Abstract
Early persistent negative symptoms (PNS) following a first episode of psychosis (FEP) are linked to poor functional outcome. Reports of reduced amygdalar and hippocampal volumes in early psychosis have not accounted for heterogeneity of symptoms. Age is also seldom considered in this population, a factor that has the potential to uncover symptom-specific maturational biomarkers pertaining to volume and shape changes within the hippocampus and amygdala. T1-weighted volumes were acquired for early (N=21), secondary (N=30), non-(N=44) PNS patients with a FEP, and controls (N=44). Amygdalar–hippocampal volumes and surface area (SA) metrics were extracted with the Multiple Automatically Generated Templates (MAGeT)-Brain algorithm. Linear mixed models were applied to test for a main effect of group and age × group interactions. Early PNS patients had significantly reduced left amygdalar and right hippocampal volumes, as well as similarly lateralized negative age × group interactions compared to secondary PNS patients (P<0.017, corrected). Morphometry revealed decreased SA in early PNS compared with other patient groups in left central amygdala, and in a posterior region when compared with controls. Early and secondary PNS patients had significantly decreased SA as a function of age compared with patients without such symptoms within the right hippocampal tail (P<0.05, corrected). Significant amygdalar–hippocampal changes with age are linked to PNS after a FEP, with converging results from volumetric and morphometric analyses. Differential age trajectories suggest an aberrant maturational process within FEP patients presenting with PNS, which could represent dynamic endophenotypes setting these patients apart from their non-symptomatic peers. Studies are encouraged to parse apart such symptom constructs when examining neuroanatomical changes emerging after a FEP.
Introduction
Negative symptoms are a cluster of symptoms that represent a disabling feature for many psychiatric and neurological conditions, characterized by the absence of goal-directed behaviour, and related cognitive and emotional states underlying motivation.1, 2 It is clear that for individuals who have experienced a first episode of psychosis (FEP), the manifestation of persistent negative symptoms (PNS) underlines a significant subgroup of patients with unmet therapeutic needs.3, 4 Early persistent negative symptoms (ePNS) are defined by the presence of anhedonia-asociality, alogia, affective flattening and/or avolition-apathy for at least six consecutive months in the absence of therapeutically significant levels of positive, extrapyramidal and/or depressive symptomatology. Patients presenting concurrently with the latter symptom cluster comprise patients with PNS due to secondary factors (sPNS), and are argued to be distinct from ePNS.5 Evidence suggests that there are pronounced cortical changes specific to ePNS compared with other FEP patients.3, 6, 7 Within limbic circuitry, the structure of the amygdala and hippocampus may be particularly informative, given the significant level of communication between these two structures8, 9 and external brain networks,10, 11 and their crucial roles in various cognitive processes8, 12 thought to be compromised in PNS patients.5, 13
The link between PNS and amygdalar–hippocampal (AG–HC) structure in FEP is yet to be examined longitudinally, although earlier work has examined relevant themes cross-sectionally.6, 14 There is a considerable amount of literature on volumetric differences in these structures, with evidence supporting lower hippocampal volumes in chronic schizophrenia,15, 16 albeit the strength and direction of this result is contested in earlier stages of the illness.17
The heterogeneity among psychosis samples may contribute to such inconsistencies. Studies have addressed this by subdividing patients into subgroups according to various characteristics. For instance, previous work has pinpointed lower left hippocampal volumes localised to the tail region in first-episode schizophrenia patients who do not achieve remission after 6 months of treatment.18 In relation to negative symptoms, several studies19, 20 have found a relationship between functional activation patterns in the amygdala and severity of affective flattening in schizophrenia. Symptom severity has also been shown to be associated with the size and shape of the amygdala in psychosis and mood disorders.21, 22 In particular, negative symptoms may be linked to hippocampal structure, as demonstrated recently in an investigation pinpointing CA1 atrophy to worsening negative symptoms.23 If these relationships hold true, one might expect that patients with ePNS may exhibit differential AG–HC structure in relation to their non-PNS peers.
In 2014, Arnett et al.24 discussed a later sociodevelopmental maturational period (‘emerging adulthood’), encompassing the age range of 18–29 years. This has vast implications for psychosis, given the emergence of a FEP within this time frame. It is feasible that neurobiological trajectories may be altered in PNS patients showing poor functional outcome, and alterations may emerge as a function of age. There has been a great deal of literature vetting for the neurodevelopmental hypothesis of schizophrenia, but surprisingly few studies emphasising age in these patient populations. One developmental study of emotional processing suggested longitudinal patterns of amygdalar–prefrontal (AG–PFC) connectivity with age (particularly implicating connections to anterior cingulate and medial prefrontal cortices), where during childhood (that is, ages 7–12), positive associations between AG–PFC connectivity strength and response to emotional faces were evident, followed by a progressive shift to negative associations in adulthood (that is, ages 19–25).25 This was postulated to reflect top–down inhibitory control of prefrontal regions on amygdalar function in response to emotional stimuli in normal development. The idea of aberrancies in these connections in schizophrenia being more closely related to negative symptoms is still speculative; however, previous work from our group has shown altered cortical trajectories in various prefrontal regions in ePNS with age,7 which provides a foundation to justify further investigation of highly interconnected limbic structure. At the level of the hippocampus, a recent review proposed that the underconnectivity of AG–PFC in schizophrenia underlying emotional/sociocognitive processing deficits may be linked to hyperactivation of the hippocampus.26 The hippocampus has been dubbed as a key anatomical region in the initiation of schizophrenia, with aberrations strongly supported by altered neurodevelopmental mechanisms.27 Thus, changes in structure of the amygdala and hippocampus in the early phase of psychotic disorders may provide important neurodevelopmental information differentiating subgroups of patients with different clinical profiles. Of note, differences with age might be best captured by methods aside from conventional volumetry, as demonstrated by Voineskos et al.28 Neurodevelopmental changes within subcortical structures have also been depicted by several other studies, including an investigation of a child-onset schizophrenia sample.29, 30
The current study combines the power of a longitudinal design conducted at a single site with clinically well-characterised patients, with minimal to no prior exposure to antipsychotic medication, to begin to address pertinent questions of differential limbic structure trajectories in subgroups of FEP. To test for specificity of results to ePNS, we also compare against a subgroup of non-PNS patients with sPNS. It is hypothesised that ePNS patients will have lower volumes within the amygdala and hippocampus compared with both sPNS and non-PNS patients and controls. Merging knowledge from the AG–HC circuitry31, 32 and previously reported results,18, 33 morphometric differences within the amygdala are postulated to be localised at both lateral and medial aspects of the amygdala, involved in sensory integration and control of outputs, respectively. For the hippocampus, differences are hypothesised to be associated with the output region of the structure (for example, subiculum; closer to the hippocampal tail), as this region has previously been pinpointed in first-episode psychosis. Finally, we expect morphometric differences to vary as a function of age between ePNS and other FEP subgroups.
Materials and methods
Participants
Ninety-five patients and forty-four controls were included. See Supplementary Figure 1 for visualisation of the sample distribution by age. All patients were recruited from the Prevention and Early Intervention Program for Psychoses (PEPP-Montréal), at the Douglas Institute, and were part of a longitudinal naturalistic outcome study. PEPP is a specialised early intervention service for individuals between the ages of 14 and 35 who are experiencing a FEP within a local catchment area of Southwest Montréal, Canada. Details are outlined elsewhere.34 The programme involves a comprehensive approach with intensive medical and psychosocial interventions provided within the context of a modified assertive case management model. Given the nature of the study design, no statistical methods were used to predetermine sample sizes.
