Abstract
Attention deficit hyperactivity disorder (ADHD), Tourette syndrome (TS) as well as obsessive compulsive disorder (OCD) are co-occurring neurodevelopmental diseases that share alterations of frontocortical neurometabolites. In this longitudinal study we investigated the behavioral and neurochemical effects of aripiprazole and riluzole treatment in juvenile spontaneously hypertensive rats (SHR), a model for ADHD. For neurochemical analysis we employed in vivo magnetic resonance spectroscopy (MRS). Spectra from voxels located at the central striatum and prefrontal cortex were acquired postnatally from day 35 to 50. In the SHR strain only, treatments reduced repetitive grooming and climbing behavior. The absolute quantification of cerebral metabolites in vivo using localized 1H-MRS at 11.7T showed significant alterations in SHR rats compared to controls (including glutamine, aspartate and total NAA). In addition, drug treatment reduced the majority of the detected metabolites (glutamate and glutamine) in the SHR brain. Our results indicate that the drug treatments might influence the hypothesized ‘hyperactive’ state of the cortico-striatal-thalamo-cortical circuitries of the SHR strain. Furthermore, we could show that behavioral changes correlate with brain region-specific alterations in neurometabolite levels in vivo. These findings should serve as reference for animal studies and for the analysis of neurometabolites in selected human brain regions to further define neurochemical alterations in neuropsychiatric diseases.
Introduction
Attention deficit hyperactivity disorder (ADHD), Tourette syndrome (TS) and obsessive compulsive disorder (OCD) are neurodevelopmental psychiatric disorders known to be accompanied by alterations of neurometabolites in the frontocortical area affecting mainly glutamatergic circuits. These disorders are often comorbid in TS and converge during school age and adolescence in a phenotype with hyperkinetic symptoms, motor and/or phonic tics.1, 2, 3, 4, 5 A general cortical disinhibition has been hypothesized as a possible explanation of these shared pathophysiological features, however, a well-defined etiology of the co-occurrence is still missing.6
There is no cure for TS, however, there are treatments to help manage the tics including pharmacological interventions. In this respect there has been a long-lasting debate about a potential worsening of tic symptoms during stimulant medication.7 Although current guidelines recommend the treatment of ADHD and co-occurring tics with stimulants, German guidelines for tic management in Tourette patients8, 9, 10 recommend the use of aripiprazole due to its therapeutic influence on ADHD symptoms and mild side effect profile.11, 12 Aripiprazole is a ‘dopaminergic stabilizer’ in use for the treatment of schizophrenia patients but can also be used as first choice to treat ADHD in patients with TS. Riluzole is an approved drug for the treatment of amyotrophic lateral sclerosis (ALS) leading to a prolongation of patient’s survival and providing a long-term safety profile. It has already been used in open and randomized clinical trials for OCD symptoms13, 14, 15, 16, 17 and very recently also for tic management.18
Co-occurring ADHD, OCD and TS symptoms are oftentimes worsening during adolescence leading to changes in drug regimens and to polypharmacy. The changes of symptoms might be caused by neurochemical alterations affecting only certain brain regions or systems. To analyze these developmental changes longitudinal studies are needed. With respect to neurometabolic adaptions, modern techniques like Proton magnetic resonance spectroscopy (1H-MRS)19 are unique in their ability to gain repeated access to multiple brain regions at defined developmental stages providing a longitudinal set of data on an extensive neurometabolite battery. 1H-MRS is a non-invasive in vivo method capable of measuring neurometabolite concentrations in a selected voxel (volumetric pixel) placed within a specific region of interest (ROI). By this, even subtle concentration changes can be detected that might have a potential pathophysiological significance for brain development.20, 21
Our study provides for, we believe, the first time a detailed overview of the absolute concentration changes during the adolescent period of 12 neurometabolites within the juvenile male rat striatum and prefrontal cortex. These regions resemble the main inhibitory and excitatory areas of the cortico-striatal-thalamic-cortical circuit thought to be affected during neurodevelopment in TS, ADHD and OCD patients.1 Moreover, an age- and strain-related detailed neurochemical profile of the spontaneously hypertensive rat (SHR), the most-commonly used animal model for ADHD, and its normotensive control, the Wistar Kyoto rat (WKY)22 is provided. Interestingly, we could identify a neurometabolomic fingerprint for each of the strains that can be used as reference for further studies. Finally, we investigated the effect of aripiprazole and riluzole, two newly proposed compounds to treat the hyperkinetic symptomatology in young patients affected by TS. To that end we generated neurochemical profiles of hyperactive and control juvenile animals and could correlate those with a thorough analysis of behavior. In summary, this study provides (I) a detailed developmental neurochemical profile of the strains investigated under control conditions, (II) under subchronic pharmacological treatment and (III) a correlation analysis between the absolute quantification of neurometabolites and the behavioral profiles.
Materials and methods
Animals
Behavioral assessments and MRS measurements were carried out within a period of 13 months on six cohorts of juvenile male Wistar Kyoto (WKY) and SHR (Charles River Laboratories, Sulzfeld, Germany). Up to 4 pups per cage were housed together with the mother animals until post-natal day (PND) 21. For the entire duration of the experiment the rats had free access to standard laboratory rodent chow and water, and were housed in a room with 12 h light-dark cycle. Temperature and humidity were maintained at 22±1 °C and 45–55%, respectively.
The study design is to analyze treated and untreated animals in a longitudinal manner (PND 35, 42, 46 and 50) in terms of behavior and neurochemical alteration during the period of adolescence.
All experiments were approved by the Committee for Animal Experimentation of the University of Ulm and the regional administrative authority in Tübingen (TVA No. 1200).
