Abstract
The establishment of mechanism-driven peripheral markers is important for translational psychiatry. Many groups, including ours, have addressed molecular alterations in peripheral tissues in association with symptomatic changes in major illnesses. Oxidative stress is implicated in the pathophysiology of schizophrenia (SZ) and bipolar disorder (BP) through studies of patient peripheral tissues and animal models. Although the relationship between peripheral changes and brain pathology remain elusive, oxidative stress may bridge such translational efforts. Nonetheless, the molecular substrates of oxidative stress are not well defined in mental conditions. Glutathione (GSH) is a non-enzymatic antioxidant that eliminates free radicals, and has been suggested to have a role in SZ. We performed a cross-sectional study of 48 healthy controls (CON), 52 SZ patients and 62 BP patients to compare the levels of peripheral GSH by a biochemical enzyme assay. We show a significant reduction of plasma GSH in both SZ and BP patients compared with CON. We evaluated possible influences of clinical characteristics on the level of GSH in SZ and BP. A decrease in GSH level correlated with Positive and Negative Syndrome Scale (PANSS) total and positive scores for SZ and correlated with the PANSS general for BP. Taken together, we provide evidence that SZ and BP display a common molecular signature in the reduction of peripheral GSH in the psychosis dimension.
Introduction
One of the challenges that face psychiatry today is the absence of mechanistic-driven diagnostic biomarkers.1 Psychiatric diagnosis is still made based on symptomatology and lacks any molecular foundation. The inability to directly access the brain limits the development of diagnostic measures in psychiatry. As a result, peripheral tissues such as blood and cerebrospinal fluid (CSF) from patients are now being used to study the underlying molecular mechanisms of psychiatric disease. The molecular mediators identified from these studies could serve as potential biomarkers.
Another current challenge in psychiatry is the validity of categorizing mental conditions into separate diseases.2 Modern psychiatry, influenced by the views of Emil Kraepelin, distinguishes schizophrenia (SZ) from bipolar disorder (BP). However, in the past decade, multiple lines of scientific evidence challenged this traditional dichotomy. Psychiatric genetics provides compelling data that SZ and BP share common genetic etiologies.3, 4 Neuropsychology suggests that cognitive deficits in SZ and BP are similar, although the severity is worse in SZ compared with BP.5, 6 Brain imaging studies demonstrated an overlap of neuroanatomical changes, such as gray matter reductions in both SZ and BP.7 It remains elusive whether common key molecular mediators exist in the pathophysiology of both SZ and BP, and if they connect shared genetic risks with gross phenotypic manifestations at the anatomical and clinical levels.
Oxidative stress has been studied in the context of severe mental disorders.8, 9, 10, 11 Oxidative stress occurs as the result of an accumulation of free radicals generated by normal metabolism and various environmental exposures to elicit cellular dysfunction.12, 13 Enzymatic and non-enzymatic antioxidants eliminate these free radicals to achieve a balance between free-radical generation and extinction.12, 13 These antioxidants include superoxide dismutases (SODs), catalase and glutathione (GSH).14, 15, 16, 17, 18, 19, 20 GSH is a representative non-enzymatic antioxidant that exists at low intracellular levels and functions as an important regulator of redox balance and oxidative stress.21, 22
It still remains elusive whether and how changes in peripheral oxidative stress markers reflect an alteration of these molecules in the brain. Some reports suggest the existence of oxidative stress in patients’ brains in parallel with peripheral tissues.14, 23, 24, 25, 26 In preclinical studies, excess oxidative stress is consistently observed in the brains of animal models with etiological triggers relevant to mental illness, in particular SZ. These models include those of neonatal hippocampal lesion, expression of mutant DISC1 protein and genetic deletion of the key GSH-synthesizing enzyme.27, 28, 29, 30, 31 Most importantly, prophylactic administration of antioxidants can ameliorate behavioral deficits in adulthood in animal models.28, 31 Thus, a possible mechanism of how excess oxidative stress and possibly GSH cascades may underlie behavioral changes has been explored. Parvalbumin-positive interneurons in the cortex and hippocampus are believed to have a key role in cognition and other higher brain functions,32, 33, 34 and these neurons are particularly sensitive to oxidative stress.27, 30, 35 Thus, these specific interneurons may be a candidate substrate of oxidative stress in the pathophysiology of mental illness.
