Abstract
Introduction
Olfactory dysfunction affects about 85-90% of Parkinson's disease (PD) patients with severe deterioration in the ability of discriminate several types of odors. In addition, studies reported declines in olfactory performances during a short period of sleep deprivation. Besides, PD is also known to strongly affect the occurrence and maintenance of rapid eye movement (REM) sleep.
Methods
Therefore, we investigated the mechanisms involved on discrimination of a social odor (dependent on the vomeronasal system) and a non-social odor (related to the main olfactory pathway) in the rotenone model of PD. Also, a concomitant impairment in REM sleep was inflicted with the introduction of two periods (24 or 48 h) of REM sleep deprivation (REMSD). Rotenone promoted a remarkable olfactory impairment in both social and non-social odors, with a notable modulation induced by 24 h of REMSD for the non-social odor.
Results
Our findings demonstrated the occurrence of a strong association between the density of nigral TH-ir neurons and the olfactory discrimination capacity for both odorant stimuli. Specifically, the rotenone-induced decrease of these neurons tends to elicit reductions in the olfactory discrimination ability.
Conclusions
These results are consistent with the participation of the nigrostriatal dopaminergic system mainly in the olfactory discrimination of a non-social odor, probably through the main olfactory pathway. Such involvement may have produce relevant impact in the preclinical abnormalities found in PD patients.
Keywords: Olfactory discrimination, Dopamine, Rotenone, REM sleep deprivation, Parkinson disease
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disease that affects a growing segment of the population with the progression of age1,2. Currently, diagnosis occurs only in the presence of motor symptoms, although, several non-motor signs have been recognized as important features of this disease, and precedes motor symptoms. Two of these well-documented prodromal signs are the sleep and olfactory disturbances. In fact, studies demonstrated the occurrence of rapid eye movement (REM) sleep suppression inflicted by the nigrostriatal lesion3,4, pharmacological dopaminergic blockade5 or dopamine (DA) transporter knockout (DAT-KO)6. In addition, olfactory dysfunction affects about 85-90% of PD patients7,8 with remarkable deterioration of detection, discrimination and odor identification9-11.
Such impairment in PD, originally described by Ansari and Johnson12, is supported by neuropathological findings of Lewy bodies presence in the olfactory bulb, olfactory tract and anterior olfactory nucleus in preclinical Braak stages prior to significant nigral degeneration13. Moreover, it has been reported worsening of olfactory function in the smelling of certain odors in detriment of others, using the odor identification test14. Regarding experimental studies in rodents, two distinct odors may be categorized: social and non-social. Accordingly, social odors present a more intricate processing, requiring the participation of the main olfactory pathway and mostly the vomeronasal system15,16. Whereas, non-social odors are exclusively processed by the main olfactory pathway16.
Interestingly, sleep deprivation adversely affected the olfactory performance in rats17,18. Disturbances in olfactory function of people with REM sleep disturbances are also found17,19-22. In this sense, various studies have observed and discussed the involvement of the dopaminergic system in olfaction, since DA seems to operate as a key player in the modulation of the glomerular activity generated from the sensory afferents to mitral/tuffed cells8,23-26. Conjointly, it has been found an enormous increase in the number of tyrosine hydroxylase immunoreactive (TH-ir) interneurons compared to controls in the glomerular layer of the olfactory bulb from PD patients24,27. It is discussed that this increment could be responsible for the inhibition of glomerular activity, promoting the hyposmia28,29. Such mechanism is attributed to the inhibitory effect of DA24,27, mediated by D2 receptors activity30, causing the suppression of the olfactory information. A very similar deficit has been recently described after the intranigral administration of rotenone in rats, reinforcing such process31. In fact, the mechanisms linking these findings remain unclear. However, a recent study reported a direct axonal dopaminergic projection from the substantia nigra pars compacta (SNpc) to the olfactory bulb of rats32. Therefore, it is suggested that the degeneration of the nigro-olfactory dopaminergic fibers contribute to the occurrence of hyposmia in PD32. However, the neurobiological basis of olfactory deficits produced by REMSD alone or associated to a nigrostriatal lesion remains to be clarified.