Drug treatment
In this gender- and age-matched vehicle-controlled designed study, each cohort randomly underwent a subchronic pharmacological intervention with vehicle (saline, 1% Tween80 (Sigma Aldrich, Taufkirchen, Germany, cat. No. P1754)), aripiprazole (Sigma Aldrich, cat. No. SML0935; 1.5 mg kg−1 i.p.) or riluzole (Sigma Aldrich, cat. No. R3772; 6 mg kg−1 i.p.). The subchronic daily drug treatment started on PND 35, when rats were weighing 100 g ±30 g, and ended on PND 50. Investigations were carried out within both WKY (9 vehicle, 7 aripiprazole and 8 riluzole) and SHR (10 vehicle, 9 aripiprazole and 9 riluzole) strain. The sample size was determined on the basis of a prior publication where sensitivity and reproducibility of pharmacoMRS at 9.4T was used for detecting changes of the neurochemical profile in two neurobiologically distinct regions of rat brain, i.e. the striatum and the prefrontal cortex as representatives with strong GABAergic and glutamatergic innervation, respectively. (Waschkies et al., 2014).
Behavioral assessment
Spontaneous locomotor activity in standard cage type III analysis
All behavioral experiments were conducted on PND 35, 42, 46 and 50 immediately before the MRS acquisition session. On each test day animals were weighed before testing and placed in the center of the individual test cage (Plexiglas standard cage type III, 425 × 266 × 155 mm) to record locomotor activity. The recording was performed for 10 min. at 50 fps rate using two cameras (Panasonic HC-V160 High Definition Video Camera) placed above (60 cm distance) and on the side (50 cm distance) of the cage, respectively. Each video was analyzed using the Ethovision 10.XT software (Noldus, Wageningen, The Netherlands) for animal behavior quantification. Horizontal locomotor activity was tracked during 5 of the 10 videotaped minutes from minute 2 to 7 and it was analyzed in terms of mean distance moved (cm), velocity (cm s−1), time spent moving/not moving (s).
Repetitive behavior analysis
The same videos were used for stereotypic behavior quantification blindly conducted by a trained observer. Within the same 5 min. of locomotion tracking, repetitive behaviors were counted: Grooming (behavioral chain composed of paw-licking, paw-rubbing over the head, licking and rubbing the side of the body, the ano-genital region and the tail), rearing (vertical locomotion up or down a vertical surface using the forepaws only) and climbing behavior (vertical locomotion up on a vertical surface using both forepaws or pull and hind paws or push to grasp footholds and haul themselves up). Each behavior was analyzed in terms of frequency (counts) and total time spent (s) performing the specific behavior.
Proton (1H) magnetic resonance spectroscopy session
MRS was carried out on a 11.7T animal scanner (Bruker, Ettlingen, Germany) equipped with a volume resonator for excitation and a phased-array surface coil for signal reception. Two regions of interest of 18.5 and 27.5 mm3 volume respectively positioned in the left striatum and the left medial prefrontal cortex were investigated. MRS was performed with a short-echo-time STEAM (STimulated Echo Acquisition Mode)23 sequence at TR/TE/TM: 5000/3.5/10 ms, 5 kHz spectral width, 2048 data points and 256 averages. Water-suppressed spectra were collected over a total acquisition time of 21 min. Molar metabolite concentration was quantified by LC Model (Version 6.3-1C, S. Provencher, Oakville). The unsuppressed water signal was used as an internal reference.24
All spectra were visually inspected for the presence of spurious resonances or artefacts prior to the statistical analysis. Cramer-Rao lower bounds (CRLB) of LC Model analysis were used to assess accuracy and reliability of the CRLB higher than 50% was considered as exclusion criterion for metabolite evaluation. Other rejection criteria, such as poor SNR (<6), existence of strong baseline distortions and line widths (full-width at half-maximum peak height, FWHH) were applied. MRS investigation immediately followed behavioral analysis. Spectra were acquired longitudinally from the same subject on PND 35, 42, 46 and 50 under anesthesia (0.8–1.8% isoflurane: air mixture supplied by face mask). Consistent with published recommendations for prolonged anesthesia exposure, breath rate was monitored throughout the scanning and isoflurane was adjusted to maintain respiration within specified target range (35-45 c.p.m.). Body temperature was maintained using a heated hydro system. The total duration of MRS including animal preparation was 90–110 min.
Statistical analysis
Data are reported as mean±s.e.m. unless otherwise stated. Behavioral data analysis was done by Mann–Whitney U-test since the normal assumption was questionable. For each metabolite detected using 1H MRS technique, two-tailed paired t-test was used to compare changes in neurometabolite absolute concentrations between the two rat strains treated with vehicle. Respective data checks supported the normal assumption here. Finally, time- and treatment-related changes in neurometabolite absolute concentrations were assessed separately for each metabolite by two-way analysis of variances with treatment as grouping factor and time (PND 35, 42, 46 and 50) as within-group factor. The presence of variance heterogeneity was excluded by the Levene-test. Data were again tested for normal distribution and Bonferroni post hoc tests were applied to account for multiple testing arising from pairwise comparisons. Specifically, each time point of the drug treated groups were compared to the respective vehicle-treated group within the same strain. All differences were considered significant when P<0.05. The analyses were conducted in GraphPad Prism 6.
Results
Strain differences of WKY and SHR rats under vehicle and drug treatment conditions during adolescence.
Body weight during treatment and brain weight at the end of the treatment period
The body weight of the rats steadily increased during the whole experimental course according to the age of both strains and there were no significant differences in body weight between SHR and WKY at any developmental time point. Rat brains were collected at the end of the experimental period and weighed and there were no strain-related differences (Supplementary Figure S1a). Moreover, at any developmental stage both drug treatments did not affect the physiological body weight gain, the daily drug treatment did also not affect the total brain weight after 15 days of subchronic administration (Supplementary Figure S1b).
