Abstract
Clinical evidence suggests that symptoms in premenstrual dysphoric disorder (PMDD) reflect abnormal responsivity to ovarian steroids. This differential steroid sensitivity could be underpinned by abnormal processing of the steroid signal. We used a pharmacometabolomics approach in women with prospectively confirmed PMDD (n=15) and controls without menstrual cycle-related affective symptoms (n=15). All were medication-free with normal menstrual cycle lengths. Notably, women with PMDD were required to show hormone sensitivity in an ovarian suppression protocol. Ovarian suppression was induced for 6 months with gonadotropin-releasing hormone (GnRH)-agonist (Lupron); after 3 months all were randomized to 4 weeks of estradiol (E2) or progesterone (P4). After a 2-week washout, a crossover was performed. Liquid chromatography/tandem mass spectrometry measured 49 steroid metabolites in serum. Values were excluded if >40% were below the limit of detectability (n=21). Analyses were performed with Wilcoxon rank-sum tests using false-discovery rate (q<0.2) for multiple comparisons. PMDD and controls had similar basal levels of metabolites during Lupron and P4-derived neurosteroids during Lupron or E2/P4 conditions. Both groups had significant increases in several steroid metabolites compared with the Lupron alone condition after treatment with E2 (that is, estrone-SO4 (q=0.039 and q=0.002, respectively) and estradiol-3-SO4 (q=0.166 and q=0.001, respectively)) and after treatment with P4 (that is, allopregnanolone (q=0.001 for both PMDD and controls), pregnanediol (q=0.077 and q=0.030, respectively) and cortexone (q=0.118 and q=0.157, respectively). Only sulfated steroid metabolites showed significant diagnosis-related differences. During Lupron plus E2 treatment, women with PMDD had a significantly attenuated increase in E2-3-sulfate (q=0.035) compared with control women, and during Lupron plus P4 treatment a decrease in DHEA-sulfate (q=0.07) compared with an increase in controls. Significant effects of E2 addback compared with Lupron were observed in women with PMDD who had significant decreases in DHEA-sulfate (q=0.065) and pregnenolone sulfate (q=0.076), whereas controls had nonsignificant increases (however, these differences did not meet statistical significance for a between diagnosis effect). Alterations of sulfotransferase activity could contribute to the differential steroid sensitivity in PMDD. Importantly, no differences in the formation of P4-derived neurosteroids were observed in this otherwise highly selected sample of women studied under controlled hormone exposures.
Introduction
Premenstrual dysphoric disorder (PMDD) is a clinically distinct affective disorder characterized by recurrent and distressing mood and behavioral symptoms during the luteal phase of the menstrual cycle that remit within a few days from the onset of menses.1, 2, 3 Levels of circulating ovarian steroids, estradiol (E2) and progesterone (P4) and hypothalamic–pituitary–ovarian axis function are normal.4 However, when ovarian steroid secretion is suppressed by gonadotropin-releasing hormone (GnRH) receptor agonists, women with PMDD experience remission of symptoms, which recur when physiologic doses of E2/P4 are added back. In contrast, asymptomatic controls undergoing identical hormone manipulations experience no changes in mood.5 Thus, in women with PMDD, clinical evidence suggests that symptoms are triggered by a differential central nervous system (CNS) response to physiologically normal changes in E2/P4. Furthermore, imaging studies identified brain regions modulated by E2/P4 (or their neuroactive metabolites), some of which respond differently in women with PMDD despite similar exposures to E2/P4, including the amygdala, striatum, medial orbitofrontal cortex, anterior cingulate cortex and prefrontal cortex.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 One candidate hormone that could mediate the differential regulation of brain circuitry in PMDD, and the triggering of symptoms by at least progesterone, is the ring A-reduced neurosteroid allopregnanolone. Despite the absence of consistent demonstrations of abnormal peripheral levels of allopregnanolone in PMDD compared with control women,16, 17, 18, 19, 20, 21, 22 there is preliminary evidence to support the hypothesis that changes in the production of or alterations in the metabolism of allopregnanolone from progesterone could serve as an 'affective switch' in susceptible women (reviewed in Schiller.16, 23 These observations not only suggest that the symptoms of PMDD are accompanied by ovarian steroid-related alterations in the activity of neuronal circuits underlying reward, social cognition and affective states,4 but also that ovarian steroids could be processed differently in women with PMDD in peripheral tissues and potentially in the CNS (or alternatively reflect differential signal transduction within the CNS).
Abnormalities in steroid metabolism are present in several conditions, including prostate cancer, breast cancer and polycystic ovary syndrome,24, 25, 26, 27 and metabolomics platforms have been employed to investigate biochemical systems (for example, lipids and neurotransmitter metabolites) in metabolic and psychiatric conditions.28, 29, 30
We hypothesized that women with PMDD would display altered steroid metabolic profiles/signatures compared with asymptomatic control women during controlled and standardized exposures to ovarian steroids. To test this, we used a steroid-based metabolomics platform to compare asymptomatic controls and women with PMDD in whom symptom remission and recurrence (that is, E2/P4 sensitivity) were confirmed by ovarian steroid manipulation.5 The main goal of this study was to examine the potential differences in the metabolic processing of standardized doses of ovarian steroids in women with PMDD and controls. Metabolomics tools enable identification and quantification of tens to thousands of compounds that represent changes in biochemical pathways31, 32 in response to treatment. Metabolomics strategies have also mapped global biochemical changes in depression, characterized the effects of selective serotonergic re-uptake inhibitors on metabolic pathways and defined several pathways implicated in individual variation in response to these medications.33, 34, 35
Materials and methods
Participant selection
We studied 15 women with PMDD aged 23–48 years. All were medication-free, with regular menstrual cycles (range, 21–35 days), not medically ill and not pregnant. Women with PMDD were self-referred in response to newspaper advertisements or were referred by their physician. Before study entry PMDD was confirmed in these women based on criteria outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).36 Women completed 3 months of prospective daily ratings using a four-item 100-mm visual-analog scale that confirmed the timing and severity of their self-reported menstrually related mood symptoms (irritability, sadness, anxiety and mood swings) as described previously.5, 37 The mean score of at least one negative mood symptoms was at least 30% higher (relative to the range of the scale used by each woman) in the week before menstruation compared with the week after the cessation of menstruation in at least two of the three cycles. Functional impairment was characterized as a daily rating form (DRF)38 score of 2 (minimal) or higher on one of 4 questions related to functional impairment (that is, stayed at home or avoided social activities, had conflicts or problems with people, symptoms interfered with relationships at work or home or symptoms interfered with work productivity) in at least 3 days out of 7 days pre-menses. Finally, DRF ratings and the results of both a semi-structured interview and a self-report questionnaire (that is, the Menstrual Screening Questionnaire and the Menstrual Assessment Form, respectively) confirmed that all women met the required number of symptoms specified in the DSM. Women with significant negative mood symptoms (on the DRF) during the follicular phase of the menstrual cycle were excluded. Thus, the diagnostic criteria for PMDD were augmented by the severity criterion of a 30% or greater increase in the mean negative mood during the week before menses compared with the week after menses—a more stringent criterion than that of DSM-IV or V.1, 36
We also recruited a group of 15 control women, none of whom had premenstrual mood symptoms using the same daily rating scales during a 2-month baseline period.
