Abstract
Severe worry includes a complex blend of maladaptive affective and cognitive processes. Contrary to other forms of anxiety, there is no consensus in the field regarding the neural basis of worry. To date, no study has looked at neural patterns associated specifically with in-scanner induction and reappraisal of worry. In this study, we attempt to describe distinct components of the ‘neural phenomenology’ of worry: induction, maintenance, severity and reappraisal, by using a personalized, in-scanner worry script. Twenty older, non-anxious participants and twenty late-life generalized anxiety disorder (GAD) participants were included. Whole-brain axial pseudo-continuous arterial spin-labeling scans were collected. We used a voxel-wise two-way ANOVA to test the group-by-block interaction. Worry induction was associated with greater cerebral blood flow (CBF) in the visual cortex, thalamus, caudate and medial frontal cortex compared with the rest. Reappraisal was associated with greater CBF in similar regions, whereas the orbital frontal gyrus showed lower CBF relative to rest. Relative to non-anxious participants, GAD had greater CBF in multiple regions during worry induction (visual and parietal cortex, middle and superior frontal) and lower CBF during reappraisal in the supplemental motor area, middle cingulate gyrus, insula and putamen. Except for the thalamus, there was no change in CBF throughout the five blocks of worry induction and reappraisal. Severe worry is distinctly associated with increased CBF in several neocortical regulatory regions. We present new data supporting the view of worry as a complex process, engaging multiple regions in the initiation, maintenance and reappraisal of worry.
Introduction
Pathologic worry is defined as a complex affective and cognitive process, negative-affect laden and relatively uncontrollable.1, 2 Pathologic worry in late-life is surprisingly prevalent in the community, with 20% of older adults reporting severe worry.3 The notable cardiovascular morbidity associated with anxiety and pathologic worry has only recently been recognized.4, 5, 6 Thus, severe worry has been associated with increased risk of stroke and other cardiovascular events, even after controlling for depression and other vascular risk factors.7, 8, 9 Recent studies have also described the association of late-life anxiety and worry with mild cognitive impairment and an increased risk of conversion to Alzheimer’s disease.10, 11, 12 Severe worry is described in several disorders, including generalized anxiety disorder (GAD), social anxiety and major depressive disorder.13
The complex blend of maladaptive affective and cognitive processes underlying worry may explain why, contrary to other forms of anxiety (for example, simple and social phobias, panic, somatic anxiety, post-traumatic stress), there is less consensus in the field regarding the neural basis of worry. Several functional magnetic resonance imaging (fMRI) studies have investigated both activation and functional connectivity among various brain regions involved in GAD—in adolescents,14, 15 young adults16, 17 and older adult participants18, 19 (see Mochcovitch et al. for a review20). However, very few studies used fMRI paradigms specifically tailored to induce worry19, 21 or analyzed specifically the effect of worry severity at rest22 or during task23 and, to our knowledge, none has explored the in-scanner reappraisal of worry.
Describing the neural basis of worry induction and reappraisal may prove consequential, especially in older adults, for further understanding the malignant effect of worry on both vascular and neural health.4, 9, 12
In this study, we will describe distinct components in the ‘neural phenomenology’ of worry: induction, maintenance, severity and reappraisal. Although worry induction is most likely a ubiquitous phenomenon associated with the universal human experience of worrying,24, 25, 26 maintenance and severity characterize the pathological worry associated with GAD, major depressive disorder (MDD) and other disorders. The maintenance component describes the tonic aspect of pathologic worry, characterized by the perpetuation of the negatively laden affective and thought processes associated with active worrying.24 The severity component describes the quantitative difference in worry intensity between normal and pathologic worry.24, 27 Recent studies found quantitative (more severe) but not qualitative differences in the worry process between normal and pathological worriers.26, 28 Another key component of pathologic worry involves the difficult to control worry process, with significant interference of the worry process in daily life described by GAD participants compared with the healthy control participants. This difficulty in controlling the worry process has been associated with emotion dysregulation, including difficulties using cognitive reappraisal to mitigate the worry process.29, 30 Cognitive reappraisal (changing a situation’s meaning in a way that alters its emotional impact31, 32) and specifically deficits in reappraisal have been implicated in the GAD/worry pathology, especially as failures to implement adaptive reappraisal strategies while rigidly using poor compensatory strategies such as worry.30 Our previous work supports the emotion dysregulation theory by indicating that normative cognitive reappraisal strategies may actually trigger somatic anxiety in participants with pathologic worry.33
We hypothesize that induction of worry will engage similar neural regions in GAD and healthy participants, but that severe worry will engage additional regions than those associated with normal or less severe worry. With regard to worry maintenance, we hypothesize that there will be sustained changes in brain regions associated with generation of worry across the worry induction blocks in participants with GAD, but not in healthy controls. We also hypothesize that, compared with healthy participants; GAD participants will have difficulties engaging regulatory prefrontal regions during reappraisal.
To explore the neural changes associated with worry induction, maintenance and reappraisal, we used a personalized, in-scanner worry induction task (see ‘Materials and Methods’). We also chose a method—arterial spin-labeling—better designed to capture the slow changes in neural activity associated with a lengthy process such as worry.18, 34
Materials and methods
Study design and participants
Magnetic resonance imaging data was collected in a total of 40 participants: 20 older participants with GAD (13F, mean age 67) and 20 non-anxious older healthy controls (10F, mean age 68). The primary inclusion criteria for anxiety participants was a primary diagnosis of GAD for at least 6 months according to the structured clinical interview for DSM-IV (SCID) and a score of 17 or higher on the Hamilton Anxiety Rating Scale (HARS) at the time of scanning. Patients with other anxiety disorders were included if GAD was the principal diagnosis (based on severity and duration), as were patients with a past history of alcohol or substance abuse that was in full remission for at least 3 months. Patients with lifetime history of comorbid unipolar depression were included if GAD was the primary diagnosis (based on duration), but participants with current major depressive disorder at the time of scanning were excluded. In addition, the Penn State Worry Questionnaire (PSWQ), a measure that was specifically developed to quantify worry, was used to assess worry severity.35 As this was a sample of older participants, we used the Cumulative Illness Rating Scale for geriatrics (CIRS-G) to quantify chronic medical illness burden (including cardiovascular health),36 as well as a comprehensive neuropsychological battery to describe cognitive status (the Repeatable Battery for the Assessment of Neuropsychological Status, RBANS,37 and select subtests of the Delis–Kaplan Executive Function Scale, D–KEFS38).
