Abstract
We examined putative microglial activation as a function of illness course in schizophrenia. Microglial activity was quantified using [11C](R)-(1-[2-chrorophynyl]-N-methyl-N-[1-methylpropyl]-3 isoquinoline carboxamide (11C-(R)-PK11195) positron emission tomography (PET) in: (i) 10 individuals at ultra-high risk (UHR) of psychosis; (ii) 18 patients recently diagnosed with schizophrenia; (iii) 15 patients chronically ill with schizophrenia; and, (iv) 27 age-matched healthy controls. Regional-binding potential (BPND) was calculated using the simplified reference-tissue model with four alternative reference inputs. The UHR, recent-onset and chronic patient groups were compared to age-matched healthy control groups to examine between-group BPND differences in 6 regions: dorsal frontal, orbital frontal, anterior cingulate, medial temporal, thalamus and insula. Correlation analysis tested for BPND associations with gray matter volume, peripheral cytokines and clinical variables. The null hypothesis of equality in BPND between patients (UHR, recent-onset and chronic) and respective healthy control groups (younger and older) was not rejected for any group comparison or region. Across all subjects, BPND was positively correlated to age in the thalamus (r=0.43, P=0.008, false discovery rate). No correlations with regional gray matter, peripheral cytokine levels or clinical symptoms were detected. We therefore found no evidence of microglial activation in groups of individuals at high risk, recently diagnosed or chronically ill with schizophrenia. While the possibility of 11C-(R)-PK11195-binding differences in certain patient subgroups remains, the patient cohorts in our study, who also displayed normal peripheral cytokine profiles, do not substantiate the assumption of microglial activation in schizophrenia as a regular and defining feature, as measured by 11C-(R)-PK11195 BPND.
Introduction
Multiple lines of evidence implicate altered immune function in schizophrenia, including epidemiological, biomolecular, genetic studies and clinical trials of adjunctive anti-inflammatory treatments.1, 2, 3 Recent reviews1, 4 summarize a still inconclusive body of work suggesting that in some cases of psychosis, activated microglia, the brain’s normally innate immune effector cells,5 may be found in the brain parenchyma using immunohistochemical staining techniques. In response to brain insult, microglial cells become activated by changing shape and function to upregulate molecules involved in cytokine release, phagocytosis and antigen presentation.6
Seminal experimental studies have shown that activation of microglia in the wake of a neuronal lesion (without blood–brain barrier damage) induces a preferential de novo expression of the 18 kD translocator protein (TSPO) in the mitochondria of activated microglia.7, 8 Using 11C-(R)-PK11195 positron emission tomography (PET), estimates of TSPO expression levels of above those seen in control subjects and indicative of the presence of activated microglia have been reported for brain diseases with known inflammatory, vascular or neurodegenerative tissue pathology.9
A number of TSPO-binding studies have examined microglial activation in schizophrenia,10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 producing mixed results. The initial studies in recent-onset patients10, 11 revealed increased 11C-(R)-PK11195 ligand binding to TSPO, providing impetus for further research. However, subsequent TSPO investigations have generally failed to replicate these findings, including recent studies using 11C-(R)-PK1119515, 16, 21 and second-generation tracers,12, 13, 17 as well as studies investigating antipsychotic-naive patient cohorts.15, 20 Despite accumulating evidence for unchanged TSPO levels in schizophrenia, both increased,10, 11, 19 and, more recently, decreased18, 20 TSPO levels have also been reported. Thus, the presence and nature of microglial abnormalities in schizophrenia remains unclear.
Differences in the pharmacokinetic and dynamic properties of TSPO radioligands represent a potential reason for these conflicting results. Apart from discrepant radioligands across studies, patient heterogeneity, medication, methodological considerations and, importantly, stage of illness may also contribute. Notably, increased TSPO binding has been reported in patient populations with shorter illness duration11 and individuals at high clinical risk of psychosis,19 providing preliminary evidence of TSPO-binding declining with illness chronicity. Alternatively, two recent investigations19, 20 using the same ligand, [11C]PBR-28, have produced conflicting results, with TSPO increased in individuals at risk of psychosis,19 yet decreased in first-episode psychosis.20 Notably, both patient cohorts were antipsychotic-naive, suggesting that antipsychotic medication effects did not explain the findings. Although differences in methodology may have accounted for the discrepant results,20, 22 it remains possible that microglial expression fluctuates throughout the course of illness, with increased expression occurring before the manifestation of overt symptoms. Nevertheless, to date, no single study has examined TSPO binding in pre-psychotic, or across stages in the course of schizophrenia, and, with the exception of Bloomfield et al.,19 prodromal or at-risk cohorts have not been further investigated.
Therefore, we tested whether the presence of activated microglia varies dynamically as a function of illness stage, by mapping TSPO expression in individuals at ultra-high risk (UHR) of psychosis, recently diagnosed and with chronic schizophrenia. Regions previously associated with gray matter loss in early stages of schizophrenia were tested for altered microglial activation in these three groups of individuals, as indexed by 11C-(R)-PK11195-binding potential, and compared to age-matched comparison subjects. We also tested whether 11C-(R)-PK11195-binding was associated with gray matter volume, peripheral cytokine levels and/or clinical symptoms. We hypothesized that increased 11C-(R)-PK11195, indicating TSPO expression by activated microglia, might occur in frontal and temporal brain regions in individuals at risk and in early stages of schizophrenia, compared to healthy controls and patients with prolonged illness.