The women with PMDD had no current Axis I psychiatric diagnosis within the past 2 years per Structured Clinical Interview for DSM-IV (SCID),39 while controls had neither current nor past Axis I diagnoses (also confirmed by SCID).
This study was approved by the Central Neuroscience Institutional Review Board within the NIMH IRP. All women provided written informed consent, and all received payment for participation according to the NIH intramural guidelines.
Hormone manipulation protocol
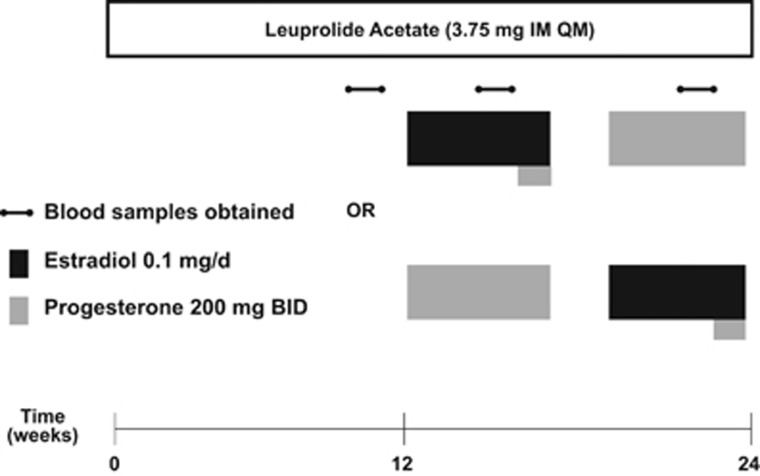
Participants received six monthly injections of the GnRH agonist leuprolide acetate, Lupron (3.75 mg intramuscularly). Plasma follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol and progesterone levels were measured at study visits every 2 weeks to confirm adequate gonadal suppression. Following 3 months of Lupron alone, participants were randomly assigned in a double-blind, crossover manner to receive either E2 (100 mcg daily by skin patch, Noven Pharmaceuticals Jersey City, NJ, USA, Watson Pharmaceuticals, Parsippany, NJ, USA) or P4 (200 mg vaginal suppository twice daily, Upsher-Smith Laboratories; NIH Pharmacy, Bethesda, MD, USA) replacement lasting for 5 weeks (with a 2-week washout between hormone administration periods) while continuing to receive Lupron injections (Figure 1). All women used a patch and a suppository each day during the hormonal addback to maintain the patency of the double-blind. This standardized the exposures to physiologic levels of ovarian steroids to avoid the confound of varying levels of estradiol and progesterone across the normal menstrual cycle. All women used the appropriate placebo patch or suppository daily during the 12-week addback.
Figure 1.
Protocol schematic for clinical hormone suppression/addback protocol. Between 2 and 5 days after onset of menses, women with PMDD and control women received six monthly intramuscular injections of 3.75-mg leuprolide (Lupron), which after an initial stimulation suppresses ovarian function. Clinic visits occurred every 2 weeks. Plasma FSH, LH, estradiol and progesterone levels were measured at each visit to confirm ovarian suppression. Following 3 months of leuprolide alone, all participants (while continuing to receive monthly leuprolide injections for another 3 months) were randomly assigned in a double-blind, crossover manner to receive either E2 (100 mg daily by skin patch) or P4 (200 mg vaginal suppository twice daily) replacement lasting for 5 weeks each (with a 2-week washout between hormone administration periods). All women used a patch and a suppository each day during the hormonal addback to maintain the patency of the double-blind. After 4 weeks of E2 all women received 1 week of P4 to induce menses (at the end of a 4-week E2 exposure). Significant recurrence of PMDD symptoms was defined by a weekly average DRF score of greater than three (moderate severity) in irritability, anxiety or sadness.40, 41 Controls were defined by an absence of affective symptoms throughout the 6-month hormone-manipulation protocol (that is, no weekly average score >2). DRF, daily rating form; E2, estradiol; P4, progesterone; PMDD, premenstrual dysphoric disorder.
Symptom ratings and criteria for response
Women completed a modified DRF,38 daily to measure symptom severity during the 6-month hormone-manipulation study. A significant recurrence of PMDD symptoms was defined by a weekly average DRF score of greater than three (moderate severity) in irritability, anxiety or sadness.40, 41 Asymptomatic controls had no affective symptoms during the 6 months (that is, no weekly average score ⩾2). At each clinic visit, all women completed the Rating for Premenstrual Tension self-ratings,42 a self-report rating that measures mood, behavior and physical symptoms on a 36-point scale, with scores ⩾ 10 consistent with PMDD symptoms.
Blood samples
Serum was obtained and stored at −80 °C for metabolomics analysis after at least 8 weeks of GnRH agonist-induced ovarian suppression (hypogonadal state and PMDD symptoms in remission), after 2–3 weeks of estradiol and after 2–3 weeks of progesterone addback (when symptoms recur in PMDD but not in control women).
Selection of steroid metabolites
We tested 49 metabolites with a focus on estradiol, progesterone and pregnenolone biosynthesis (see Table 1 and Supplementary Table S1, and the Kyoto Encyclopedia of Genes and Genomes: http://www.genome.jp/kegg/pathway.html). We selected direct precursors or metabolites of estradiol (estrone, estriol and so on), progesterone (pregnenolone, pregnenolone sulfate (3b-hydroxy-5-pregnen-20-one-3-SO4), allopregnanolone (3a-hydroxy-5a-pregnan-20-one)) and testosterone (androstenedione, dehydroepiandrosterone (DHEA)) and metabolites with neuroactive potential.43, 44 Of particular interest were steroids with direct actions at the GABAA receptor, including pregnenolone and DHEA (direct or indirect positive modulators) or their sulfated metabolites, pregnenolone sulfate and dehydroepiandrosterone-SO4 (DHEAS; direct or indirect negative modulators).43, 44, 45 The sulfation of several neuroactive steroids converts agonist actions at GABAA receptors to antagonist effects, whereas removal of the sulfate group has the opposite effect.43, 44, 45
Table 1. Restricted neurosteroid panel.
| Androgens | Estrogens | Progestogens | Corticosteroids |
|---|---|---|---|
| Dehydroepiandrosterone | Estrone | 9-Dehydroprogesterone | Cortexolone |
| DHEAS | Estradiol | Progesterone | Cortisol |
| Dehydroepiandrosterone glucuronide | Estradiol-3-SO4 | 3a-Hydroxy-5a-pregnan-20-one (allopregnanolone) | Cortexone |
| Androstenedione | Estrone-3-SO4 | 17a,20a-Dihydroxyprogesterone | Corticosterone |
| Testosterone | Estrone-3-glucuronide | 3b-Hydroxy-5-pregnen-20-one-3-SO4 (pregnenolone sulfate) | |
| 2-hydroxyestrone | Pregnanediol |
Abbreviations: DHEAS, dehydroepiandrosterone-SO4; LOD, limit of detectability; SO4, sulfate group.