Spontaneous locomotor activity
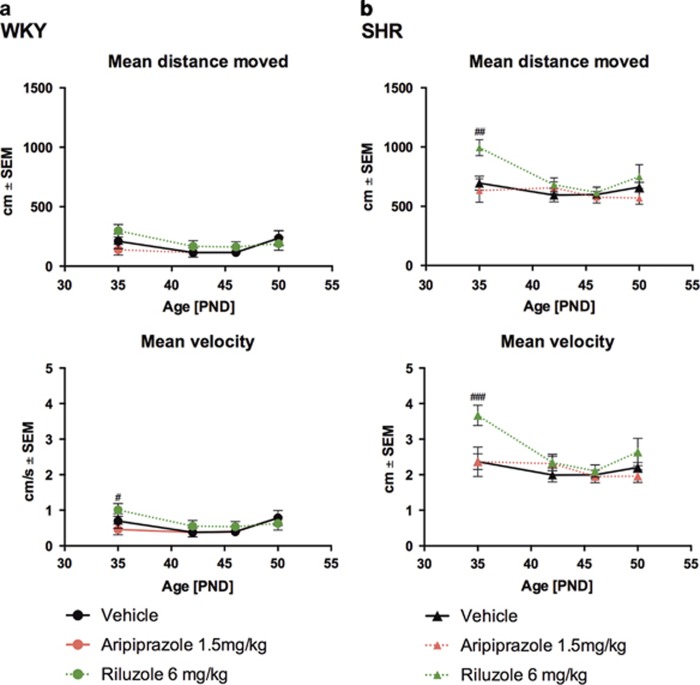
During the entire duration of the experiment the spontaneous locomotor activity was analyzed. The SHR strain showed the typical hyperactive phenotype compared to WKY rats (P<0.0001, Supplementary Figure S2a–d). Treatment with both drugs did not affect the strain-specific spontaneous locomotor activity (mean distance travelled as well as mean velocity). After an initial and significant increase of activity in the SHR strain treated with riluzole the effect was lost to the end of the treatment period (Figures 1a and b).
Figure 1.
Spontaneous locomotor activity profile of juvenile WKY- and SHR- rats under vehicle (black filled line), aripiprazole (red dotted line) and riluzole (green dotted line) treatment during adolescence. Stars indicate levels of significance (#P<0.05, ##P<0.01, ###P<0.001 and ####P<0.0001) between treated animals compared to the vehicle-treated group tested with 2 ways analysis of variance followed by Bonferroni post hoc test. Circles and triangles refer to the WKY (a) and SHR strain (b) respectively.
Repetitive behavior analysis
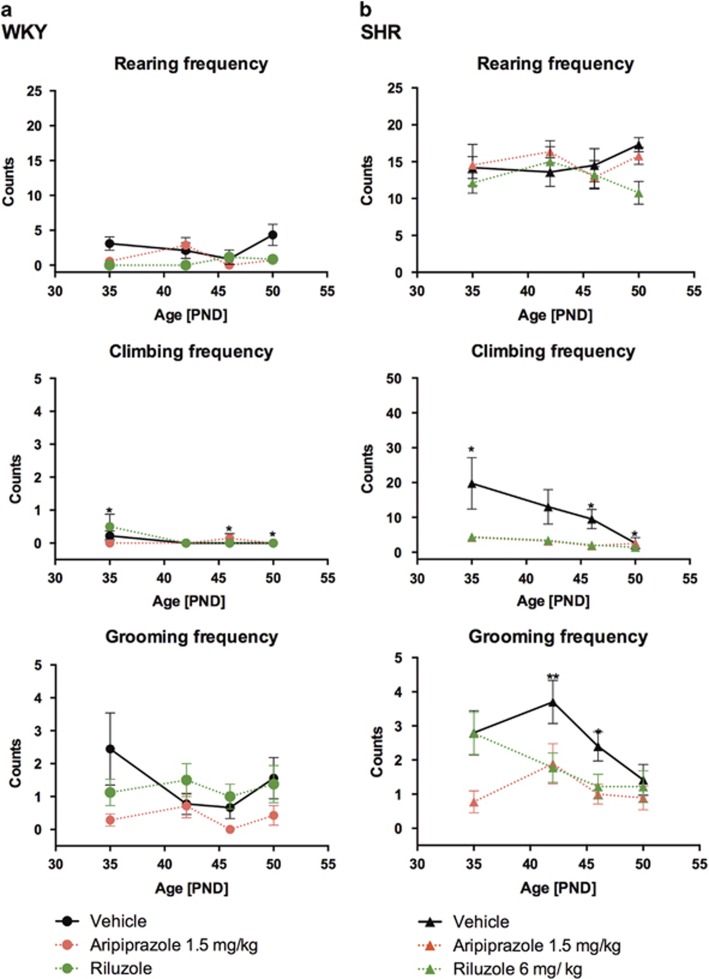
Spontaneous repetitive behavior like grooming, climbing and rearing behavior (mean frequency and duration) were analyzed. As shown in the Supplementary Material (Supplementary Figure S3a and b) the SHR strain showed an age-related, significantly increased frequency of rearing (PND 35, 46, 50, P<0.001; PND 42, P<0.01) and climbing (PND 35, 42, 50, P<0.05; PND 46, P<0.01) behavior compared to WKY rats in which such a climbing behaviour cannot be recognized. Grooming frequency was shown to be significantly different between strains only on PND 42 (P<0.01) and 46 (P<0.05). SHRs also show a significant increased time spent rearing (PND 35, P<0.05; PND 42, 46, 50 P<0.001), climbing (PND 42, 50 P<0.05, PND 46, P<0.01), but not grooming compared to WKY (Supplementary Figure S4).