Neuroimaging component
The neuroimaging study began in 2003, in which patients partook in three scheduled visits: baseline, 1-year follow-up (FUP1) and 2-year follow-up (FUP2). Inclusion criteria included the following: age above 18 years, diagnosis of affective or non-affective psychosis, IQ>70, no past antipsychotic medication treatment for more than 1 month before entry to PEPP, no major medical disorders and sufficient stability for the scanning procedure. Note that, although exposure to antipsychotic medication was restricted before acceptance to PEPP, most patients were prescribed antipsychotic medication before their first scan, and thus some patients did in fact have more than 1 month of cumulative exposure to antipsychotic medication for the neuroimaging portion of the study. Exclusion criteria include the following: a history of neurological illnesses and head trauma resulting in loss of consciousness that could affect cognition, presence of neurological disorder as by medical record examination, lifetime diagnosis of substance dependence and/or any potential contraindication for the magnetic resonance imaging scan. See Supplementary Methods for detailed information on patients excluded from the neuroimaging study.
Non-clinical controls were recruited through advertisements within the same local catchment area. In addition to exclusion criteria listed for FEP patients, controls were excluded if they had any current/past history of Axis I disorders, and/or a first-degree relative suffering from a schizophrenia spectrum disorder. All participants provided written informed consent, and the protocol was approved by the Research Ethics Board of the Douglas Mental Health University Institute and the McGill University Faculty of Medicine.
Clinical assessment and demographic data
Diagnosis was made using the Structured Clinical Interview for the Diagnostic Statistical Manual, Version IV (SCID-IV),35 performed by a trained interviewer and confirmed by a research clinician psychiatrist. Depression was assessed with the Calgary Depression Scale for Schizophrenia.36 Positive and negative symptoms were assessed with the Scale for the Assessment of Positive Symptoms (SAPS)37 and Scale for the Assessment of Negative Symptoms (SANS).38 Antipsychotic medication dosages were converted to chlorpromazine equivalents according to the literature,39 and multiplied by percent medication adherence.40 Parental socioeconomic status,41 handedness42 and full-scale IQ43, 44 were assessed for both controls and patients.
Following our previous work,5, 7 early PNS were defined according to the following criteria: (1) global rating of moderate or more on at least one negative symptom as measured by the SANS; (2) global rating of mild or less on all positive symptoms as measured by the SAPS; (3) a total score of four or less on the Calgary Depression Scale for Schizophrenia (CDSS); (4) absence of extrapyramidal symptoms requiring anticholinergic treatment; and (5) all above criteria are maintained for a period of at least 6 months.4, 5 Patients were classified as having PNS due to secondary factors if criteria 2, 3 and/or 4 were not met. Specifically, if moderate or worse levels of delusions or hallucinations were present from month 3 to 6 or from month 6 to month 9, severe levels of negative symptoms were considered secondary.
Magnetic resonance imaging acquisition
All scanning procedures were carried out at the Montreal Neurological Institute on a 1.5-T Siemens Sonata scanner. T1-weighted volumes were acquired using a three-dimensional gradient echo pulse sequence with sagittal volume excitation (TR)=22 ms, echo time (TE)=9.2 ms, flip angle=30, 180 1-mm contiguous sagittal slices). The rectangular field of view for the images was 256 mm (superior-inferior (SI)) and 204 mm (anterior-posterior (AP)).
Post-processing: MAGeT-brain
Amygdalar and hippocampal structures were extracted bilaterally using the Multiple Automatically Generated Templates (MAGeT)-Brain algorithm28, 30, 45 (https://github.com/CobraLab/MAGeTbrain). This technique utilises a limited number of high-resolution atlases that have been manually segmented as described previously (amygdala;46 hippocampus;47 https://github.com/CobraLab/atlases). Extensive validation of MAGeT has been done previously, as shown in several references from our group,45, 48 which have also included subsets of the described patient sample here, with data acquired on a 1.5-T scanner. Segmentations were also submitted to the shape morphometric branch of MAGeT-Brain, yielding local vertex-wise surface area (SA) maps for each subject. Information about MAGeT-Brain processing and quality control is detailed in Supplementary Methods. An example for a representative candidate of segmentation of the amygdala and hippocampus, and of vertex wise SA are depicted in Supplementary Figures 2 and 3, respectively.
Statistical analyses
Demographic and clinical variables (with a single time point) were analysed with one-way analyses of variance for continuous variables or χ2-ratio tests for nominal variables. For IQ, an analysis of covariance was used to covary for test version. SAPS/SANS sums of item scores between FEP subgroups were assessed across clinical time points with Generalised Estimating Equations. Antipsychotic dosages, CDSS scores and the time period in months between scan and nearest symptom evaluation were assessed between the three patient groups at each scan time point, using one-way analyses of variance for normally distributed variables, and Kruskall–WallisH-tests for non-normally distributed variables. Analyses of clinical variables were conducted using PASW Statistics 21 (SPSS, 2009, Chicago, IL, USA) and were two-tailed with a critical P-value of 0.05.
Neuroanatomical analyses: volume
For scans that passed quality control (see Supplementary Methods), volumetric differences between FEP subgroups and controls were assessed using linear mixed effects models applied to each structure and hemisphere separately using Matlab (2015a). Gross volumetric differences in structure were assessed with the following model:
where Y represents whole left/right amygdalar/hippocampal volume, d1is the random within-subjects effect, β1–5 represents regression coefficients and ε is residual error. Linear age effects were then examined separately by adding the following term to the above model: ‘β6(group × age)’. To control for multiple comparisons, the false discovery rate (FDR) procedure was used with q=0.05, which limits the expected proportion of incorrectly rejected null hypotheses to 5%.49
Neuroanatomical analyses: SA
To assess differences in shape morphometry between groups, statistics were performed across all vertices of bilateral amygdalar and hippocampal surfaces using the SurfStat toolbox within Matlab (http://www.math.mcgill.ca/keith/surfstat/). Each hemisphere was assessed separately, using an equivalent mixed effects model as described in the previous section, covarying for total SA of the structure by hemisphere in place of total brain volume (β5 (Total SA)). Similarly, the main effect of group was first tested, followed by linear age × group interactions. For all analyses, statistical maps were thresholded and multiple comparisons were taken into account using random field theory for non-isotropic images,50 limiting the chance of reporting a false positive finding to below P=0.05.
Supplementary linear mixed effects models with altered covariates
Four additional models were tested with altered covariates, to explore effects of different variables, in addition to the chosen covariates of sex, handedness and total brain volume/total SA. These four altered models were as follows: (A) covarying for diagnosis, (B) covarying for antipsychotic medication in the FEP patient sample only; as described above, antipsychotic dosages were converted to chlorpromazine equivalents and took into account medication adherence, (C) removal of sex and handedness and (D) covarying for IQ (note two controls were excluded from this analysis, given missing IQ information). The rationale behind analyses C was motivated by the fact that our groups did not significantly differ on sex and handedness. These variables were kept in the main model presented in this manuscript, given the well-documented and clear impact of sex and handedness on neuroanatomy.51, 52, 53 However, recent evidence has not found support for the effects of handedness on cerebral anatomy.54 With respect to sex differences, Pruessner et al.55 reported no sex differences in amygdalar and hippocampal volumes. Thus, it is of interest to investigate how significant findings may be altered when these variables are removed from the model.