Guided by many promising preclinical studies that potentially link GSH and oxidative stress with the pathophysiology of SZ, multiple groups have also conducted clinical studies and measured GSH levels in peripheral blood of patients with SZ. Although many studies report the reduction of GSH in tissues of SZ patients, there are still some inconsistencies among these reports.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 This is partly because some studies were performed in a small sample size, and further confirmation is needed.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 An alternative approach is to explore correlations between symptoms and GSH.46 Contrary to the abundance of studies in SZ, only three studies measured GSH in BP, which had conflicting results.47, 48, 49 Given that SZ and BP likely share common pathophysiological mechanisms, it is an important question whether or not GSH is altered in both SZ and BP. Such commonality and specificity of molecular changes among mental illness are to be investigated from the viewpoints in translational psychiatry.
In the present study, we address the significance of peripheral GSH in SZ and BP. We recruited BP patients that exhibited at least one psychotic episode because they share some of the neurocognitive or neuroanatomical deficits observed in SZ.50, 51 First, we examined the levels of plasma GSH in patients with SZ and BP compared to healthy controls (CON). Second, we correlated peripheral GSH levels with clinical symptoms and neurocognitive functions. Finally, we assessed the effect of types of medication, drug adherence, duration of illness and previous psychiatric hospitalization on the level of GSH in SZ and BP.
Materials and methods
Participants
All patients (n=114) were recruited as part of the Monitoring Recovery from an Episode of Severe Mental Illness (RECOVERY) study in the greater Baltimore–Washington, D.C. area from two cohorts admitted between August 2008 and December 2012 from the Johns Hopkins Hospital Community Psychiatry Program and the Johns Hopkins Bayview Medical Center Community Psychiatry Program. Participants had DSM-IV clinical diagnoses of SZ, schizoaffective disorder, schizophreniform disorder, BP (type I or II), delusional disorder or psychotic disorder not otherwise specified. All participants experienced at least one psychotic episode and were able to speak and understand English. The participants enrolled were screened for heart disease, diabetes and kidney disease and were not taking supplements. The study was approved by the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health. We obtained written informed consent from all participants after a complete description of the study. The CON (n=48) were obtained from the Prevention Research Center (PRC) trial and The Johns Hopkins Schizophrenia Center (JHSZC). A second control cohort was collected from the Baltimore Epidemiologic Catchment Area (ECA) follow-up study but was not used in the final study. None of the CON met the DSM defined criteria for psychiatric disorders.
Psychiatric symptoms were assessed in the patients using the Positive and Negative Syndrome Scale (PANSS).52 The PANSS is a 30 item rating scale designed to measure symptomatology and is completed by a trained interviewer after a semi-structured interview. The PANSS has a total and three subscale scores: positive, negative and general psychopathology. Cognitive function was assessed using the Brief Assessment of Cognition in Schizophrenia (BACS).53 The BACS composite score is calculated by averaging six subdomains: working memory, motor speed, verbal fluency, attention and speed of information processing, and executive function. We used the BACS composite score as an overall indicator for neurocognitive function. We also collected information on smoking status (smoker/non-smoker), types of medications (antipsychotics/mood stabilizers/antidepressants/minor tranquilizers), adherence to mediations (everyday/almost everyday/most days/hardly ever/never), duration of illness, years of education and previous psychiatric hospitalization. All patients completed clinical assessments except one SZ patient who refused the PANSS and BACS assessments, and one BP patient who refused the PANSS assessment. The CON did not receive any BACS assessment.
Measurement of GSH in plasma
Peripheral whole-blood samples were collected from each participant by venous puncture (Supplementary Table 1). Blood collection was not taken at the time of diagnosis. The mean delay between blood collection and diagnosis is not available. The Johns Hopkins Genetic Resources Core Facility processed samples from all cohorts; samples that arrive before 1500 hours are processed within 2 h and samples after are processed immediately the next morning (~16–18 h). Plasma was isolated by centrifugation at room temperature at 1960 × g for 15 min, aliquoted and stored at−80 °C. Total GSH (the sum of GSH and glutathione disulfide (GSSG)) was measured in plasma using modifications of the Tietze method.54, 55 Specifically, plasma samples were deproteinated by adding 50% v/v of freshly prepared 10% metaphosphoric acid (MPA), centrifuging at 2000 × g for 2 min and then the supernatant stored at −20 °C until assaying. For the assay, 5 μl of freshly prepared 4 m triethanolamine was added to 100 μl of MPA-treated plasma. GSH was then measured according to kit specifications (Cayman Chemical, Ann Arbor, MI, USA, 703002). Each plasma sample was measured in duplicate and reported as the average of the two values. We ran a standard curve from 1 to 0.016 μm that maintained linearity with a slope of 26±5 and an R2 of 0.998±0.001 (Supplementary Figure 1). The rate of increase in absorbance at 415 nm, which measures the reduction of 5–5′-dithiobis (2-nitrobenzoic acid) by GSH, reflects the total GSH content. The concentration of total GSH in plasma was reported as μm. We describe total GSH simply as GSH.