As shown in Figures 2a and b, drug treatment during adolescence with both drugs significantly reduced grooming (PND 35, P<0.01; PND 42, P<0.05) and climbing (PND 35, P<0.001; PND 42 and 46, P<0.05) frequency in the SHRs but not in the WKY strain. Rearing frequency was not affected. The same treatment does not affect the time spent grooming, climbing or rearing (Supplementary Figure S4).
Figure 2.
Grooming, climbing and rearing frequency of juvenile WKY- (a) and SHR- rats (b) treated with vehicle (black filled line), aripiprazole (red dotted line) or riluzole (green dotted line) during adolescence. Stars indicate levels of significance (*P<0.05, **P<0.01, ***P<0.001) treated animals compared to the vehicle-treated group tested with analysis of variance 2 ways followed by Bonferroni post hoc test.
In vivo (1H) MRS absolute quantification of neurometabolites during adolescence
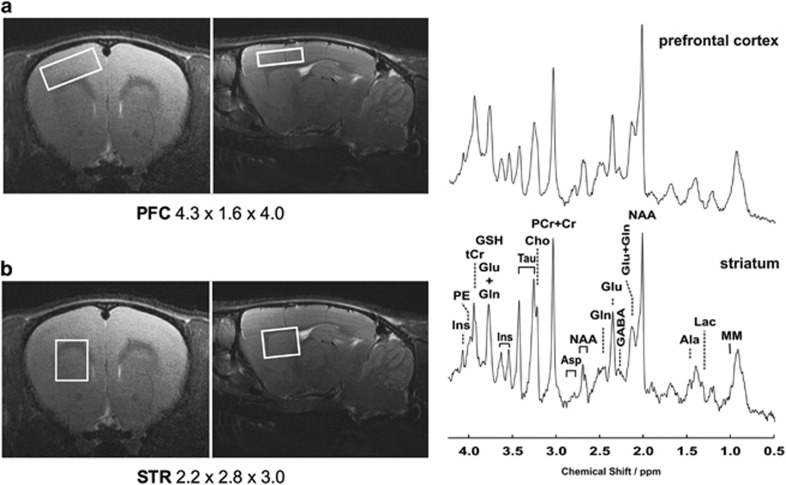
Typical locations and size of the volumes of interest (VOIs) centered in the striatum and prefrontal cortex that were selected for spectroscopic studies and a representative spectrum for each of the analyzed VOI is shown in Figures 3a and b. Metabolites used in the final analysis were GABA, glutamate, glutamine, aspartate, inositoles, taurine, PE, alanine, and GSH as absolute concentration, while combined values were reported for NAA+NAAG (total NAA), GPC+PCh (total choline), and Cr+PCr (total creatine) due to the inability to consistently resolve individual components. Data from prefrontal cortex and striatum are shown respectively in Supplementary Figure 6a and b.
Figure 3.
Volumes of interest (VOI) have been centered within the rat prefrontal cortex (a) and striatum (b) defining the selected areas for the MR spectroscopy studies. Shown are also representative spectra obtained using multislice FLASH, TR/TE=191/5 ms, 17.5° flip angle, 27 mm field-of-view, 0.75 mm slice thickness.
Region-specific neurochemical profile of WKY and SHR rats under vehicle, aripiprazole and riluzole treatment during adolescence
Neurometabolite levels quantified in both striatum and prefrontal cortex within the WKY and SHR groups treated with vehicle showed time course dependent trajectories. They display a brain region-specific profile that for some components was also strain-specific. In the striatal region, PND 50 reveals the largest strain-related differences: SHR show significant higher striatal glutamine and aspartate concentrations while in the prefrontal region higher glutamine levels are accompanied by increased glutamate, total NAA, taurine and alanine concentrations. Total creatine shows higher concentrations starting from late adolescence, while the low aspartate concentration present at the early- and mid-adolescence reach WKY levels toward the latest time points. Data are shown in the Supplementary Material (Supplementary Figure S5).
Under daily, subchronic drug treatment conditions during rats’ adolescence, we did not detect any significant neurochemical alteration within the brain region-specific profile of WKY rats, both with aripiprazole and riluzole administered intraperitoneally. On the contrary, in SHR rats both drug treatments exerted a decrease of the concentrations of neurometabolites and in general the effect was stronger in the prefrontal cortex compared to the striatum. In particular, aripiprazole treatment of the juvenile SHR strain induced a strong reduction of prefrontal glutamine, glutamate and GABA that persisted for all time points analyzed as well as a strong and stable reduction of total NAA, GSH, inositoles and creatine concentration. PE, taurine and total choline also showed initially significantly reduced prefrontal levels that then tend to stabilize over time, while alanine and aspartate levels seem not to be affected by treatment with aripiprazole.
On the other hand, riluzole treatment exerts a strong, stable and specific reduction of the prefrontal levels of glutamate over time, while glutamine levels start to decrease at PND 46 and GABA levels are not severely affected. Total creatine and inositole levels are also heavily reduced during the entire period of riluzole treatment. A reduction of glutamine, total NAA, PE, GSH and aspartate could be detected towards PND 46. Alanine levels, again, seem not to be affected by the treatment.
Finally, within the striatum, both drugs showed an overall weaker effect and significantly lower neurometabolite concentrations could only be randomly detected. Worth noticing is that following aripiprazole treatment striatal glutamate alone shows a significant and stable reduction that was not seen at PND 50 anymore. A specific and stable reduction of any of the striatal neurometabolites analyzed could not be detected as indicator of the riluzole treatment within the striatum. Graphic representation of the data related to the absolute quantification of the neurometabolites is shown in Supplementary Figure S6b.
Brain region-specific correlation overview between SHR characteristic phenotype and neurometabolite absolute concentration.