Materials and methods
Participants
The study was approved by the Melbourne Health and Austin Health ethics committees and all participants provided written informed consent. Three recent-onset patients were excluded from the study due to poor PET image quality, a history of seizures or an abnormality in the magnetic resonance imaging (MRI) T1-weighted scan. A total of 70 participants were included in this study: 10 individuals at UHR for psychosis, 18 recent-onset schizophrenia patients (illness duration<2 years), 15 chronic schizophrenia patients (illness duration>5 years) and 27 healthy controls matched on age, sex and parental education. Healthy controls were divided into mutually exclusive younger (n=15; age range=18–25) and older (n=12; age range=31–42) groups to yield healthy comparison groups that were matched in mean age to the patient groups. The older comparison group was matched in mean age to chronic patients, whereas the younger group was matched to both UHR individuals and recent-onset patients. Sample characteristics are shown in Table 1.
Table 1. Sample characteristics.
|
Young HC |
Older HC |
UHR |
Recent-onset |
Chronic |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=15 |
n=12 |
n=10 |
n=18 |
n=15 |
||||||
| Sex (male/female) |
13/2 |
9/3 |
6/4 |
16/2 |
10/5 |
|||||
| Smoking status (yes/no)a |
6/9 |
2/10 |
3/7 |
12/6 |
8/7 |
|||||
| M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | |
| Age | 21.7 | 2.1 | 36.3 | 4.2 | 20.7 | 2.2 | 20.6 | 5.5 | 35.2 | 6.6 |
| BMI | 24.3 | 4.1 | 25.5 | 5.4 | 22 | 2.4 | 27.6 | 5.4 | 27.6 | 5.5 |
| Injected activity | 335.4 | 16.8 | 350.6 | 18.1 | 330.9 | 19 | 342.4 | 23.2 | 333.8 | 26.9 |
| Specific activity | 39.5 | 16.6 | 47.7 | 34.3 | 56.3 | 17.8 | 40.0 | 12.3 | 52.4 | 43.3 |
| DOI (years) | — | — | — | — | — | — | 1.5 | 1.0 | 13.6 | 8.8 |
| Age of symptom onset | — | — | — | — | — | — | 19.8 | 2.4 | 17.8 | 2.6 |
| Symptoms | ||||||||||
| General pathology (BPRS total)b | — | — | — | — | — | — | 38.3 | 10 | 48.4 | 12.4 |
| Positive symptoms (BPRS subscore)b | — | — | — | — | — | — | 12.6 | 4.6 | 19.5 | 7.8 |
| Negative symptoms (SANS)b | — | — | — | — | 30.3 | 12.7 | 23 | 13.5 | 35.5 | 19.9 |
| General functioning (SOFAS)b, c | 83.7 | 7.3 | 78.3 | 10.7 | 57.2 | 9.6 | 56.6 | 11.2 | 42.5 | 14.6 |
| Medication | ||||||||||
| Antipsychotic dose (CPZ-EQ) | — | — | — | — | — | — | 1444.1 | 2438.6 | 1582.4 | 1853.5 |
| n | % | n | % | |||||||
| Any antipsychotics | — | — | — | — | — | — | 14 | 78 | 15 | 100 |
| Typical treatment | — | — | — | — | — | — | 2 | 11 | 9 | 60 |
| Atypical treatment | — | — | — | — | — | — | 12 | 67 | 3 | 20 |
| Combined typical and atypical | — | — | — | — | — | — | 0 | 0 | 3 | 20 |
| No antipsychotic treatment | — | — | — | — | — | — | 4 | 22 | 0 | 0 |
| Any mood medication | — | — | — | — | — | — | 4 | 22 | 6 | 40 |
| No mood medication | — | — | — | — | — | — | 14 | 78 | 9 | 60 |
Abbreviations: ACC, anterior cingulate cortex; BMI, body mass index; BPRS, Brief Psychiatric Rating Scale; CPZ-EQ, chlorpromazine-equivalent dose (mg); DOI, duration of illness; HC, healthy control; M, mean; n, number; SANS, Scale for the Assessment of Negative Symptoms; SOFAS, Social and Occupational Functioning Assessment Scale; UHR, ultra-high-risk.
Significant difference between chronic patients and older healthy controls (P<0.05).
Significant increase in chronic patients compared to recent-onset patients (P<0.05).
Significant increase in chronic patients compared to UHR patients (P<0.05).
Individuals meeting standardized ‘UHR’ criteria were approached and interviewed to confirm UHR status according to the Comprehensive Assessment of At Risk Mental State,23 a structured interview designed to assess sub-threshold psychosis symptoms. Briefly, UHR patients met criteria for one or more of the following categories, accompanied by a decline in general functioning:
First-degree relative with a history of psychosis or the individual has a schizotypal personality disorder;
Sub-threshold intensity/frequency of positive symptoms;
Brief limited intermittent psychotic symptoms with spontaneous remission within 1 week.
Eligible recent-onset and chronic patients were diagnosed with a schizophrenia-spectrum disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.24 Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).24
Exclusion criteria for all participants included a history of head injury or seizures, diagnosis of a neurological disorder, impaired thyroid functioning, diabetes, pregnancy, contraindication to MRI scanning, generalized inflammatory condition and treatment with immunosuppressive, corticoid/glucocorticoid, steroidal or non-steroidal anti-inflammatory medication within 2 weeks of scanning, due to potential interaction with 11C-(R)-PK11195. All participants were asked to refrain from alcohol consumption and tobacco use for 12 h prior to scanning. Additional exclusion criteria for healthy controls were a history of mental illness (personally or in a first degree relative) or alcohol or drug dependence. Healthy controls were interviewed by two trained investigators using the SCID to confirm participants had no history of diagnosable psychopathology.