Statistical analysis
Statistical analyses were performed using R version 3.3.0 for Windows (The R Foundation for Statistical Computing, Vienna, Austria). Group comparison of demographic and clinical data were conducted using Welch’s two sample t-test for continuous variables, except for age in which one-way ANOVA was used. Comparisons of categorical data were performed using Fisher’s exact test with Monte Carlo simulation N=1 000 000. Direct comparisons of demographic and clinical data between healthy CON vs SZ/BP were accomplished using Welch’s two sample t-test.
The Shapiro–Wilk normality test was performed on GSH and log GSH levels, and showed non-normal distributions for both SZ and BP patients, therefore subsequent correlation analysis was performed on total GSH levels. To compare GSH levels in plasma of patient and CON groups, we used ANCOVA analysis with adjustment for age, sex, ethnicity and smoking status to determine differences among the three groups. To determine differences between groups, we performed a post hoc pair-wise (Tukey–Kramer) comparison.
Correlation between GSH levels and PANSS (total, positive, negative and general) or BACS composite score was performed using Spearman’s rank correlation coefficient. Partial correlations were further tested to control for age, smoking status, gender and ethnicity. We also performed multiple regression analyses to evaluate the clinical characteristics (severity of symptoms, neurocognitive functions, previous hospitalization and comorbid health conditions) on total GSH level in SZ and BP. We performed the multiple regression analyses with and without the interaction term (clinical characteristics × diagnosis). All data were expressed as mean±standard deviation (s.d.). Statistical significance was defined as P<0.05.
Results
Study population and neuropsychiatric assessment
In the present report, we studied patients with SZ, those with BP, and CON. These three groups were not fully matched with regard to age, gender, ethnicity and smoking status (Table 1). The age of patients was significantly higher than CON. The distributions of sex, ethnicity and smoking status were significantly different between SZ and BP (Table 1). Therefore, we incorporated age, sex, ethnicity and smoking status in our regression model to adjust for these variables. We did not see a significant difference between SZ and BP in the duration of illness calculated at the age of initial psychiatric contact. The rate of past hospitalization was similar in SZ and BP. As expected, SZ patients received more antipsychotic medication compared to BP (P<0.001), and BP patients received more mood stabilizers than SZ (P<0.001). There was also increased use of benzodiazepines in BP compared with the SZ patients (P<0.016). There was no significant difference in use of antidepressant or in adherence to the medications between SZ and BP. PANSS total scores were significantly lower in BP than SZ, and BACS composite scores were significantly higher in BP than SZ (P<0.001).
Table 1. Clinical and demographic characteristics (plasma storage time<100 months).
| Characteristics | CON (N=48) | SZ (N=52) | BP (N=62) | P-valuea | CON vs SZ P-valueb | CON vs BP P-valueb |
|---|---|---|---|---|---|---|
| Age | 29.02±7.10 | 42.46±10.52 | 41.13±10.75 | <0.001* | <0.001* | <0.001* |
| Gender (male/female) | 29/19 | 28/24 | 18/44 | 0.002* | 0.549 | 0.002* |
| Ethnicityc (AA/C/H/A/other) | 31/16/0/0/1 | 35/12/1/0/4 | 22/30/1/1/8 | 0.004* | 0.332 | 0.008* |
| Smokingd (smoker/non-smoker/unknown) | 13/35/0 | 31/5/16 | 42/4/16 | <0.001* | <0.001* | <0.001* |
| Duration of Illness (years)e | — | 14.10±12.71 | 11.07±11.69 | 0.195 | — | — |
| Previous hospitalization (yes/no) | — | 41/11 | 46/16 | 0.660 | — | — |
| Antipsychotics (yes/no) | — | 42/10 | 25/37 | <0.001* | — | — |
| Mood stabilizers (yes/no) | — | 7/45 | 32/30 | <0.001* | — | — |
| Antidepressant (yes/no) | — | 16/36 | 23/39 | 0.554 | — | — |
| Benzodiazepines (yes/no) | — | 3/49 | 14/48 | 0.016* | — | — |
| Adherence to medicationf | — | 34/9/4/0/1/4 | 36/13/7/1/3/2 | 0.703 | — | — |
| PANSS total scoreg | — | 65.29±21.44 | 52.77±15.15 | <0.001* | — | — |
| BACS composite scoreh | — | 30.96±7.51 | 36.70±7.90 | <0.001* | — | — |
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; BP, bipolar disorder; CON, healthy control; PANSS, Positive and Negative Syndrome Scale; SZ, schizophrenia.