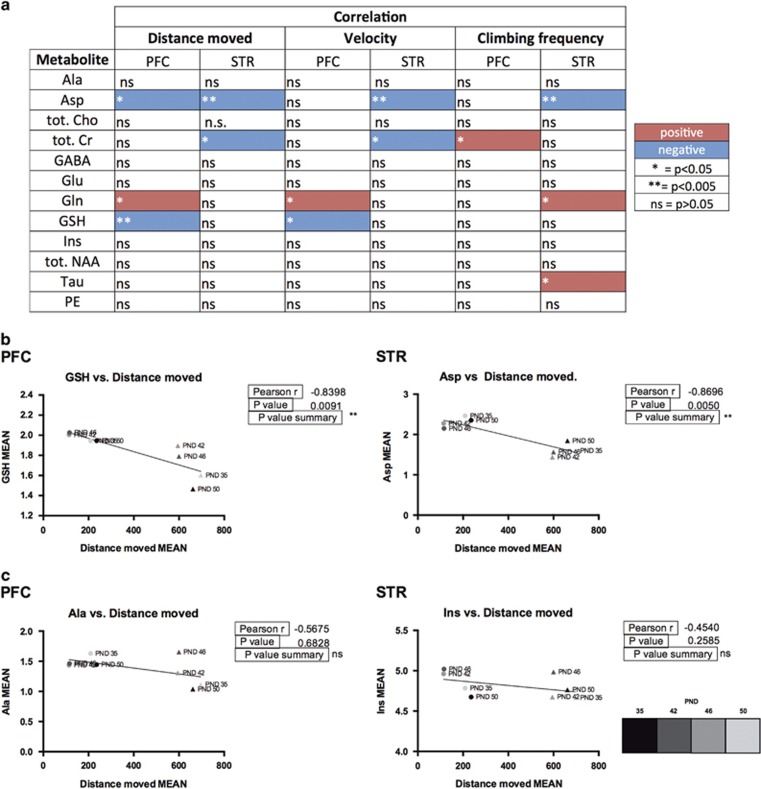
After assessing the hyperactivity in the SHR experimental group, we wanted to test whether altered concentrations of the metabolites detected correlate to specific motor activity characteristics such as total distance moved, velocity25 and spontaneous climbing frequency (Supplementary Figure S3). We used these three parameters that were shown to be significantly different between the two strains (Supplementary Figure S3 and 4).
In Figures 4a and b, we provide an overview of the correlations between hyperactivity parameters and mean concentrations of each metabolite detected within the specific VOI over time in both strains under vehicle treatment conditions. In brief, in all of the three behavioral parameters a negative correlation of striatal aspartate levels was found to be significant (P<0.005). Striatal total creatine levels also show a significant negative correlation with the mean total distance moved and velocity, but not with the climbing behavior. This behavior, however, was correlated positively with prefrontocortical total creatine levels (P<0.05). Prefrontal cortex levels of glutamine positively correlate with hyperlocomotion, while its levels in the striatum show a significant positive correlation with spontaneous climbing frequency (P<0.05). We also found a negative correlation of prefrontal GSH with the mean distance moved (P<0.005) and mean velocity (P<0.05). Finally, the mean climbing frequency, in our study a peculiarity of juvenile SHR rats’ repetitive behavior repertoire shows a unique positive correlation with total creatine in the prefrontal cortex (P<0.05).
Figure 4.
Correlation overview. (a) List of the 1H-MRS detected 12 metabolites and their correlation values to mean distance moved, mean velocity and mean climbing frequency, sorted by brain region (PFC: prefrontal cortex; STR: striatum). Red and blue colors indicate positive and negative correlations respectively. Symbols legend: *P<0.05; **P<0.005; NS, P>0.05. (b) Representative graphs of the most significant metabolite correlations to distance moved, sorted by brain region. (c) Representative graph metabolites that do not significantly correlate to mean distance moved, sorted by brain region. WKY and SHR strains are indicated by circles and triangles respectively and colored in grey scale according to age. All mean values shown refer to the vehicle-treated groups.
Discussion
The comorbidity of TS with ADHD and/or OSD is—at least in part—caused by genetic predispositions.5 Within their own complex and well-described clinical phenomenology, they share especially ‘hyperkinetic’ features that results in tics, motor disinhibition/hyperactivity and compulsive/ritualistic movements suggesting possible malfunctions within overlapping neuronal circuits. A review of transcranial magnetic stimulation (TMS) studies revealed a general cortical disinhibition6 but a pathophysiological mechanism that explains their co-occurrence is not yet defined. As hypothesized by Mol Debes4 all entities may be considered as ‘disorders of disinhibition’ to be explained by fronto-striatal dysfunctions26 that go along with a co-shared genetically susceptibility2 as well as neurotransmitter release alterations. Due to the unresolved pathophysiology and overlapping clinical phenomenology, the pharmacological interventions are still limited to symptomatic treatments. In light of recent clinical trials (for review see Ghanizadeh et al.12), we were motivated to study the in vivo effect of aripiprazole and riluzole on behavior especially on hyperkinetic movements by subchronic daily application during adolescence in an animal model.27 We employed magnetic resonance spectroscopy (1H-MRS) to analyze the concentration of brain region-specific neurometabolites of individual rats over time to also foster clinical translatability of experimental paradigms using animal models and patient cohorts.28 1H-MRS ability of non-invasively quantifying in vivo the distribution of molecules of interest in the brain has been validated by ex vivo tissue samples in both, animals and humans.29, 30, 31 However, depending on the specific metabolite, in some cases concentrations are difficult to compare due to the rapid degradation processes starting immediately after cell death.32
We used the SHR and its normotensive control (WKY) since the SHR strain meets many of the criteria defining it as an adequate animal model of ADHD as it presents the behavioral characteristics of inattention, impulsivity and hyperactivity.22 Our results show that neither aripiprazole nor riluzole increase significantly the body weight of SHR and WKY rats during adolescence, maintaining the strain-specific, slightly higher weight for the SHR compared to controls.33 Increased body weight is a common side effect often reported in studies investigating antipsychotic chronic treatments in patients,34 however, animals were only treated for about 2 weeks so that longer treatment periods are needed to finally proof that the compounds are not affecting body and/or brain weight.
