Abstract
Lithium is first-line therapy for bipolar affective disorder and has recently been shown to have protective effects in populations at risk for Alzheimer’s disease (AD). However, the mechanism underlying this protection is poorly understood and consequently limits its possible therapeutic application in AD. Moreover, conventional lithium formulations have a narrow therapeutic window and are associated with a severe side effect profile. Here we evaluated a novel microdose formulation of lithium, coded NP03, in a well-characterized rat model of progressive AD-like amyloid pathology. This formulation allows microdose lithium delivery to the brain in the absence of negative side effects. We found that NP03 rescued key initiating components of AD pathology, including inactivating GSK-3β, reducing BACE1 expression and activity, and reducing amyloid levels. Notably, NP03 rescued memory loss, impaired CRTC1 promoter binding of synaptic plasticity genes and hippocampal neurogenesis. These results raise the possibility that NP03 be of therapeutic value in the early or preclinical stages of AD.
Introduction
Lithium has been used for more than 60 years in the treatment of bipolar affective disorder and has recently shown disease-modifying properties in individuals at risk for developing Alzheimer’s disease (AD)1, 2, 3, 4 and in AD animal models.5, 6, 7 Although there have been many advances in our understanding of its mechanism of action,8 precisely how lithium confers its protective effects in AD remains poorly understood. Clinical trials support the notion that lithium’s multi-target function can modulate cerebrospinal fluid levels of Aβ, tau, GSK-3β, and stabilize cognition in individuals with AD.2, 4 However, despite appearing as an attractive therapeutic candidate for AD treatment, conventional lithium formulations (lithium carbonate and lithium citrate) have a narrow therapeutic window owing to negative side effects at higher doses and their use in the elderly requires careful monitoring.9, 10
NP03 (issued from the Aonys technology developed by Medesis Pharma, Montpellier, France) is a novel microdose lithium formulation, wherein lithium is encapsulated in reverse water-in-oil microemulsions composed of self-assembled specific polar lipids, surfactant and co-surfactants (lecithin and ethanol), allowing enhanced central nervous system (CNS) uptake.11, 12, 13, 14, 15, 16 With NP03, transmucosal administration permits a significantly lower amount of lithium to be administered (400 × fold lower; blood concentration below detection limit of 0.06 mmol l−1), as this route of administration avoids acid hydrolysis in the gastrointestinal tract and bypasses hepatic metabolism, thus leading to high bioavailability. The Aonys technology has been shown to enhance CNS penetration of the P42 peptide to prevent Huntingtin aggregation in a murine model of Huntington’s disease (HD),12 for efficient CNS targeting of lithium in the same model,11 and CNS targeting of siRNAs in a murine model of prion disease.13 NP03 has previously shown neuroprotective properties in a Huntington’s disease murine model at remarkably low doses while avoiding the well-known adverse side effects of conventional lithium formulations.11
We evaluated whether the NP03 microdose formulation of lithium (40 μg Li per kg) might be efficient in modifying the early Alzheimer-like progressive amyloid pathology in McGill-R-Thy1-APP transgenic rats.17 At the early, pre-plaque stage McGill AD transgenic rats show in vivo disruption of synaptic plasticity in Aβ-burdened neurons18 and cognitive impairment.17, 18, 19, 20, 21, 22 It has also been reported that early intraneuronal Aβ accumulation blocks CRTC1 synaptonuclear transport, resulting in impaired promoter occupancy, and impaired neuroplasticity gene expression.22 In this study, we tested NP03 at the pre-plaque stage since intervening at early pathological stages offers a more promising outcome compared to later stages, by which time the brain has suffered extensive damage.23
Here we report beneficial effects of NP03 in halting the evolving AD pathology in McGill AD transgenic rats and in rescuing early Aβ-driven deficits in learning and memory. NP03 further induced an inactivated GSK-3β profile, and dampened Bace1 (β-site APP cleaving enzyme-1) gene expression and BACE1 activity, rendering lower levels of toxic Aβ42 peptides. NP03 also restored CRTC1 promoter occupancy in synaptic plasticity genes required for learning and memory. Furthermore, it re-established hippocampal neurogenesis in the AD rat transgenic model. These findings thus suggest that NP03 reverses key AD pathologies in an in vivo AD model, and that it may have therapeutic value in the early stages of the disease.
Materials and methods
Full description of Materials and methods is available as Supplementary Materials.
Animals
Male and female homozygous McGill-R-Thy1-APP transgenic rats17 were socially housed under 12 h light–dark schedule at 21 °C with free access to food and water. Number of animals is indicated in figure legends. Procedures were approved by the Animal Care Committee of McGill University.
Drug treatment
Three-month-old rats received microdose lithium NP03 (40 μg Li per kg; 1 ml kg−1; Medesis Pharma) or vehicle formulation lacking lithium (1 ml kg−1) on rectal mucosa 5 days per week for 8 weeks. A separate group of 250–300 g wild-type rats were treated with increasing doses of lithium (0–600 μg Li per kg) NP03. No changes in weight, drinking behaviour or urination were observed.
Behavioural tests
Novel object recognition, Morris water maze and auditory fear-conditioning tasks were performed as previously described.19
Determination of brain lithium levels
Brain tissue was collected 3–5 h following NP03 or vehicle administration and processed for analysis by inductively coupled plasma mass spectrometry (ICP-MS) (ICAP-Q, Thermo Fisher Scientific, Waltham, MA, USA).
Immunohistochemistry
Immunohistochemistry procedures were performed as previously described.22 Sections were imaged using an Axio Imager 2 microscope (Carl Zeiss Canada, North York, ON, Canada) running Zen Blue software (Carl Zeiss).
Western blotting
Hippocampal tissue was homogenized and Western blot analysis was carried out as previously described.22 The following primary antibodies were applied: anti-phospho-GSK-3βSer9 (5B3; Cell Signaling Technology, Danvers, MA, USA; #9323); anti-total GSK-3β (27C10; Cell Signaling #9315); anti-β-Catenin (6B3; Cell Signaling #9582); anti-βIII Tubulin (Promega, Madison, WI, USA; #G7121); and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 6C5; EMD Millipore, Etobicoke, ON, Canada, #MAB374).
Electrochemiluminescence-linked immunoassay
Protein levels of hippocampal Wnt3a ligand were determined using the electrochemiluminescence-linked immunoassay kit from Meso Scale Discovery (K150SOD; Gaithersburg, MD, USA) following manufacturer’s instructions.
Chromatin immunoprecipitation
Chromatin immunoprecipitation and gene expression analysis procedures were as previously described.22
BACE1 activity detection assay
BACE1 activity in cortical tissue was assessed using the fluorescence resonance energy transfer (FRET)-based β-secretase (BACE1) Activity Detection Kit (Sigma-Aldrich, St Louis, MO, USA; CS0010).