Welch’s two sample t-test is used for continuous variables, except for age in which one-way ANOVA is used. Fisher’s exact test is used for categorical variables with Monte Carlo simulation N=1 000 000.
Welch’s two sample t-test was used to compare control vs SZ/BP.
African American/Caucasian/Hispanic/Asian/other.
Missing smoking status is considered unknown.
Information for 1 SZ and 1BP patient is missing.
Everyday/almost everyday/most days/hardly ever/never/not available.
One patient with SZ and 1 patient with BP refused for PANSS assessment.
One patient with SZ refused to take BACS assessment.
*P<0.05.
Measurement of GSH: influences of tissue storage
Storage duration may affect the levels of metabolites in biospecimens. To address this question, we examined the influence of total GSH level on storage time (in months) in plasma samples from the RECOVERY, PRC and JHSZC cohorts as well as the ECA cohort. The RECOVERY, PRC and JHSZC plasma samples had a storage time of <100 months at the time of the biochemical experiment, whereas the ECA cohort had a storage time >100 months. When we included all cohorts (RECOVERY, PRC, JHSZC and ECA) independent of storage time, we obtained similarly trended results in comparison to the analysis performed using plasma samples with a storage time of <100 months (RECOVERY, PRC and JHSZC). The data showed that storage time did not have a significant influence on total GSH level. This result was not affected by the inclusion of the ECA cohort.
Although storage time did not significantly influence total GSH level, we did observe a slight decrease in GSH level with increased storage time when the ECA cohort was included. To take a conservative approach, we chose to only include plasma samples with a storage time of <100 months. Therefore, the final study included plasma samples from the RECOVERY, PRC and JHSZC cohorts.
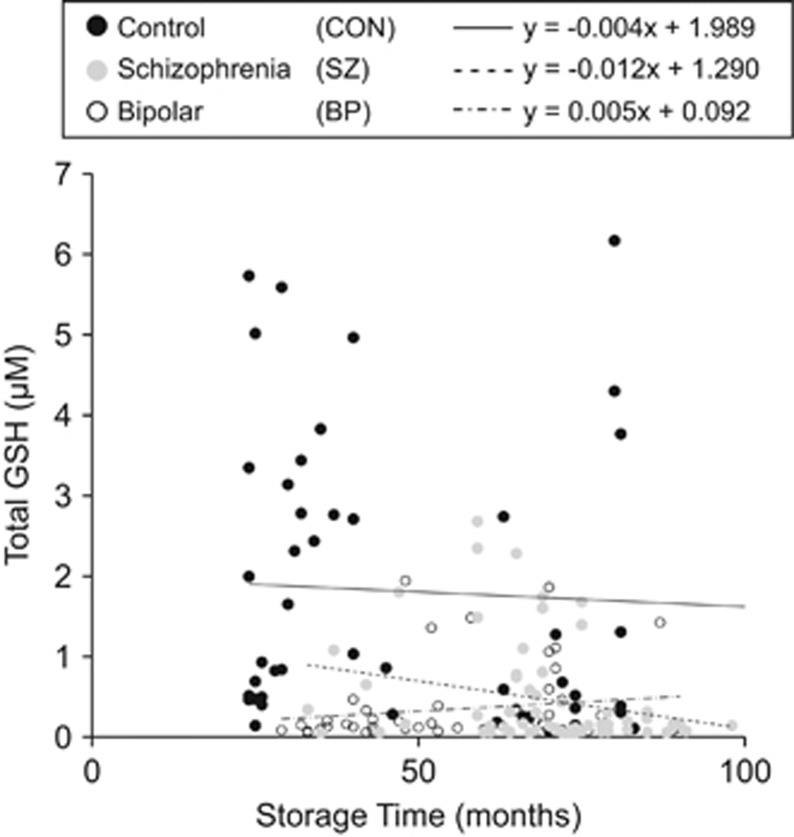
The total GSH level vs storage time in months from those cohorts are shown in Figure 1, a best fit line and slope were reported for each CON, BP and SZ group. The slopes for CON and SZ plasma samples were slightly negative showing an overall decrease in GSH level with increase in storage time (Figure 1). Conversely, the slope for BP samples was positive indicating an increase in GSH levels with increased storage time (Figure 1). A lack of a consistent trend in effect of GSH level over storage time across the three groups indicates that storage time is not an influencing factor in this study and reflects the overall distribution of GSH levels. This is also confirmed by the fact that our results were not affected by including the older control ECA cohort samples.