In view of that, in the present study we employed the intranigral rotenone model of PD leading to a massive mitochondrial inhibition and selective degeneration of dopaminergic neurons in the SNpc31,33-35. It is therefore feasible that the well-known dopaminergic supersensitivity effect induced by REMSD5,36, and also by rotenone nigral lesion35, could affect the activity of dopaminergic system, generating an increase in their inhibitory effect, therefore promoting a more pronounced disruption in olfactory function. Accordingly, the main goal of this study was to test if such pattern of olfactory dysfunction may vary for social (dependent on the vomeronasal system) and non-social (dependent on the main olfactory pathway) odors.
Experimental Procedures
Animals
Male Wistar rats weighing 280-320 g at the beginning of the protocols were used. They were housed in groups of five in polypropylene cages with wood shavings as bedding and maintained in a temperature-controlled room (22±2ºC) on a 12-h light-dark cycle (lights on at 7:00 AM) with food and water provided ad libitum. All experiments were conducted in accordance with guidelines of Brazilian Guide for Care and Use of Laboratory Animals (COBEA) and the protocol complies with the recommendations of Federal University of Paraná and was approved by the Institutional Ethics Committee (approval ID # 651).
Experimental design
First experiment - Determination of non-social odor preference Non-social olfactory preference test in a radial maze
In this experiment, we aimed to determine a pattern of olfactory preference for a non-social odor. To execute that we exposed 10 rats to different non-social odors (mint, musk, vanilla, lemon and water as a control) in a radial maze with five arms. The central zone was a pentagon (20 cm x 20 cm x 25 cm height), while the arms had a square shape (17 cm x 17cm x 25 cm height). The animals were free to enter and explore all the arms of the radial maze during the sessions. At the end of the arms the odor was presented to the animals in plastic containers (50 mL falcon tube) with several small holes (about 1 mm of diameter each). Inside the containers there was a filter paper (3 cm x 1 cm) soaked with 100 µL of the odor essence (Essências Curitiba, Brazil) or water (control). Each rat was tested in 6 different sessions of 3 minutes. All the odors (including the water - control) were presented simultaneously (1 odor/arm) during the sessions, however, the sequence of the odors within the arms was changed for each session in order to avoid a spatial learning bias. Clean sawdust was included in all of the arms to dilute odorants and work as a consistent background odor for all non-social odor containers.
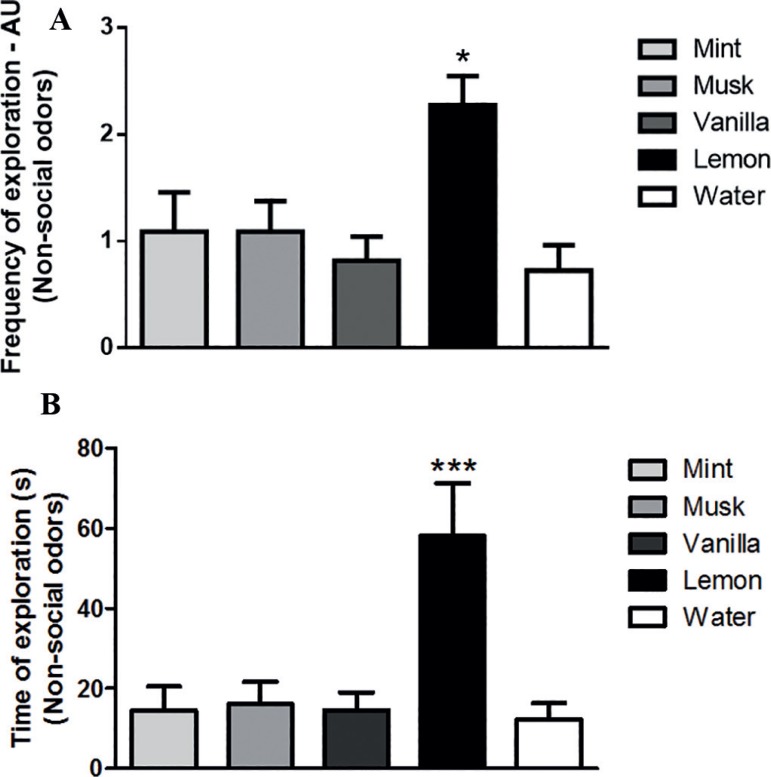
All the sessions were video-recorded for subsequent analysis of the following parameters: frequency of arms exploration and time of arms exploration. Results are presented as mean of the 6 sessions (Figure 2) and indicated that the lemon odor was preferred as an indicator of non-social odor preference; hence, we selected this odor as a non-social olfactory stimulus for the second experiment.