TBS soluble and insoluble human Aβ40 and Aβ42 ELISA
Levels of soluble and insoluble human Aβ40 and Aβ42 were assessed in cortical homogenates following the manufacturer’s directions (Invitrogen, Carlsbad, CA, USA; KHB3482 and KHB3442).
Neurogenesis analysis
Coronal sections containing anterior dorsal hippocampus (three sections per animal) were incubated with antibody against doublecortin (DCX) (1:600, C-18, sc-8066, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Alexa Rhodamine anti-goat. DCX-immunoreactive cells were counted in a blind manner and expressed as a mean number of cells per section for each condition.
Statistical analysis
Data were analysed using two-way ANOVA to examine effects of NP03 treatment and App transgene and all possible pairwise tests of the group means were examined using Tukey’s post hoc tests. Means of two groups were compared using an unpaired Student’s t-tests (GraphPad Software, La Jolla, CA, USA). Data are reported as mean±s.e.m. Statistical significance was P<0.05.
Results
The McGill-R-Thy1-APP transgenic rat model of AD-like amyloid pathology (AD rats hereafter) expresses the mutated human Aβ precursor protein (APP). It shows intraneuronal Aβ as early as 1 week postnatally, and extracellular plaques appear between 6 and 9 months of age in homozygous animals, spreading from the hippocampus to the cortex (Supplementary Figure 1).17 These AD rats show impaired cellular signaling, long-term potentiation and cognition at the pre-plaque state.17, 18, 19, 22, 24 Thus, we treated AD rats at this time point to assess the ability of NP03 to rescue established Aβ-driven pathology and related cognitive impairment.
NP03 treatment
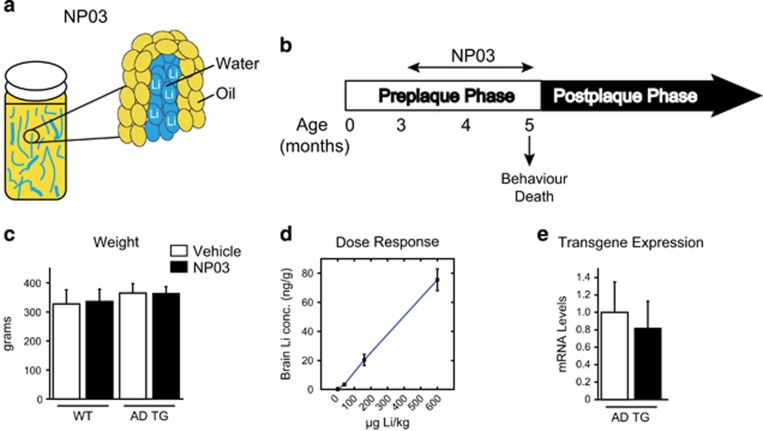
We evaluated a microdose formulation of lithium, NP03, in the treatment of AD-like amyloid pathology at the early, pre-plaque stage of the disease. NP03 contains the lithium citrate salt deposited in a water-in-oil microemulsion (Figure 1a). This formulation allows significantly lower levels of lithium to be delivered, avoiding the off-target effects associated with conventional dosing. Thus, AD transgenic rats and their wild-type littermates received either NP03 (40 μg Li per kg; 1 ml kg−1) or vehicle (1 ml kg−1) 5 days per week for 2 months (Figure 1b) starting at 3 months of age. Importantly, no weight changes, a common side effect of lithium, occurred following NP03 treatment (two-way ANOVA, P>0.05; Figure 1c). Brain lithium in rats treated with 40 μg Li per kg NP03 reached 3.36 ng g−1 of brain tissue, as measured by ICP-MS analysis, demonstrating that considerable amounts of lithium reached the CNS across the blood–brain barrier. Of note, the concentration of lithium increased with increasing doses of lithium, suggesting saturation was not reached at 40 μg Li per kg (Figure 1d). Finally, transcript production of the Thy1 promoter-driven APP transgene was not affected by NP03 treatment (t(13)=0.3601; P=0.7246; Figure 1e).
Figure 1.
Experimental design. (a) Schematic representation of the NP03 formulation with lithium incorporated as water-in-oil nanoparticles. (b) Experimental design showing that McGill-R-Thy1-APP transgenic rats and wild-type control rats were treated with NP03 or vehicle during the pre-plaque phase of the amyloid pathology, starting at 3 months of age and finishing at 5 months. Behavioural testing included Morris water maze, novel object recognition and auditory fear conditioning. (c) The absence of body weight change after NP03 treatment. (d) The incremental accumulation of lithium in brain with escalating NP03 dosage as determined by ICP-MS (e) Quantitative PCR experiments showing the NP03 treatment had no effect on the expression of the App transgene in the McGill-R-Thy1-APP animal model. Data from n⩾5 rats per condition were analysed via two-way ANOVA with Tukey’s post hoc test (c) and Student’s t-test (e). ANOVA, analysis of variance; APP, Aβ precursor protein; ICP-MS, inductively coupled plasma mass spectrometry.
NP03 reversed memory impairments in AD rats
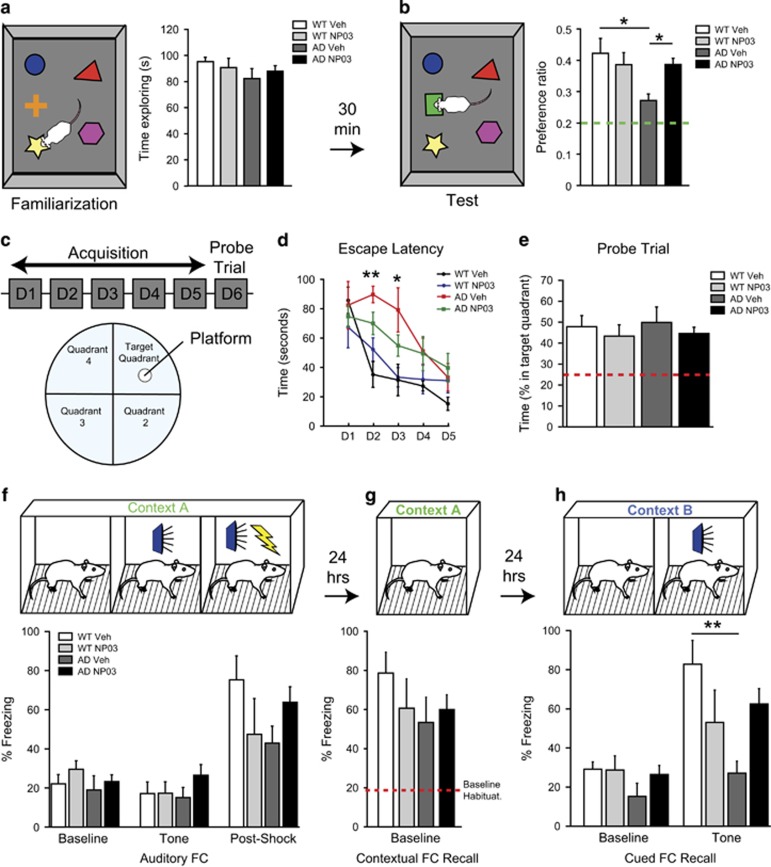
We first assessed the effect of NP03 treatment on learning and memory. During the familiarization phase of the novel object recognition (NOR) task, all rats demonstrated high exploratory behaviour, spending approximately half the allotted time exploring objects (Figure 2a). Following a 30-min intertrial interval, rats were presented with the test phase (Figure 2b) where one familiar object was replaced with a novel object. As expected, wild-type rats showed a clear preference for the novel object with a preference ratio of 40% (chance preference being 20%) while vehicle-treated AD rats demonstrated a significant impairment (Treatment × Transgene interaction: F(1,26)=5.65; P=0.0251). Tukey’s post hoc analyses revealed a significant reduction in the novel object preference for the vehicle-treated Alzheimer rats compared to the wild-type control (P<0.05). Importantly, NP03 restored novel object preference in AD rats significantly above the AD vehicle group (P<0.05), and comparable to that of the wild-type control (P>0.05).