The spontaneous locomotor activity measurements under control conditions readily confirmed the known strain-related locomotor patterns25 showing SHRs travelling a threefold increased distance at a fivefold increased velocity when compared to the WKY strain. Treatment with both drugs did not affect the juvenile spontaneous pattern of locomotion showed by control (WKY) animals. Interestingly, also the SHR strain did not show any changes in locomotion, however grooming and climbing was significantly reduced while rearing was not. These results indicate that both compounds exert a selective action on the repetitive behavioral traits but not on the spontaneous hyperlocomotion that are described as typical features of this ADHD model.35 The repetitive climbing behavior, that was observed to be specific for the SHR strain only, was also significantly affected.36
Our attempt to quantify and correlate our 1H-MRS brain-specific neurometabolites with the hyperactivity traits of the juvenile SHR strain arises from already existing clinical data. However, it needs to be mentioned that clinical and preclinical data must be interpreted with care due to technical and experimental differences in the application of the 1H-MRS technique such as magnetic field strength, whole-brain or single-voxel analysis, presence/absence of anesthesia, age or diet regime of the participants, acquisition period, readout of results (as ratio or absolute concentration).
On the neurochemical level our study revealed strain-related and brain region-specific differences. Within the striatum of vehicle-treated SHR rats, glutamine and aspartate levels were significantly higher at the end of the adolescence period compared to control animals; also glutamate concentrations are slightly higher. This finding is well in line with ADHD medication-free children that were reported to have elevated striatal glutamate levels.37 In the prefrontal cortex of SHRs we found glutamate, glutamine, total NAA, taurine and alanine to be significantly higher when compared to the control strain. In children with ADHD, an MRS study also revealed increased glutamate/glutamine in both frontal cortical areas and increased N-acetyl aspartate but also choline in the right frontal area of the ADHD-H subjects. N-acetylasparte/creatine (NAA/Creatine) ratio in the right frontal region, and myoinositol/creatine (Myo inositol/Creatine) ratio in the right and left frontal regions also appeared to be highly associated with the regulation of sensorimotor, language, and memory and learning functioning in children with ADHD.38 Carrey et al.39 also showed higher striatal glutamate, glutamate/glutamine (Glx) but also creatine (Cr) concentrations in children with ADHD compared to controls. In general, our results are very much in line with the possible increase of Glx (combination of glutamine, glutamate and GABA peaks) within the striatum and cortex in pediatric patients with ADHD and other neurodevelopmental disorders suggesting changes in fronto-striatal glutamatergic circuits across the lifespan.20 Finally, 1H-MRS investigations analyzing bilateral dorsolateral prefrontal cortex and white matter in children affected by ADHD showed a correlation between several metabolite concentrations and symptoms.40 This, together with the higher concentration of excitatory neurotransmitters found within the frontal brain area could represent one of the reasons explaining the hyperactive nature of the SHR animal model investigated.
The neurochemical scenario that has been measured in treated animals showed a strain-specific effect on the SHR rats that was in general stronger in the prefrontal cortex compared to the striatum. Aripiprazole treatment induced a strong reduction of prefrontocortical glutamine, glutamate and GABA as well as a stable reduction of total NAA, GSH, inositoles and creatine. Values for PE, taurine and total choline were also lower and got more stable over time. In contrast to these results, a spectroscopic study in rats performed by McLoughlin et al.41 demonstrated that antipsychotic drugs (including aripiprazole) consistently increased N-acetyl aspartate (NAA) levels in at least one brain area, suggesting a common therapeutic response. Children with ADHD, however, treated with stimulants and healthy children were found to have higher choline ratios in the left prefrontal region and lower N-acetyl-aspartate ratios in the left striatum region as well as lower glutamate–glutamine ratios in the left cerebellum when compared with drug naive children with ADHD. In these three regions, there was no difference between treated children with ADHD and typically developed children.42 In line with our result, decreased NAA in the dorsolateral prefrontal cortex was found by Husarova et al. after atomoxetine medication supporting the hypothesis that atomoxetine could decrease hyperactivation of dorsolateral prefrontal cortex neurons.43 Our results should help to understand the mechanism by which aripiprazole exerts its function as a dopaminergic stabilizer being also able to influence the glutamine-glutamate-GABA cycle. This effect has also been shown in a recently published paper showing that aripiprazole treatment normalized patient values of striatal glutamate to control levels.44
In good correlation with the data shown by the in vivo pharmaco-(1H)MRS study on adults female Sprague Dawley rats, in our study riluzole heavily reduced glutamate in the PFC and to some extent also glutamine, but not GABA.45 Together with its function as glutamatergic modultator, riluzole was found to readily reduce also total creatine and inositoles level indicating its role in cell plasticity. Given the higher levels of choline, total NAA and creatine were found within the right prefrontal white matter of OCD patients, correlating with the severity of symptoms,46 our results support the hypothesis that riluzole is an effective drug for the treatment of OCD symptoms.