This table shows the restricted neurosteroid panel, which outlines the steroid metabolites meeting the threshold for inclusion (<40% of values below LOD) in all of the three hormone conditions (total 21 metabolites, out of 49 metabolites tested).
The complete neurosteroid panel (see Supplementary Table S1) included 49 selected steroid hormones that are direct precursors or metabolites of testosterone, estradiol and progesterone, as well as their sulfated derivatives. We also measured corticosteroid levels, in order to rule out decreased activity in the synthetic enzymes for sex steroids leading to a diversion of cholesterol precursors toward corticosteroid synthesis.
Finally, we measured levels of cortexolone, cortisol, corticosterone and cortexone to evaluate possible alternate pathways of steroid metabolism (analogous to the diversion of cholesterol precursors from corticosteroids toward sex steroid synthesis in congenital adrenal hyperplasia46, 47).
Sample preparation and analysis
Serum samples were extracted and subjected to ultraperformance liquid chromatography tandem mass spectrometry analysis for measurement of neurosteroids in serum samples as described previously27 (see Supplementary Methods for detail).
Statistical analyses
Demographic and clinical differences across diagnostic groups
To compare demographic and clinical characteristics of women with PMDD and control women (that is, age, body mass index (BMI), days of exposure to Lupron, E2 and P4, storage times,48, 49 parity and race), we used Student’s t-tests and χ2-tests (or Fisher’s exact test when appropriate), with a significance threshold of P<0.05, uncorrected for multiple comparisons. We employed analysis of variance with repeated measures (ANOVA-R) to examine potential differences in serum levels of estradiol and progesterone with diagnosis (that is, PMDD and control women) as a between-group factor and hormone condition (that is, Lupron alone, estradiol addback and progesterone addback) as the within-subject factor. Finally, we also used ANOVA-Rs to examine differences in symptom severity across hormone conditions or between diagnoses.
Metabolomic differences across diagnostic groups
We did not use metabolite data with greater than 40% of values below the individual assay’s limits of detectability (LOD) in any of the two diagnostic groups or in any of the three hormone conditions. These non-detectable metabolites were distributed across both diagnostic groups and all hormone conditions. Thus, metabolites meeting this criterion in any hormone condition were not included in analyses examining the change in the metabolite level from Lupron to E2 or Lupron to P4, or the differences across diagnoses in basal metabolite levels within each hormone condition.
To analyze differences in metabolite concentrations between the women with PMDD and control women, we used the open-source statistical software, R (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0 http://www.R-project.org/).
Women were first stratified by diagnostic group (PMDD and controls), and within each diagnostic group we tested for significant metabolite differences between each of the three hormone conditions using a non-parametric, paired Wilcoxon rank-sum test. Subsequently, changes in metabolite levels between PMDD and control women (that is, diagnosis-related differences in the 'delta' metabolite level) from Lupron to E2 and from Lupron to P4 were tested using an unpaired Wilcoxon rank-sum test. All results were corrected for multiple comparisons using a false-discovery rate approach (q<0.2).50, 51
Results
Sample characteristics
Demographics and clinical characteristics of women with PMDD and control women are listed in Table 2. Women with PMDD were significantly older and had higher BMI compared with control women (both comparisons P<0.05). There were no between-group differences in racial distribution, parity, the durations of exposure to Lupron, E2 or P4 prior to obtaining the serum samples, storage times or race (P>0.05 for all comparisons). Only one woman with PMDD had a past major depression, the rest had no past Axis I psychiatric illness. Ten women with PMDD experienced symptom recurrence only after exposure to the P4, four women only after E2 exposure and one under both P4 and E2 exposures. There was a significant diagnosis-by-hormone interaction in severity scores on the Premenstrual Tension-rater (P=0.02) reflecting significantly greater symptom severity in PMDD during addback compared with Lupron alone and compared with control women during addback of E2 or P4 treatment.
Table 2. Demographics and clinical characteristics of women with PMDD and control women.
| Control | PMDD | |
|---|---|---|
| Age (years), mean (s.d.)* | 32.9 (8.1) | 39.1 (7.3) |
| BMI (kg/m2), mean (s.d.)* | 24.2 (3.5) | 27.9 (5.6) |
| Lupron exposure (days), mean (s.d.) | 68.9 (4.0) | 71.5 (4.6) |
| Estradiol exposure (days), mean (s.d.) | 20.5 (4.2) | 21.8 (6.7) |
| Progesterone exposure (days), mean (s.d.) | 21.9 (4.7) | 19.9 (4.1) |
| Storage times (years) | 5.7 (1.9) | 6.2 (2.4) |
| Race (numbers of women) | ||
| White | 7 | 5 |
| Black | 3 | 8 |
| Hispanic | 4 | 1 |
| Asian | 0 | 1 |
| Other | 1 | 0 |
| Parity | ||
| Parity=0 | 9 | 5 |
| Parity=1–5 | 6 | 10 |
| Past Axis I Psychiatric Illness# (numbers of women) | 0 | 1 |
| Symptom trigger (numbers of women)* | ||
| Progesterone | 0 | 10 |
| Estradiol | 0 | 4 |
| Both | 0 | 1 |
| PMDD symptom severity (Steiner–Carroll scale: self-report)* mean (s.d.) | ||
| Lupron | 0.6 (1.1) | 1.8 (1.6) |
| E2 | 0.1 (0.3) | 2.8 (3.1) |
| P4 | 0.3 (0.8) | 6.1 (7.1) |
Abbreviations: ANOVA-R, analysis of variance with repeated measures; BMI, body mass index; E2, estradiol; P4, progesterone; PMDD, premenstrual dysphoric disorder.
Demographics and clinical information of the sample are shown in this table. Women with PMDD were significantly older, had higher BMI and showed significant differences in PMDD symptom severity compared with controls under all hormone conditions, as well as across hormone conditions (significant comparisons indicated with an asterisk*, P<0.05). One woman with PMDD had a past major depression (indicated with a pound sign#). There were no between-group differences in the durations of exposure to Lupron, E2 or P4 prior to obtaining the serum samples, storage times, parity or race (P>0.05 for all comparisons). Ten women with PMDD experienced PMDD symptom recurrence only after exposure to the P4, 4 women only after E2 exposure only and 1 after both P4 and E2 exposures. There was a significant diagnosis-by-hormone interaction in severity scores on the Premenstrual Tension-rater (F2, 56=4.1, P=0.02) reflecting significantly greater symptom severity in PMDD during addback compared with Lupron alone and compared with control women during addback of E2 or P4 treatment. ANOVA-R showed no significant effects of diagnosis (PMDD versus control women) or a diagnosis-by-hormone condition interaction between diagnosis and hormone condition for serum levels of either E2 (diagnosis: F1,28=1.3, P=0.3; diagnosis-by-hormone interaction: F2,56=0.5, P=0.6) or P4 (diagnosis: F1,28=0.03, P=0.9; diagnosis-by-hormone interaction: F2,56=0.04, P=0.9).