Figure 2.
NP03 rescued learning and memory deficits in transgenic rats modelling AD-like amyloid pathology. (a) Novel object recognition familiarization phase showed AD rats were unimpaired in exploratory behaviour. (b) Vehicle-treated AD rats showed an impairment in novel object recognition on the test phase which was rescued with NP03 treatment. (c, d) Escape latency in Morris water maze task was higher for AD-vehicle rats on Days 2 and 3 compared to the wild-type vehicle rats, although all rats showed improved performance after 5 days of training as indicated by reduced escape latency. (e) Accordingly, there was no difference in percentage time spent by each group in the target quadrant. (f) Rats placed into Context A show baseline freezing of ~20%, and elevation following a mild footshock but not elicited by the presentation of a 5 kHz tone. (g) All groups froze at levels above baseline when returned to Context A 24-h later for contextual fear-conditioning recall. (h) Rats demonstrated baseline-freezing levels in novel Context B, supporting specificity of the contextual fear response. Presentation of 5 kHz tone failed to induce an elevation in freezing in AD-vehicle rats. This deficit is prevented in NP03-treated AD rats. Data are from n⩾5 rats per condition. * P<0.05, **P<0.01 determined using repeated measures two-way ANOVA (d) or two-way ANOVA with Tukey’s post hoc test (a, b, e, f, g, h). AD, Alzheimer’s disease; ANOVA, analysis of variance.
Rats were then tested on the Morris water maze task.25, 26 On training Day 1 (Figure 2c), mean platform latencies were not significantly different, ranging 67.00 –85.75 s (Figure 2d), and all groups showed improved performance with training (two-way ANOVA: time effect F(4,135)=12.28; P<0.0001). However, AD rats performed significantly worse on acquisition phase performance on Days 2 (AD vehicle vs WT vehicle: P<0.01) and 3 (AD vehicle vs WT vehicle: P<0.05) compared to the wild type. Again, NP03 rescued performance on Days 2 and 3 in AD rats that performed at the same level as wild-type rats (AD NP03 vs WT vehicle: P>0.05). On the Probe Trial (Figure 2e), AD rats made just as many platform crosses compared to the wild-type rats, indicating that while they took longer over training Days 1–5 to learn the task, they were eventually able to locate the platform, and thus, showed no deficit on the subsequent Probe Trial.
Finally, in the auditory fear-conditioning task, all rats displayed equal baseline freezing in training Context A (CXT-A; Figure 2f; Baseline) and the presentation of a 5-kHz tone elicited no change in freezing, verifying that the tone was initially innocuous (Figure 2f; Tone). As expected, a mild footshock co-terminating with the tone led to an elevation in freezing for all rats (Figure 2f; Post-Shock). When rats were returned to training context (CXT-A) 24 h later they all showed high levels of freezing indicating AD rats were unimpaired in contextual fear memory (Figure 2g).19 When we tested rats in a novel context (Context B, CXT-B) 24 h later all groups again showed baseline freezing (Figure 2h). However, presentation of the 5-kHz tone in CXT-B elicited a high level of freezing in wild-type but not vehicle-treated AD rats. NP03 rescued this cued recall in impaired AD rats. These observations were supported by the results of two-way ANOVA, which revealed a significant Transgene × Treatment interaction (F(1,24)=9.19; P=0.0058), as well as a significant main effect for Treatment (F(1,24)=4.66; P=0.0411), but not for Transgene (F(1,24)=0.07; P=0.7939). Post hoc analyses revealed that vehicle-treated TG rats had lower levels of freezing compared to vehicle-treated WT (P<0.05), while there was no difference between control animals and NP03-treated TG animals (P>0.05). Together our results reveal that NP03 rescued Aβ-induced memory impairments in novel object recognition, spatial learning and amygdala-dependent fear memory.