Results
Sociodemographic and clinical
In the FEP group, baseline scans were performed on average 4.1 (s.d.=1.9) months after entry to PEPP. For the entire group, including controls, interscan intervals were ~13.1 (s.d.=1.3) months between baseline and FUP1, and 12.5 (s.d.=1.7) months between FUP1 and FUP2. Nine participants (six FEP and controls) were not scanned at FUP1 but were scanned at FUP2; average interscan interval was 26.7 (s.d.=3.1) months between baseline and FUP2.
The groups did not significantly differ in sex ratio, handedness, parental socioeconomic status or age at scanning time (see Table 1). However, controls significantly differed from all patient groups on Full-Scale IQ and years of education. Within the three patient groups, there were no significant differences in CDSS scores or time elapsed between the magnetic resonance imaging scan and symptom evaluation. As expected, the sPNS patient subgroup had significantly higher SAPS totals compared with the ePNS and non-PNS subgroups across baseline and 1-year follow-up. In addition, the ePNS and sPNS subgroups had significantly higher SANS totals compared with the non-PNS subgroup across all time points. See Supplementary Table 1 and Supplementary Figure 4 for breakdown of SAPS/SANS scores across clinical time points and relevant statistics. FEP subgroups differed in distribution of diagnosis, with a higher proportion of non-PNS diagnosed with affective psychotic disorders (major depression, bipolar), and higher proportions of schizophrenia/schizophreniform diagnoses in the sPNS and ePNS subgroups. In addition, amount of antipsychotic prescribed at the second scanning time point was significantly higher for sPNS patients compared with non-PNS patients; thus, diagnosis and antipsychotic medication were included as covariates in supplemental analyses (see Supplementary Figure 5).
Table 1. Demographic and clinical information for longitudinal sample.
|
FEP |
|||||||
|---|---|---|---|---|---|---|---|
|
Non-ePNS |
|||||||
| ePNS | sPNS | Non-PNS | Controls | Statistic(df) | P-value | ||
| General demographics | N(+ subset with three scans) | 21 (18) | 30 (15) | 44 (27) | 44 (24) | ||
| Male, N (%) | 15 (71.4) | 21 (70.0) | 31 (70.5) | 25 (56.8) | χ2(3)= 2.5 | 0.5 | |
| Education in years | 11.1 (2.5) | 11.6 (2.4) | 12.7 (2.4) | 14.2 (2.5) | F(3,138)=10.4 | <0.001a | |
| Socioeconomic status | 3.4 (1.0) [16] | 3.4 (1.1) [29] | 3.0 (1.0) [42] | 3.4 (0.9) [41] | χ2(3)= 6.1 | 0.1 | |
| Right handed, N (%) | 17 (81.0) | 25 (83.3) | 38 (86.4) | 38 (86.4) | χ2(3)= 0.5 | 0.9 | |
| Full scale IQb | 96.9 (15.3) | 97.8 (15.3) | 100.3 (15.3) | 111.5 (15.3) [42] | F(3,136)=7.1 | <0.0001a | |
| Diagnosis**, N (%) | χ2(6)= 13.1 | 0.017 | |||||
| Schizophrenia/schizophreniformc | 16 (76.2) | 26 (86.7) | 24 (54.5) | ||||
| Affective disorder | 3 (14.3) | 1 (3.3) | 15 (34.1) | ||||
| Delusional disorder | 0 (0) | 1 (3.3) | 2 (4.5) | ||||
| Psychosis not otherwise specified | 2 (9.5) | 2 (6.7) | 3 (6.8) | ||||
| Scan 1 | Age | 23.2 (3.6) | 24.5 (4.0) | 4.6 (0.7) | 23.8 (3.5) | F(3,138)=1.0 | 0.4 |
| Window |Scan−Symptom Eval| (months) | 0.6 (0.4) | 0.8 (0.5) | 0.7 (0.6) | F(2,94)=0.5 | 0.6 | ||
| SAPS global | 3.6 (3.8) | 6.6 (3.9) | 2.4 (2.6) | χ2(2)= 22.2 | <0.0001d | ||
| SANS global | 9.6 (2.9) | 9.0 (3.5) | 5.9 (3.2) | F(2,94)=13.3 | <0.0001e | ||
| CDSS | 2.4 (2.7) | 3.1 (3.2) | 1.7 (2.5) [43] | χ2(2)= 3.4 | 0.2 | ||
| CPZ equivalent (in mg) | 758.4 (671.3) | 965.9 (844.6) | 774.2 (707.9) | χ2(2)=9.7 | 0.6 | ||
| Adherence (%) | 86.6 (21.3) | 87.9 (19.1) | 84.6 (27.5) | χ2(2)= 0.2 | 0.9 | ||
| Antidepressant, N(%) | 2 (9.5) | 5 (16.7) | 9 (20.5) | ||||
| Benzodiazepine, N (%) | 1 (4.8) | 4 (13.3) | 2 (4.5) | ||||
| Anticholinergic, N (%) | 1 (4.8) | 5 (16.7) | 6 (13.6) | ||||
| Mood stabiliser, N (%) | 1 (4.8) | 0 (0) | 7 (15.9) | ||||
| Scan 2 | N | 19 | 28 | 41 | 41 | ||
| Age | 24.3 (3.8) | 25.5 (4.1) | 25.6 (4.3) | 24.7 (3.4) | F(3,128)=0.7 | 0.5 | |
| Window |Scan−Symptom Eval| (months) | 1.8 (1.5) | 2.1 (1.7) | 1.8 (1.2) | χ2(2)= 0.3 | 0.9 | ||
| SAPS global | 2.7 (2.5) | 5.7 (4.0) | 1.6 (2.5) | χ2(2)= 24.3 | <0.0001d | ||
| SANS global | 8.4 (3.5) | 7.9 (3.6) | 3.3 (3.2) | F(2,87)=22.8 | <0.0001e | ||
| CDSS | 1.0 (1.5) | 1.9 (3.0) [27] | 1.4 (2.7) | χ2(2)= 1.7 | 0.4 | ||
| CPZ equivalent (in mg) | 2875.2 (2059.7) | 4434.7 (3337.6) | 2656.8 (2187.0) | χ2(2)=10.3 | 0.006f | ||
| Adherence (%) | 87.0 (16.0) | 80.0 (19.9) | 81.1 (25.5) | χ2(2)=1.3 | 0.5 | ||
| Antidepressant, N (%) | 4 (23.5) [17] | 6 (22.2) [27] | 10 (25.0) [40] | ||||
| Benzodiazepine, N(%) | 0 (0) [16] | 3 (11.1) [27] | 1 (2.4) | ||||
| Anticholinergic, N (%) | 1 (6.3) [16] | 1 (3.7) [27] | 2 (4.9) | ||||
| Mood stabiliser, N(%) | 2 (12.5) [16] | 0 (0) [27] | 5 (12.2) | ||||
| Scan 3 | N | 20 | 17 | 29 | 27 | ||
| Age | 25.5 (3.7) | 26.2 (3.7) | 26.3 (4.4) | 26.9 (3.5) | F(3,92)=0.5 | 0.6 | |
| Window |Scan−Symptom Eval| (months) | 1.0 (1.9) | 0.4 (0.5) | 0.5 (0.7) | χ2(2)= 0.8 | 0.7 | ||
| SAPS global | 3.0 (3.0) | 4.1 (4.1) | 2.0 (2.4) | χ2(2)= 3.1 | 0.2 | ||
| SANS global | 7.2 (3.6) | 5.6 (4.0) | 3.0 (3.5) | χ2(2)= 16.9 | <0.0001e | ||
| CDSS | 2.5 (3.3) [18] | 2.1 (2.5) | 1.6 (2.1) [28] | χ2(2)= 0.7 | 0.7 | ||
| CPZ equivalent (in mg) | 4216.5 (3906.2) | 6753.8 (6368.0) | 5177.0 (4994.3) | χ2(2)= 2.0 | 0.4 | ||
| Adherence (%) | 78.4 (26.2) | 78.3 (27.9) | 77.1 (28.7) | χ2(2)= 0.05 | 0.98 | ||
| Antidepressant, N (%) | 5 (26.3) [19] | 3 (21.4) [14] | 3 (11.5) [26] | ||||
| Benzodiazepine, N(%) | 0 (0) [19] | 0 (0) [14] | 0 (0) [26] | ||||
| Anticholinergic, N (%) | 2 (10.5) [19] | 0 (0) [15] | 0 (0) [27] | ||||
| Mood stabiliser, N (%) | 1 (5.3) [19] | 1 (7.1) [14] | 6 (25.0) [24] | ||||
Abbreviations: CDSS, Calgary Depression Scale for Schizophrenia; CPZ, chlorpromazine; ePNS, early persistent negative symptom; FEP, first-episode of psychosis; FUP, follow-up; SAPS/SANS, Scale for Assessment of Positive/Negative Symptoms; sPNS, persistent negative symptoms due to secondary factors.