Figure 1.
Effect of total glutathione (GSH) levels upon storage duration of control and patient plasma samples. The levels of GSH are plotted against storage time for healthy control (CON) (●), bipolar disorder (BP) (○) and schizophrenia (SZ) ( ) plasma samples. All CON and patient plasma samples had a storage time of <100 months. A trend line of the data was obtained for each sample (CON (–), y=−0.004x+1.989; BP (–·–·–) y=0.005x+0.092; SZ (– – –) y=−0.012x+1.290). The variability of slopes across the three sample populations indicates storage time is not a significant influencing factor on total GSH levels and is more an effect of the random overall distribution of GSH levels within each group.
) plasma samples. All CON and patient plasma samples had a storage time of <100 months. A trend line of the data was obtained for each sample (CON (–), y=−0.004x+1.989; BP (–·–·–) y=0.005x+0.092; SZ (– – –) y=−0.012x+1.290). The variability of slopes across the three sample populations indicates storage time is not a significant influencing factor on total GSH levels and is more an effect of the random overall distribution of GSH levels within each group.
Comparison of GSH levels in plasma in patient and control groups
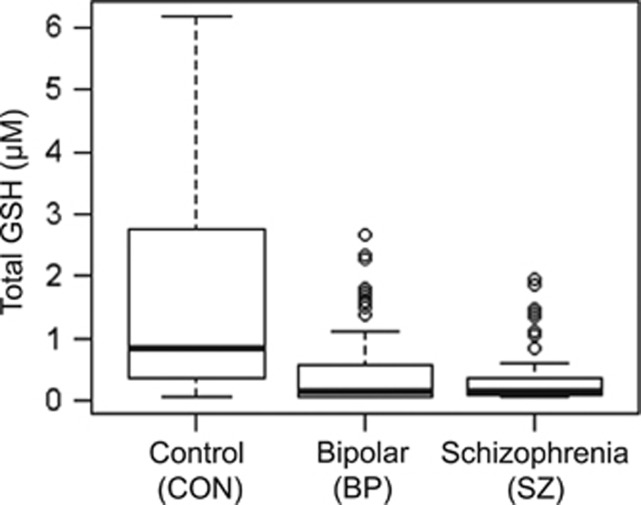
We examined the levels of GSH in plasma from the three groups (CON, SZ and BP). We observed decreased GSH in SZ compared to CON (CON, 1.736±1.766; SZ, 0.365±0.478) (Figure 2). We also observed decreased GSH in BP compared to CON (CON, 1.736±1.766; BP, 0.476±0.655) (Figure 2). Levels of GSH in SZ and BP patients (SZ, 0.365±0.478; BP, 0.476±0.655) were comparable.
Figure 2.
Reduction of plasma glutathione (GSH) levels in schizophrenia (SZ) and bipolar disorder (BP) compared with healthy controls (CON). The levels of plasma GSH were measured in CON, SZ and BP. The GSH levels in CON, SZ and BP are depicted as box-plots. The GSH level was significantly lower in both patient groups compared with CON (CON, 1.737±1.776; SZ, 0.365±0.478; BP, 0.476±0.655; CON vs SZ, P<0.001; CON vs BP, P<0.001).
We used ANCOVA analysis with adjustment for age, sex, ethnicity and smoking status to observe differences among the three groups. We observed a significant difference among the groups (P<0.001). We performed a post hoc pair-wise (Tukey–Kramer) comparison adjusting for age, sex, ethnicity and smoking to determine differences between groups. We obtained a significant decrease between SZ and CON groups (P<0.001), and BP and CON groups (P<0.001). There was not a significant difference between the SZ and BP groups (P=0.802).