Clinical measures
The following scales were administered within 1 week of the PET scan: the SCID24 to confirm diagnoses and assess exclusion criteria; the Expanded Brief Psychiatric Rating Scale (BPRS)25 to provide a rating for general psychopathology and positive symptoms, the Scale for the Assessment of Negative Symptoms (SANS)26 to measure negative symptoms and the Social and Occupational Functioning Assessment Scale (SOFAS)27 to index social functioning.
Positron emission tomography acquisition
To assess regional microglial activation, PET scans utilizing the ligand 11C-(R)-PK11195 were obtained on a Philips Gemini TF64 scanner at the Department of Molecular Imaging and Therapy, Austin Hospital, Melbourne, VIC, Australia. To minimize head movement, a thermoplastic facemask was fixed to the head of each participant prior to scanning. A low dose CT scan was acquired to allow correction for tissue attenuation. PET acquisition commenced with the injection of 11C-(R)-PK11195, which was delivered as a bolus into the antecubital vein of each participant. Emission data were acquired in listmode over 60 min and reconstructed post-acquisition into 22 temporal frames (6 × 30 s, 7 × 60 s, 4 × 150 s, 2 × 300 s and 3 × 600 s) using List mode Time-of-Flight 3D Line of Response RAMLA (Listmode TF-MLEM algorithm). Images were reconstructed with the following parameters: image voxel size=2 × 2 × 2 mm, FOV=256 × 256 × 180 mm and slice thickness=2.0 mm.
Magnetic resonance imaging acquisition
MRI scans were acquired within 2 weeks of PET scanning on a 3-T Siemens Trio at the Murdoch Childrens Research Institute, Royal Children’s Hospital, Parkville, VIC, Australia. Scanning parameters were as follows: 3D SPGR spoiled gradient T1 weighted, echo time=3 ms, repetition time=14 ms, 256 contiguous slices covering whole brain, 1 × 1 × 1 mm voxels.
Image pre-processing
PET and T1 anatomical scans were processed using FMRIB's Software Library (FSL).28 For each subject, dynamic PET volumes were reoriented to standard Montreal Neurological Institute (MNI152) images and realigned to the middle volume. T1-weighted volumes were skull-stripped and manually checked to optimize extraction. Skull-stripped T1-weighted volumes were first registered to native PET space, using an averaged PET volume (frames 1–8) as the registration target (FLIRT).29 Next, T1 volumes were registered to MNI152 standard space, following a two-step procedure: first, the affine transformation was computed to match the T1 volume to the template (FLIRT);29 and second, nonlinear normalization applied the deformation field that warps the original T1 volume to the template, setting the previous affine transformation as the starting estimate (FNIRT).30
Regional binding potential
Six bilateral regions of interest (ROI) were selected based on prior research reporting marked gray matter loss in the early stages of schizophrenia:31, 32 dorsal frontal (superior and middle frontal cortex), orbital frontal, anterior cingulate, medial temporal (hippocampus and parahippocampus), thalamus and insula. Regions were delineated using the automated anatomical labeling atlas (AAL)33 projected onto individual PET space using the linear affine from T1 to PET and the inverse of the nonlinear warp from T1 to MNI space.
Parametric maps of binding potential (BPND) were computed using the basis-function implementation of the simplified reference-tissue model.34 For each individual, BPND was averaged over all voxels comprising each ROI to yield a regionally averaged BPND estimate for each of the six ROIs. Four alternative reference regions were investigated to define a reference time-activity curve for the simplified reference-tissue model: (i) cerebellar gray matter;15 (ii) a data-driven supervised clustering approach;35, 36 (iii) gray matter voxels with the lowest (10%) standard uptake values; and (iv) white matter.37 Results pertaining to the cerebellar reference are presented here, with other results relegated to Supplementary Material. Support for the cerebellar reference is three-fold: (i) extracting reference data from plasma is not ideal for use with 11C-(R)-PK11195, due to the highly variable kinetic behavior of this tracer in plasma;38 (ii) previous post-mortem examination detected lower [3H]PK11195-binding densities in cerebellar gray matter relative to our ROIs;15, 39 and (iii) there were no significant differences in cerebellar standard uptake values between patient and control groups (f=2.5, P>0.05; Supplementary Figure S1).
Measurement of gray matter volume
Gray matter volume maps were calculated for each participant using voxel-based morphometry, as implemented using the computational anatomy toolbox (CAT12) in statistical parametric mapping (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) running in Matlab R2014b. In brief, T1 images were normalized to a template space and segmented into gray matter, white matter and cerebrospinal fluid. Native-space gray matter segments were then spatially aligned to a high-dimensional Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra template and normalized to MNI space. Gray matter voxels were multiplied by the linear and nonlinear components of this deformation to provide a measure of the absolute amount of GM tissue corrected for individual brain sizes. All images passed the voxel-based morphometry quality check based on the ‘display one slice’ and ‘check sample homogeneity of covariance’ modules. Images were smoothed with an 8mm full-width-half-maximum Gaussian kernel. Mean gray matter volume was extracted from the resulting modulated, normalized and smoothed T1 volumes for the 6 cortical ROIs, using the AAL parcellation.
Measurement of peripheral cytokines
All subjects were fasting and blood samples were obtained between 0800 and 1100 hours within 1 week of the PET scan. Peripheral blood was collected from participants in serum separating tubes (SSTII), which were then stored at −80 °C, pending analysis.