Exclusion criteria included lifetime psychosis or bipolar disorder, dementia, Mini-Mental State Examination (MMSE) score <24, high suicide risk, ongoing psychotherapy, current antidepressant or anxiolytic use, as well as issues related to entering the MR environment. Participants were psychotropic-free at the time of the scan and underwent a washout period of 2 weeks if previously on antidepressants (6 weeks if on fluoxetine) (three participants were tapered off antidepressants). Participants were allowed non-psychotropic medications. Non-anxious participants had no history of psychiatric disorders. The University of Pittsburgh Institutional Review board approved the study. All participants provided written informed consent.
Magnetic resonance imaging data collection
Imaging data were collected at the University of Pittsburgh Magnetic Resonance Research Center using a 3-Tesla Siemens (Siemens Medical Solutions, Erlangen, Germany) Trio TIM scanner with a 32-channel head coil. Whole-brain axial pseudo-continuous arterial spin-labeling scans were collected (15 min, echo time (TE)=25, repetition time (TR)=4000 ms, flip angle (FA)=90°, 120 labeled and unlabeled volumes, FOV=64 × 64, slices=24, slice thickness=5 mm). High-resolution anatomical images (T1-weighted magnetization-prepared rapid gradient echo MPRAGE) were collected over 4 min and 43 s using the following parameters: FOV=256 × 254 mm, voxel size 1 × 1 × 1 mm, TI=900 ms, TR/TE=2/3.43 ms, flip angle=9°.
Worry induction and reappraisal task
The worry induction task used a personalized worry script. The script consisted of individualized worry-generating statements alternating with instructions to reappraise worry. During the participants’ initial evaluation, we elicited specific worry themes, which were then used to create sentences that instructed the participant to worry ‘as hard as s/he can, as s/he usually does it’ about the specific theme (for example, ‘worry about your husband’s health’, ‘worry about your back pain getting worse’). Participants were also trained in reappraisal strategies by a master-level instructor (SW), and practiced both worry induction and worry reappraisal prior to the in-scanner experiment, and they offered feedback regarding the accuracy of each worry induction and each worry reappraisal sentence (for example, ‘my husband has an excellent medical team’, ‘physical therapy always helps with my back pain’). Five worry induction statements and three worry reappraisal statements were selected in no particular order for each participant for the in-scanner experiment (this was done as it maximized the number of participants that had that number of each statement). The order of each statement was random and was counterbalanced (due to the wide variety of themes in the statements). Each block (5 worry blocks and 3 reappraisal blocks) included a single worry/reappraisal statement presented for the entire duration of the block. Each reappraisal block followed one of the worry induction blocks and the instruction was to reappraise that specific worry statement. Although in the scanner, each worry induction/reappraisal statement remained on the screen for 1 min (15 s fixation+45 s of active task). The in-scanner worry induction paradigm consisted of a block design of worry induction blocks alternating with worry reappraisal blocks, preceded by a 5 min resting state. After each in-scanner induction/reappraisal block, participants evaluated the severity of experienced worry on a scale from 1 (none) to 5 (very severe).
Image preprocessing
All preprocessing was done in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Worry induction pseudo-continuous arterial spin-labeling sequences were first motion-corrected (rigid registration to the mean using a least squares approach with 4th degree spline interpolation). Labeled and unlabeled volumes were motion-corrected separately at first and then motion-corrected together (by correcting to the mean). The data were then smoothed in native space using a Gaussian kernel with full-width at half-maximum of 8 mm. The structural image was skull-stripped manually using ITK-SNAP. The skull-stripped structural image was used to coregister the regular structural image to the mean functional image (affine coregistration with normalized mutual information as the cost function and 4th degree B-spline interpolation). The anatomical image was segmented into six tissue classes: gray matter, white matter, cerebrospinal fluid, skull, soft-tissue and air. This process outputs gray matter segmentation in native functional space and a deformation field, which can be used to normalize the functional images to a standard anatomical space (MNI).
Cerebral blood flow (CBF) estimates were generated using a MatLab script developed by JJ Wang.34, 39 We input the following parameters into the script: simple subtraction, label time of 0.7, delay time of 1.2, slice time of 45, labeling efficiency of 0.85 and TE of 25. We used an individualized gray matter mask (threshold at a probability of 0.05) in the analysis. After CBF was calculated at each volume (120 volumes), the deformation field was used to normalize the CBF volumes into MNI space. Using a custom MatLab script, we calculated the mean CBF across the rest block, mean CBF across all five worry induction blocks, mean CBF for each worry induction block, mean CBF across each reappraisal block and mean CBF across all three reappraisal blocks. Two quality control checks were performed on each individual image to confirm that the functional images were properly co-registered to structural images and then to the standard space. Stripped structural images were subsequently normalized to MNI space and an average structural image was generated to overlay all neuroimaging results.
Owing to the nature of the participants being studied (older participants with anxiety), we expected a fair degree of motion within the scanner. Thus, special care was taken to minimize motion within the scanner, as well as during our analysis. We calculated maximum motion (using motion parameters from motion correction) in x, y, z directions for translational and rotational motion. We found that there existed no group differences in x (T(25.1)=−1.2, P=0.26), y (T(29.2)=−0.8, P=0.42) and z (T(38)=0.2, P=0.82) translation or x (T(38)=−0.7, P=0.48), y (T(25.2)=−0.8, P=0.41) and z (T(34.5)=−0.9, P=0.37) rotation, however as expected (although not significant), the GAD group showed higher maximum motion than the healthy control (HC) except for z-translations. Two participants were excluded due to excessive motion (one in each group), whereas another was excluded due to falling asleep in the scanner during the worry induction task (HC group). Four participants (2 from each group) did not perform the reappraisal blocks due to differences in early scanning procedures (reappraisal not included in those tasks).
Statistical analysis
Statistical analyses were conducted in SPM12 (voxel-wise) and SPSS (clinical and demographic characteristics).
We first tested the main effect of worry induction and reappraisal (as compared to rest) by performing a paired t-test to test whether there was a significant difference between CBF during worry induction or reappraisal and rest. In all subsequent analyses, we subtracted the rest blocks from the worry induction and reappraisal blocks to adjust for the effect of rest.
We performed a voxel-wise, whole-brain 2-way ANOVA to test whether there was a significant interaction between group (HC and GAD) and block (5 blocks of worry and 3 blocks of reappraisal), with group as an independent factor and block as a repeated factor. To test for effects of block independent of group, we conducted a 1-way repeated measure ANOVA (in regions where the interaction was not significant), which tested whether there were any regions that changed across the worry or reappraisal blocks independent of group. The input for these previous two analyses was the mean CBF maps for the worry induction blocks (minus rest) and the mean CBF maps for the worry reappraisal blocks (minus rest). To test for group effects (independent of block), we conducted an independent samples t-test, which tested for significant differences (in regions where the interaction was not significant) between HC and GAD, where inputs were the mean CBF maps during worry induction or reappraisal. Due to group differences in cognitive function (see ‘Results’), we also adjusted for cognitive function. In the next step, we tested a regression model to identify the regions where the mean CBF (during worry induction or reappraisal) was significantly correlated with worry severity, as measured by PSWQ. Although testing for group differences and association with worry severity, we also adjusted for depression severity, as measured by core depressive Hamilton depression rating scale (HDRS) items (excluding anxiety items from HDRS40).