NP03 inactivated hippocampal GSK-3β and restored native β-catenin
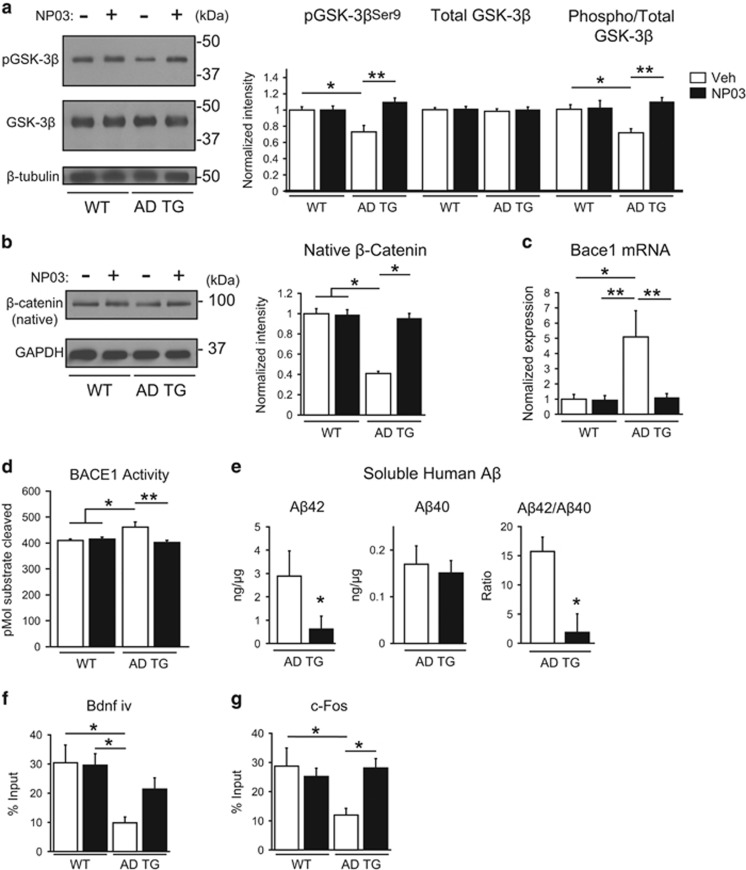
We next investigated the biochemical changes that might account for the observed behavioural restoration by NP03 in AD rats. We first investigated the phosphorylation status of GSK-3β, a multifunctional serine/threonine kinase widely expressed in brain and linked to AD pathology.27, 28, 29 We assessed relative phosphorylation of GSK-3β at serine 9 compared to total GSK-3β as an indicator of GSK-3β inactivation.30 Using quantitative Western blotting we found a significant reduction in the levels of phospho-GSK-3βSer9 in Alzheimer transgenic rats compared to the wild-type (Treatment x Transgene interaction: F(1,23)=8.91; P=0.0066; WT:Veh vs TG:Veh, P<0.05). NP03 reversed this deficit in the AD transgenic rats (TG:Veh vs TG:NP03, P<0.01; WT:Veh vs TG:NP03, P>0.05. Importantly, the ratio of phospho-GSK-3βSer9 to total GSK-3β was significantly reduced in the hippocampus of AD rats relative to wild-type rats, reflecting a release of inhibitory phosphorylation on GSK-3β (Treatment x Transgene interaction: F(1,23)=6.42; P=0.0186; WT:Veh vs TG:Veh, P<0.05). NP03 restored the ratio of phospho-GSK-3βSer9 to total GSK-3β in AD rats (TG:Veh vs TG:NP03, P<0.01; Figure 3a), a finding that is consistent with a previous report that showed NP03 inhibiting GSK-3β in a mouse model of Huntington’s disease.11 GSK-3β phosphorylates several components of the Wnt/β-catenin transduction pathway, including β-catenin, which is subsequently recognized by ubiquitin and targeted for proteasomal degradation.31 We measured levels of the Wnt ligand and found no significant difference between any of the conditions (two-way ANOVA: Treatment: F(1,23)=0.02; P=0.8793; Transgene: F(1,23)=0.28; P=0.5991; interaction: F(1,23)=1.20; P=0.2856; Supplementary Figure 2). However, we did observe a significant decrease in native β-catenin (a GSK-3β substrate) protein in the hippocampus of AD rats (significant main effect for Transgene (F(1,21)=5.82; P=0.0251; WT:Veh and WT:NP03 vs TG:Veh, P<0.05; Figure 3b), suggesting a facilitation of degrading mechanisms of the native form of β-catenin in these rats. We found that NP03 treatment rescued the steady-state levels of β-catenin, coinciding with inactivation of GSK-3β (TG:Veh vs TG:NP03, P<0.05).
Figure 3.
NP03 mediates elevation of hippocampal GSK-3β phosphorylation, restoration of β-catenin levels, reduction of BACE1 levels and of its catalytic activity, along with reduction of Aβ42 levels in AD rats. (a) AD rats presented a profile of hyperactive GSK-3β, as measured by the ratio of phospho-GSK-3βSer9/total GSK-3β in hippocampal protein extracts examined by Western blotting. Quantification of phospho-GSK-3βSer9 and total GSK-3β band intensity demonstrated that the significant decline in the ratio of phospho-GSK-3βSer9/total GSK-3β (indicating increased GSK-3β activity) in AD rats vs wild-type rats was restored to control levels with NP03. (b) Hippocampal tissue from wild-type and AD rats receiving vehicle or NP03 microdose lithium was analysed by Western blot for levels of β-catenin. Quantification of native β-catenin (normalized to wild-type vehicle) showed significant reduction in AD rats vs wild type. Reduction of native β-catenin was reversed by NP03 treatment. (c) Levels of Bace1 gene expression and (d) BACE1 activity were elevated in AD rats. This increase was reduced with NP03 treatment. (e) Consequently, the levels of soluble human Aβ42 measured by ELISA were reduced with NP03 treatment, as was the Aβ42/Aβ40 ratio. n⩾5 rats per condition. (f, g) Chromatin immunoprecipitation analysis revealed significant reduction in CRTC1 occupancy in promoters of synaptic plasticity-associated genes Bdnf iv and c-fos in AD rats. These deficits were overcome with NP03 treatment. *P<0.05, **P<0.01 determined by two-way ANOVA with Tukey’s post hoc test (a, b, c, d, f, g) and Student’s t-test (e). AD, Alzheimer’s disease; ANOVA, analysis of variance.
NP03 reduced BACE1 activity and the ratio of Aβ42/Aβ40
Wnt/β-catenin signalling supports transcriptional repression of Bace1 by promoting the binding of T-cell factor-4 at Bace1 genetic promoters.32 Thus, in accordance with the observed reduction of native β-catenin, we observed a five-fold increase in Bace1 mRNA in the hippocampus of AD transgenic rats relative to the wild type (Figure 3c) that was diminished to normal levels with NP03 treatment (Treatment × Transgene interaction: F(1,23)=6.91; P=0.0150; WT:Veh vs TG:Veh, P<0.05; TG:Veh vs TG:NP03, P<0.01). In addition, using a FRET-based activity assay, we observed increased substrate cleavage activity by BACE1 in vehicle-treated AD transgenic rats compared to the wild type (Figure 3d). NP03 treatment reduced BACE1 activity in AD rats to baseline levels, a reduction of 12.96%. These observations were supported by the results of a two-way ANOVA, which revealed a significant Transgene × Treatment interaction (F(1,25)=9.547; P=0.0050), as well as a significant main effect for treatment (F(1,25)=6.65; P=0.0162), but not for Transgene (F(1,25)=3.28; P=0.0822). Post hoc analyses revealed that vehicle-treated TG rats had significantly elevated BACE1 activity compared to vehicle- and NP03-treated WT (P<0.05). NP03 reduced BACE1 activity in Alzheimer rats compared to the vehicle-treated AD rats (P<0.01) to a level not significantly different from the WT (P>0.05).
BACE1 cleaves APP to generate Aβ peptides and other APP-derived fragments.33, 34 Accordingly, BACE1 inhibitors have been considered as putative therapeutic agents for the treatment of AD.35 Given the increase in Bace1 gene expression and activity in AD transgenic rats, we subsequently analysed the levels of TBS- and guanidine-soluble human Aβ40 and Aβ42 peptides (Figure 3e). In the TBS-soluble fraction, as expected, Aβ42 peptides and Aβ40 peptides were highly expressed in AD transgenic rats. Importantly, NP03 reduced the levels of soluble Aβ42 in AD transgenic rats (t(12)=2.028; P=0.0327), a decrease of 4.5-fold, while levels of soluble Aβ40 were unchanged (t(12)=6.197; P>0.05). The reduction in Aβ42 led to a significant decrease in the soluble Aβ42/Aβ40 ratio (t(12)=2.187; P=0.0247). Insoluble Aβ42 and Aβ40 peptides were not detected at this early, 5-month time point. Thus, NP03 effectively reduced Bace1 mRNA and activity, and diminished levels of soluble Aβ42 in AD transgenic rats.