Figure 2.
Determination of non-social odor preference. A. Frequency of exploration (arbitrary units - AU) spent in each odorant stimuli. B. Time (s) of exploration spent in each odorant stimuli. The bars represent the mean ± standard error of the mean, n=10 per group, *P≤0.05, ***P≤0.001. One-way ANOVA followed by the Newman-Keuls test.
Second experiment - Possible olfactory impairment generated by SNpc lesion associated with REMSD
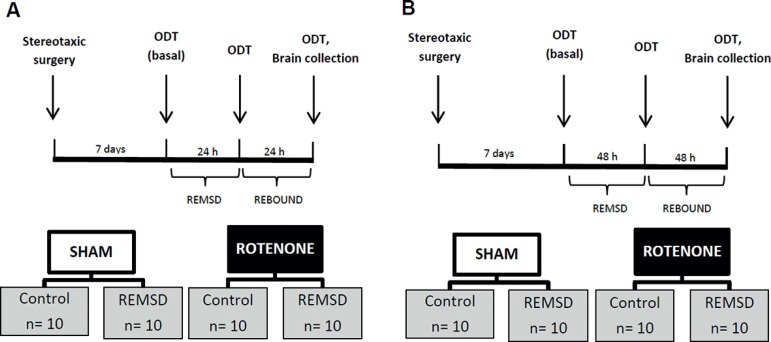
Before the stereotaxic surgeries, the rats were randomly distributed in two groups: sham (n=10) and rotenone (n=10). Seven days after the rotenone nigral infusion the animals were subjected to the olfactory discrimination task (ODT), for both social and non-social odors, in three different time-points: 24 h before REMSD (Baseline); immediately after 24 or 48 h of REMSD (REMSD) and 24 or 48 h after that (Rebound) (Figure 1). We executed this protocol twice, each time for a different period of REMSD tested: 24 h (sham n=10; rotenone n=10) (Figure 1A) and 48 h (sham n=10; rotenone n=10) (Figure 1B). Immediately, after the last time-point tested, the rats had their brains perfused and fixed for subsequent immunohistochemical analysis of SNpc TH-ir neurons.
Figure 1.
Schematic representation of the second experiment. A. 24 h of REMSD. B. 48 h of REMSD. Olfactory discrimination task (ODT), REM sleep deprivation (REMSD).
Stereotaxic surgery
Rats were sedated with intraperitoneal xylazine (10 mg/kg; Syntec do Brasil Ltda, Brazil) and anesthetized with intraperitoneal ketamine (90 mg/kg; Syntec do Brasil Ltda, Brazil). The following coordinates were used to the bilateral injury, bregma as a reference: SNpc (AP)=−5,0 mm, (ML)=± 2,1 mm e (DV)=−8,0 mm (Paxinos and Watson, 2005). Needles were guided to the region of interest for a bilateral infusion of 1 µL of rotenone (12 µg/µL), or of dimethylsulfoxide - DMSO (Sigma-Aldrich(r), United States) for the sham group. Using an electronic infusion pump (Insight Instruments, Ribeirão Preto, Brazil) at a rate of 0.33 µL/min(31,35,37,38).