Multiple comparisons correction
To correct for multiple comparisons in the voxel-wise analysis, we used 3dClustSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). All voxel-wise analyses were corrected to P<0.05 and had the following minimum cluster sizes: main effect k=429 voxels, interaction k=643 voxels, block effect independent of group k=647 voxels, group effect independent of block k=418 voxels and regression with PSWQ k=408 voxels. All brain images were generated using xjview and BrainNet viewer.41
Owing to the recent literature on multiple comparison corrections in the neuroimaging field and especially the concern regarding cluster-wise correction approaches, we also present another multiple comparison correction suggested by Eklund et al.42 Although we have used an updated ClustSim version that corrected previous bugs reported by Eklund et al., we also report the results of a multiple comparison correction via permutation testing. Using statistical non-parametric mapping (SnPM) toolbox, we used 5000 permutations to estimate the voxel-wise P-values and then performed multiple comparisons correction (cluster-wise inference with P<0.005, uncorrected threshold) to control the family-wise error rate at α=0.05. We highlight in the text, tables and figures when the results pass correction via this method.
Results
Supplementary Table 1 presents the demographic, clinical and neuropsychological data. There were no differences in age, gender, race, education, global physical health (as measured by CIRS-G) and overall cognition (as measured by MMSE) between healthy and older GAD participants. As expected, compared with healthy participants, the older GAD participants had higher HARS, HDRS (as well as HDRS core depressive symptoms defined as HDRS minus the anxiety items40) and PSWQ scores. GAD also had lower performance in two cognitive tests (D–KEFS Trail Making, set-switching minus motor speed and D–KEFS color word inhibition).38
The in-scanner worry severity ratings (1–5, low–high) showed that there was no significant interaction between group and the five worry induction blocks (F(3,101)=1.13, P=0.341). There were also no group differences across any of the five inductions (F(1,34)=0.66, P=0.422) as well as no differences across block (F(3,101)=0.23, P=0.875). The mean (across all five blocks) of the worry severity scores for the healthy participants was 2.80 (95% confidence interval (CI)=0.33). For the GAD participants the mean was 2.97 (95% CI=0.37). Following reappraisal, the mean was 1.10 (95% CI=0.14) for healthy participants and 1.13 (95% CI=0.20) in GAD participants, which was not significantly different. There was on average an increase in worry rating when comparing each worry block to the previous non-worry block (147% (s.d.=59%) in HC and 155% (s.d.=61%) in GAD). This increase indicates that participants engaged in worry during the worry induction blocks.
Main effect of worry induction and reappraisal
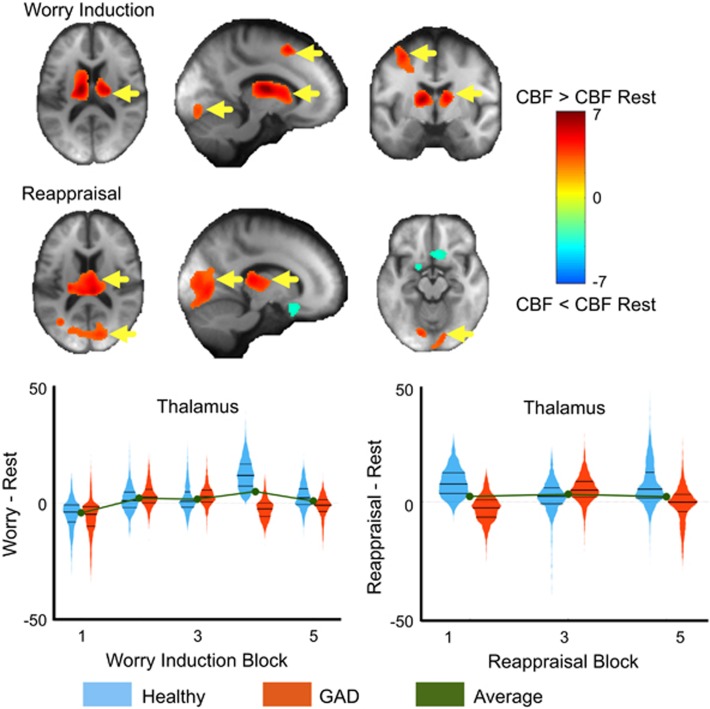
Compared to the rest trial, worry induction was associated with greater CBF in the following regions: left medial frontal cortex (supplemental motor area (BA8), dorsal ACC (BA32), middle, superior frontal cortex), bilateral visual cortex, bilateral thalamus and caudate (Figure 1 and Table 1).
Figure 1.
Main effect of worry induction and reappraisal. Paired t-tests comparing the mean cerebral blood flow (CBF) during worry induction (top) and reappraisal (bottom) compared to resting CBF. Areas where CBF was greater during worry or reappraisal than rest are shown in red/warm colors and the reverse effect in blue/cool colors. The colors indicate T-statistics from the paired t-test. Yellow arrows indicate regions that survived multiple comparisons correction via a non-parametric method (SnPM). The thalamus is plotted across the five worry induction blocks as well as the three reappraisal blocks. Violin plots are the mirrored histograms of the entire voxel-wise data in that region (to show variation across voxels). Healthy participants are shown in blue and generalized anxiety disorder (GAD) in red. The average is shown in green. Note: Bilateral thalamus showed a significant block effect independent of group.