NP03 rescued CRTC1 binding at synaptic plasticity-associated gene promoters
In rodent models of AD, the transcription co-regulator CRTC1 is dysregulated early, when Aβ peptides are still intraneuronally confined.22, 36, 37, 38 In a previous study, we showed an Aβ-mediated impairment in CRTC1-dependent gene transcription that was reflected by reduced CRTC1 nuclear translocation, reduced CRTC1 occupancy at synaptic plasticity-associated gene promoters and reduced transcription of plasticity-associated genes in AD transgenic rats.22 As it has been reported that higher concentrations of lithium enhances CRTC1 oligomer formation as well as the interaction between CREB co-activating factors CRTC1 and CREB-binding protein,39, 40, 41 we tested whether NP03 lithium microdoses could reverse the Aβ-driven impairment in CRTC1 promoter occupancy. Thus, consistent with our previous report,22 we found that in AD transgenic rats, genomic promoter occupancy was significantly reduced for Bdnf iv (brain-derived neurotrophic factor) (Figure 3f). These observations were supported by the results of a two-way ANOVA, which revealed a significant main effect for Transgene (F(1,30)=9.50; P=0.0044). Post hoc analyses revealed that vehicle-treated Alzheimer transgenic rats had significantly reduced CRTC1 promoter occupancy at Bdnf iv compared to vehicle- and NP03-treated wild type (P<0.05). NP03 restored CRTC1 Bdnf iv promoter occupancy to a level not significantly different from the wild type (P>0.05). CRTC1 promoter occupancy was also reduced at c-fos in AD transgenic rats (Transgene × Treatment interaction (F(1,29)=5.77; P=0.0229; Figure 3g). Post hoc analyses revealed that vehicle-treated Alzheimer transgenic rats had significantly lower CRTC1 occupancy of c-fos promoters compared to the wild-type vehicle group (P<0.05). NP03 significantly increased CRTC1 occupancy in Alzheimer transgenic rats compared to the vehicle-treated Alzheimer transgenic rats (P<0.05) to a level not significantly different from the wild type (P>0.05). Thus, it is likely that NP03-driven restoration in CRTC1 genomic occupancy contributed to behavioural recovery in AD rats treated with NP03 by facilitating expression of synaptic plasticity-related genes.
NP03 rescued adult hippocampal neurogenesis
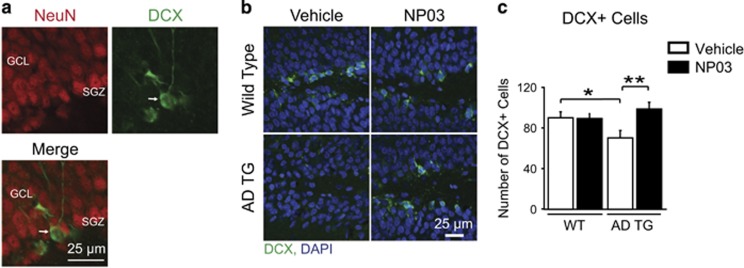
The subgranular zone (SGZ) of the hippocampus exhibits persistent neurogenesis into adulthood—a process shown to be impaired with amyloid deposition.42, 43 As Wnt/β-catenin signalling has been reported to regulate adult hippocampal neurogenesis,44 we processed tissue sections containing the SGZ using an antibody specific for the protein doublecortin (Figure 4a), a reliable and specific marker of adult neurogenesis.45 Vehicle-treated AD rats showed impaired SGZ neurogenesis compared to the wild-type groups, as demonstrated by a reduction in the number of DCX+ cells (Treatment × Transgene interaction F(1,12)=8.65; P=0.0124; WT:Veh and WT:NP03 vs TG:Veh, P<0.05; Figures 4b and c). Similarly to the effects reported for conventional lithium in mice,46 and in fitting with its effects on Aβ and the Wnt/β-catenin signalling pathway, we found that NP03 lithium microdoses rescued loss of neurogenesis in AD rats (TG:Veh vs TG:NP03, P<0.01).
Figure 4.
NP03 rescues hippocampal neurogenesis. (a) Adult-generated DCX-expressing cells (arrow, green) in SGZ of the dentate gyrus of the hippocampus do not overlap with NeuN (red), a marker of mature neurons. (b) DCX-expressing cells (green) in the SGZ of the dentate gyrus of vehicle- and NP03-treated rats, counterstained with nuclear marker DAPI (blue). Scale bar, 25 μm. (c) Analysis revealed a significant reduction in DCX-immunoreactive cell numbers in vehicle-treated AD transgenic rats. NP03 restored SGZ neurogenesis in AD rats. *P < 0.05, **P < 0.01 determined by two-way ANOVA followed by Tukey’s post hoc test. AD, Alzheimer’s disease; ANOVA, analysis of variance; DCX, doublecortin; GCL, granule cell layer; SGZ, subgranular zone.
Discussion
This study indicates that microdose lithium NP03 can efficiently reach the brain to rescue cognitive impairments at early stages of AD-like amyloid pathology in AD rats. Importantly, this effect coincided with the inactivation of GSK-3β and a reduction in Aβ42 levels. Furthermore, NP03 blunted BACE1 hyperactivity in AD rats while restoring hippocampal neurogenesis and CRTC1 Bdnf iv and c-fos promoter occupancy. These aspects are critical for the effective CRTC1 transcription as required for learning and memory mechanisms.47, 48 In this regard, our results coincide with recent findings where subclinical concentrations of lithium provoked higher intracellular and extracellular BDNF concentration in hippocampal cell cultures.49 Of note, NP03 treatment did not produce side effects relating to lithium toxicity nor did it provoke adverse effects in wild-type animals. These results support the hypothesis that microdoses of lithium are sufficient to impact Aβ neuropathology by affecting multiple components of the Wnt/β-catenin signalling pathway, among others.
Despite wide use in treatment of bipolar disorder, a major issue with the application of lithium remains its severe side effect profile.9, 10, 50 Lithium has a narrow therapeutic window, and severe side effects preclude its long-term use in elderly patients. Adverse effects of lithium include tremor, nausea, polyuria, polydipsia and weight gain,9 and because of these side effects, lower plasma concentrations are intentionally targeted for maintenance treatment. Here we evaluated a novel lipidic nano-particle formulation of lithium (NP03) that delivers significantly lower amounts of lithium, while allowing enhanced CNS uptake of lithium, avoiding adverse effects without loss of therapeutic efficacy.