General demographics for whole sample are presented, followed by information corresponding to each scan. All data represented as mean (s.d.), unless otherwise specified. Levene’s test revealed no significant differences in variance between subgroups. Square brackets [] include adjusted sample size included in statistical analysis because of missing data points. All antipsychotic totals are presented as cumulative chlorpromazine equivalents in mg, as prescribed by a psychiatrist, and are reported along with corresponding medication adherence percentages. SAPS/SANS totals are presented as the mean scores of the sum of item-level scores. Note that ‘SANS total’ excludes the ‘attention’ subscale.
Post hoc comparisons showed that controls differed from all FEP patient groups in years of education (P<0.005) and IQ (P<0.01). IQ differences were covaried by test version. No differences existed between patient groups.
IQ means and s.d. are presented as adjusted values, covaried by test version (WAIS-III versus WASI). There was no difference between different test versions on IQ (F1,136=0.9, P=0.3).
Assessed using Fisher’s exact test of independence.
Tukey’s post hoc comparisons revealed significant differences in SAPS scores between sPNS and other two patient groups (P<0.005).
Tukey’s post hoc comparisons revealed significant differences in SANS scores between ePNS and sPNS and remaining non-PNS patients (P<0.001) for Scans 1 and 2. For Scan 3, non-PNS still significantly differed from ePNS (P=0.001), but there was only a trend-like difference between non-PNS and sPNS (P=0.08).
Post hoc analyses indicated that sPNS patients were prescribed significantly more antipsychotic medication (in CPZ equivalent dosage) cumulatively compared with non-PNS patients at Scan 2 (P=0.02), and was still significant when taking into consideration medication adherence (multiplying CPZ equivalent by percent adherence), with χ2(2)=6.2, P=0.046 (post hoc sPNS>non-PNS P=0.03). No significant differences emerged between the ePNS group and other FEP subgroups at Scan 2.
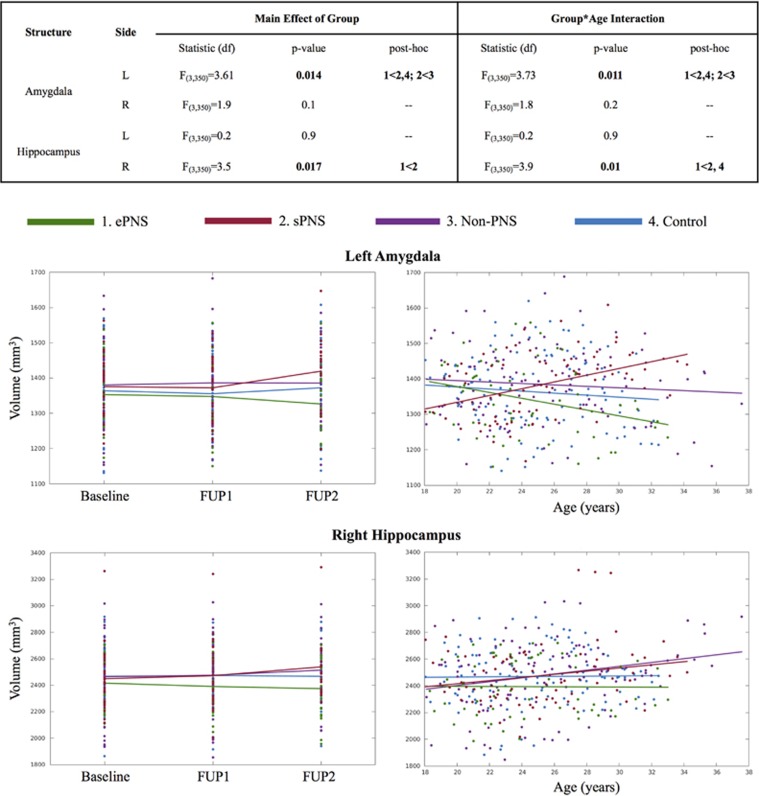
Amygdalar and hippocampal volumetry
Descriptive statistics of left/right amygdalar and hippocampal volumes adjusted for age, sex, total brain volume and handedness are outlined in Table 2. After FDR correction, linear mixed models revealed significant group differences for right amygdalar volumes (F3,350=3.61, P=0.014) and hippocampal volumes (F3,350=3.5, P=0.017). Significant effects also emerged for age × group interactions for left amygdalar volumes (F3,350=3.73, P=0.011), as well as the right hippocampus (F3,350=3.9, P=0.010). Post hoc tests (all P<0.05) showed that within the left amygdala, the sPNS group had a significantly different and positive correlation with age compared with ePNS patients and controls. The non-PNS group also had a significantly different slope with age compared with ePNS patients. For the right hippocampus, the ePNS group had a significantly different negative correlation with age compared with sPNS patients and Controls. No significant group or age × group differences emerged for the right amygdala or left hippocampus (Figure 1).