The main result in vehicle-treated condition is the significant negative correlation of striatal aspartate with distance moved, velocity and climbing behavior, as well as a positive correlation between prefrontal glutamine levels and distance moved and velocity. In general, correlations seems to be heavily driven by strain differences, but results do not change when we observe the strains separately even with the given small sample size. In particular, GSH prefrontal levels negatively correlated with distance moved and velocity, reminding us of the results of Brennan’s study in which lower posterior cingulate cortex GSH/Cr ratio were found in OCD participants compared with non-OCD participants.47
In conclusion we were able to provide an in vivo MRS protocol showing specific temporal and spatial changes of metabolites during brain maturation and under different drug treatments in a rat model for ADHD indicating a ‘hyperactive’ state of cortico-striatal-thalamo-cortical circuitries. The non-invasive use of preclinical 1H-MRS technique might serve as reference for future studies and encourage the 1H-MRS-based neurochemical analysis of human brain regions to further define neuropathology and control compound testing in neuropsychiatric diseases.
Acknowledgments
This work was supported by the Marie Curie ITN TS-EUROTRAIN (FP7-PEOPLE-2012-ITN, under the REA grant agreement n°316978). This manuscript is dedicated to Prof. Andrea G. Ludolph who passed away during the time period when the experiments were conducted. She designed and motivated this study, was essential for the EUROTRAIN network and was sharing her outstanding clinician expertise in translating clinical questions to preclinical level and back. We also thank Anne Subgang for the spectra acquisition and technical support during scanning time; Barbara Commisso and Silvia Cursano for their help with animal treatment; Simona Rizzo for her help and expertise in graphic design and Benjamin Mayer for professional statistical support.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
During the last 5 years Professor JMF received research funding from the EU, DFG (German Research Foundation), BMG (Federal Ministry of Health), BMBF (Federal Ministry of Education and Research), BMFSFJ (Federal Ministry of Family, Senior Citizens, Women and Youth), German armed forces, several state ministries of social affairs, State Foundation Baden-Württemberg, Volkswagen Foundation, European Academy, Pontifical Gregorian University, RAZ, CJD, Caritas, Diocese of Rottenburg-Stuttgart. Moreover, he received travel grants, honoraria and sponsoring for conferences and medical educational purposes from DFG, AACAP, NIMH/NIH, EU, Pro Helvetia, Shire, several universities, professional associations, political foundations, and German federal and state ministries. Clinical trials for Lundbeck, BMBF, Servier. Professor Fegert holds no stocks of pharmaceutical companies. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T et al. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome. Mov Disord 2004; 19: 416–425. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Grados MA. Familiality of tourette syndrome, obsessive-compulsive disorder, and attention-deficit/hyperactivity disorder: Heritability analysis in a large sib-pair sample. J Am Acad Child Adolesc Psychiatry 2011; 50: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry 2012; 21: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol Debes NMM. Co-morbid disorders in Tourette syndrome. Behav Neurol Hindawi Publishing Corporation 2013; 27: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015; 72: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunse T, Wobrock T, Strube W, Padberg F, Palm U, Falkai P et al. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimul 2014; 7: 158–169. [DOI] [PubMed] [Google Scholar]
- Erenberg G. The relationship between tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol 2005; 12: 217–221. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Roessner V, Münchau A, Müller-Vahl K. Tourette syndrome and other tic disorders in childhood, adolescence and adulthood. Dtsch Arztebl Int 2012; 109: 821–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo R, Gulisano M, Calì PV, Curatolo P. Tourette Syndrome and comorbid ADHD: Current pharmacological treatment options. Eur J Paediatr Neurol 2013; 17: 421–428. [DOI] [PubMed] [Google Scholar]
- Termine C, Selvini C, Rossi G, Balottin U. Emerging treatment strategies in tourette syndrome: What’s in the pipeline? Int Rev Neurobiol 2013; 112: 445–480. [DOI] [PubMed] [Google Scholar]
- Safavi P, Hasanpour-Dehkordi A, AmirAhmadi M. Comparison of risperidone and aripiprazole in the treatment of preschool children with disruptive behavior disorder and attention deficit-hyperactivity disorder: A randomized clinical trial. J Adv Pharm Technol Res 2016; 7: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A. Systematic review of clinical trials of aripiprazole for treating attention deficit hyperactivity disorder. Neurosciences (Riyadh) 2013; 18: 323–329. [PubMed] [Google Scholar]
- Emamzadehfard S, Kamaloo A, Paydary K, Ahmadipour A, Zeinoddini A, Ghaleiha A et al. Riluzole in augmentation of fluvoxamine for moderate to severe obsessive compulsive disorder: randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci 2016; 70: 332–341. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Wasylink S, Billingslea E, Simpson R, Jakubovski E et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: A pilot randomized placebo-controlled trial. J Clin Psychiatry 2015; 76: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRX 2006; 3: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PJ, Joseph LA, Farmer CA, Luckenbaugh DA, Lougee LC, Zarate CA et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology 2014; 39: 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 2005; 58: 424–428. [DOI] [PubMed] [Google Scholar]