Table 1. Results of all voxel-wise statistical analyses.
| Analysis | Condition | Region name | Cluster size | Max T/F | X | Y | Z | Passed SnPM |
|---|---|---|---|---|---|---|---|---|
| Main effect | Worry | Bilateral lingual/cuneus/MOG/fusiform/IOG | 1717 | T(39)=5.7 | 20 | −90 | −22 | Yes |
| Bilateral thalamus/caudate/ACC | 2822 | T(39)=7.2 | −14 | −10 | 12 | Yes | ||
| Left SMA (BA8)/mPFC/dACC (BA32) | 1503 | T(39)=5.3 | −8 | 12 | 56 | Yes | ||
| Reappraisal | Bilateral lingual/calcarine/MOG | 3619 | T(31)=5 | −18 | −78 | −8 | Yes | |
| Bilateral thalamus | 2210 | T(31)=6 | 6 | −26 | 12 | Yes | ||
| Bilateral MeFG/olfactory/SFG (orbital) | 521 | T(31)=−4 | 8 | 18 | −18 | No | ||
| Group-by-block interaction | Worrya | Left posterior insula | 332 | F(1,152)=15 | −34 | −16 | 22 | No |
| Right anterior hippocampus | 494 | F(1,152)=16.2 | 34 | −20 | −20 | No | ||
| Reappraisal | Not significant | |||||||
| Block effect | Worry | Bilateral thalamus/parahippocampus/hippocampus | 4801 | F(4,156)=8.7 | 12 | −30 | 6 | No |
| Reappraisal | Not significant | |||||||
| Group differences (GAD−HC) | Worry | Left MTG/STG | 520 | T(38)=3.8 | −64 | −18 | −4 | No |
| Right MFG/SFG | 483 | T(38)=5.2 | 38 | 62 | −2 | No | ||
| Left PoCG/PreCG/MFG/IPL | 2005 | T(38)=4.8 | −36 | −26 | 64 | Yes | ||
| Right SPL/precuneus/PoCG/IPL/angular/SOG | 3575 | T(38)=5.0 | −16 | −84 | 50 | Yes | ||
| Right SFG/MeFG | 462 | T(38)=4.2 | 6 | 38 | 62 | No | ||
| Reappraisal | Right putamen/insula | 499 | T(30)=−3.7 | 34 | 4 | 10 | No | |
| Left MTG/STG | 696 | T(30)=4.4 | −64 | −34 | 4 | No | ||
| Left insula/IFG (oper) | 1928 | T(30)=−6.3 | −30 | 0 | 22 | Yes | ||
| Right MCC/SFG/SMA | 2254 | T(30)=−7.5 | 28 | 16 | 68 | Yes | ||
| Right PreCG/PoCG | 541 | T(30)=4.5 | 12 | −32 | 76 | No | ||
| Group differences adjusted for HDRS | Worry | Not significant | ||||||
| Reappraisal | Not significant | |||||||
| Group differences adjusted for D–KEFS set-switching and inhibition | Worry | Left PoCG/PreCG/IPL | 626 | T(36)=3.9 | −50 | −36 | 60 | No |
| Reappraisal | Left lentiform nucleus/putamen/pallidum | 443 | T(36)=−5.1 | −22 | −12 | 6 | Yes | |
| Right SFG/MCC/SMA | 992 | T(28)=−7.1 | 28 | 16 | 68 | Yes | ||
| Left MFG/PreCG | 472 | T(28)=−5.1 | −30 | 18 | 64 | No | ||
| Association between CBF and PSWQ | Worry | Right parahippocampus | 482 | T(38)=4 | 8 | 10 | −26 | Yes |
| Right MFG/SFG/IFG | 1650 | T(38)=5.3 | 38 | 62 | −2 | Yes | ||
| Left MTG/STG | 496 | T(38)=4 | −68 | −20 | −4 | Yes | ||
| Bilateral PoCG/precuneus/SPL/PreCG/IPL/MFG/angular/SOG/cuneus | 12 653 | T(38)=5.5 | −16 | −84 | 50 | Yes | ||
| Right MFG/SFG/PreCG | 1273 | T(38)=5.2 | 32 | 14 | 66 | Yes | ||
| Right SFG/SMA/MeFG | 659 | T(38)=4.3 | 6 | 38 | 62 | No | ||
| Reappraisal | Left insula | 769 | T(30)=−5.1 | −32 | −2 | 22 | No | |
| Association between CBF and PSWQ adjusted for HDRS | Worry | Right parahippocampus/amygdala/hippocampus | 620 | T(38)=3.9 | 18 | −2 | −18 | No |
| Right SFG/MeFG | 545 | T(38)=3.7 | 22 | 62 | −8 | Yes | ||
| Left SPL/PoCG | 658 | T(38)=3.5 | 26 | −46 | 62 | Yes | ||
| Reappraisal | Not significant |
Abbreviations: BA, Brodmann area; CBF, cerebral blood flow; dACC, dorsal anterior cingulate; GAD, generalized anxiety disorder; HC, healthy controls; HDRS, Hamilton depression rating scale; IFG, inferior frontal; IOG, inferior occipital; IPL, inferior parietal; MCC, middle cingulate; MeFG, medial frontal; MFG, middle frontal; MOG, middle occipital; mPFC, medial prefrontal cortex; MTG, middle temporal; PoCG, postcentral gyrus; PreCG, pre, central gyrus; SFG, superior frontal; SMA, supplemental motor; SOG, superior occipital; SPL, superior parietal; STG, superior temporal.
Each voxel-wise analysis and the significant regions with cluster sizes, max T/F-statistics (with respective degrees of freedom) and x, y, z-coordinates in MNI space. ‘Not significant’ indicates that the results did not pass multiple comparisons correction. The final column indicates whether the cluster passed a non-parametric multiple comparisons correction (SnPM).
Not significant. See Supplementary Figure 1 for analysis of effect sizes.
Compared with the rest trial, worry reappraisal was associated with greater CBF in: bilateral visual cortex, and bilateral thalamus and lower CBF in: bilateral anterior cingulate, medial frontal and orbital gyrus (Figure 1 and Table 1).
Group × block ANOVA
These whole-brain, voxel-wise statistical results are shown in Table 1 and Figure 2 (as well as Supplementary Figure 1).
Group-by-Block interaction. After correcting for multiple comparisons, the interaction did not reach statistical significance (threshold cluster size Ke=643). However, we performed a separate analysis in the two most prominent clusters (right hippocampus, parahippocampal gyrus (Ke=440) and left posterior insula BA13 (Ke=332)) to establish the magnitude and direction of the effect size (Supplementary Figure 1). No regions showed group-by-block interactions across the reappraisal blocks.
Block effect independent of group. Across the five worry induction blocks, we found significant increases in CBF in the bilateral thalamus as well as the hippocampus and parahippocampal gyrus (Figure 1). There were no regions that significantly changed across the three reappraisal blocks.