REMSD and Rebound procedures
REMSD was performed as previously described by Tufik et al.(36), using the single platform method. Rats were individually placed on a circular platform (6.5 cm in diameter) in a cage (23×23×30 cm) filled with water up to 1 cm below the platform level. At the onset of each REM sleep episode, the animal experiences a loss of muscle tonus and falls into the water, thus being awakened. When platforms of this size are used, REM sleep is completely eliminated(39). Throughout the study, the experimental room was maintained at controlled conditions (22 ± 2ºC, 12:12 h light/dark cycle, lights on 7:00 a.m.). Food and water were provided ad libitum by placing chow pellets in a dispenser positioned inside the cage and water bottles on a grid located on top of the tank. The duration of REMSD periods was equivalent to the duration of the respective rebounds.
Social and non-social odor discrimination task (ODT)
This test was previously described by Soffié and Lamberty and subsequently modified by Prediger et al.(40-42) and recently used by Rodrigues et al.(31). A rectangular arena (60 x 40 x 50 cm) bisected by a dividing, with door allowing free passage between the two compartments was used. There was a period of adaptation in the apparatus for 5 minutes, during which the animals were free to explore both compartments with fresh sawdust. For the social discrimination, during the test, one compartment presented sawdust loaded with the familiar odor of the animal (obtained from its exposure to this sawdust during the preceding 48 h).
The other compartment presented new clean sawdust, designated as a non-familiar odor. For the non-social discrimination, during the test, both compartments presented clean sawdust, however, in one compartment there was lemon essence (100 µL in a filter paper inside of the pierced 50 mL falcon tube) and in the opposite compartment water as a control (equally presented).
The test started by placing the animal in the middle of the discrimination box, and the exploratory behavior in the compartments was recorded during 5 minutes. It is expected that the animal with olfactory impairment tends to explore both compartments equally, indicating absence of discrimination. The opposite is also expected if the olfactory function is intact, i.e., when animals prefer to explore a particular compartment(43).
As a parameter of discrimination, the "discrimination index (DI)" was calculated by dividing the difference in exploration time between the two compartments (non-familiar compartment - familiar compartment) by the total amount of exploration for both compartments (non-familiar compartment + familiar compartment). DI was then multiplied by 100 to express it as a percentage(31,35). DI equals to zero corresponds to a full preference towards non-familiar odor. Negative scores correspond to a preference towards familiar odor.
TH-ir immunohistochemistry
Density of TH-ir neurons was estimated within the SNpc. Animals were deeply anesthetized, with ketamine, immediately after the behaviors tests, and were transcardially perfused with saline first, then with 4% of the fixative solution formaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed from the skulls and were immersed for 48 h in that fixative solution at 4ºC. Subsequently, the brains were placed in 30% sucrose solution for 3 days and were frozen at -80ºC before sectioning. Series of 40 µm thick sections were cut on a cryostat (-20°C) in the frontal plane, and collected at the -4.92 mm to -5.52 mm from the bregma(44).
Tissue sections were incubated with primary mouse anti-TH antibody, diluted in phosphate-buffered saline containing 0.3% Triton X-100 (1:500; Chemicon, CA, USA) overnight at 4ºC. Biotin-conjugated secondary antibody incubation (1:200 anti-mouse # Vector Laboratories, USA), was performed for 2 h at room temperature. After several washes in phosphate-buffered saline, antibody complex was localized using the ABC system (Vectastain ABC Elite kit, Vector Laboratories, USA) followed by 3,3-diaminobenzidine reaction with nickel enhancement.
The sections were then mounted onto gelatin-coated slides and coverslipped after dehydration in ascending concentrations of ethanol-xylene solutions. Cell density counts were conducted making use of the software ImageJ (https://imagej.nih.gov/ij/). Counts were done on twelve sections (corresponding to the 480 µm interval), and an average density per section (and consequently for each animal) was obtained. For each group a mean value was calculated and converted to a percentage relative to the sham group, and compared with rotenone group. The mean density of TH-ir neurons in each hemisphere was considered representative of the SNpc neuronal cells in each animal. The images were obtained using a motorized Axio Imager Z2 microscope (Carl Zeiss, Jena, DE), equipped with an automated scanning VSlide (Metasystems, Altlussheim, DE).