As outlined in Tables 3, 4, 5, all women (PMDD and control) treated with E2, showed a significant increase in estradiol with E2 treatment (PMDD: median change (Median)=72 pg/ml; q=0.056; control: Median=61 pg/ml; q=0.014), and a significant increase in progesterone with P4 treatment (PMDD: Median=7850 pg/mL; q=0.001; control: Median=8530 pg/ml; q=0.001), relative to the Lupron condition.
Levels of serum estradiol and progesterone during Lupron alone and after each hormonal addback condition
There were no significant effects of diagnosis or a diagnosis-by-hormone condition interaction for levels of either estradiol or progesterone. All women (PMDD and control) treated with E2 showed significant increases in serum estradiol after E2 treatment compared with Lupron, and significant increases in serum progesterone levels after P4 treatment (Table 3).
Table 3. Serum levels of steroid metabolites (pg ml−1) in PMDD (n=15) and controls (n=15) during three hormone conditions—mean (s.d.).
| Treatment; group | Lupron alone | Estradiol | Progesterone | |||
|---|---|---|---|---|---|---|
| NC | PMD | NC | PMD | NC | PMD | |
| DHEA | 1597.5 (2197.5) | 1379.5 (1104.3) | 1389.1 (1144.9) | 815.8 (774.2) | 1947.9 (1766.4) | 1127.3 (666.0) |
| DHEAS | 977 766.1 (432 417.6) | 765 185.6 (485 557.4) | 1 101 474.9 (800 163.7) | 511 647.2 (321 591.3) | 1 231 969.7 (723 437.8) | 553 833.9 (339 282.8) |
| DHEA-Glu | 42 015.6 (16 337.3) | 34 679.2 (24 736.1) | 47 252.7 (25 905.6) | 24 304.7 (22 416.4) | 47 073.3 (24 028.9) | 28 650.9 (21 361.5) |
| 9-Dehydroprogesterone | 2535.9 (2141.0) | 2732.3 (2133.7) | 2052.9 (1507.0) | 1570.8 (1588.6) | 2288.0 (1273.9) | 1748.0 (1297.8) |
| 7a-Hydroxyandrostenediol | 1912.3 (1878.5) | 2858.8 (6265.4) | NA | NA | 1163.7 (1025.7) | 1730.6 (2602.7) |
| Progesterone | 67.9 (112.3) | 56.7 (100.0) | 38.6 (32.0) | 64.0 (54.1) | 8409.2 (3052.7) | 8202.8 (3035.3) |
| Cortexolone | 79.8 (123.9) | 105.4 (93.9) | 65.6 (82.7) | 163.9 (205.7) | 86.8 (117.7) | 171.7 (142.5) |
| Cortisol | 12 000.2 (10 545.0) | 17 695.5 (11 174.2) | 11 048.2 (10 547.3) | 14 754.7 (12 930.9) | 18 922.8 (13 818.5) | 21 248.8 (16 876.5) |
| Cortexone | 18.3 (25.9) | 31.6 (19.1) | 19.9 (22.5) | 28.8 (31.0) | 49.9 (45.1) | 68.3 (51.1) |
| Corticosterone | 614.9 (766.8) | 615.5 (668.8) | 423.3 (634.5) | 657.6 (1170.6) | 504.7 (376.2) | 911.9 (1390.9) |
| Androstenedione | 383.0 (205.1) | 406.1 (264.2) | 431.0 (249.8) | 343.5 (345.7) | 518.3 (253.0) | 314.0 (265.5) |
| Testosterone | 110.2 (90.8) | 94.8 (85.9) | 117.6 (145.7) | 67.1 (63.7) | 88.6 (69.7) | 69.8 (82.2) |
| Estrone | 333.4 (301.8) | 405.0 (505.6) | 438.6 (678.4) | 219.0 (118.1) | 264.3 (253.9) | 305.4 (180.8) |
| Estradiol | 67.4 (82.3) | 65.4 (65.6) | 156.1 (90.3) | 282.2 (567.5) | 35.8 (44.8) | 86.5 (134.6) |
| Estradiol-3-SO4 | 39.2 (23.9) | 70.2 (52.1) | 179.1 (82.0) | 118.7 (94.7) | 37.1 (20.5) | 46.4 (29.2) |
| Estrone-SO4 | 333.8 (118.2) | 502.4 (295.6) | 2116.6 (1378.0) | 1542.1 (1347.9) | 349.5 (155.6) | 496.9 (375.4) |
| Estradiol-3-Glu | 54.6 (126.9) | 31.4 (35.0) | 40.3 (44.9) | 23.0 (33.1) | NA | NA |
| Estrone-3-Glu | 835.9 (368.6) | 1361.7 (780.6) | 1103.4 (551.6) | 1201.6 (885.4) | 829.4 (506.9) | 1050.2 (469.2) |
| 2-Hydroxyestrone | 96.0 (150.6) | 72.4 (90.8) | 233.8 (646.8) | 49.6 (49.1) | 68.8 (90.3) | 46.5 (42.2) |
| 2-Hydroxyestradiol | NA | NA | NA | NA | NA | NA |
| 2-Methoxy-3 OH-estrone | NA | NA | NA | NA | NA | NA |
| 6b-Hyroxyestradiol | NA | NA | NA | NA | NA | NA |
| 16a-Hydroxyestrone | NA | NA | NA | NA | NA | NA |
| Estriol | NA | NA | NA | NA | NA | NA |
| 3a-Hydroxy-5a-pregnan-20-one | 425.8 (163.6) | 367.9 (224.2) | 370.4 (134.5) | 366.3 (138.3) | 744.2 (187.7) | 686.1 (268.8) |
| 5a-Dihydroprogesterone | NA | NA | NA | NA | NA | NA |
| Allopregnanediol | NA | NA | NA | NA | NA | NA |
| 11a-Hydroxy-4-pregnene-3,20-dione | NA | NA | NA | NA | NA | NA |
| 11b-Hydroxy-4-pregnene-3,20-dione | NA | NA | NA | NA | NA | NA |
| 17-Hydroxypregnenolone | NA | NA | NA | NA | NA | NA |
| 17-Hydroxyprogesterone | NA | NA | NA | NA | NA | NA |
| 21-Hydroxypregnanolone | 712.2 (715.2) | 516.4 (510.6) | NA | NA | NA | NA |
| 21-Hydroxypregnenolone | NA | NA | NA | NA | NA | NA |
| 7a-Hydroxypregnenolone | NA | NA | NA | NA | NA | NA |
| 20a-Hydroxy-5a-pregnan-3-one | NA | NA | NA | NA | NA | NA |
| 20a-Dihydroprogesterone | NA | NA | NA | NA | NA | NA |
| 17a,20a-Dihydroxyprogesterone | 90.6 (58.7) | 93.8 (65.1) | 83.5 (71.5) | 73.1 (72.6) | 144.5 (67.6) | 103.1 (57.9) |
| 3b-Hydroxy-5-pregnen-20-one-3-SO4 | 17 038.1 (12 188.9) | 20 008.5 (12 422.2) | 17 864.9 (19 268.8) | 12 428.5 (11 518.0) | 24 646.4 (18 675.7) | 21 174.9 (12 905.6) |
| Eltanolone (pregnanolone) | NA | NA | NA | NA | NA | NA |
| Pregnanediol | 72.2 (129.4) | 111.4 (171.2) | NA | NA | 503.5 (400.3) | 418.7 (318.1) |
| 5b-Dihydroprogesterone | NA | NA | NA | NA | NA | NA |
| 5a-Dihydrotestosterone | NA | NA | NA | NA | NA | NA |
| 17b-Dihydroandrosterone | NA | NA | NA | NA | NA | NA |
| 17b-DihydroEPIandrosterone | NA | NA | NA | NA | NA | NA |
| 7a-Hydroxytestosterone | NA | NA | NA | NA | NA | NA |
| 7a-Hydroxyandrostenedione | 190.3 (194.1) | 284.1 (244.1) | NA | NA | 209.2 (322.9) | 363.5 (531.6) |
| 27-Hydroxycholesterol | NA | NA | NA | NA | NA | NA |
| 24-Hydroxycholesterol | NA | NA | NA | NA | NA | NA |
Abbreviations: DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone-SO4; NA, not applicable since values of steroid metabolite was below limits of detectability of the assay; NC, negative control; PMDD, premenstrual dysphoric disorder.