Three cytokines (interleukin 1 beta [IL-1β], tumor necrosis factor alpha [TNF-α] and interleukin 6 [IL-6]) from the Human High-sensitivity T cell assay (HST-CMAG28SPMX13, Merck Millipore, Billerica, MA, USA) were quantified in duplicate using the Luminex Magpix assay in a subset of participants (data not available for three participants), cytokine data were not obtained in two older controls and one recent-onset patient). Inter-assay co-efficients were below 15% for all analytes assayed (CV<15%) and intra-assay co-efficients of variation (CV) were below 5% (CV<5%). The average minimum detectable value across all plates was 0.23 pg ml−1 for 1 l-1beta, 0.05 pg ml−1 for IL-6 and 0.12 pg ml−1 for TNF-α. Serum samples were thawed at 4 °C and centrifuged at 1400 g for 5 min in order to remove any aggregate protein. The supernatant was then transferred to a new tube and diluted 1:2 in assay buffer supplied in the kit. A 7-point standard curve was generated using the standards supplied in the assay, which were reconstituted and serially diluted according to manufacturer’s instructions. The data was generated using a 5-parameter logistic standard curve fit corrected for sample dilutions in the Millipore Analyst Software (Merck Millipore).
Statistical analyses
Between-group differences
Regional BPND was normally distributed within each group, as confirmed by Shapiro–Wilk’s test (>0.05) and homogeneity of variances was checked by Levene’s test. Two-sample t-tests were used to independently test the null hypothesis of equality in group means between: (i) UHR individuals and the younger control group; (ii) recent-onset patients and the younger control group; and (iii) chronic patients and the older control group. These three null hypotheses were independently tested for age, body mass index, amount of injected activity, specific activity of the ligand and regional 11C-(R)-PK11195 BPND for each of the six ROIs. The false discovery rate (FDR)40 was used to provide error control across the six ROIs. Sex and smoking status were compared between groups using Fisher’s exact test (two-tailed).
Peripheral cytokines and gray matter
Partial correlation analysis was used to test for an association across all individuals (patients and controls) between: (i) regional BPND (six regions) and gray matter volume (six regions); as well as, (ii) BPND (six regions) and three peripheral serum cytokines (IL1-β, TNF-α and IL-6). The FDR was used to provide error control across the six correlations tested in the former correlation analysis, and the 18 correlations tested in the latter (6 regions × 3 cytokines). These correlations controlled for age, as an association was detected between age and thalamic BPND.
Medication and clinical symptoms
Among the pooled schizophrenia groups (recent-onset and chronic patients), a partial correlation was used to examine the relationship between regional BPND and chlorpromazine-equivalent antipsychotic dose, while controlling for age. FDR correction was applied across the six regional tests. Correlations of regional BPND with positive (BPRS-positive subscale) and negative (SANS total) symptom severity were examined separately in UHR, recent-onset and chronic patients, with the FDR correction performed across the 12 tests in each cohort (6 regions × 2 symptom scales). All reported P-values associated with regional analyses were FDR corrected unless otherwise specified and FDR<0.05 was considered statistically significant. A trend was defined as FDR<0.1.
Results
Demographics, clinical and biological parameters
There were no significant differences in age, sex, body mass index, injected or specific radioactivity or levels of cytokine markers (Supplementary Figure S2) between patients and their respective healthy control groups (FDR<0.05). There was a greater proportion of smokers in the chronic patient group (53%), compared to the age-matched control group (17% χ2(1, n=27)=3.84, P=0.0499). Compared to age-matched controls, UHR patients displayed significantly increased gray matter volume compared to healthy controls in the orbital frontal cortex (P=0.008; Supplementary Figure S3). In addition, recent-onset patients displayed significantly decreased gray matter volume in the insular cortex relative to age-matched controls (P=0.008; Supplementary Figure S3). Gray matter volume did not significantly differ between chronic patients and older healthy controls in any region examined (FDR<0.05).
Compared to recent-onset patients, chronic patients exhibited significantly greater general psychopathology (BPRS total) scores (t(31)=2.5, P<0.05), positive symptom (BPRS subscale) scores (t(31)=3.1, P<0.01), negative symptom (SANS total) scores (t(31)=2.1, P<0.05), as well as significantly decreased general functioning (SOFAS) scores (t(31)=3.1, P<0.01). Furthermore, chronic patients displayed significantly decreased general functioning (SOFAS scores) compared to UHR patients (t(23)=2.8, P<0.05). Therefore, potential associations between regional BPND and positive and negative symptom scores were examined separately in UHR, recent-onset and chronic patients.
Between-group comparisons of regional 11C-(R)-PK11195 BPND
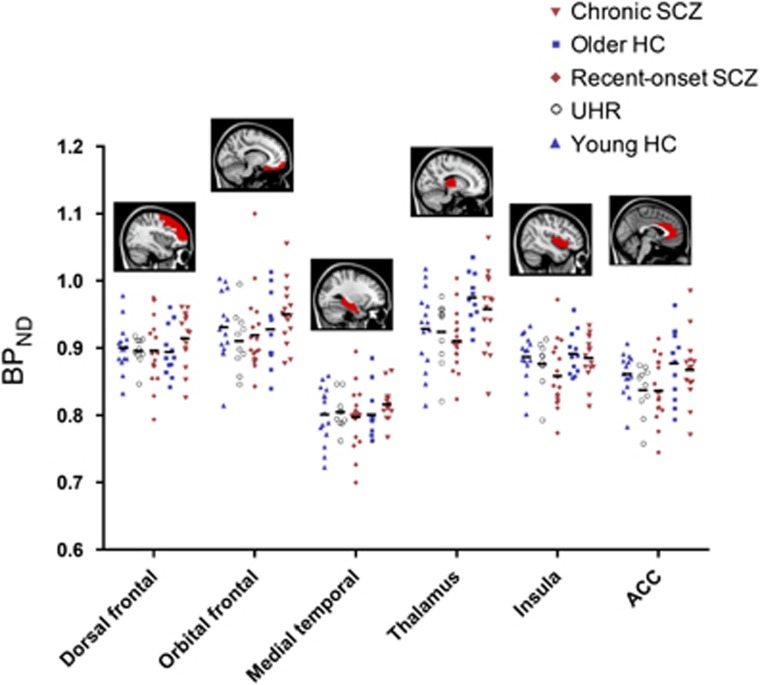
No significant differences in 11C-(R)-PK11195 BPND were found between patients (UHR, recent-onset and chronic) and their respective healthy control groups (younger and older; Table 2 and Figure 1). Inclusion of smoking status and sex as nuisance covariates did not change these results. In secondary analyses examining the effects of medication, unmedicated patients (n=4) displayed lower BPND compared to antipsychotic-treated patients (n=29) and healthy controls (n=27), after controlling for age (Supplementary Figure S4). In supplementary analyses, no significant differences were also found using whole-brain, voxel-wise analysis and BPND estimates derived using three alternative reference regions (Supplementary Figure S5). With voxel-wise analysis, each gray matter voxel was independently tested for a between-group difference and cluster-based inference was used to provide error control over the family of all voxels.41 This data-driven supplementary analysis avoided the need for anatomically defined ROIs.