Table 2. Amygdalar and hippocampal volumes: descriptives.
|
FEP |
||||||
|---|---|---|---|---|---|---|
|
Non-ePNS |
||||||
| Time point | Structure | Side | (1) ePNS | (2) sPNS | (3) Non-PNS | (4) Controls |
| Baseline (scan 1) | Amygdala | L | 1386.9 (22.6) | 1358.7 (18.6) | 1362.2 (15.4) | 1377.4 (15.5) |
| R | 1404.5 (22.0) | 1370.5 (18.1) | 1381.7 (15.0) | 1384.6 (15.0) | ||
| Hippocampus | L | 2488.3 (52.0) | 2452.1 (42.8) | 2517.4 (35.4) | 2536.1 (35.5) | |
| R | 2471.0 (52.7) | 2421.4 (43.4) | 2433.9 (35.9) | 2490.9 (36.0) | ||
| FUP1 (scan 2) | Amygdala | L | 1384.6 (23.4) | 1359.9 (19.0) | 1365.7 (15.8) | 1367.3 (15.8) |
| R | 1398.5 (22.9) | 1362.9 (18.6) | 1383.7 (15.4) | 1395.8 (15.5) | ||
| Hippocampus | L | 2486.5 (52.2) | 2485.6 (42.4) | 2530.3 (35.1) | 2530.8 (35.2) | |
| R | 2446.7 (54.5) | 2453.8 (44.3) | 2443.2 (36.7) | 2492.1 (36.8) | ||
| FUP2 (scan 3) | Amygdala | L | 1371.1 (25.7) | 1363.7 (27.5) | 1366.1 (20.7) | 1395.2 (21.7) |
| R | 1394.2 (24.3) | 1379.2 (26.0) | 1390.4 (19.5) | 1403.9 (20.5) | ||
| Hippocampus | L | 2499.5 (52.6) | 2457.5 (56.3) | 2557.4 (42.3) | 2567.8 (44.3) | |
| R | 2448.7 (53.2) | 2453.7 (56.9) | 2483.4 (42.7) | 2499.3 (44.8) | ||
Abbreviations: ePNS, early persistent negative symptom; FEP, first-episode of psychosis; FUP, follow-up; L, left; R, right; sPNS, persistent negative symptoms due to secondary factors.
Mean hippocampal and amygdalar volumes are adjusted for total brain volume, age, sex and handedness, with s.e. in brackets. There were no differences in volumes between groups when looking at the data cross-sectionally.
Figure 1.
Amygdalar and hippocampal volumes: significant group main effects and group × age interactions. Table presents statistics from linear mixed effects analyses. Significant results that survived FDR correction for multiple comparisons are indicated in bold. Post hoc contrasts were based on the four groups: (1) ePNS, (2) sPNS, (3) non-PNS and (4) Controls. Significant group and group × age contrasts are depicted in the corresponding graphs below the table. ePNS, early persistent negative symptoms; FUP, follow-up; sPNS, persistent negative symptoms due to secondary factors.
Hippocampal and amygdalar shape morphometry—vertex-wise results
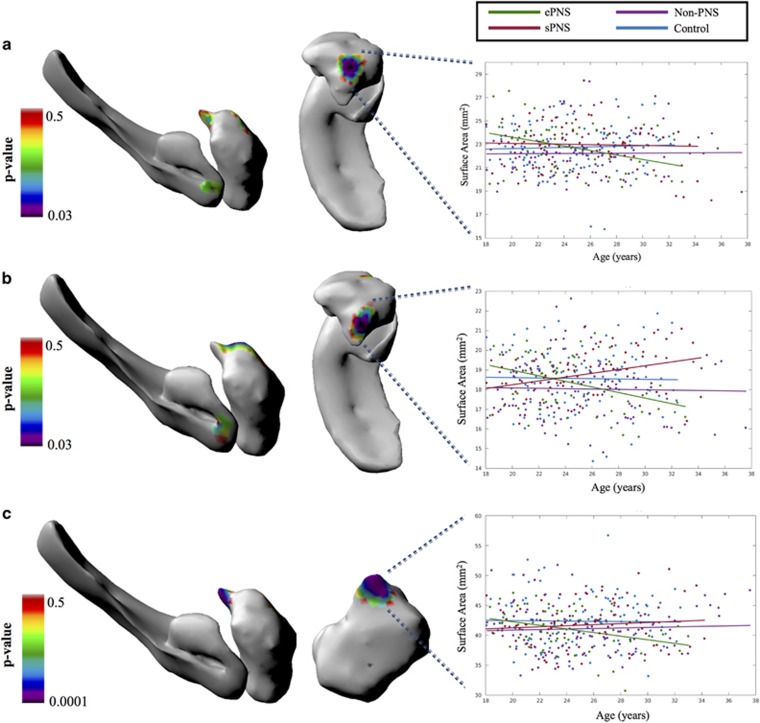
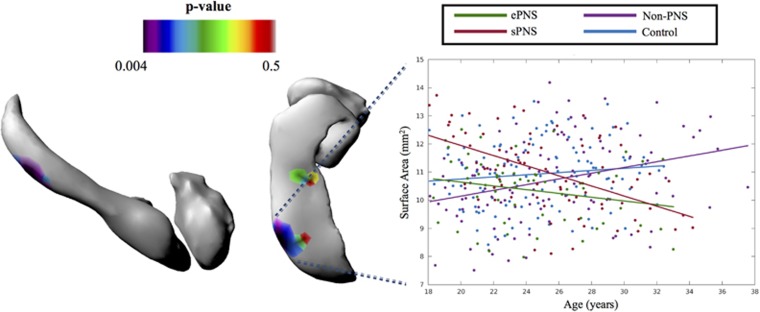
There were no significant main effects of group for either structure bilaterally. However, significant findings emerged with the age × group interaction. For the left amygdala, the following contrasts and regions had significantly different SA trajectories with age comparing groups on vertex-wise SA: (1) ePNS<non-PNS within a central/anterior cluster (Figure 2a), (2) ePNS<sPNS and Controls in a more dorsocentral region (Figure 2b) and (3) ePNS<Controls within a posterior/centromedial portion (Figure 2c). For the right hippocampus, a significant cluster emerged in a portion of the hippocampal tail comparing SA trajectories with age between non-PNS patients and the other FEP subgroups; specifically, the ePNS group had a significant negative relationship with age in this cluster compared with sPNS and non-PNS subgroups, and the sPNS group also exhibited a significantly different and opposite trajectory compared with non-PNS patients and controls (P⩽0.001; Figure 3). No significant age × group interactions were found for SA across the left hippocampus or right amygdala. Controlling for diagnosis, antipsychotic medication and IQ did not significantly change the interpretation of results. Similarly, removing handedness and sex as covariates in the linear mixed effects model did not alter results, with the exception of one SA cluster of the left central amygdala, which did not survive correction for multiple comparisons with random field theory after removing sex and handedness as covariates, namely when comparing age trajectories between ePNS and non-PNS patients. See Supplementary Table 2 and Supplementary Figure 5 for results with altered covariates for volumetry and shape morphometry, respectively.
Figure 2.
Significant group × age interactions in surface area of the left hemisphere. Statistical maps overlaid on left hippocampal and amygdalar 3D surface renderings represent RFT-corrected P-values. Surface area metrics from clusters with a corrected P-value less than 0.05 were extracted and plotted against age for each group. (a) Significantly decreased surface area with age in the ePNS group compared with non-PNS within a central/anterior region of the left amygdala (292 df, P=0.03). Comparison of regression slopes with age reveals similar effects comparing with controls as well (F(3,350)=5.01, P=0.002; post hoc ePNS<Controls P=0.0011). (b) Significantly decreased surface area with age in the ePNS group compared with sPNS in a more dorsal region (compared with A) of the central amygdala (292 df, P=0.03). Further comparison with other groups revealed significant differences in regression slopes with age (F(3,350)=4.43, P=0.0045), such that the ePNS had a significantly reduced relationship between age and surface area in this cluster compared with both non-PNS and Controls (P⩽0.01). (c) Significantly decreased surface area with age in the ePNS group compared with Controls in a posterior (centromedial) portion of the amygdala (298 df, P=0.0001). Mixed effects statistics for comparison of regression slopes yielded F(3,350)=4.19, P=0.006, where no other contrasts apart from Controls versus ePNS were significant. No significant interaction effects with age emerged for the left hippocampus. Orientation: from left to right, surfaces depict left medial view and dorsal view of the hippocampus and amygdala, with the exception of c, where the dorsal view has been replaced by a posterior view of the amygdala for better visualisation of the significant cluster. df, degree of freedom; ePNS, early persistent negative symptom; RFT, random field theory; sPNS, persistent negative symptoms due to secondary factors.