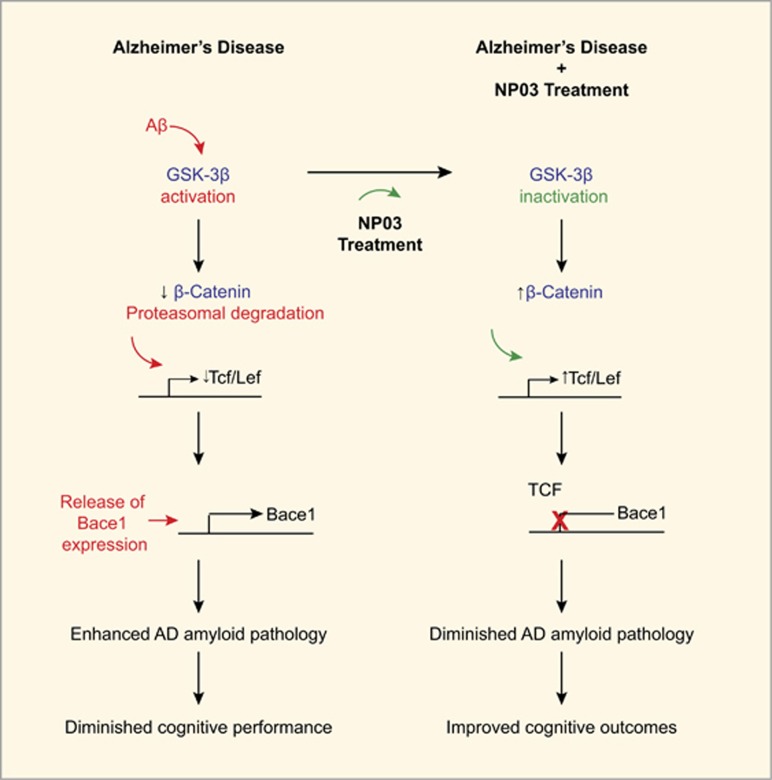
The Wnt/β-catenin pathway has been implicated in AD pathogenesis51, 52, 53, 54 and accordingly modulation of this pathway is thought to represent a possible therapeutic strategy in AD. Our results support the hypothesis that lithium modulates Wnt/β-catenin signalling via inactivation of GSK-3β, contributing to a reduction of Bace1 mRNA expression, inhibition of BACE1 and reduction of Aβ neuropathology. Recently, Parr et al.32 showed that activation of Wnt/β-catenin signalling pathway promotes binding of T-cell factor-4, a Bace1 gene repressor, and multiple lines of evidence point to a role of clinical doses of lithium in mimicking Wnt/β-catenin signalling through the inactivation of GSK-3β.51, 55, 56, 57 Furthermore, BACE1 activity is consistently shown to be elevated in AD and in models of Alzheimer amyloidosis.58, 59, 60 NP03 reduced Bace1 expression and activity and it is likely through this mechanism that soluble Aβ42 levels were reduced. The interpretations of the present results are summarized in Figure 5, showing that Aβ-driven hyperactivity of GSK-3β would lead to reduced stability of β-catenin, thereby removing the transcriptional repression of Bace1 by TCF/LEF. This would favour Aβ production via the sequential cleavage of APP by BACE1. Conversely, NP03 inactivates GSK-3β through phosphorylation at Ser 9, restoring β-catenin signalling components, and transcriptional repression of Bace1. This ultimately leads to reduced production of Aβ42 peptides and the consequential diminution of the Alzheimer’s-like amyloid pathology, resulting in improved cognitive outcomes (Figure 5). This model is supported by a recent report showing that inhibition of Wnt signalling induces amyloidogenic processing and aggregation of Aβ42 peptides.61 Thus, notwithstanding the limitations of transgenic models, the reduction in soluble Aβ42 observed here is significant, as dementia severity correlates more closely with presence of soluble Aβ, rather than with fibrillar Aβ deposits in the brain.62
Figure 5.
Schematic interpretation of the neurochemical consequences of microdose lithium (NP03 treatment) leading to lesser Alzheimer’s disease (AD)-like pathology and improved cognitive outcomes. NP03 mediates BACE1 inhibition via β-catenin signalling. Native β-catenin is stabilized by Wnt and initiates transcription of Tcf/Lef, a transcriptional repressor of Bace1. In AD, a release of inhibitory phosphorylation of GSK-3β permits the enzyme to phosphorylate β-catenin at residues Thr41/Ser37/Ser33. Phosphorylated β-catenin is rapidly ubiquitinated and targeted for proteasomal degradation. In the absence of TCF repression, Bace1 gene expression is increased, promoting BACE1 hyperactivity, and the sequential proteolytic cleavage of APP by beta and gamma secretases. Such a mechanism would contribute towards increased levels of Aβ thus diminishing the Aβ-driven pathology. The present observation indicates that NP03 therapy inhibits GSK-3β activity to maintain normal β-catenin levels. Scheme adapted from Llorens Martin et al.63 APP, Aβ precursor protein.
Aβ deposition is a negative regulator of adult hippocampal neurogenesis.42, 43 We found that NP03 can prevent loss of hippocampal neurogenesis at early stages of Aβ neuropathology, consistent with earlier reports using App mutant mice.46 Although it is not known what exact role adult neurogenesis plays in behaviour,64, 65 incorporation of adult-born neurons in the hippocampus SGZ is important for inhibitory modulation of mature granule cells.66 Furthermore, adult neurogenesis has been implicated in pattern separation and the normal processing of information in the hippocampal circuit.67 Thus, it is likely that NP03-rescued cognition in AD rats results in part by the preservation of hippocampal neurogenesis.
Effects of lithium on AD in clinical populations have been reported, although with conflicting results.1, 3 A study administering lithium carbonate (serum levels 0.3–0.8 mmol l−1) for up to 1 year showed no cognitive improvement.68 A second study administering lithium sulphate (serum levels 0.5–0.8 mmol l−1) for 10 weeks, including a 6-week titration phase50 also failed to show benefit. However, a trial assessing lithium at earlier stages of the pathology2 revealed a significant decrease in cerebrospinal fluid p-tau181 and improvement on the ADAS-Cog subscale after 12-month lithium treatment (0.25–0.5 mmol l−1). In a low-dose trial, 300 μg of lithium was given once daily for 15 months4 and while the placebo group deteriorated in MMSE scores, the lithium-treated group remained stable. Together, the clinical studies reveal that the possibility of beneficial effects of lithium in the treatment of AD should not be excluded. Rather, further clinical trials with lithium microdoses within well-defined clinical populations should be undertaken,69 for instance, at early Alzheimer pathological stages and/or in Down syndrome (DS), at the transition from DS-AD asymptomatic to DS-AD symptomatic. Such studies should include biomarker measures, along with ‘self-to-self’ cognitive assessment70 to determine treatment efficacy.