Although we adjusted for age in our ANCOVA analysis, we were interested in observing the effect of total GSH levels upon age. We plotted total GSH level vs age for CON, BP and SZ (Supplementary Figure 2). As observed, the trend line for the CON was consistently approximately twofolds higher than the corresponding SZ and BP trend line when considering total GSH level over the entire age distribution.
GSH levels and clinical characteristics
Next, we determined whether the levels of peripheral GSH were correlated with PANSS scores or BACS scores in SZ and BP, respectively, correcting for age, sex, ethnicity and smoking (Table 2). We observed significant correlation of the PANSS total (P=0.033) and PANSS positive (P=0.013) scores with total GSH level within the SZ patient population. We also observed significant correlation between the PANSS general score (P=0.035) and total GSH level within the BP patient population. We did not observe significant correlation between total GSH level and BACS scores in SZ and BP.
Table 2. Partial correlation of GSH levels with PANSS and BACS scores in SZ and BP, corrected for age, sex, ethnicity and smoking.
| ρc | P-value | |
|---|---|---|
| SZa(n=52) | ||
| PANSS total | −0.311 | 0.033* |
| PANSS positive | −0.359 | 0.013* |
| PANSS negative | −0.203 | 0.172 |
| PANSS general | −0.262 | 0.075 |
| BACS average | 0.245 | 0.097 |
| BPb(n=62) | ||
| PANSS total | −0.204 | 0.127 |
| PANSS positive | −0.140 | 0.298 |
| PANSS negative | −0.107 | 0.427 |
| PANSS general | −0.279 | 0.035* |
| BACS average | −0.062 | 0.645 |
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; BP, bipolar disorder; GSH, glutathione; PANSS, Positive and Negative Syndrome Scale; SZ, schizophrenia.
One SZ patient refused to take PANSS and BACS assessment.
One BP patient refused to take PANSS.
Shapiro–Wilk normality test was performed on GSH and log GSH levels, and showed non-normal distributions in both SZ and BP patients, so Spearman’s rank correlation coefficient was used for GSH levels, with partial correlation corrected for age, sex, ethnicity and smoking status. *P<0.05.
Finally, we performed multiple linear regression analyses to assess the effect of each clinical characteristic of severity of disease, previous hospitalization, illness duration and medication adherence on the level of total GSH. We found no significant effect on total GSH level from any of these clinical characteristics.
Discussion
The present study demonstrates a significant reduction in the level of plasma GSH in both SZ and BP patients compared to CON. Our data, from a reasonably large sample size, validates the significance of GSH deficits in SZ pathology, which has been suggested previously.36, 37, 38, 39, 40, 41, 42 In contrast, it has been unclear whether GSH had a role in BP, because only three previous studies showed conflicting results.47, 48, 49 This study provides evidence that GSH deficits are also present in patients with BP, indicating that the reduction of peripheral GSH is a common molecular signature of SZ and BP. Given that the BP patients in the present study experienced psychosis, GSH may be involved in psychosis-associated pathophysiology that is shared by both SZ and BP.
As we propose that this reduction is related to psychosis, we do not exclude that this reduction may be associated with other conditions such as neurodegenerative disorders. This study did not include the quantification of GSH levels in BP without psychosis, but we view it is an important topic to investigate in a future study. Although blood collection was taken after diagnosis and not during a period of psychosis, we do not believe the reduction of total GSH level is a direct indicator of a patient’s psychotic state, but is associated with a predisposition to psychosis. Finally, we report total GSH values in the present study, and acknowledge the significance in understanding the contribution of the reduced (GSH) and oxidized (GSSG) forms of GSH to the overall blood level observed and will address this in the future.
We further addressed how the GSH changes are related to clinical features. The levels of plasma GSH were significantly correlated with PANSS scores in both SZ and BP, which may support the idea that the changes in the GSH levels are associated with the psychosis dimension commonly underlying these two diseases. The type of medication administered to each patient group was significantly different. Thus, the reduction of GSH may be an intrinsic pathophysiological change common to SZ and BP rather than an effect of medication. By studying SZ, BP and CON in the same study, we are now able to propose an important role for GSH in both SZ and BP.
Establishing high-throughput, objective biomarkers is important to the field of translational psychiatry. Towards this goal, molecules that are associated with pathophysiological mechanisms (for example, pathophysiological mediators altered in the disease conditions) are promising candidates for such biomarkers. More practically, such candidates are scalable in measurement, with reasonable sensitivity and specificity. Although it is too early to state that GSH is a promising biomarker under the criteria, the data in the present study provide an optimistic perspective that molecules in GSH signaling (even it is not GSH itself) may be candidates for biomarkers in the future.