Figure 3.
Significant group × age interactions in surface area of the right hemisphere. Statistical maps overlaid on left hippocampal and amygdalar 3D surface rendering represent RFT-corrected P-values. Significant cluster emerged with increased surface area with age in non-PNS compared to sPNS and ePNS (P<0.01) in a posterior/ventral portion of the hippocampus. Further comparison to other groups revealed significant differences in regression slopes with age (F(3,350)=7.04, P<0.001), such that the ePNS group had a significantly negative relationship between surface area in this cluster and age compared to sPNS and non-PNS (P⩽0.001). In addition to sPNS differing from non-PNS, sPNS patients also had a significantly different positive slope compared with Controls (P<0.001). No significant interaction effects with age emerged for the right amygdala. Orientation: from left to right, surfaces depict right lateral view, followed by ventral view of hippocampal and amygdalar structures. ePNS, early persistent negative symptoms; RFT, random field theory; sPNS, persistent negative symptoms due to secondary factors.
Hippocampal and amygdalar shape morphometry—post hoc region-of-interest results
For the four significant clusters that emerged through a vertex-wise investigation of SA described above, total SA values were extracted for each of the regions and regression slopes were calculated for each of the four groups (ePNS, sPNS, non-PNS and Controls), to see whether any other groups differed at the regional level. For the first central/anterior amygdalar cluster, in addition to differing from non-PNS patients, the ePNS group had significantly different regression slopes from Controls (Omnibus: F(3,350)=5.01, P=0.002; post hoc ePNS<Controls P=0.0011; Figure 2a). For the second amygdalar region (dorsal to the first), ePNS had a significantly reduced relationship between age and SA in this cluster compared with both non-PNS and Controls (Omnibus: F3,350=4.43, P=0.0045; post hoc P⩽0.01), in addition to the previously reported vertex-wise difference between ePNS and sPNS (Figure 2b). Finally, the last significant amygdalar cluster, localised more posteriorly and centromedially, did not uncover any additional significant relationships apart from the previously reported difference between ePNS and Controls (Omnibus: F3,350=4.19, P=0.006; Figure 2c).
For the single significant right hippocampal cluster, furtherpost hoc analyses of regression slopes revealed additional differences between sPNS patients and controls (Omnibus: F3,350=7.04, P<0.001; post hoc sPNS<Control, P<0.001; Figure 3).
Discussion
The current study provides evidence for changes in AG–HC structural trajectories, specifically in FEP patients presenting with PNS. Volumetric findings within the left amygdala and right hippocampus indicated that ePNS patients had significantly different/decreased relationships with age compared with non-PNS patients and controls, in addition to having significantly reduced volumes within these structures. SA findings were similarly lateralised, where the most prominent direction of findings emerged with significant contraction with age in ePNS across several amygdalar regions and a posterior hippocampal cluster. Furthermore, the sPNS group showed significantly decreased SA with age within the latter hippocampal region in opposition to the notable expansion with age examined in non-PNS patients and controls. Noteworthy, non-PNS patients never differed from healthy controls. Results remained largely unaltered when covarying for IQ, diagnosis and antipsychotic dosage, and removing sex and handedness from the model.
The differential and striking trajectories uncovered in relation to negative symptom presentation within AG–HC structure encourage further exploration of dynamic brain changes in different psychiatric samples, as others have suggested.56, 57 Nacewicz et al.58 explored such age effects on the amygdala in an autistic sample, and found significantly lower amygdalar volumes in older individuals with autism, not unlike the amygdalar trajectories found within our ePNS group. Given the parallels that can be drawn between flattened affect and social impairments in autism with symptom presentation in ePNS, the amygdala represents a plausible target for future transdiagnostic work. At the level of the hippocampus, associations have been previously drawn between lower hippocampal volumes and poor functioning in FEP,59 lending support to our findings of decreased right hippocampal volumes in patients with ePNS and corresponding negative trajectories with age.
Notably, only age × group interactions on specific regions of AG–HC shape morphometry-yielded significant results, concordant with previous claims that differences in SA morphology may represent a dynamic and neurodevelopmental endophenotype.29, 30, 60, 61, 62, 63 Few studies have looked at AG–HC shape morphology in psychosis, although relevant objectives were investigated in the work of Qiu et al.22 The authors found significant surface alterations within the left hippocampal tail and right hippocampal body, with first-episode schizophrenia patients exhibiting greater inward deformations compared with first-episode mania and controls. This is consonant with the significant inward deformations consistently seen in our ePNS group, containing a higher distribution of patients diagnosed with schizophrenia/schizophreniform (as opposed to an affective disorder). In fact, controlling for diagnosis in our analyses strengthened the statistical significance of shape deformation clusters.
The consistent lateralisation of results observed across volumetric and morphometric analyses deserves discussion. Although studies in FEP and schizophrenia have uncovered differences bilaterally, many findings in psychosis have been skewed to the left hemisphere.18, 64, 65, 66 In contrast, our findings within the hippocampus were right-lateralised and were specific to patients with PNS. Witthaus et al.67 reported similarly lateralised findings in an investigation of patients at ultra-high risk for psychosis and those who transitioned to having a FEP. Specifically, this study pinpointed lower volumes in a subset of ultra-high risk patients who transitioned to psychosis within the left amygdala, and lower right hippocampal volumes in FEP patients. These consistent findings at similar stages of psychosis suggest that lateralisation of limbic structural volume differences may reflect an early biomarker underlying the manifestation of psychosis and subsequent negative symptomatology. The localisation of deformation differences also overlapped with our initial hypotheses, where significant differences emerged in a medial region of the amygdala, a region posited to have ‘striatal-like’ features with GABAergic-containing neural circuitry.32, 68 We also observed hippocampal morphometric differences closer to the output region of the structure, in line with previous studies.22, 31, 33 Although not initially hypothesised, significant differences were uncovered within central regions of the amygdala, which has an integral role in forming associations between stimuli on the basis of their motivational salience, ultimately shaping emotional behaviours.69, 70 Thus, aberrancies in the development of these key regions involved in emotion and motivational behaviours may contribute to the differential expression of negative symptoms exhibited by ePNS and sPNS patients, although further work is needed to understand the mechanisms underlying the manifestation of symptoms in these two groups.
