching of yeast cells to branched filamentous cells. Moreover, cultures exposed to low-pressure CO2 (3%) exhibited more yeast and bud cells without any pseudohyphae formation. Different signaling pathways and transcription factor genes are essential for morphological changes in yeast cells. The STE11, STE12, and STE20 proteins are indicated to be at play in morphological changes of S. cerevisiae and adaptation of C. glabrata to hypertonic stress [23-26]. To determine whether C. glabrata STE genes are responsible for these morphological changes, future studies are required.
Acknowledgments
We wish to thank the Institute of Biophysics and Biochemistry, Tehran University of Medical Sciences, Tehran, Iran for providing laboratory facilities for performing SEM. This study was financially supported by the School of Public Health, Tehran University of Medical Sciences (TUMS; grant No: 9411352004).
Author’s contribution
S. R. and S. K. designed and managed the project. E. S. performed the tests. S. AKA. was project partner and wrote the first draft of the manuscript. S. R. edited the final manuscript. S. D. was project partner.
Conflict of interest
None declared.
Financial disclosure
No financial interests related to the material of this manuscript has been declared.
References
- 1.Paluchowska P, Tokarczyk M, Bogusz B, Skiba I, Budak A. Molecular epidemiology of Candida albicans and Candida glabrata strains isolated from intensive care unit patients in Poland. Mem Inst Oswaldo Cruz. 2014;109(4):436–41. doi: 10.1590/0074-0276140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36(2):288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger M, Leibovici L, Perez S, Samra Z, Ostfeld I, Levi I, et al. Characteristics of candidaemia with Candida-albicans compared with non-albicans Candida species and predictors of mortality. J Hosp Infect. 2005;61(2):146–54. doi: 10.1016/j.jhin.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Bialkova A, Šubík J. Biology of the pathogenic yeast Candida glabrata. Folia Microbiol. 2006;51(1):3–20. doi: 10.1007/BF02931443. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33(5):673–88. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 6.Jayatilake JA, Samaranayake YH, Cheung LK, Samaranayake LP. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non‐albicans Candida species in reconstituted human oral epithelium. J Oral Pathol Med. 2006;35(8):484–91. doi: 10.1111/j.1600-0714.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 7.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5(4):366–71. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 8.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68(6):1077–90. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MC, Cutler NS, Heitman J. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11(1):183–99. doi: 10.1091/mbc.11.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csank C, Haynes K. Candida glabrata displays pseudohyphal growth. Fems Microbiol Lett. 2000;189(1):115–20. doi: 10.1111/j.1574-6968.2000.tb09216.x. [DOI] [PubMed] [Google Scholar]
- 11.Bahn YS, Mühlschlegel FA. CO2 sensing in fungi and beyond. Curr Opin Microbiol. 2006;9(6):572–8. doi: 10.1016/j.mib.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Ko YJ, Maeng S, Floyd A, Heitman J, Bahn YS. Comparative transcriptome analysis of the CO2 sensing pathway via differential expression of carbonic anhydrase in Cryptococcus neoformans. Genetics. 2010;185(4):1207–19. doi: 10.1534/genetics.110.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahn YS, Molenda M, Staab JF, Lyman CA, Gordon LJ, Sundstrom P. Genome-wide transcriptional profiling of the cyclic AMP-dependent signaling pathway during morphogenic transitions of Candida albicans. Eukaryot Cell. 2007;6(12):2376–90. doi: 10.1128/EC.00318-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth CC, Johnson E, Baker ME, Haynes K, Mühlschlegel FA. Phenotypic identification of Candida albicans by growth on chocolate agar. Med Mycol. 2005;43(8):735–8. doi: 10.1080/13693780500265998. [DOI] [PubMed] [Google Scholar]
- 15.Wayne P. Clinical and laboratory standards institute: reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-; CLSI document M27-A3. CLSI. 2008;28:6–12. [Google Scholar]
- 16.Flegler SL, Heckman Jr JW, Klomparens KL. Scanning and transmission electron microscopy: an introduction. London, UK: Oxford University Press; 1993. [Google Scholar]
- 17.Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. Scanning electron microscopy. Curr Protoc Microbiol. 2012;2:2B. doi: 10.1002/9780471729259.mc02b02s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadtländer CT. Scanning electron microscopy and transmission electron microscopy of mollicutes: challenges and opportunities. Modern Res Educ Topics Microsc. 2007;1:122–31. [Google Scholar]
- 19.Rüping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections. Drugs. 2008;68(14):1941–62. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- 20.Coelho MA, Belo I, Pinheiro R, Amaral AL, Mota M, Coutinho JA, et al. Effect of hyperbaric stress on yeast morphology: study by automated image analysis. Appl Microbiol Biotechnol. 2004;66(3):318–24. doi: 10.1007/s00253-004-1648-9. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda M, Yamamoto Y, Cocunubo-Castellanos J, Tonoike H, Kawano T, Ishikawa H, et al. Antimicrobial effects of pressured carbon dioxide in a continuous flow system. J Food Sci. 1998;63(4):709–12. [Google Scholar]
- 22.Shimoda M, Cocunubo‐Castellanos J, Kago H, Miyake M, Osajima Y, Hayakawa I. The influence of dissolved CO2 concentration on the death kinetics of Saccharomyces cerevisiae. J Appl Microbiol. 2001;91(2):306–11. doi: 10.1046/j.1365-2672.2001.01386.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8(24):2974–85. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 24.Calcagno AM, Bignell E, Rogers TR, Jones MD, Mühlschlegel FA, Haynes K. Candida glabrata Ste11 is involved in adaptation to hypertonic stress, maintenance of wild-type levels of filamentation and plays a role in virulence. Med Mycol. 2005;43(4):355–64. doi: 10.1080/13693780400006088. [DOI] [PubMed] [Google Scholar]
- 25.Calcagno AM, Bignell E, Warn P, Jones MD, Denning DW, Mühlschlegel FA, et al. Candida glabrata STE12 is required for wild‐type levels of virulence and nitrogen starvation induced filamentation. Mol Microbiol. 2003;50(4):1309–18. doi: 10.1046/j.1365-2958.2003.03755.x. [DOI] [PubMed] [Google Scholar]
- 26.Calcagno AM, Bignell E, Rogers TR, Canedo M, Mühlschlegel FA, Haynes K. Candida glabrata Ste20 is involved in maintaining cell wall integrity and adaptation to hypertonic stress, and is required for wild‐type levels of virulence. Yeast. 2004;21(7):557–68. doi: 10.1002/yea.1125. [DOI] [PubMed] [Google Scholar]