The unit of measurement for all steroid metabolites is pg ml−1.
Comparisons of basal steroid metabolite levels between women with PMDD and controls
There were no differences in absolute steroid metabolite levels between women with PMDD and controls in the Lupron, E2 or P4 addback conditions (Table 3).
Differences in steroid metabolite levels from Lupron to E2 or Lupron to P4 treatment
Changes in steroid metabolite levels observed in both PMDD and Controls
Compared with the Lupron condition, treatment with E2 (Table 4) resulted in significant increases, in both PMDD and control women, in levels of estrone-SO4 and estradiol-3-SO4 levels (Table 4).
Table 4. Changes in steroid metabolites during ovarian steroid replacement of E2.
| Metabolite |
Median Δ |
P-value |
Q-value |
|||
|---|---|---|---|---|---|---|
| PMDD | NC | PMDD | NC | PMDD | NC | |
| Estrone-SO4 | 888 | 1425 | 0.002 | 1.22E−04 | 0.042 | 0.001 |
| Estradiol | 72 | 61 | 0.005 | 0.002 | 0.056 | 0.014 |
| Estrone | −73 | 0 | 0.012 | 0.561 | 0.065# | 0.978 |
| DHEAS | −119 657 | 49 563 | 0.012 | 0.720 | 0.065# | 0.978 |
| 3b-Hydroxy-5-pregnen-20-one-3-SO4 | −6176 | 959 | 0.018 | 0.847 | 0.076# | 0.978 |
| DHEA | −324 | 311 | 0.028 | 0.890 | 0.098# | 0.978 |
| Estradiol-3-SO4* | 47 | 140 | 0.055 | 6.10E−05 | 0.166 | 0.001 |
Abbreviations: DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone-SO4; E2, estradiol; Median Δ, median change; NC, normal control; PMDD, premenstrual dysphoric disorder.
The unit of measurement for all steroid metabolites is pg ml−1.
This table shows the significant changes in steroid levels in women with PMDD and controls with E2 addback relative to Lupron. Significant differences indicated by Q<0.2.
Only estradiol-3-SO4 levels showed significant between-group differences following E2 treatment (*P=0.002; q=0.035), with a significantly more blunted response to E2 in PMDD compared with control women. Direct comparisons of other E2-related changes in steroid levels between PMDD and control women did not reach significance.
Within diagnostic groups, we observed significant effects of hormone condition in the magnitude of the change in steroid metabolite levels from Lupron to E2 in PMDD but not controls, as follows: women with PMDD showed significant decreases in serum levels of estrone (median Δ: PMDD=−73 pg ml−1 (decreased), #q=0.065; controls=0 pg ml−1, q=0.978), pregnenolone sulfate (3b-hydroxy-5-pregnen-20-one-3-SO4; median Δ: PMDD=−6176 pg ml−1(decreased), #q=0.076; controls=959 pg ml−1, q=0.978), DHEAS (median Δ: PMDD=−119 657 pg ml−1(decreased), #q=0.065; controls=49 563 pg ml−1, q=0.978) and DHEA (median Δ: PMDD=−324pg ml−1, #q=0.098 (decreased); controls=311 pg ml−1, q=0.978).
Compared with the Lupron condition, replacement of P4 (Table 5) resulted in significant increases, in both PMDD and control women, in serum levels of allopregnanolone and pregnanediol. Serum levels of cortexone also were significantly increased in both groups.
Table 5. Changes in steroid metabolites during ovarian steroid replacement of P4.
| Metabolite |
Median Δ |
P-value |
Q-value |
|||
|---|---|---|---|---|---|---|
| PMDD | NC | PMDD | NC | PMDD | NC | |
| Progesterone | 7850 | 8530 | 6.10E−05 | 6.10E−05 | 0.001 | 0.001 |
| 3a-Hydroxy-5a-pregnan-20-one | 344 | 318 | 1.22E−04 | 6.10E−05 | 0.001 | 0.001 |
| Pregnanediol | 342 | 506 | 0.010 | 0.005 | 0.077 | 0.030 |
| DHEAS* | −91 935 | 159 968 | 0.018 | 0.048 | 0.104 | 0.157 |
| Cortexone | 31 | 31 | 0.026 | 0.041 | 0.118 | 0.157 |
| 2-Hydroxyestrone | −5.4 | −10.7 | 0.060 | 0.135 | 0.183# | 0.387 |
| Estradiol-3-SO4 | −26 | −12 | 0.064 | 0.649 | 0.183# | 0.727 |
| Cortexolone | 69 | 0 | 0.064 | 0.727 | 0.183# | 0.727 |
| Androstenedione | −101 | 123 | 0.252 | 0.035 | 0.447 | 0.157# |
| 17a,20a-Dihydroxyprogesterone | 13 | 40 | 0.599 | 0.002 | 0.726 | 0.015# |
Abbreviations: DHEA, dehydroepiandrosterone; Median Δ, median change; NC, normal control; P4, progesterone; PMDD, premenstrual dysphoric disorder.
The unit of measurement for all steroid metabolites is pg ml−1.
This table shows the significant changes in steroid levels in women with PMDD and controls with P4 addback relative to Lupron. Significant differences indicated by Q<0.2.
Only DHEAS levels showed significant between-group differences following P4 treatment (*P=0.003; q=0.073), decreasing in women with PMDD treated with P4 but increasing in control women subjected to the same hormonal treatment. Direct comparisons of other P4-related changes in steroid levels between PMDD and control women did not reach significance.