Although GSH is scalable in measurement by using peripheral blood, it remains elusive how the changes in peripheral GSH reflect brain pathology. The variability in the levels of peripheral GSH, although the absolute differences in GSH levels between patient and control groups are significant, is high. Measurement of blood GSH may be a useful indicator to estimate psychosis but this cannot stand as a biomarker by itself. Instead, similar to the C-reactive protein, which is still regarded as useful in monitoring the pathological status of immune-related conditions (for example, rheumatoid arthritis), we propose that GSH can be used as a candidate objective marker for mental disorders, in combination with other markers. These markers have smaller variability with their sensitivity and specific complementary to those of GSH that are associated with psychotic status in general.
We acknowledge potential limitations in the present study design. First, the present study was designed in a cross-sectional manner. Thus, it is difficult to determine a causal relationship between the levels of GSH and clinical outcomes. A prospective study that measures GSH and its relationship with clinical features is crucial to assess the potential clinical utility of GSH in these pathological conditions. Second, the participants in this study were recruited in the context of public health and as a result medical histories, including information on liver disease, were limited. All patient participants were screened for heart conditions, diabetes and kidney disease. Third, fasting state and age are not perfectly controlled, and there is variability in blood processing time and time of blood collection following diagnosis; nevertheless, as these confounding factors underlie all groups, it is unlikely that these factors affect the conclusion. Fourth, we focused on patients with severe mental disorders who experienced at least one psychotic episode and the measures were limited to common assessment tools that can be applied to both SZ and BP. Therefore, we did not rate BP patients with the Young Mania Rating Scale or the Montgomery Asberg Depression Rating Scale.56, 57 BACS does not include the assessment for some cognitive constructs, such as ideational fluency and visual memory, which are known to be impaired in patients with SZ and BP.5 Thus, the negative results in the correlation of the GSH levels and the BACS composite scores do not necessarily mean that GSH and cognitive deficits are unrelated in the diseases. For example, neurocognitive batteries such as the calibrated ideational fluency assessment word/design fluency or the brief visuospatial memory test will complement this limitation in future studies.
In previous reports, we observed molecular changes in SOD1 and inflammatory-related molecules in CSF only in the early phase of disease but not in the chronic phase.19, 20 Changes in many of these molecules were consistently observed in another cohort for early psychosis.58 In contrast, the present study highlights the change of an oxidative stress-associated molecule in the chronic stage of SZ and BP. These results imply two different but potentially overlapping interpretations. First, a reduction in plasma GSH may be an enduring trait after onset of disease, which contrasts with the reduction of SOD1 in CSF that preferentially manifested in the early stages of psychosis.19 Second, reduction in plasma GSH may represent a general risk of mental illness, such as SZ and BP, which may exist even before the onset of disease. Future studies in a longitudinal design may provide an answer to the first question. To address the second question, a systematic study of plasma GSH that includes high-risk cohorts and first-degree relatives will be useful. In addition, we may need to compare the potential differences between data from different tissues (for example, plasma and CSF). Although peripheral biomarkers are important in translational and clinical psychiatry, it remains mechanistically unclear how molecular changes in the blood and brain are related. Therefore, preclinical animal models may aid in further understanding this link and the underlying mechanisms of oxidative stress in disease.