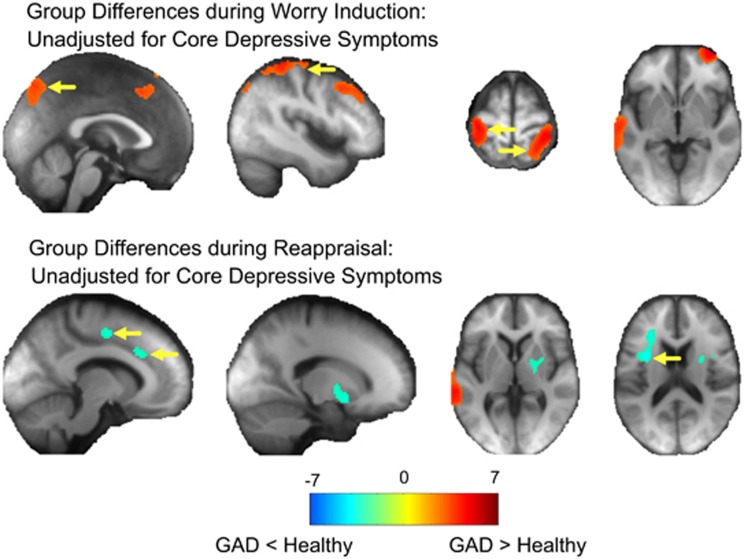
Group effect independent of block. Compared to HC, GAD participants had greater CBF during worry induction in bilateral frontal areas, in the left temporal gyrii, bilateral visual, bilateral motor and sensory cortex and bilateral parietal cortex (Figure 2). This effect was not significant after we adjusted for core depressive symptoms. As GAD and HC differed significantly in two cognitive tests, we adjusted for both D–KEFS Trail Making and Color Word Interference tests (which differed by group), we found that GAD continued to have greater CBF than HC in the left post-, pre-central and inferior parietal (Table 1 and Supplementary Figure 2).
Figure 2.
Group differences during worry induction and reappraisal (unadjusted for core depressive symptoms). Independent t-tests comparing the mean cerebral blood flow (CBF) in the healthy compared to the generalized anxiety disorder (GAD) group during worry induction (minus rest, top) and reappraisal (bottom). Areas where CBF was greater in the GAD group are shown in red/warm colors and healthy greater than GAD in blue/cool colors. The colors indicate T-statistics from the independent t-test. Yellow arrows indicate regions that survived multiple comparisons correction via a non-parametric method (SnPM). These results did not pass significance threshold after adjusting for core depressive symptoms (HDRS).
Compared to HC, GAD participants had greater CBF during reappraisal in the left middle, superior temporal and right motor and sensory cortex. However, HC had greater CBF during reappraisal in the middle cingulate, supplemental motor area, left inferior frontal gyrus, right putamen and bilateral insula (Figure 2). This effect was not significant after we adjusted for core depressive symptoms. We further adjusted for both D–KEFS Trail Making and Color Word Interference tests. We found that HC had greater CBF than GAD in left lentiform nucleus, putamen, pallidum, middle frontal, pre-central, and right superior frontal, middle cingulate and supplemental motor area (Table 1 and Supplementary Figure 2).
Effect of worry severity
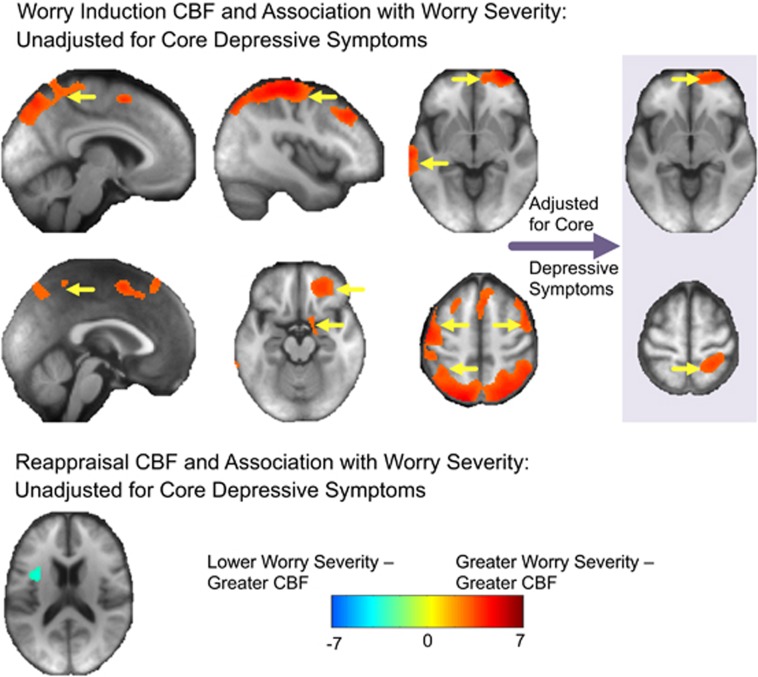
Worry severity was significantly correlated with increased CBF in a large number of regions (Figure 2). Greater worry severity was associated with greater CBF in the middle and superior temporal gyrus, bilateral visual, bilateral motor and sensory, bilateral parietal, as well as bilateral frontal areas and parahippocampus. Adjusting for core depressive symptoms showed this association was robust to depressive symptoms in the following regions: right parahippocampus, hippocampus and amygdala, as well as superior frontal gyrus and left superior parietal lobe (Figure 3).
Figure 3.
Worry severity (PSWQ) association with cerebral blood flow (CBF) during worry induction and reappraisal (unadjusted and adjusted for core depression severity). Association between worry severity (as measured by PSWQ) and CBF during worry (top) and reappraisal (bottom) CBF (minus rest). The colors indicate the T-statistic from the regression analysis; the positive associations (greater worry severity associated with greater CBF) are shown in red/warm colors, whereas the negative associations (greater worry severity associated with lower CBF) are shown in blue/cool colors. Yellow arrows indicate regions that survived multiple comparisons correction via a non-parametric method (SnPM). Importantly, two regions remained significant even after adjusting for core depressive symptoms. There were no regions during reappraisal that significantly associated with worry severity after adjusting for core depressive symptoms.
During reappraisal, lower CBF was significantly associated with greater worry severity in the anterior insula, but this result was not significant after adjusting for core depressive symptoms (Figure 3).
Discussion
In conclusion, our results show a complex pattern of changes in CBF, with different components associated with worry induction, maintenance, severity and reappraisal. Worry induction was associated with higher CBF in multiple regions, but maintaining the worry process involved mainly a sustained increased in the CBF in the thalamus and hippocampus. The group differences during worry induction, initially showing multiple regions with increased CBF in GAD vs HC, were rendered non-significant once controlling for core depressive symptoms. Worry severity results were robust to the effect of depressive symptoms and were localized in right parahippocampus, hippocampus and amygdala, as well as superior frontal gyrus and left superior parietal lobe. With regard to reappraisal, HC had greater CBF than GAD in several regulatory regions, which were mostly robust to the effect of cognitive differences between groups. However, this effect was not significant after adjusting for core depressive symptoms.