Table 2. Comparison of 11C-(R)-PK11195 BPND across the five study groups.
|
Young HC |
UHR |
Recent-onset SCZ |
Older HC |
Chronic SCZ |
UHR vs young HC |
Recent-onset vs young HC |
Chronic vs older HC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | t | P | d | t | P | d | t | P | d | |
| Dorsal frontal | 0.90 | 0.04 | 0.9 | 0.02 | 0.9 | 0.05 | 0.89 | 0.04 | 0.91 | 0.04 | 0.38 | 0.71 | 0.08 | 0.28 | 0.78 | 0.05 | −1.34 | 0.19 | −0.26 |
| Orbital frontal | 0.93 | 0.05 | 0.91 | 0.04 | 0.92 | 0.06 | 0.93 | 0.05 | 0.95 | 0.05 | 1.05 | 0.30 | 0.21 | 0.63 | 0.53 | 0.11 | −1.16 | 0.26 | −0.22 |
| Medial temporal | 0.80 | 0.04 | 0.80 | 0.03 | 0.80 | 0.04 | 0.8 | 0.04 | 0.82 | 0.02 | −0.23 | 0.82 | −0.05 | 0.24 | 0.81 | 0.04 | −1.32 | 0.20 | −0.25 |
| Thalamus | 0.93 | 0.06 | 0.92 | 0.05 | 0.91 | 0.04 | 0.98 | 0.04 | 0.96 | 0.06 | 0.19 | 0.85 | 0.04 | 1.01 | 0.32 | 0.18 | 0.88 | 0.39 | 0.17 |
| Insular cortex | 0.89 | 0.04 | 0.88 | 0.03 | 0.86 | 0.05 | 0.89 | 0.03 | 0.89 | 0.03 | 0.74 | 0.47 | 0.15 | 1.91 | 0.06 | 0.33 | 0.48 | 0.64 | 0.09 |
| ACC | 0.86 | 0.03 | 0.84 | 0.04 | 0.84 | 0.04 | 0.88 | 0.05 | 0.87 | 0.05 | 1.73 | 0.10 | 0.35 | 1.82 | 0.08 | 0.32 | 0.50 | 0.62 | 0.10 |
Abbreviations: ACC, anterior cingulate cortex; BPND, binding potential; d, effect size (Cohen’s d) HC, healthy control; M, mean; P, uncorrected P-value; SCZ, schizophrenia; s.d., standard deviation; t, t statistic; UHR, ultra-high-risk. Equal variances not assumed.
Figure 1.
Comparison of 11C-(R)-PK11195 BPND in individuals at ultra-high risk, recently diagnosed and chronically ill with schizophrenia, compared to age-matched comparison subjects. BPND did not differ between patients and controls. Bars denote the mean value of BPND. Controls are shown in blue and patients in red. Sagittal slices show AAL regions investigated. ACC, anterior cingulate cortex; AAL, automated anatomical labeling; BPND, binding potential; HC, healthy controls; SCZ, schizophrenia; UHR, ultra-high risk.
Correlations with regional 11C-(R)-PK11195 BPND
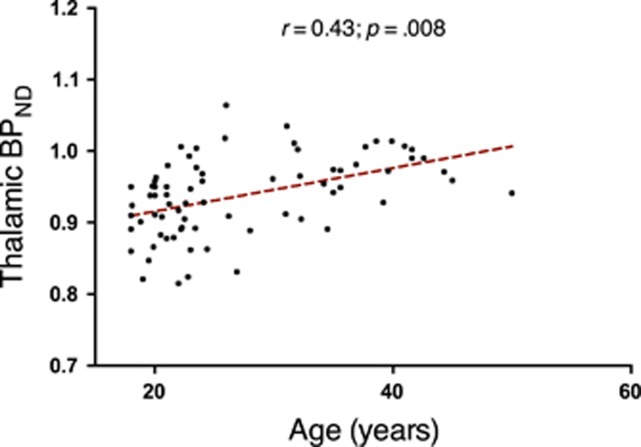
There was no relationship between chlorpromazine-equivalent antipsychotic dose and BPND in any ROI (FDR <0.05). In contrast, there was a positive correlation between age and BPND in the thalamus of all groups combined (r=0.43, P=0.008, FDR; Figure 2). Therefore, cytokine and clinical correlations with BPND were examined with partial correlations, controlling for age. No relationships were found with peripheral pro-inflammatory serum cytokine levels, regional gray matter volumes or symptom severity scores (positive and negative symptoms) in UHR, recent-onset or chronic patients.
Figure 2.
Correlation between 11C-(R)-PK11195 BPND and age. BPND was positively correlated to age in the thalamus. BPND, binding potential.