Within diagnostic groups, we observed trend effects of hormone condition in the magnitude of the change in steroid metabolite levels from Lupron to P4 in PMDD but not controls, as follows: 2-hydroxyestrone (median Δ: PMDD=−5.4 pg ml−1 (decreased), #q=0.183; controls=−10.7 pg ml−1, q=0.387), estradiol-3-SO4 (median Δ: PMDD=−26 pg ml−1(decreased), #q=0.183; controls=−12 pg ml−1, q=0.727) and cortexolone (median Δ: PMDD=69 pg ml−1, #q=0.183; controls=0 pg ml−1, q=0.727). In addition, we also observed significant effects of hormone condition in the magnitude of the change in steroid metabolite levels from Lupron to P4 in controls but not in PMDD, as follows: 17a, 20a-dihydroxyprogesterone (median Δ: PMDD=13 pg ml−1, q=0.726; controls=40 pg ml−1, #q=0.015) and androstenedione (median Δ: PMDD=−101 pg ml−1(decreased), q=0.447; controls=123 pg ml−1, #q=0.157).
Changes in steroid metabolite levels that differed between PMDD and Controls
Only estradiol-3-SO4 levels showed a significant diagnostic difference between PMDD and control women after E2 treatment compared with Lupron (Table 4). Women with PMDD had a significantly attenuated (that is, blunted) increase in estradiol-3-sulfate after E2 compared with control women. Other E2-related changes in steroid metabolite levels did not differ between PMDD and control women. Despite the absence of other significant between-group differences, significant within-group differences between Lupron and hormone-replaced were observed in one group but not in the other. Within-group differences in E2-treated women with PMDD included significant decreases in estrone, pregnenolone sulfate (3b-hydroxy-5-pregnen-20-one-3-SO4), DHEAS and DHEA levels, compared with no significant change, or a trend toward increased levels of these metabolites in controls (Table 4).
Only DHEAS levels showed a significant diagnosis-related difference between PMDD and control women after P4 treatment (Table 5), with a decrease in serum levels in PMDD and an increase in controls. Notably, none of the four progesterone-related neurosteroid metabolites successfully measured (9-dehydroprogesterone, 3a-hydroxy-5a-pregnan-20-one (allopregnanolone), 17a, 20a-dihydroxyprogesterone and pregnanediol) showed significant diagnostic differences after P4. In particular, the magnitude of the increases in allopregnanolone levels was almost identical in PMDD and controls (Table 5).
Despite the absence of other significant between-group differences, significant within-group differences between Lupron and hormone-replaced conditions were observed in one group but not in the other. Estradiol-3-SO4 levels and 2-hydroxyestrone decreased significantly in P4-treated women with PMDD but not in controls. The significant increases in 17a, 20a-dihydroxyprogesterone and androstenedione levels observed in control women were not seen in women with PMDD. Finally, only women with PMDD showed a significant increase in cortexolone levels (Table 5).
Discussion
We examined the hypothesis that altered metabolic processing of ovarian steroids after E2/P4 exposure would distinguish women with PMDD from matched, asymptomatic controls. The women with PMDD met clinical criteria for PMDD and, of particular relevance, were also confirmed to be behaviorally sensitive to E2/P4.5 Controls lacked any significant menstrual cycle or hormone-related behavioral symptoms. Both groups received identical hormone manipulations to standardize exposures. We used a targeted metabolomics platform to detect differences in steroid metabolites in these highly selected clinical phenotypes exposed to identical doses of E2 and P4 while receiving Lupron.
Despite several studies reporting abnormal levels of P4-derived neurosteroids in PMDD, our analysis revealed few significant differences between women with PMDD and controls. Notably, serum levels of the ring A-derived neurosteroid metabolites of P4 were virtually identical in the groups during all hormonal conditions. These similar serum levels also suggest that a larger sample size would be unlikely to reveal significant differences. These findings contrast with several,16, 19, 20, 21, 22 but not all, naturalistic studies across the menstrual cycle17, 18 and suggest that basal abnormalities of neurosteroid production do not contribute to the pathophysiology of PMDD. Thus, our data confirm, under controlled conditions, the absence of metabolic differences in the formation of 5-alpha-reduced (and 3-beta-hydroxy steroid dehydrogenated) metabolites of P4 in PMDD compared with controls. The neurosteroid hypothesis for PMDD has been very attractive but has been inadequately tested, to date, for the following two reasons: (1) the actions are largely determined by the integrated neurosteroid signal from the profile of metabolites, which, until now, have not been accessible for measure; and (2) samples of women with PMDD are heterogeneous with respect to the critical variable—hormone sensitivity. Both of these obstacles have been overcome in the current study, thus enabling us to answer, albeit negatively, a critical etiological question (and one that is all the more important to address, given recent demonstration of the efficacy of a neurosteroid in another reproductive endocrine-related mood disorder, postpartum depression (PPD;52, 53 despite the lack of evidence of abnormal levels of allopregnanolone in PPD). Nonetheless, our findings of otherwise normal P4-derived neurosteroid levels in PMDD are consistent with recent evidence that the luteal-phase increase (that is, change) in neurosteroid levels—not basal levels—mediates the onset of symptoms in women with PMDD,23 as blockade of 5-alpha reductase activity mitigated the PMDD symptom onset. Finally, it is possible that our inability to demonstrate abnormalities of neurosteroid levels or profiles may reflect our selection of a distinct, homogeneous subphenotype—women with documented ovarian hormone-induced behavioral sensitivity—and therefore may not generalize to the larger and more heterogeneous population of women with PMDD.
Our findings suggest that after a short-term exposure, women with PMDD process ovarian steroids differently from control women. Specifically, women with PMDD had relatively lower serum levels of the sulfated metabolites of the neurosteroids DHEA, pregnenolone and estradiol after exposures to either E2 or P4. Sulfation of hydrophobic steroids reduces steroid binding to its receptor and facilitates the transfer of the steroid from the tissues into the circulation (in favor of excretion).54 The relative balance of sulfated and non-sulfated neurosteroid metabolites determines the actions of these compounds at excitatory and inhibitory neuronal systems. Thus, the reduction in sulfated metabolites observed in PMDD could contribute to the development of PMDD symptoms, and, therefore, reflect the presence of a differential processing of the steroid signal. Nonetheless, decreased levels of sulfated neurosteroids would be predicted to reduce glutamatergic activity, promote GABA agonism and, therefore, favor anxiolytic effects instead of the symptom provocation that occurs in the women with PMDD during the addback phase of the study.
The decreased peripheral levels of sulfated neurosteroids in PMDD occurred in the absence of detectable differences in the non-sulfated forms of these same steroids (that is, estradiol, DHEA and pregnenolone). These differences also are not explained by differential hormone exposure as no diagnosis or diagnosis-by-hormone condition effects were observed in peripheral levels of E2/P4, and there were no differences in sulfated steroids during Lupron alone.