The main effect of worry induction, regardless of group or block, was associated with higher CBF in three distinct groups of regions: regions involved in visual processing (lingual, fusiform, occipital), regions involved in emotion generation and implicit emotion processing (caudate and thalamus) and regions involved in explicit emotion regulation (medial prefrontal cortex, supplemental motor, dorsal ACC).43
When adding group, we initially found several regions that differentiate between older GAD and HC, which is contrary to our initial hypothesis that worry induction would not differ between the two groups. However, these results were not significant when adjusting for core depressive symptoms, which may indicate that ‘primary’ induction of worry (not associated with depression) may actually not differ between groups. These results support the importance of controlling even for sub-threshold depressive symptoms when studying GAD, given the high depression-GAD comorbidity (see below for a discussion regarding the advantages of a dimensional approach focused on worry severity).
Although worry has been classically associated with verbal-linguistic activity, the association of visual processing areas with worry generation is supported by more recent literature44, 45, 46, 47 as recent meta-analyses link the visual cortex with emotion-generation processes.43, 48 The subcortical hub of thalamus and caudate acts a sensory and motor information hub that relays information to both core emotion generation (for example, limbic and orbitofrontal cortex (OFC)) and emotion-regulation regions (mPFC, SMA, dACC).43, 49 The caudate has long been recognized as a region involved in emotion regulation49, 50 and generalized anxiety.51, 52
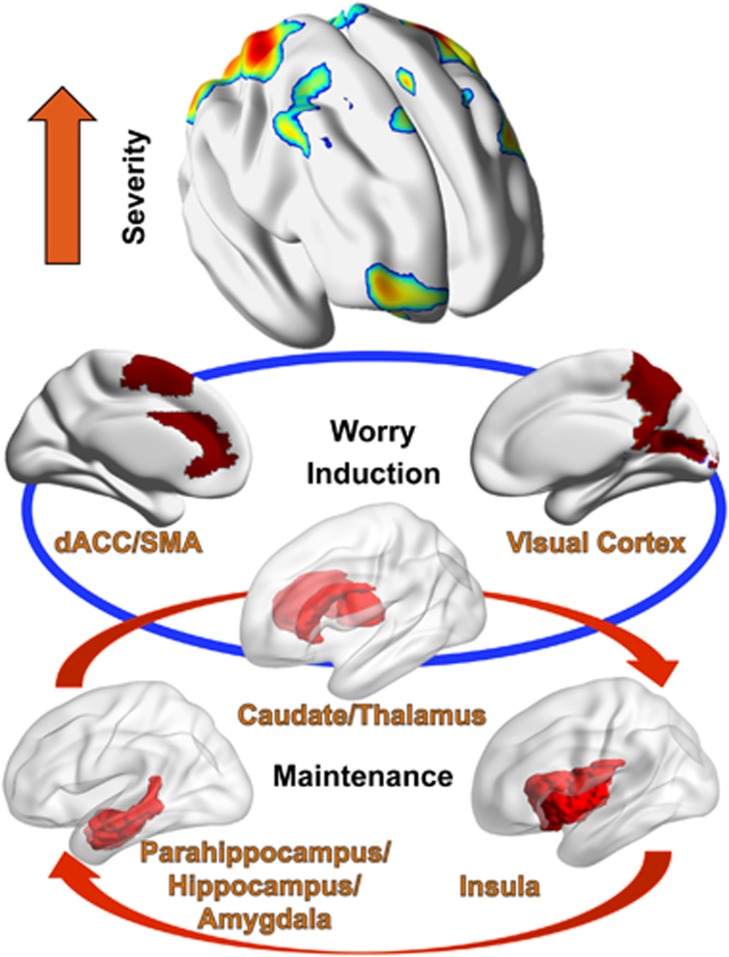
Overall, our results indicate that induction of worry is associated with three major hubs: an emotion-generation hub (related to visual imagery), a subcortical ‘relay’ (thalamus and caudate) and an emotion-regulation hub (SMA, vmPFC, dACC) (Figure 4).
Figure 4.
Model of induction, maintenance and severity of worry. Three distinct layers and multiple hubs associated with (1) initiation of worry (visual cortex; caudate/thalamus; SMA/dACC); (2) maintenance/lack of habituation (thalamus/parahippocampus/hippocampus/amygdala, posterior insula); (3) worry severity (visual areas BA 7/19, SMA/mPFC, orbitofrontal cortex (OFC), dlPFC, supramarginal gyrus, inferior parietal). Some regions contribute to two different phenomena (e.g., Thalamus appears to be involved in both induction and maintenance of worry, the visual cortex is involved in both induction and severity of worry).
The measure of worry maintenance—the main effect of block—identifies regions (bilateral thalamus and hippocampus) whose perfusion changes over the course of multiple worry induction blocks. Looking at the course of perfusion across blocks in these regions (Figure 1), we may infer that these areas are associated with a pattern of ‘worry maintenance’: they ramp up after the first induction block and maintain high perfusion for the remaining blocks (in the thalamus block we notice a further ‘bump’ up in CBF by block four).
The direction of the change in CBF indicates that—regardless of group—repeated worry induction does not attenuate the blood flow in several regions. One may speculate this is associated with a lack of habituation, which may ultimately contribute to the relatively mediocre effect of cognitive behavioral therapies in late-life GAD, especially with regard to reducing worry severity.53, 54
This presentation becomes more complex when adding the effect of worry severity (Figure 2). Participants with higher worry severity show activity in some of the previously described areas (visual areas such as BA 19 and BA 7, and emotion-regulation regions (SMA and mPFC)), but they recruit additional regions involved in emotion regulation such as OFC (BA10, 11), dlPFC (BA 9), supramarginal gyrus (BA4), inferior parietal (BA40). Most of these regions have been systematically associated with executive attention (volitional selective attention and working memory)43 and explicit emotion regulation.55, 56 Once controlling for core depressive symptoms, the medial frontal and superior parietal cortical regions remain significant (which indicates that a categorical diagnosis such as GAD may actually ‘dilute’ the effect related to pathologic worry), but in addition, we also note a limbic cluster addition. Thus, it appears that higher worry severity is associated with additional recruitment of neocortical regulatory regions as well as potential limbic activation. This may support the emotion dysregulation theory of worry as a maladaptive regulation strategy.30, 57, 58 Taken together, these results endorse the view of worry as a complex process, supported by several distinct hubs, with discrete components for induction and maintenance (Figure 4).