- Lemmon ME, Grados M, Kline T, Thompson CB, Ali SF, Singer HS. Efficacy of glutamate modulators in tic suppression: A double-blind, randomized control trial of D-serine and riluzole in tourette syndrome. Pediatr Neurol 2015; 52: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry 2008; 47: 1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaijen J, Lythgoe DJ, Amiri H, Buitelaar JK, Glennon JC. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: A review of magnetic resonance spectroscopy studies. Neurosci Biobehav Rev 2015; 52: 74–88. [DOI] [PubMed] [Google Scholar]
- Lee MR, Denic A, Hinton DJ, Mishra PK, Choi D-S, Pirko I et al. Preclinical (1)H-MRS neurochemical profiling in neurological and psychiatric disorders. Bioanalysis 2012; 4: 1787–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Wøien G, Walaas SI, Storm-Mathisen J, Bergersen LH et al. The spontaneously hypertensive rat model of ADHD–the importance of selecting the appropriate reference strain. Neuropharmacology 2009; 57: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Michaelis T, Merboldt KD, Hänicke W, Gyngell ML, Chien D et al. Localized NMR spectroscopy in vivo. Progress and problems. NMR Biomed 1989; 2: 188–195. [DOI] [PubMed] [Google Scholar]
- De Souza SW, Dobbing J. Cerebral edema in developing brain. I. Normal water and cation content in developing rat brain and postmortem changes. Exp Neurol 1971; 32: 431–438. [DOI] [PubMed] [Google Scholar]
- Knardahl S, Sagvolden T. Open-field behavior of spontaneously hypertensive rats. Behav Neural Biol 1979; 27: 187–200. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Mallet L, Golmard J-L, Béhar C, Durif F, Jalenques I et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: tics, compulsions, or both? PLoS One 2010; 5: e12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med 2013; 4: 624–630. [PMC free article] [PubMed] [Google Scholar]
- Hoyer C, Gass N, Weber-Fahr W, Sartorius A. Advantages and challenges of small animal magnetic resonance imaging as a translational tool. Neuropsychobiology 2014; 69: 187–201. [DOI] [PubMed] [Google Scholar]
- Liubinas SV, Drummond KJ, Desmond PM, Bjorksten A, Morokoff AP, Kaye AH et al. Glutamate quantification in patients with supratentorial gliomas using chemical shift imaging. NMR Biomed 2014; 27: 570–577. [DOI] [PubMed] [Google Scholar]
- Barker PB, Breiter SN, Soher BJ, Chatham JC, Forder JR, Samphilipo MA et al. Quantitative proton spectroscopy of canine brain: in vivo and in vitro correlations. Magn Reson Med 1994; 32: 157–163. [DOI] [PubMed] [Google Scholar]
- Burri R, Bigler P, Straehl P, Posse S, Colombo JP, Herschkowitz N. Brain development: 1H magnetic resonance spectroscopy of rat brain extracts compared with chromatographic methods. Neurochem Res 1990; 15: 1009–1016. [DOI] [PubMed] [Google Scholar]
- Battistuta J, Bjartmar C, Trapp BD. Postmortem degradation of N-acetyl aspartate and N-acetyl aspartylglutamate: an HPLC analysis of different rat CNS regions. Neurochem Res 2001; 26: 695–702. [DOI] [PubMed] [Google Scholar]
- Hsu J-W, Lee L-C, Chen R-F, Yen C-T, Chen Y-S, Tsai M-L. Striatal volume changes in a rat model of childhood attention-deficit/hyperactivity disorder. Psychiatry Res 2010; 179: 338–341. [DOI] [PubMed] [Google Scholar]
- Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Bruno KJ, Hess EJ. Rodent models of ADHD. Curr Top Behav Neurosci 2011; 9: 273–300. [DOI] [PubMed] [Google Scholar]
- Alves R, Barbosa De Carvalho G, Antonio M, Venditti C. High-and low-rearing rats differ in the brain excitability controlled by the allosteric benzodiazepine site in the GABA A receptor. J Behav Brain Sci 2012; 2: 315–325. [Google Scholar]
- MacMaster FP, Carrey N, Sparkes S, Kusumakar V. Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol Psychiatry 2003; 53: 184–187. [DOI] [PubMed] [Google Scholar]
- Courvoisie H, Hooper SR, Fine C, Kwock L, Castillo M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsychiatry Clin Neurosci 2004; 16: 63–69. [DOI] [PubMed] [Google Scholar]
- Carrey N, MacMaster F, Gaudet L, Schmidt M. Striatal creatine and glutamate/glutamine in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2007; 17: 11–17. [DOI] [PubMed] [Google Scholar]
- Husarova V, Bittsansky M, Ondrejka I, Dobrota D. Correlations of ADHD symptoms with neurometabolites measured by 1H magnetic resonance spectroscopy. Bratisl Lek Listy 2014; 115: 635–642. [DOI] [PubMed] [Google Scholar]
- McLoughlin GA, Ma D, Tsang TM, Jones DNC, Cilia J, Hill MD et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res 2009; 8: 1943–1952. [DOI] [PubMed] [Google Scholar]
- Benamor L. (1)H-Magnetic resonance spectroscopy study of stimulant medication effect on brain metabolites in French Canadian children with attention deficit hyperactivity disorder. Neuropsychiatr Dis Treat 2014; 10: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husarova V, Bittsansky M, Ondrejka I, Dobrota D. Prefrontal grey and white matter neurometabolite changes after atomoxetine and methylphenidate in children with attention deficit/hyperactivity disorder: a (1)H magnetic resonance spectroscopy study. Psychiatry Res 2014; 222: 75–83. [DOI] [PubMed] [Google Scholar]
- Kanaan AS, Gerasch S, García-García I, Lampe L, Pampel A, Anwander A et al. Pathological glutamatergic neurotransmission in Gilles de la Tourette syndrome. Brain 2016; 21: 90–97. [DOI] [PubMed] [Google Scholar]
- Waschkies CF, Bruns A, Müller S, Kapps M, Borroni E, von Kienlin M et al. Neuropharmacological and neurobiological relevance of in vivo1H-MRS of GABA and glutamate for preclinical drug discovery in mental disorders. Neuropsychopharmacology 2014; 39: 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AM, Soreni N, Stanley JA, Greco A, Mendlowitz S, Szatmari P et al. Proton magnetic resonance spectroscopy of prefrontal white matter in psychotropic naïve children and adolescents with obsessive–compulsive disorder. Psychiatry Res Neuroimaging 2014; 222: 67–74. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Jensen JE, Perriello C, Pope HG, Jenike MA, Hudson JI et al. Lowerposterior cingulate cortex glutathione levels in obsessive compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.