Discussion
In the largest TSPO PET study in schizophrenia to date, we found no evidence for differential 11C-(R)-PK11195-binding in individuals at risk for psychosis, or patients recently diagnosed and chronically ill with schizophrenia. In addition, 11C-(R)-PK11195 binding was not related to peripheral cytokines, gray matter volume or clinical severity. Our study is consistent with an increasing number of studies reporting no difference in TSPO expression at various stages of schizophrenia.12, 13, 15, 16, 17, 21 However, we report this finding across three distinct stages of illness in the same study, with all individuals scanned and analyzed at the same site.
In agreement with prior studies, thalamic BPND was positively correlated to age across all subjects.8, 16, 19 This correlation was observed in patients and controls separately (data not shown), suggesting that TSPO expression may be more sensitive to normal age-related increases than illness-related processes. Increased thalamic TSPO expression may be sensitive to age by virtue of the dense reciprocal thalamic connections with cortical and subcortical brain areas allowing a significant cumulative effect of TSPO expression, while subtle more spatially distributed increases may remain below detection levels in manifest areas of tissue destruction or neurodegeneration.
Contrary to our hypothesis, we found no evidence of altered 11C-(R)-PK11195 BPND in either our UHR or recent-onset cohorts relative to age-matched controls, suggesting that differences in illness stage do not likely account for the discrepant findings of recent studies using the same tracer.19, 20 While statistically significant differences were not evident, 11C-(R)-PK11195 BPND tended to be reduced in both UHR and recent-onset patients in the insula and ACC, with an effect size of d=0.35 and 0.32, respectively; a notable preliminary observation given findings of decreased TSPO expression in a recent study of unmedicated first-episode psychosis patients.20 Interestingly, in agreement with this study, we found significantly reduced TSPO in antipsychotic-naive patients, suggesting that absence of TSPO elevation in our study is not due to antipsychotic medication. These findings challenge the assumption of increased TSPO expression in prodromal and early stages of psychosis, and suggest that a downregulation of TSPO may be involved in the pathophysiology of the illness, possibly normalizing with antipsychotic treatment.
The possibility remains however that increased microglial activation occurs in discrete subpopulations of patients42 defined by illness duration, symptom exacerbation,43 inflammation or gray matter loss,44 which were not captured at the time of scanning or by the current sample population. For example, although 11C-(R)-PK11195 BPND did not differ across all three stages of illness and was not related to duration of illness (data not shown), increased (or decreased) TSPO expression could occur during specific time-windows, such as during very early (that is, <2 months) illness. Our recent-onset patients were scanned, on average, within the first 1.5 years of their illness, while microglial activation might have been present only at the time of their first psychotic episode. Likewise, although we found no relationship between symptom severity and TSPO expression, consistent with five previous studies,10, 11, 14, 16, 20 our patient cohorts were relatively symptomatically stable, limiting our ability to assess the contribution of symptom exacerbation on TSPO availability.
Alternative to clinical factors, TSPO upregulation may be associated with a patient subgroup defined by high inflammation, a notion supported by post-mortem studies in chronic schizophrenia.42, 45, 46, 47, 48 Of note however, we found no BPND differences between high and low inflammatory groups of recent-onset patients, based on a median split of averaged and scaled cytokine levels (data not shown). Nevertheless, a subgroup possibility remains as our patient groups did not display elevated pro-inflammatory cytokines relative to controls across the markers examined in serum (IL1-β, TNF-α and IL-6) and hence, may not represent ‘high inflammatory’ cohorts. Therefore, differences in patient immune profiles, including peripheral cytokine levels, may explain discrepancies between our study and previous findings of increased TSPO expression. However, previous work by Coughlin et al.17 found no association between [11C]DPA-713 distribution volume and plasma IL-6 levels in recent-onset patients with significantly increased IL-6 levels, suggesting that increased peripheral inflammation may occur without increases in TSPO. In line with this proposition, we did not detect a relationship between peripheral cytokine markers and TSPO expression. Further work is required to understand the relationship between peripheral and central markers of inflammation, including TSPO.
Given the well-established findings of gray matter loss in schizophrenia32, 49 and the proposed link between gray matter loss and microglial activation during acute illness,1 patients displaying significant gray matter loss may represent an alternative subpopulation of patients with increased TSPO expression. Notably, we did not detect significantly reduced gray matter in chronic patients relative to controls, despite a trend of decreased volume across all regions examined, with an average effect size of d=0.3 (Supplementary Figure S3). Therefore, the absence of between-group BPND differences may be due to our patient populations, which did not display increased inflammation or gray matter loss. Alternatively, our sample size may have been insufficient to identify such biological shifts, including a changed TSPO signal. Nevertheless, it should be recognized that the sample size of our patient cohorts were similar to, or greater than, previous studies and, together, our patient sample represents the largest PET TSPO study in schizophrenia to date.
It is lastly noteworthy that reduced TSPO levels in an infection-mediated mouse model was recently associated with increased cytokine expression,18 which challenges the assumption that low-grade neuroinflammation (such as that possibly occurring in schizophrenia), is reflected by an increase in TSPO. This suggests that TSPO may not be the optimal target for imaging microglial activation and neuroinflammation in schizophrenia. This might partly be due to other TSPO mechanisms related to astrocytes, TSPO density,50 glucose metabolism,51 glial mitochondria52 or transcriptional events53 that contribute to the PET TSPO signal, potentially underlying the mixed results and heterogeneity observed across and within studies.18, 20 In addition, microglial activation is a highly diverse and dynamic process, involving classically pro-inflammatory (M1) and neuroprotective (M2) phenotypes, which may change in disease-specific contexts.54 Due to the heterogeneity of microglial function,54 it remains possible that neuroinflammatory processes are present in schizophrenia but are unable to be captured with current PET techniques using radioligands for the TSPO. As the TSPO signal does not solely represent microglial activation or neuroinflammation, in vivo PET studies would benefit from the development of markers specific to microglia phenotype and activation in order to determine their role in the pathophysiology of schizophrenia.