Another noteworthy point is the significantly different trajectories uncovered within the sPNS group, particularly within the hippocampus. We had initially hypothesised that the ePNS group would show the most significant changes, and we had not expected such marked effects in the sPNS group, meriting additional dialogue on the potential effect of positive symptoms on the limbic structure, and differential changes with age. Links between hippocampal shape and positive symptomatology were recently addressed by Mamah et al.,33 in which higher levels of disorganised positive symptoms were significantly correlated with surface contraction in the lateral CA1 hippocampal subregion. Depressive symptomatology also may have contributed to results within the sPNS group. For instance, previous work71 has suggested that amygdalar volume reductions seem to be specific to the intersection of psychosis and depression. Other work has corroborated evidence for hippocampal shape changes in depression.72 Further longitudinal investigation of positive and depressive symptomatology in relation to amygdalar and hippocampal structure is warranted to disentangle the specific contributions of different symptom domains.
Several studies have refuted the idea of progressive structural changes in the hippocampus and amygdala after the onset of psychosis.17, 73, 74 However, our findings suggest that null findings may be a result of pooling together patients into a unitary group and simply comparing with healthy controls, which would be similarly found in our sample if our three FEP subgroups were merged. The age window at which a FEP is experienced has large consequences for the social developmental stage of the individual, and it comes as no surprise that these effects may be manifested differentially with age at the level of limbic structure. Neurodevelopmental models of psychosis-related disorders are increasingly beginning to interlace psychosocial and biological factors into a coherent model to facilitate treatment,75 which requires further understanding of potential gene × environment interactions on neurobiology to help us fully understand the psychological and biological mechanisms contributing to PNS following a FEP.
It is worth discussing the chosen categorical approach, as opposed to the often-used ‘dimensional’ approach of regressing symptom severity against neuroanatomical measures. Although meaningful information can certainly be derived by the latter approach, symptom data are often not normally distributed, and the amount of clinical information used in such brain–behaviour relationships is often limited by the imaging data. Given that the current study design had a greater number of clinical time points (that is, 5+) compared with imaging time points (that is, 2–3), regression analyses would have restricted the presented analysis to the available imaging data, and important dynamic information regarding the longitudinal course of symptoms across different domains would have been lost. Thus, the adopted approach capitalises on the clinical data available in linking symptoms to neuroanatomical trajectories within the amygdala and hippocampus. Finally, our findings suggest that the resultant subgroups of patients do indeed seem to have distinct biological underpinnings in AG–HC structure, and such an approach in defining patient subgroups independent of diagnostic categories may inform and/or contribute to pending changes in diagnostic categories that have often been criticised for lacking biological validity. Although future work is certainly required to gain more confidence in the validity of the proposed subgroups, this approach holds promise in bringing to the forefront meaningful clinical subtypes of patients who have experienced a FEP and addressing the clinical picture surrounding negative symptom presentation.
The current study offers several strengths and limitations. Recruitment strategies have been optimised for this large sample of FEP patients such that all patients were recruited from a single well-defined catchment area in the absence of other competing services. Furthermore, a wealth of longitudinal data is available for these patients, including data from structured clinical assessments to complement neuroimaging data. However, there are inherent limitations to the manner by which early and secondary PNS groups were separated. Given that the latter group exhibits treatment resistance, and arguably, poor outcome similarly to ePNS patients, future investigations should try to incorporate additional behavioural and clinical data to disentangle neurobiological findings. It is possible that these two subgroups of patients have overlapping neuroanatomical features that were not directly addressed by this study. There was also an uneven drop-out rate among the FEP subgroups for the neuroimaging portion of the study, with the highest attrition observed in the sPNS group. Finally, imaging was acquired on a 1.5-T scanner, which prevented us from reliably resolving hippocampal subfield structure, an emerging interest in the study of neuropsychiatric disorders.65, 76, 77, 78, 79
There have been a wide array of findings pinpointing aberrant AG–HC structure in psychotic disorders, with scant research looking at specific symptom constructs in psychosis, and further localising changes with surface morphometry. The current study addresses these gaps in the literature and elucidates differential volumetric and shape morphometric trajectories with age within lateralised regions of the amygdala and hippocampus, in relation to persistent negative symptoms in psychosis. These findings suggest potential neurodevelopmental aberrations that coincide with negative symptom presentation, and could represent dynamic endophenotypes underlying patient subgroups within heterogeneous first-episode psychosis populations. As alluded to, current pharmacological interventions have poor efficacy on negative symptoms, and a better understanding of the biological mechanisms and anatomical/functional consequences underlying such symptom presentation may allow for better and more targeted design of future medications. In parallel with an improved description of what is occurring at the neural level, it will be important to test concrete behavioural measures, such as verbal memory, to unravel the effects of therapeutic interventions in early psychosis and other implicated neuropsychiatric conditions on potential improvement of negative symptoms. Improved models of brain–behaviour relationships alongside clinical descriptors of negative symptoms hold promise in translational research and dissipating the status of negative symptoms as a largely unmet therapeutic need in psychotic disorders, especially for more prevalent domains of negative symptoms encompassing avolition, asociality and anhedonia.
Acknowledgments
We would like to thank Raihaan Patel, Gabriel Devenyi and Min Tae M Park (Cerebral Imaging Centre, Douglas Mental Health Institute, McGill University, Montreal, Canada) for their help with image processing, quality control procedures and analysis. We also thank all PEPP-Montreal research staff for their efforts in recruitment and clinical data collection. Finally, we are grateful to all patients and families for participating in the study. The study was supported by operating grants from the Canadian Institutes of Health Research (CIHR; #68961) and the Sackler Foundation to Drs M Lepage/AK Malla. Salary awards include: Fonds de la Recherche en Santé du Québec (FRSQ; CM, ML, MMC and RJ), James McGill Professorship (ML) and Canada Research Chairs Programme (AKM).
Disclaimer
The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; nor in the decision for publication.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp).
M Lepage reports having received financial assistance/compensation for research and educational events from Janssen-Ortho, Eli Lilly, Roche and Otsuka/Lundbeck Alliance. AK Malla reports having received financial assistance/compensation for research and educational activities from Pfizer, Janssen-Ortho, AstraZeneca and Bristol-Myers Squibb. R Joober reports having received consultancy honorariums from Pfizer and Janssen-Ortho. The remaining authors declare no conflicts of interest.