The group-by-block interaction in the whole-brain ANOVA rendered two regions marginally non-significant: the right hippocampus and the left posterior insula (BA13) (Supplementary Figure 1). The analysis of effect sizes indicates that for both regions there is an opposite pattern in the GAD and non-anxious participants. For left posterior insula there was an increased effect size from block one minus two (Cohen’s d=0.2) to block one minus five (Cohen’s d=0.7) in GAD, whereas healthy participants had a decrease from a low effect size at block one minus two (d=0.06) to a moderate but negative effect size (d=−0.4) at block one minus five. Thus, we may speculate that posterior insula seems to ‘disengage’ in healthy non-anxious participants, while becoming increasingly more active across worry induction blocks in GAD. An opposite pattern was noticed for the right anterior hippocampus (Supplementary Figure 1).
The reappraisal results were relatively surprising. Although we confirmed the involvement of the prefrontal cortex, the main effect shows a large effect of occipital and thalamic areas, which we can only explain through ‘cross-contamination’ (participants may have continued to worry during reappraisal blocks). Thus, a closer examination of block differences during reappraisal, shows an initial increase in CBF at block one, followed by flattened CBF throughout the following blocks. An alternative explanation is that participants, who were trained in reappraisal strategies prior to scanning, may have reappraised more economically59 while in the scanner, or that, despite instructions, they used an alternative emotion-regulation strategy such as distraction or detachment (see ‘limitations’). At this time, the limitations in study design do not allow for a definite conclusion.
Group differences during reappraisal revealed that older GAD participants have less engagement of frontal regulatory regions than healthy controls, which confirms our hypothesis regarding reappraisal difficulties in older GAD. Interestingly, GAD participants were also significantly different from HC in two executive function cognitive processes, although there were no global cognitive differences between the groups. When controlling in the group analysis for these two cognitive abilities, the results strengthen the ‘advantage’ of HC by adding two additional clusters with higher CBF during reappraisal (left middle frontal gyrus and left striatum). Impairments in several cognitive domains—including inhibition—were reported in late-life GAD on a much larger sample.60 As these results are not significant when controlling for core depressive symptoms and given that none of the older GAD participants were clinically depressed, we may speculate that the failure to engage reappraisal regions may represent a common denominator of late-life depression and GAD.
Overall, based on the results of the current study we can advance a model of induction, maintenance and severity of worry (Figure 4). This model involves different phenomenological layers and several distinct regions—some (like the visual cortex, bilateral thalamus, caudate and medial PFC) associated with the initiation of worry, others with maintenance or lack of habituation (for example, thalamus, posterior hippocampus, posterior insula), and some with excessive, severe worry. In addition, although not included in the model, our results indicate a deficit of older GAD in engaging regions classically associated with cognitive reappraisal.
The specific neural characteristics associated with severe worry (greater lateral PFC, OFC and parietal involvement) may prove critical for future development of targeted treatments. Thus, while worry in itself is a universal phenomenon experienced by researchers and laymen alike, and may even confer an evolutionary advantage by modifying threat-related decision-making,61 severe worry carries a significant public health burden and has proven difficult to treat with standard pharmaco- or psychotherapies. The current study points toward two types of areas that may benefit from targeted interventions such as transcranial magnetic stimulation:62 the regions associated exclusively in severe worry (for example, lateral PFC and parietal cortex) and those associated in protracted quality of worry (for example, left OFC, left posterior insula).
Our study has several limitations. First, given the small sample some analyses were underpowered and the results will need further replication. As we did not have a midlife group, we cannot generalize the results in younger individuals. Especially regarding reappraisal, the limited but distinct cognitive domain deficits may point toward age-related changes in late-life GAD signaling a prodromal neurocognitive disorder.10 Although not clinically depressed, the GAD sample had a significantly higher burden of depression symptoms, which may have interfered with some of the results. Thus, when controlling for core depressive symptoms, the group differences between GAD and controls were lost, but the worry severity regression results were largely maintained. Given the high GAD-MDD comorbidity, the effects of controlling for depressive symptoms are not surprising. These results advocate for a dimensional approach focused on worry severity and less for a categorical approach where results are contaminated by non-specific symptoms shared by GAD and MDD (for example, fatigability, poor concentration, sleep disturbances). The in-scanner worry induction task has several limitations: the longer blocks (design to capture the lengthier worry induction process) may have weakened the observed CBF response during reappraisal; the pre-scanning reappraisal training may have diminished the in-scanner intensity of worry induction response; the lack of a reappraisal efficacy assessment prevents the evaluation of the type of emotion regulation use in the scanner; and finally there is the possibility of cross-contamination between the blocks (for example, participants who reappraised a worry theme in a previous block may have spontaneously use reappraisal during the next worry induction block). As we acknowledge this possibility, we note that worry themes varied among blocks and the severity of worry appears to increase during worry induction blocks when compared with previous non-worry induction blocks. A final limitation involves the use of arterial spin labeling, which allowed us to explore a lengthier and slower phenomenon such as worry induction. However, compared with blood oxygen-level dependent response, arterial spin labeling is challenged by a poorer signal-to-noise ratio63, 64 and, although we attempted to reduce the noise (improved estimation and use of the subject-specific gray matter), our results need further confirmation.
To our knowledge, this is the first study exploring worry induction and reappraisal in correlation with changes in cerebral blood flow. We present new data supporting the view of worry as a complex process, associated with multiple regions involved sequentially in the initiation, maintenance, severity and reappraisal of the worry process.
Acknowledgments
This work was supported by the National Institute of Mental Health (Grants MH 086686, MH 071944 and MH 076079) and the Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (to Dr Andreescu).