Several aspects of our experimental design require consideration. First, the ligand used in this study, 11C-(R)-PK11195, has shown lower affinity for the TSPO relative to second-generation ligands.22 Therefore, it could be argued that the lower signal-to-noise of 11C-(R)-PK11195 in comparison to second-generation ligands might have precluded the detection of subtle alterations in TSPO. In turn, our results are contingent on 11C-(R)-PK11195, and different conclusions might be reached with other tracers, including those yielding higher-binding affinity, such as [18F]-FEPPA and [11C]PBR28 [ref 55, 56, 57] and lower non-specific binding, such as [11C]-DPA-713.58 On the other hand, it is unlikely that our findings are ligand specific, as increased TSPO has been detected in prior investigations using 11C-(R)-PK11195,10, 11 and studies using second-generation ligands have reported negative findings.12, 13, 14, 16, 17 Second, it might be argued that our use of a reference-tissue method resulted in inaccurate estimates of 11C-(R)-PK11195 BPND. We used a pseudo-reference region; specifically, cerebellar gray matter, to derive the input. Although this approach has been criticized on the basis that TSPO is ubiquitous throughout the brain and within venous sinuses around the cerebellum,36 cerebellar gray matter represents a reasonable compromise.15 Importantly, we found no significant difference in cerebellar BPND between patients and controls. Our findings were also verified using simplified reference-tissue model estimates applied with three additional reference inputs (Supplementary Material), indicating robustness to the choice of reference input. Moreover, supplementary analyses were performed at the level of individual voxels to test for differential BPND expression across the entire gray matter volume, without reference to any prior regional hypotheses. These whole-brain, data-driven analyses confirmed our main findings, in that the null hypothesis of equality in BPND between patient and control groups was not rejected for any gray matter voxels.
In summary, we found no evidence of 11C-(R)-PK11195-binding differences in groups of individuals at high risk, recently diagnosed and chronically ill with schizophrenia. We conclude that altered microglial activation, indexed by 11C-(R)-PK11195, is stable across stages in the course of illness and does not significantly differ compared to healthy comparison subjects. Therefore, illness duration is unlikely to be a confounding factor in previous PET studies. It is possible however, that inflammatory signals vary in patient subpopulations characterized by peripheral inflammation, gray matter loss or symptom exacerbation. Furthermore, our findings do not discount the possibility of new-generation TSPO tracers and alternative imaging methodologies yielding significant between-group differences in microglial activation. Therefore, assessment of TSPO combined with complimentary MRI measures of neuroinflammation59 and other biomakers in larger samples is warranted.
Acknowledgments
We gratefully acknowledge all participants for making this study possible. In addition, we kindly thank Steven Tahtalian, Eleni Ganella and Cassandra Wannan for assisting with data collection, Drs Lee and Kumar for assisting with patient recruitment and Dr Phassouliotis for study coordination. We are grateful to A/Prof Fornito for his intellectual input and Prof Paul Cumming for many fruitful discussions on PET analysis. We thank Kunthi Pathmaraj for providing technical PET support on this study. Finally, we are grateful to Melbourne Health and Austin Health for providing access to participant information. This study was supported by an Australian National Health and Medical Research Council (NHMRC) Project Grant (1065742), a Royal Melbourne Hospital Grant in Aid (GIA-030-2016), a NARSAD Distinguished Investigator Grant (18722 to CP), a University of Melbourne Early Career Researcher grant (601253 to VC), Australian Rotary Health, Ian Scott PhD Scholarship in Mental Health (to MAD), an NHMRC Early Career Fellowship (628880 to VC), a University of Melbourne Faculty Fellowship (to VC and BN), an NHMRC Senior Principal Research Fellowship to CP (628386, 1105825) and NHMRC Principal Research Fellowship to CSW (1117079), a NARSAD Independent Investigator Award (to BN), the Schizophrenia Research Institute (utilizing infrastructure funding from the NSW Ministry of Health and the Macquarie Group Foundation), the University of New South Wales (to CSW) and Neuroscience Research Australia.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Laskaris L, Di Biase M, Everall I, Chana G, Christopoulos A, Skafidas E et al. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 2016; 173: 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Derkits E. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167: 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol 2009; 31: 706–715. [DOI] [PubMed] [Google Scholar]
- Trepanier M, Hopperton K, Mizrahi R, Mechawar N, Bazinet R. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 2016; 21: 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn NA. Microglia dysfunction in schizophrenia: an integrative theory. Med Hypotheses 2000; 54: 198–202. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- Banati R, Myers R, Kreutzberg G. PK (‘peripheral benzodiazepine’)–binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H] PK 11195 binding to activated microglia. J Neurocytol 1997; 26: 77–82. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE et al. In-vivo measurement of activated microglia in dementia. Lancet 2001; 358: 461–467. [DOI] [PubMed] [Google Scholar]
- Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T et al. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport 2001; 12: 3439–3442. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: A PET Study. J Nucl Med 2009; 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. Microglia activation in recent-onset schizophrenia: a Quantitative (R-[11C]PK11195 Positron Emission Tomography Study. Biol Psychiatry 2008; 64: 820–822. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET Study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Tseng H-H, Rao N, Selvanathan T, Kenk M, Bazinet RP et al. Imaging microglial activation in untreated first-episode psychosis: a PET Study With [18F] FEPPA. Am J Psychiatry 2016; 174: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET Study With [18F]-FEPPA. Schizophr Bull 2015; 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry 2016; 21: 1672–1679. [DOI] [PubMed] [Google Scholar]
- van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R et al. In vivo (R)-[11C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr 2016; 2: 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [lsqb]11C[rsqb]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 2016; 6: e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry 2017; doi:10.1038/mp.2016.248. [DOI] [PubMed]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C] PBR28 PET brain imaging study. Am J Psychiatry 2015; 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C] PBR28. Mol Psychiatry 2017; 22: 850–856. [DOI] [PubMed] [Google Scholar]
- Banati R, Hickie IB. Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med J Aust 2009; 190: S26. [DOI] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A et al. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans 2015; 43: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung A, Phillips L, McGorry P, Ward J, Donovan K, Thompson K Comprehensive Assessment of At-risk Mental States (CAARMS). University of Melbourne, Department of Psychiatry, Personal Assessment and Crisis Evaluation Clinic: Melbourne, Australia, 2002.