Supplementary Material
References
- Brown RG, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci 2000; 23: 412–417. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry 1982; 39: 784–788. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Hovington CL, Buchy L, Malla AK, Joober R, Lepage M. Cortical thinning in temporo-parietal junction (TPJ) in non-affective first-episode of psychosis patients with persistent negative symptoms. PLoS ONE 2014; 9: e101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 2007; 33: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M. Identifying persistent negative symptoms in first episode psychosis. BMC Psychiatry 2012; 12: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit A, Bodnar M, Malla AK, Joober R, Lepage M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry 2012; 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski C, Bodnar M, Malla AK, Joober R, Lepage M. Age-related cortical thickness trajectories in first episode psychosis patients presenting with early persistent negative symptoms. NPJ Schizophr 2016; 2: 16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004; 14: 198–202. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: effects of emotion. Emot Rev 2009; 1: 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS et al. A validated network of effective amygdala connectivity. Neuroimage 2007; 36: 736–745. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 2011; 223: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DR, Bai F, Barrett SL, Turkington A, Rushe TM, Mulholland CC et al. Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav 2012; 6: 49–60. [DOI] [PubMed] [Google Scholar]
- Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M. Impairment in verbal memory observed in first episode psychosis patients with persistent negative symptoms. Schizophr Res 2013; 147: 223–229. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F et al. Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150: 59–65. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 2004; 21: 1563–1575. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 2004; 174: 151–162. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Velakoulis D, Smith DJ, Bond D, Stuart GW, McGorry PD et al. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res 2001; 52: 37–46. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Malla AK, Czechowska Y, Benoit A, Fathalli F, Joober R et al. Neural markers of remission in first-episode schizophrenia: a volumetric neuroimaging study of the hippocampus and amygdala. Schizophr Res 2010; 122: 72–80. [DOI] [PubMed] [Google Scholar]
- Lepage M, Sergerie K, Benoit A, Czechowska Y, Dickie E, Armony JL. Emotional face processing and flat affect in schizophrenia: functional and structural neural correlates. Psychol Med 2011; 41: 1833–1844. [DOI] [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry 2007; 64: 1356–1366. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Hoschl C. Amygdala volumes in mood disorders—meta-analysis of magnetic resonance volumetry studies. J Affect Disord 2009; 115: 395–410. [DOI] [PubMed] [Google Scholar]
- Qiu A, Gan SC, Wang Y, Sim K. Amygdala-hippocampal shape and cortical thickness abnormalities in first-episode schizophrenia and mania. Psychol Med 2013; 43: 1353–1363. [DOI] [PubMed] [Google Scholar]
- Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry 2017; 22: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ, Žukauskienė R, Sugimura K. The new life stage of emerging adulthood at ages 18–29 years: implications for mental health. Lancet Psychiatry 2014; 1: 569–576. [DOI] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD et al. Age-related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp 2016; 37: 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K. Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum disorder and schizophrenia. J Pharmacol Exp Ther 2016; 358: 504–515. [DOI] [PubMed] [Google Scholar]
- Kalmady SV, Venkatasubramanian G, Shivakumar V, Gautham S, Subramaniam A, Jose DA et al. Relationship between interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naïve schizophrenia: evidence for differential susceptibility? PLoS ONE 2014; 9: e96021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Winterburn JL, Felsky D, Pipitone J, Rajji TK, Mulsant BH et al. Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan. Hum Brain Mapp 2015; 36: 3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci USA 2014; 111: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Rapoport JL, Giedd JN, Raznahan A, Shaw P, Collins DL et al. Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: a longitudinal study. Hum Brain Mapp 2015; 36: 1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 2011; 12: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH. Inhibitory networks of the amygdala for emotional memory. Front Neural Circ 2013; 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Alpert KI, Barch DM, Csernansky JG, Wang L. Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. Neuroimage Clin 2016; 11: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Jordan G, MacDonald K, Joober R, Malla A. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis 2015; 203: 356–364. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P V and SCID-I/NP Version 20). Biometric Research Department: New York, NY, USA, 1998. [Google Scholar]
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res 1990; 3: 247–251. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). University of Iowa: Iowa City, IA, USA, 1984. [Google Scholar]
- Andreasen NC. Modified Scale for the Assessment of Negative Symptoms (SANS). University of Iowa: Iowa City, IA, USA, 1984. [Google Scholar]
- Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull 2015; 41: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy CM, Rabinovitch M, Schmitz N, Joober R, Malla A. A comparison study of multiple measures of adherence to antipsychotic medication in first-episode psychosis. J Clin Psychopharmacol 2010; 30: 64–67. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-Factor Index of Social Position. Yale University Press: New Haven, CT, USA, 1965. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wecshler Adult Intelligence Scale. 3rd edn, The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Pipitone J, Park MT, Winterburn J, Lett TA, Lerch JP, Pruessner JC et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 2014; 101: 494–512. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 2015; 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN et al. A novel in vivo atlas of human hippocampal subfields using high-resolution 3T magnetic resonance imaging. Neuroimage 2013; 74: 254–265. [DOI] [PubMed] [Google Scholar]
- Makowski C, Béland S, Kostopoulos P, Bhagwat N, Devenyi GA, Malla AK et al. Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: comparing automated approaches to manual delineation. NeuroImage 2017; S1053-8119: 30180-5. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289–300. [Google Scholar]
- Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage 2004; 23(Suppl 1): S189–S195. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage 2001; 14: 685–700. [DOI] [PubMed] [Google Scholar]
- Li W, van Tol M-J, Li M, Miao W, Jiao Y, Heinze H-J et al. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp 2014; 35: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, der Haegen LV, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci 2014; 15: 193–201. [DOI] [PubMed] [Google Scholar]
- Guadalupe T, Willems R, Zwiers M, Arias Vasquez A, Hoogman M, Hagoort P et al. Differences in cerebral cortical anatomy of left- and right-handers. Front Psychol 2014; 5: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci 2001; 21: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Pantelis C. Using longitudinal imaging to map the 'relapse signature' of schizophrenia and other psychoses. Epidemiol Psychiatr Sci 2014; 23: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Nimgaonkar VL, Phillips ML, Kupfer DJ. All the world's a (clinical) stage: rethinking bipolar disorder from a longitudinal perspective. Mol Psychiatry 2015; 20: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry 2006; 63: 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M, Lepage M, Collins DL, Pruessner JC, Joober R, Malla AK. Reduced hippocampal volume and hypothalamus-pituitary-adrenal axis function in first episode psychosis: evidence for sex differences. Neuroimage Clin 2015; 7: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Felsky D, Tampakeras M, Lerch JP, Mulsant BH, Kennedy JL et al. DISC1 and striatal volume: a potential risk phenotype for mental illness. Front Psychiatry 2012; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, De Rossi P, Watson B, Wharton A, Greenstein D, Raznahan A et al. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2014; 53: 7809 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp W, Sudre G, Wharton A, Greenstein D, Raznahan A et al. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol Psychiatry 2015; 20: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Chakravarty MM, Joober R, Lepage M. Dynamic endophenotypes and longitudinal trajectories: capturing changing aspects of development in early psychosis. J Psychiatr Neurosci 2016; 41: 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 2005; 57: 633–639. [DOI] [PubMed] [Google Scholar]
- Kawano M, Sawada K, Shimodera S, Ogawa Y, Kariya S, Lang DJ et al. Hippocampal subfield volumes in first episode and chronic schizophrenia. PLoS ONE 2015; 10: e0117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry 2002; 59: 839–849. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Mendes U, Brüne M, Ozgürdal S, Bohner G, Gudlowski Y et al. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci 2010; 35: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009; 62: 757–771. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Murray JE, Milton AL. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci 2013; 7: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 2002; 26: 321–352. [DOI] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A et al. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry 2008; 165: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isıklı S, Ugurlu O, Durmusoglu E, Kizilates G, Kitis O, Ozan E et al. Altered hippocampal formation shape in first-episode depressed patients at 5-year follow-up. J Psychiatr Res 2013; 47: 50–55. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry P, Dudgeon P, Brewer W, Cook M et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56: 133–141. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188: 510–518. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sideli L, La Cascia C, La Barbera D. Bridging the gap between research into biological and psychosocial models of psychosis. Shanghai Arch Psychiatry 2015; 27: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry 2014; 71: 769–777. [DOI] [PubMed] [Google Scholar]
- Haukvik UK, Westlye LT, Morch-Johnsen L, Jorgensen KN, Lange EH, Dale AM et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry 2015; 77: 581–588. [DOI] [PubMed] [Google Scholar]
- de Flores R, La Joie R, Chetelat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience 2015; 309: 29–50. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry 2010; 67: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.