The relative balance between sulfated and non-sulfated steroids is maintained by two families of enzymes, the sulfotransferases (SULT) and sulfatases (STS) working in concert with sulfate group donor molecules, 3’-phospho-adenosine-5’-phosphosulfate (PAPS) and its synthetic enzyme PAPS-synthase, as well as organic anion transporter proteins that control influx and efflux into and out of the cell, respectively.54, 55 In humans, both abnormalities in the regulation of this system and mutations of enzymes and transporter-protein genes are associated with disease states including behavioral conditions such as autism and attention-deficit disorder.54 The presence of lower E2-3-sulfate in PMDD suggests an abnormality within the SULT family of enzymes, as in humans E2-3-sulfate is not a substrate for STS (see http://www.genome.jp/kegg/pathway.html#metabolism (humans)). Similarly, the absence of diagnostic differences in either estrone or its sulfated metabolite suggests that the potential abnormality in the regulation of sulfation is not uniformly present in PMDD.
The regulatory complexity of steroid sulfation prevents a more precise localization of the source for the diagnostic differences observed in this study. Members of the SULT family are differentially localized within the brain, regulated by a wide range of nuclear receptors and steroids (for example, by P4 and therefore could show increased SULT activity in the luteal phase of the menstrual cycle56), and exhibit substrate-induced feedback inhibition.54 Although there was no clear evidence of an effect of progesterone in this study, the difference in DHEAS levels after P4 addback (that is, decreasing in PMDD and increasing in control women) might reflect differential processing of P4. Alternatively, P4-induced changes in SULT could have been obscured by changes in STS. Nonetheless, the current data seem to contradict any significant luteal phase effect directly related to P4.
Abnormalities of both DHEAS and pregnenolone sulfate have been suggested to contribute to abnormal affective states,45, 57, 58 and, therefore, it is possible that the altered profile of sulfated neurosteroids relative to controls directly contributed to PMDD symptom recurrence. Few studies have evaluated blood levels of sulfated DHEA, E2 and pregnenolone during the luteal phase in women with PMDD and controls. One study17 reported no significant differences in pregnenolone sulfate levels between women with PMDD and controls, and, in contrast to our findings, higher plasma levels of pregnenolone sulfate were associated with more severe symptoms in PMDD. Similarly, although DHEAS may have potent affective state altering effects in women, no study has observed significant diagnostic differences in DHEAS in women with PMDD. Alternatively, as sulfation is regulated by both estrogen and progesterone receptors (and their cognate ligands), and women with PMDD exhibit altered luteal-phase responsivity to E2/P4 in a range of phenomena,5, 59, 60, 61, 62 the differences in sulfated metabolites could reflect further evidence of abnormal steroid signaling in PMDD that is not directly causing symptoms. Finally, as glucocorticoids and other stress-related factors (that is, soluble cytokines) can regulate steroid sulfation,54 it is possible that the altered levels of sulfated steroids occur secondary to the effects of stress or the presence of a negative affective state in women with PMDD during E2/P4 addback.
Several limitations in this study deserve mention. It comprised a small sample, so that metabolite analysis did not predict which women with PMDD were progesterone responders (that is emergence of PMDD symptoms on progesterone) versus estrogen responders, or precisely localize sulfation pathway abnormalities in PMDD. Our selection criterion excluded over 50% of metabolites with low levels, thus limiting the number of analyses. The main goal of this study was to examine the potential differences in the metabolic processing of standardized doses of ovarian steroids in women with PMDD and controls. Although potential confounding factors (for example, age, race, parity and BMI) were not a concern for testing metabolite changes within diagnostic groups, because of the paired study design, this was not the case for testing metabolite changes between PMDD and control groups. For these tests, the small sample size did not allow for adequate statistical control for age, race and BMI, and additional research is needed to determine the impact of these factors on metabolite levels in women with PMDD. Women with PMDD and control women did not significantly differ in either racial distribution or parity. Levels of sulfated steroids can decline with age; however, the role of declining age on SULT activity remains unclear with one study describing age-related changes in SULT2A1 expression,54 although others report no changes with age.46, 54, 63, 64 Similarly, few studies have documented an effect of age on 5-alpha-reductase activity.65 Thus, it is unlikely, albeit, possible that the 6-year difference in age between the two groups underlies their differences in E2/P4-induced sulfated steroid levels. Similarly, evidence in humans suggests that at least SULT1E1 is pro-adipogenic, and, therefore, the higher average BMI observed in the women with PMDD would be consistent with increased (not decreased) sulfotransferase activity.54, 66 The sample size also limited our ability to examine whether race and ethnicity or parity contributed to the observed differences in hormone levels. Although women with PMDD and controls did not significantly differ in these characteristics, nonetheless, we should include the caveat that the literature does not allow one to conclude that these factors (age, BMI, race and parity) in women significantly regulate the synthetic enzymes relevant to our findings; that is, STS and SULT (differential effects) and 5-alpha reductase (no differential effects). In addition, the design of this study was deliberately constructed to use the GnRH agonist-induced ovarian suppression to create a hormonally uniform hypogonadal 'baseline', a controlled condition permitting better assessment of the effects of a physiologically relevant and standardized ovarian steroid challenge. Our data suggest but do not definitively demonstrate that these inferred alterations in sulfation enzymes are relevant to the onset of PMDD symptoms during the luteal phase of the menstrual cycle when levels of estradiol and progesterone increase. Clearly, these pilot data need to be followed-up in the naturalistic setting during the course of the normal menstrual cycle in a sufficiently large sample of women to control for differences in substrate (that is, estradiol and progesterone secretion) as well as more specific evaluations of SULT and STS enzyme activities. A more comprehensive assessment of those steroid metabolites in which very low levels of several metabolites could not be reliably measured (as the levels were below the limits of detection for the steroid assays employed in this study) is also necessary to pursue these potential diagnostic-related differences in steroid metabolism. Finally, the generalization of our findings to the larger population of women with PMDD is limited by the strict research criteria employed.
This is the first study, to our knowledge, to employ a pharmacometabolomics approach to investigate the pattern of both basal and stimulated levels of steroid metabolites in women with well-characterized PMDD and controls. Notably, the same dose of P4 was not metabolized into P4-derived neurosteroids differently in PMDD and control women. However, we found differences in the sulfation of estradiol, DHEA and pregnenolone in women with PMDD when exposed to either E2 or P4, suggesting that differences in the metabolism of sulfated neurosteroids could be a source of the observed differential steroid sensitivity in PMDD, and, therefore, could contribute to the underlying pathophysiology of this condition.
Acknowledgments
We would like to thank Karla Thompson and Linda Schenkel of NIMH for their clinical support and data management. This work was written as part of Peter J Schmidt’s official duties as a Government employee. The views expressed in this article do not necessarily represent the views of the NIMH, NIH, HHS or the United States Government. This research was supported by the Intramural Research Program of the NIMH, NIH. NIMH Protocols NCT00001259 and NCT00001322; Project # MH002865.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp).
The authors declare no conflict of interest.