Acknowledgments
This work was supported by the National Institute of Mental Health MH-084018 (AS), MH-094268 Silvio O. Conte center (AS), MH-069853 (AS), MH-085226 (AS), MH-088753 (AS), MH-092443 (AS), MH-105660 (AS) and MH-100776 (WE), as well as grants from Stanley (AS), S-R (AS), RUSK (AS), NARSAD (AS), MSCRF (AS) and anonymous donor to the Department of Mental Health, Bloomberg School of Public Health. TT is supported by the Japanese young psychiatrists fellowship program organized by Dr Teruhiko Higuchi at the National Center for Neurology and Psychiatry in Japan. A fund from Mitsubishi Tanabe Pharma Corporation was partly used for recruitment of 12 healthy controls.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science 2015; 348: 499–500. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry 2009; 66: 988–989. [DOI] [PubMed] [Google Scholar]
- Kavanagh DH, Tansey KE, O'Donovan MC, Owen MJ. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry 2015; 20: 72–76. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry 2007; 62: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Pena J, Aretouli E, Orue I, Cascella NG, Pearlson GD et al. Confirmatory factor analysis reveals a latent cognitive structure common to bipolar disorder, schizophrenia, and normal controls. Bipolar Disord 2013; 15: 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 2013; 170: 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya CD, Howell KR, Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2013; 46: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 2013; 74: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry 2015; 21: 10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet 2016; 388: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 2002; 30: 620–650. [DOI] [PubMed] [Google Scholar]
- Emiliani FE, Sedlak TW, Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr Opin Psychiatry 2014; 27: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature 2000; 404: 190–193. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct 2002; 20: 171–175. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci 2009; 34: 263–271. [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Gama CS, Andreazza AC, Salvador M, Cereser KM, Gomes FA et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1677–1681. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LM, Nardin P et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res 2007; 41: 523–529. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry 2013; 18: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Hayes LN, Tanaka T, Xiao M, Yolken RH, Worley P et al. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr Res 2016; 183: 64–69. [DOI] [PubMed] [Google Scholar]
- Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G et al. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin Chem 2001; 47: 1467–1469. [PubMed] [Google Scholar]
- Lu SC. Glutathione synthesis. Biochim Biophys Acta 2013; 1830: 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008; 11: 851–876. [DOI] [PubMed] [Google Scholar]
- Boskovic M, Vovk T, Kores Plesnicar B, Grabnar I. Oxidative stress in schizophrenia. Curr Neuropharmacol 2011; 9: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima G, Benz B, Parpura V, Cuenod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophr Res 2003; 62: 213–224. [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets 2005; 16: 386–391. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 2007; 318: 1645–1647. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014; 83: 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci USA 2013; 110: 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 2013; 73: 574–582. [DOI] [PubMed] [Google Scholar]
- das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry 2012; 71: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459: 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 2012; 17: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology 2012; 62: 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuntas I, Aksoy H, Coskun I, Caykoylu A, Akcay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med 2000; 38: 1277–1281. [DOI] [PubMed] [Google Scholar]
- Ballesteros A, Jiang P, Summerfelt A, Du X, Chiappelli J, O'Donnell P et al. No evidence of exogenous origin for the abnormal glutathione redox state in schizophrenia. Schizophr Res 2013; 146: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich-Muszalska A, Olas B, Glowacki R, Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology 2009; 59: 1–7. [DOI] [PubMed] [Google Scholar]
- Mico JA, Rojas-Corrales MO, Gibert-Rahola J, Parellada M, Moreno D, Fraguas D et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry 2011; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011; 11: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1178–1183. [DOI] [PubMed] [Google Scholar]
- Samuelsson M, Skogh E, Lundberg K, Vrethem M, Ollinger K. Taurine and glutathione in plasma and cerebrospinal fluid in olanzapine treated patients with schizophrenia. Psychiatry Res 2013; 210: 819–824. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y et al. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res 2006; 81: 291–300. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Haile CN et al. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry 2007; 68: 754–760. [DOI] [PubMed] [Google Scholar]
- Akyol O, Herken H, Uz E, Fadillioglu E, Unal S, Sogut S et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 995–1005. [DOI] [PubMed] [Google Scholar]
- Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS ONE 2008; 3: e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhan Tuncel O, Sarisoy G, Bilgici B, Pazvantoglu O, Cetin E, Unverdi E et al. Oxidative stress in bipolar and schizophrenia patients. Psychiatry Res 2015; 228: 688–694. [DOI] [PubMed] [Google Scholar]
- Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 371–375. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Singh N, Whitaker E, de Brito M, Lewis AM, Vieta E et al. Altered plasma glutathione levels in bipolar disorder indicates higher oxidative stress; a possible risk factor for illness onset despite normal brain-derived neurotrophic factor (BDNF) levels. Psychol Med 2014; 44: 2409–2418. [DOI] [PubMed] [Google Scholar]
- Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry 2013; 74: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry 2013; 170: 886–898. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68: 283–297. [DOI] [PubMed] [Google Scholar]
- Koga M, Serritella AV, Messmer MM, Hayashi-Takagi A, Hester LD, Snyder SH et al. Glutathione is a physiologic reservoir of neuronal glutamate. Biochem Biophys Res Commun 2011; 409: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969; 27: 501–522. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435. [DOI] [PubMed] [Google Scholar]
- Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull 2014; 40: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.