- First MB, Gibbon M The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Hersen M (ed), Comprehensive Handbook of Psychological Assessment, vol. 2. John Wiley & Sons Inc: Hoboken, NJ, USA, 2004, pp 134–143.
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962; 10: 799–812. [Google Scholar]
- Andreasen NC Scale for the Assessment of Negative Symptoms. University of Iowa Press: Iowa City, IA, USA, 1983.
- Morosini P, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM‐IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatr Scand 2000; 101: 323–329. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004; 23(Supp 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Technical Report. FMRIB Centre: Oxford, UK, 2007.
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB Centre: Oxford, UK, 2010.
- Pantelis C, Velakoulis D, Wood S, Yücel M, Yung A, Phillips L et al. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry 2007; 19: 371–379. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz CF, Cropley VL, Wannan C, Di Biase M, McGorry PD, Pantelis C. Structural neuroimaging across early-stage psychosis: aberrations in neurobiological trajectories and implications for the staging model. Aust N Z J Psychiatry 2016; 51: 455–476. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage 1997; 6: 279–287. [DOI] [PubMed] [Google Scholar]
- Yaqub M, van Berckel BNM, Schuitemaker A, Hinz R, Turkheimer FE, Tomasi G et al. Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)-[lsqb]11C[rsqb]PK11195 brain PET studies. J Cereb Blood Flow Metab 2012; 32: 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 2007; 48: 158–167. [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol 2005; 11: 346–355. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Davis B, Matthews JC, Rahmoune H, Hong G, Gee A et al. The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant α1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl Med Biol 2003; 30: 199–206. [DOI] [PubMed] [Google Scholar]
- Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A et al. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H] PK 11195: anatomical and subcellular distribution. Brain Res Bull 1987; 18: 49–61. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 2002; 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. NeuroImage 2004; 23: 54–63. [DOI] [PubMed] [Google Scholar]
- Fillman S, Cloonan N, Catts V, Miller L, Wong J, McCrossin T et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2012; 18: 206–214. [DOI] [PubMed] [Google Scholar]
- Cropley V, Wood SJ, Pantelis C. Brain structural, neurochemical and neuroinflammatory markers of psychosis onset and relapse: Is there evidence for a psychosis relapse signature? Int Clin Psychopharmacol 2013; PMID:23669508.
- Zhang Y, Catts V, Sheedy D, McCrossin T, Kril J, Weickert CS. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 2016; 6: e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepień T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 2005; 43: 81–89. [PubMed] [Google Scholar]
- Rao JS, Kim H-W, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res 2013; 147: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca's area volume. Mol Psychiatry 2015; 21: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett 1999; 271: 126–128. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Patti J, Wood S, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res 2009; 108: 104–113. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G, Leschiner S, Spanier I, Veenman L, Weizman A et al. Acute and repeated swim stress effects on peripheral benzodiazepine receptors in the rat hippocampus, adrenal, and kidney. Neuropsychopharmacology 2001; 25: 669–678. [DOI] [PubMed] [Google Scholar]
- Momosaki S, Imamoto N, Hosoi R, Sawada Y, Abe K, Zhang M-R et al. PK11195 might selectively suppress the quinolinic acid-induced enhancement of anaerobic glycolysis in glial cells. Brain Res 2010; 1340: 18–23. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 2010; 9: 971–988. [DOI] [PubMed] [Google Scholar]
- Fan J, Papadopoulos V. Transcriptional regulation of translocator protein (Tspo) via a SINE B2-mediated natural antisense transcript in MA-10 leydig cells. Biol Reprod 2012; 86, Article 147 141–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Boddeke H, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol 2017; 79: 619–643. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Zoghbi SS, Simèon FG, Taku A, Pike VW, Innis RB et al. Quantification of translocator protein (18 kDa) in the human brain with PET and a novel radioligand, 18 F-PBR06. J Nucl Med 2009; 50: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi M, Briard E, Zoghbi SS, Gourley JP, Hong J, Fujimura Y et al. Brain and whole-body imaging in nonhuman primates of [11 C] PBR28, a promising PET radioligand for peripheral benzodiazepine receptors. NeuroImage 2008; 39: 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S et al. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol 2008; 35: 305–314. [DOI] [PubMed] [Google Scholar]
- Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EFJ. [11C]-DPA-713 and [18F]-DPA-714 as new PET tracers for TSPO: a comparison with [11C]-(R)-PK11195 in a rat model of herpes encephalitis. Mol Imag Biol 2009; 11: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Granziera C, Hooker JM, Loggia ML. In vivo imaging of human neuroinflammation. ACS Chem Neurosci 2016; 7: 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.