Supplementary Material
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th edn. American Psychiatric Association Press: Arlington, VA, 2013. [Google Scholar]
- Yonkers KA, O'Brien PMS, Eriksson E. Premenstrual syndrome. Lancet 2008; 371: 1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry 2012; 169: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Johnson AL, Abate AC, Rubinow DR, Schmidt PJ. Reproductive steroid regulation of mood and behavior. Compr Physiol 2016; 6: 1135–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338: 209–216. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA 2005; 102: 16060–16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M et al. Hippocampal structural changes across the menstrual cycle. Hippocampus 2008; 18: 985–988. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affective Disord 2007; 108: 87–94. [DOI] [PubMed] [Google Scholar]
- Baller EB, Wei SM, Kohn P, Rubinow DR, Alarcon G, Schmidt PJ et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry 2013; 170: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage 2010; 53: 1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 2010; 30: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M, Bannbers E, Wikstrom J, Fredrikson M, Sundstrom-Poromaa I. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol 2013; 23: 1474–1483. [DOI] [PubMed] [Google Scholar]
- Sundstrom PI, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 2014; 8: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 2007; 104: 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 1997; 94: 8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology 2014; 231: 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab 1996; 81: 1076–1082. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH Jr., Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab 1994; 79: 1256–1260. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 1997; 90: 709–714. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W et al. Cortical γ-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 2002; 59: 851–858. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol 2000; 142: 269–273. [DOI] [PubMed] [Google Scholar]
- Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol 2004; 18: 79–87. [DOI] [PubMed] [Google Scholar]
- Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE et al. 5alpha-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology 2016; 4: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe CC, Goldman MM, Zhang K, Clarke N, Reitz RE, Welt CK. Simultaneous measurement of thirteen steroid hormones in women with polycystic ovary syndrome and control women using liquid chromatography-tandem mass spectrometry. PLoS ONE 2014; 9: e93805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzker J, Hofer D, Trummer C, Ulbing M, Harger A, Pieber T et al. Testosterone to dihydrotestosterone ratio as a new biomarker for an adverse metabolic phenotype in the polycystic ovary syndrome. J Clin Endocrinol Metab 2015; 100: 653–660. [DOI] [PubMed] [Google Scholar]
- Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther 2013; 140: 223–238. [DOI] [PubMed] [Google Scholar]
- Gaikwad NW. Ultra performance liquid chromatography-tandem mass spectrometry method for profiling of steroid metabolome in human tissue. Anal Chem 2013; 85: 4951–4960. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology 2009; 34: 173–186. [DOI] [PubMed] [Google Scholar]
- Sas KM, Karnovsky A, Michailidis G, Pennathur S. Metabolomics and diabetes: analytical and computational approaches. Diabetes 2015; 64: 718–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Hill M, Ripova D, Mohr P, Hampl R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J Steroid Biochem Mol Biol 2013; 133: 77–83. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Weinshilboum R. Metabolomic signatures for drug response phenotypes: pharmacometabolomics enables precision medicine. Clin Pharmacol Ther 2015; 98: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Weinshilboum RM. Pharmacometabolomics: implications for clinical pharmacology and systems pharmacology. Clin Pharmacol Ther 2014; 95: 154–167. [DOI] [PubMed] [Google Scholar]
- Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV et al. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry 2016; 21: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Bogdanov MB, Wikoff WR, Zhu H, Boyle SH, Churchill E et al. Pharmacometabolomic mapping of early biochemical changes induced by sertraline and placebo. Transl Psychiatry 2013; 3: e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo R, Hebbring S, Ji Y, Zhu H, Zeng ZB, Batzler A et al. Merging pharmacometabolomics with pharmacogenomics using '1000 Genomes' single-nucleotide polymorphism imputation: selective serotonin reuptake inhibitor response pharmacogenomics. Pharmacogenet Genomics 2012; 22: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 4th edn, American Psychiatric Association: : Washington, DC, USA, 1994. [Google Scholar]
- Rubinow DR, Roy-Byrne PP, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry 1984; 141: 684–686. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Cohen J, Halbreich U. Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord 1986; 10: 127–135. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-IP). Biometrics Research, New York State Psychiatric Institute: : New York, NY, USA, 2002. [Google Scholar]
- Halbreich U, Backstrom T, Eriksson E, O'Brien S, Calil H, Ceskova E et al. Clinical diagnosis criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecol Endocrinol 2007; 23: 123–130. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder - clinical procedures and research perspectives. Gynecol Endocrinol 2004; 19: 320–334. [DOI] [PubMed] [Google Scholar]
- Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand 1980; 62: 177–190. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res 1999; 830: 72–87. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 2013; 230: 151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M. Neurosteroids and potential therapeutics: focus on pregnenolone. J Steroid Biochem Mol Biol 2015; 160: 78–87. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011; 32: 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med 2003; 349: 776–788. [DOI] [PubMed] [Google Scholar]
- Kugler KG, Hackl WO, Mueller LA, Fiegl H, Graber A, Pfeiffer RM. The impact of sample storage time on estimates of association in biomarker discovery studies. J Clin Bioinformatics 2011; 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolelli G, Muti P, Micheli A, Sciajno R, Franceschetti F, Krogh V et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev 1995; 4: 509–513. [PubMed] [Google Scholar]
- Reiner-Benaim A. FDR control by the BH procedure for two-sided correlated tests with implications to gene expression data analysis. Biom J 2007; 49: 107–126. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003; 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes SJM, Colquhoun HM, Doherty JP, Raines SM, Hoffman EB, Rubinow DR et al. Open-label, proof-of-concept study of SAGE-547 (allopregnanolone) in the treatment of severe postpartum depression. Hum Psychopharmacol Clin Exp 2017; 32: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A et al. Brexanolone in postpartum depression: a randomized controlled trial. Lancet 2017; pii: S0140-6736(17)31264-3. [DOI] [PubMed]
- Mueller JW, Cilligan LC, Ilkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev 2015; 36: 526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL et al. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci 2006; 90: 5–22. [DOI] [PubMed] [Google Scholar]
- Koizumi M, Momoeda M, Hiroi H, Hosokawa Y, Tsutsumi R, Osuga Y et al. Expression and regulation of cholesterol sulfotransferase (SULT2B1b) in human endometrium. Fertil Steril 2010; 93: 1538–1544. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 2009; 30: 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res 2010; 186: 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 2003; 88: 3057–3063. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Smith MJ, Schenkel LA, Schmidt PJ, Dancer K. Facial emotion discrimination across the menstrual cycle in women with premenstrual dysphoric disorder (PMDD) and controls. J Affect Disord 2007; 104: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask K, Gulinello M, Backstrom T, Geyer MA, Sundstrom-Poromaa I. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology 2008; 33: 2283–2290. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology 2007; 32: 2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005; 26: 171–202. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA, Falany CN. Regulation of the cytosolic sulfotransferases by nuclear receptors. Drug Metab Rev 2013; 45: 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem 1994; 63: 25–61. [DOI] [PubMed] [Google Scholar]
- Ihunnah CA, Wada T, Philips BJ, Ravuri SK, Gibbs RB, Kirisci L et al. Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis. Mol Cell Biol 2014; 34: 1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.