It must be noted that while cognitive impairments and AD-like pathology were reversed by NP03 treatment, our work cannot completely rule out the possibility that the effect of NP03 is of preventative nature and thus NP03 administration would prevent pathology progression, rather than rescuing it. These aspects will be addressed in future studies.
Conventional lithium dosage is associated with a significant adverse side-effect profile including nausea, dizziness, nephrotoxicity, polyuria, polydipsia and tremor.9, 10 Serum lithium levels achieved here were below detection limit of 0.06 mmol l−1. By comparison, this is significantly lower than the levels which are typically targeted for individuals with bipolar disorder, which range between 0.5 and 1.5 mmol l−1.71 This is also lower than the doses targeted in Alzheimer patients, which range from 0.3 to 0.8 mmol l−1.68, 72 Therefore, the justification of NP03 as a less-toxic alternative to conventional lithium stems from the microdose nature of the formulation and its capacity to reach the brain. This is especially important given the long-term treatment required for AD, and its application in elderly populations.50
In our studies, NP03 formulation restored spatial learning, recognition memory and associative learning in a rat model of AD-like amyloid pathology. NP03 reduced brain Aβ42, and proved to be an efficacious inhibitor of BACE1 activity. Finally, NP03 restored neurogenesis and CRTC1 promoter occupancy in synaptic plasticity genes. NP03 did not provoke adverse side effects. With a unique formulation allowing controlled microdose delivery setting it apart from current conventional lithium therapy, NP03 represents a promising therapeutic agent for mild-moderate and foremost for prodromal AD. The emerging literature would indicate that prodromal AD will be safely identified for earlier therapeutic intervention73, 74, 75 and, as such, these cohorts should be suitable candidates for NP03 and alternative microdose lithium therapies.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research grant number: 102752 and Centres of Excellence in Neurodegeneration grant number: 01074. We thank Dr A Frosst, the Frosst family and Merck Canada for their continuing support. We thank Dr. Jean-Benoit Charron for his help with chromatin immunoprecipitation experiments. SDC is the holder of the Charles E Frosst/Merck Research Associate position. MFI was the recipient of a Biomedical Doctoral Award from the Alzheimer Society of Canada (2011–2014). HH is the holder of a Fonds de Recherche Sante Quebec Postdoctoral Fellowship. ACC is the holder of the Charles E Frosst/Merck-endowed Chair in Pharmacology and is a Team Leader for the Canadian Consortium on Neurodegeneration in Aging.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry 2008; 65: 1331–1335. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Bri J Psychiatry 2011; 198: 351–356. [DOI] [PubMed] [Google Scholar]
- Mauer S, Vergne D, Ghaemi SN. Standard and trace-dose lithium: a systematic review of dementia prevention and other behavioral benefits. Aust N Z J Psychiatry 2014; 48: 809–818. [DOI] [PubMed] [Google Scholar]
- Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease. Curr Alzheimer Res 2013; 10: 104–107. [DOI] [PubMed] [Google Scholar]
- Zhang X, Heng X, Li T, Li L, Yang D, Du Y et al. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-beta production in an aged Alzheimer's disease transgenic mouse model. J Alzheimers Dis 2011; 24: 739–749. [DOI] [PubMed] [Google Scholar]
- Nunes MA, Schowe NM, Monteiro-Silva KC, Baraldi-Tornisielo T, Souza SI, Balthazar J et al. Chronic microdose lithium treatment prevented memory loss and neurohistopathological changes in a transgenic mouse model of Alzheimer's disease. PLoS ONE 2015; 10: e0142267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci 2007; 27: 1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N Engl J Med 2004; 351: 476–486. [DOI] [PubMed] [Google Scholar]
- Azab AN, Shnaider A, Osher Y, Wang D, Bersudsky Y, Belmaker RH. Lithium nephrotoxicity. Int J Bipolar Disord 2015; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ, Jefferson JW. Lithium tremor. J Clin Psychiatry 1995; 56: 283–287. [PubMed] [Google Scholar]
- Pouladi MA, Brillaud E, Xie Y, Conforti P, Graham RK, Ehrnhoefer DE et al. NP03, a novel low-dose lithium formulation, is neuroprotective in the YAC128 mouse model of Huntington disease. Neurobiol Dis 2012; 48: 282–289. [DOI] [PubMed] [Google Scholar]
- Arribat Y, Talmat-Amar Y, Paucard A, Lesport P, Bonneaud N, Bauer C et al. Systemic delivery of P42 peptide: a new weapon to fight Huntington's disease. Acta Neuropathol Commun 2014; 2: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Relano-Gines A, Resina S, Brillaud E, Casanova D, Vincent C et al. Systemic delivery of siRNA down regulates brain prion protein and ameliorates neuropathology in prion disorder. PLoS ONE 2014; 9: e88797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Diat O, Lerner DA, El Ghzaoui A, Ajovalasit A, Dorandeu C et al. Water solubilization capacity of pharmaceutical microemulsions based on Peceol(R), lecithin and ethanol. Int J Pharm 2014; 475: 324–334. [DOI] [PubMed] [Google Scholar]
- Mouri A, Diat O, El Ghzaoui A, Bauer C, Maurel JC, Devoisselle JM et al. Phase behavior of reverse microemulsions based on Peceol((R)). J Colloid Interface Sci 2014; 416: 139–146. [DOI] [PubMed] [Google Scholar]
- Mouri A, Legrand P, El Ghzaoui A, Dorandeu C, Maurel JC, Devoisselle JM. Formulation, physicochemical characterization and stability study of lithium-loaded microemulsion system. Int J Pharm 2016; 502: 117–124. [DOI] [PubMed] [Google Scholar]
- Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, DeWilde A et al. A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis 2010; 20: 113–126. [DOI] [PubMed] [Google Scholar]
- Qi Y, Klyubin I, Harney SC, Hu N, Cullen WK, Grant MK et al. Longitudinal testing of hippocampal plasticity reveals the onset and maintenance of endogenous human Aβ-induced synaptic dysfunction in individual freely behaving pre-plaque transgenic rats: rapid reversal by anti-Aβ agents. Acta Neuropathol Commun 2014; 2: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iulita MF, Allard S, Richter L, Munter LM, Ducatenzeiler A, Weise C et al. Intracellular Abeta pathology and early cognitive impairments in a transgenic rat model overexpressing human amyloid precursor protein: a multidimensional study. Acta Neuropathol Commun 2014; 2: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano P, Martino Adami PV, Do Carmo S, Blanco E, Rotondaro C, Capani F et al. Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer's disease. Front Behav Neurosci 2014; 8: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino Adami PV, Quijano C, Magnani N, Galeano P, Evelson P, Cassina A et al. Synaptosomal bioenergetic defects are associated with cognitive impairment in a transgenic rat model of early Alzheimer's disease. J Cereb Blood Flow Metab 2015; 37: 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EN, Abela AR, Do Carmo S, Allard S, Marks AR, Welikovitch LA et al. Intraneuronal amyloid beta accumulation disrupts hippocampal CRTC1-dependent gene expression and cognitive function in a rat model of Alzheimer disease. Cereb Cortex 2017; 27: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014; 71: 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard S, Ducatenzeiler A et al. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer's disease. Neurobiol Aging 2014; 35: 2249–2262. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial navigation does not require the presence of local cues. Learn Motiv 1981; 12: 236–260. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47–60. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116(Pt 7): 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 2004; 29: 95–102. [DOI] [PubMed] [Google Scholar]
- Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A et al. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest 2013; 123: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789. [DOI] [PubMed] [Google Scholar]
- Wu G, He X. Threonine 41 in beta-catenin serves as a key phosphorylation relay residue in beta-catenin degradation. Biochemistry 2006; 45: 5319–5323. [DOI] [PubMed] [Google Scholar]
- Parr C, Mirzaei N, Christian M, Sastre M. Activation of the Wnt/beta-catenin pathway represses the transcription of the beta-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J 2015; 29: 623–635. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature 1992; 360: 672–674. [DOI] [PubMed] [Google Scholar]
- Das U, Wang L, Ganguly A, Saikia JM, Wagner SL, Koo EH et al. Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat Neurosci 2015; 19: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol 2014; 13: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Damas A, Valero J, Chen M, Espana J, Martin E, Ferrer I et al. Crtc1 activates a transcriptional program deregulated at early Alzheimer's disease-related stages. J Neurosci 2014; 34: 5776–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarin M, Salih DA, Yasvoina M, Cummings DM, Guelfi S, Liu W et al. A genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Rep 2015; 10: 633–644. [DOI] [PubMed] [Google Scholar]
- Parra-Damas A, Chen M, Enriquez-Barreto L, Ortega L, Acosta S, Perna JC et al. CRTC1 function during memory encoding is disrupted in neurodegeneration. Biol Psychiatry 2016; 81: 111–123. [DOI] [PubMed] [Google Scholar]
- Heinrich A, von der Heyde AS, Boer U, Phu do T, Tzvetkov M, Oetjen E. Lithium enhances CRTC oligomer formation and the interaction between the CREB coactivators CRTC and CBP—implications for CREB-dependent gene transcription. Cell Signal 2013; 25: 113–125. [DOI] [PubMed] [Google Scholar]
- Boer U, Eglins J, Krause D, Schnell S, Schofl C, Knepel W. Enhancement by lithium of cAMP-induced CRE/CREB-directed gene transcription conferred by TORC on the CREB basic leucine zipper domain. Biochem J 2007; 408: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich A, Boer U, Tzvetkov M, Oetjen E, Knepel W. Stimulation by lithium of the interaction between the transcription factor CREB and its co-activator TORC. Biosci Rep 2009; 29: 77–87. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol 2007; 204: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med 2002; 1: 125–135. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005; 437: 1370–1375. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 2004; 19: 234–246. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 2010; 5: e14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng TH, Uzgil B, Lin P, Avliyakulov NK, O'Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell 2012; 150: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA et al. Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 2012; 32: 17857–17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Paula VJ, Gattaz WF, Forlenza OV. Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar Disord 2016; 18: 692–695. [DOI] [PubMed] [Google Scholar]
- Hampel H, Ewers M, Burger K, Annas P, Mortberg A, Bogstedt A et al. Lithium trial in Alzheimer's disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 2009; 70: 922–931. [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res Brain Res Rev 2000; 33: 1–12. [DOI] [PubMed] [Google Scholar]
- Anderton BH, Dayanandan R, Killick R, Lovestone S. Does dysregulation of the Notch and wingless/Wnt pathways underlie the pathogenesis of Alzheimer's disease? Mol Med Today 2000; 6: 54–59. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Avila ME, Medina MA, Perez-Palma E, Bustos BI, Alarcon MA. Wnt/beta-catenin signaling in Alzheimer's disease. CNS Neurol Disord Drug Targets 2014; 13: 745–754. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Varela-Nallar L. Wnt signaling in the nervous system and in Alzheimer's disease. J Mol Cell Biol 2014; 6: 64–74. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry 2010; 15: 272–285, 228. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 1996; 6: 1664–1668. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 1997; 185: 82–91. [DOI] [PubMed] [Google Scholar]
- Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology. J Neuroinflammation 2012; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 2011; 34: 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Hanzel CE, Jacobs ML, Machnes Z, Iulita MF, Yang J et al. Rescue of early bace-1 and global DNA demethylation by S-adenosylmethionine reduces amyloid pathology and improves cognition in an Alzheimer's model. Sci Rep 2016; 6: 34051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rojas C, Burgos PV, Inestrosa NC. Inhibition of Wnt signaling induces amyloidogenic processing of amyloid precursor protein and the production and aggregation of Amyloid-beta (Abeta)42 peptides. J Neurochem 2016; 139: 1175–1191. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 1999; 46: 860–866. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Jurado J, Hernandez F, Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci 2014; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 2011; 70: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 2011; 70: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV et al. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 2015; 26: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014; 344: 598–602. [DOI] [PubMed] [Google Scholar]
- Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S. A feasibility and tolerability study of lithium in Alzheimer's disease. Int J Geriatr Psychiatry 2008; 23: 704–711. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M. The putative use of lithium in Alzheimer's disease. Curr Alzheimer Res 2016; 13: 853–861. [DOI] [PubMed] [Google Scholar]
- Iulita MF, Ower A, Barone C, Pentz R, Gubert P, Romano C et al. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: relation to cognitive decline and longitudinal evaluation. Alzheimers Dement 2016; 12: 1132–1148. [DOI] [PubMed] [Google Scholar]
- Severus WE, Kleindienst N, Seemuller F, Frangou S, Moller HJ, Greil W. What is the optimal serum lithium level in the long-term treatment of bipolar disorder—a review? Bipolar Disord 2008; 10: 231–237. [DOI] [PubMed] [Google Scholar]
- Leyhe T, Eschweiler GW, Stransky E, Gasser T, Annas P, Basun H et al. Increase of BDNF serum concentration in lithium treated patients with early Alzheimer's disease. J Alzheimers Dis 2009; 16: 649–656. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 2016; 12: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio R, Quiroz YT et al. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated beta-amyloid. Alzheimers Dement 2016; 12: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Genthon R, Cavedo E, Habert MO, Lamari F, Gagliardi G et al. Preclinical Alzheimer's disease: a systematic review of the cohorts underlying the concept. Alzheimers Dement 2017; 13: 454–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.