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Borkovec TD, Shadick RN, Hopkins M. The nature of normal and pathological worry. In: Rappe RM, Barlow DH (eds). Chronic Anxiety: Generalized Anxiety Disorder and Mixed Anxiety-Depression. Guilford: New York, NY, 1991, pp 29–51. [Google Scholar]
- Mennin DS, Heimberg RG, Fresco DM, Ritter MR. Is generalized anxiety disorder an anxiety or mood disorder? Considering multiple factors as we ponder the fate of GAD. Depress Anxiety 2008; 25: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J, Conroy RM, Bruce I, Denihan A, Greene E, Kirby M et al. The spectrum of worry in the community-dwelling elderly. Aging Ment Health 2011; 15: 985–994. [DOI] [PubMed] [Google Scholar]
- Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BH. Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol 2016; 118: 511–519. [DOI] [PubMed] [Google Scholar]
- Batelaan NM, Seldenrijk A, Bot M, van Balkom AJ, Penninx BW. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry 2016; 208: 223–231. [DOI] [PubMed] [Google Scholar]
- Denollet J, Maas K, Knottnerus A, Keyzer JJ, Pop VJ. Anxiety predicted premature all-cause and cardiovascular death in a 10-year follow-up of middle-aged women. J Clin Epidemiol 2009; 62: 452–456. [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Kubzansky LD, Thurston RC. Prospective study of anxiety and incident stroke. Stroke 2014; 45: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EJ, de Jonge P, Na B, Cohen BE, Lett H, Whooley MA. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease: The Heart and Soul Study. Arch Gen Psychiatry 2010; 67: 750–758. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Spiro A 3rd, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation 1997; 95: 818–824. [DOI] [PubMed] [Google Scholar]
- Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry 2016; 24: 823–842. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Teverovsky E, Fu B, Hughes TF, Chang CC, Ganguli M. Old worries and new anxieties: behavioral symptoms and mild cognitive impairment in a population study. Am J Geriatr Psychiatry 2014; 22: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook K, Kim KH, Suh SY, Lee KS. Intolerance of uncertainty, worry, and rumination in major depressive disorder and generalized anxiety disorder. J Anxiety Disord 2010; 24: 623–628. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adoles Psychiatry 2013; 52: 290–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry 2012; 72: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009; 66: 1361–1372. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety 2011; 28: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L et al. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry 2015; 23: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J Affect Disord 2014; 167: 336–342. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G et al. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med 2010; 40: 117–124. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J, Smith S, Forster S, John OP, Bishop SJ. Resting state correlates of subdimensions of anxious affect. J Cogn Neurosci 2014; 26: 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster S, Nunez Elizalde AO, Castle E, Bishop SJ. Unraveling the anxious mind: anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb Cortex 2015; 25: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD. The nature, functions, and origins of worry. In: Tallis GDF (ed). Worrying: Perspectives on Theory, Assessment, and Treatment. Wiley & Sons: Sussex, England, 1994. [Google Scholar]
- May R. The Meaning of Anxiety. W.W. Norton & Co: New York, NY, 1996. [Google Scholar]
- Ruscio AM, Borkovec TD, Ruscio J. A taxometric investigation of the latent structure of worry. J Abnorm Psychol 2001; 110: 413–422. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Broman-Fulks JJ, Bergman SM, Green BA, Zlomke KR. A taxometric investigation of the latent structure of worry: dimensionality and associations with depression, anxiety, and stress. Behav Ther 2010; 41: 212–228. [DOI] [PubMed] [Google Scholar]
- Goncalves DC, Byrne GJ. Who worries most? Worry prevalence and patterns across the lifespan. Int J Geriatr Psychiatry 2013; 28: 41–49. [DOI] [PubMed] [Google Scholar]
- Tsypes A, Aldao A, Mennin DS. Emotion dysregulation and sleep difficulties in generalized anxiety disorder. J Anxiety Disord 2013; 27: 197–203. [DOI] [PubMed] [Google Scholar]
- Mennin DS, McLaughlin KA, Flanagan TJ. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J Anxiety Disord 2009; 23: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 2002; 39: 281–291. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ (ed). Handbook of Emotion Regulation. Guilford Press: New York, NY, 2007. [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L et al. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry 2015; 23: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 2008; 26: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry questionnaire. Behav Res Therapy 1990; 28: 487–495. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41: 237–248. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998; 20: 310–319. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System Examiner's Manual. The Psychological Corporation: San Antonio, TX, 2001. [Google Scholar]
- Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imaging 2012; 30: 1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Blakesley-Ball RE, Mulsant BH, Mazumdar S, Houck PR, Szanto K et al. Speed of improvement in sleep disturbance and anxiety compared with core mood symptoms during acute treatment of depression in old age. Am J Geriatr Psychiatry 2006; 14: 550–554. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE 2013; 8: e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 2012; 35: 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Ormel J, Aleman A. The neural correlates of worry in association with individual differences in neuroticism. Hum Brain Mapp 2014; 35: 4303–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch CR, Hayes S, Mathews A, Perman G, Borkovec T. The extent and nature of imagery during worry and positive thinking in generalized anxiety disorder. J Abnorm Psychol 2012; 121: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkenhorst E, Crowe SF. The effect of state worry and trait anxiety on working memory processes in a normal sample. Anxiety Stress Coping 2009; 22: 167–187. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L et al. The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Res 2015; 234: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci 2010; 22: 2864–2885. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008; 59: 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opialla S, Lutz J, Scherpiet S, Hittmeyer A, Jancke L, Rufer M et al. Neural circuits of emotion regulation: a comparison of mindfulness-based and cognitive reappraisal strategies. Eur Arch Psychiatry Clin Neurosci 2015; 265: 45–55. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Pine DS, Muehlhan M, Lueken U, Steudte-Schmiedgen S, Beesdo-Baum K. Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization. Psychiatry Res 2015; 234: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CM, Yang JC, Jeong GW. Functional neuroanatomy associated with the interaction between emotion and cognition in explicit memory tasks in patients with generalized anxiety disorder. Acta Radiol 2016; 58: 98-106. [DOI] [PubMed]
- Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry 2009; 24: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp SR, Ayers CR, Nuevo R, Stoddard JA, Sorrell JT, Wetherell JL. Meta-analysis comparing different behavioral treatments for late-life anxiety. Am J Geriatr Psychiatry 2009; 17: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Neurosci 2015; 16: 693–700. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot 2011; 25: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Ritter M, Heimberg RG. An open trial of emotion regulation therapy for generalized anxiety disorder and cooccurring depression. Depress Anxiety 2015; 32: 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27: 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc Cogn Affect Neurosci 2012; 7: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Andreescu C, Wetherell JL, Mantella R, Begley AE et al. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. Br J Psychiatry 2011; 199: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloyan B, Bulley A, Suddendorf T. Episodic foresight and anxiety: Proximate and ultimate perspectives. Br J Clin Psychol 2016; 55: 4–22. [DOI] [PubMed] [Google Scholar]
- Paes F, Machado S, Arias-Carrion O, Velasques B, Teixeira S, Budde H et al. The value of repetitive transcranial magnetic stimulation (rTMS) for the treatment of anxiety disorders: an integrative review. CNS Neurol Disord Drug Targets 2011; 10: 610–620. [DOI] [PubMed] [Google Scholar]
- Ewing JR, Cao Y, Knight RA, Fenstermacher JD. Arterial spin labeling: validity testing and comparison studies. J Magn Reson Imaging 2005; 22: 737–740. [DOI] [PubMed] [Google Scholar]
- Perthen JE, Bydder M, Restom K, Liu TT. SNR and functional sensitivity of BOLD and perfusion-based fMRI using arterial spin labeling with spiral SENSE at 3T. Magn Reson Imaging 2008; 26: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.