Statistical Analysis
Differences between groups in the ODT were analyzed by two-way analysis of variance (ANOVA) with lesion as the between-subjects factor, REMSD as the within-subjects factor and interaction between these factors as the interaction factor - followed by the Bonferroni post hoc test. The pattern of olfactory preference for a non-social odor was analyzed by one-way ANOVA followed by the Newman-Keuls multiple comparison test. TH-immunohistochemistry was analyzed by unpaired two-tailed t Test. Pearson's correlation coefficients (r) were calculated to establish relationships between the percentage of TH-ir neurons density and the DI obtained from social and non-social odors. Values were expressed as mean ± standard error of mean (SEM). The level of significance was set at p≤0.05.
RESULTS
First experiment - Determination of non-social odor preference
As can be seen in Figure 2, the animals were exposed to a number of different non-social odors. According to the frequency of exploration parameter (Figure 2A) the rats exhibited a significant preference for the lemon odor (p<0.05) in comparison to the others tested [F(4,54)=4.84; p=0.002]. In addition, considering the time of exploration (Fig. 2B), the animals showed an equal increment (p<0.001) of this parameter for the lemon odor compared to the others [F(4,54)=6.88; p=0.0002].
Second experiment - Possible olfactory impairment generated by SNpc lesion associated with REMSD
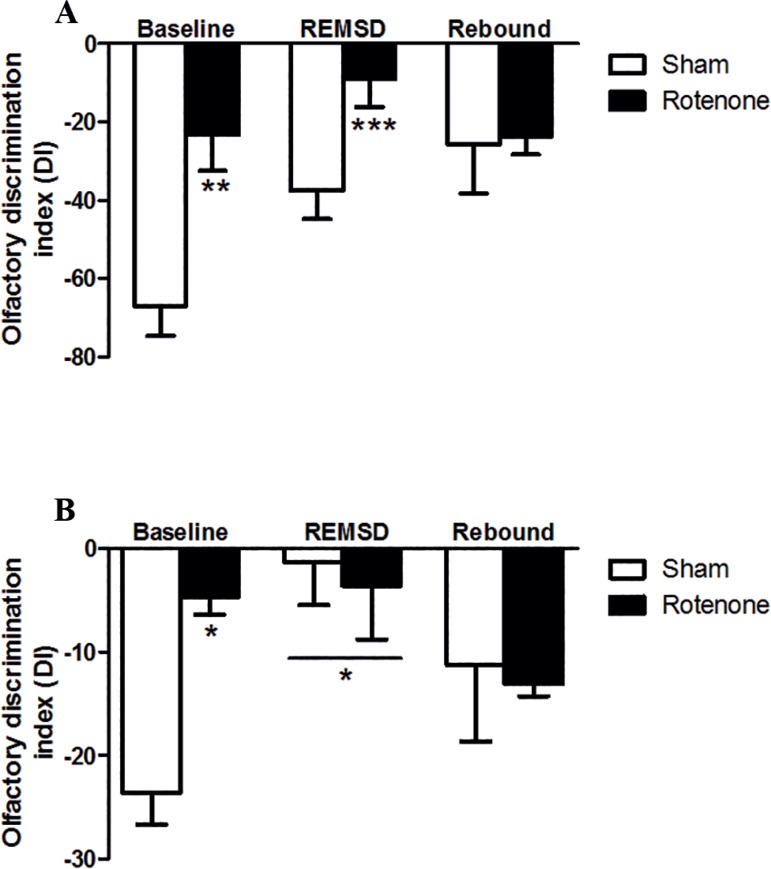
Figure 3 shows the DI obtained from the ODT of social (Figure 3A) and non-social odors (Figure 3B). Accordingly, the rotenone group demonstrated a significant increase (p<0.01) in the DI compared to the sham group in the baseline. Likewise, the rotenone group remained exhibiting an increased DI after 24 h of REMSD (p<0.001) compared to the sham baseline group as indicated by the lesion [F(2,30)=1.74; p=0.19], time [F(2,28)=0.84; p=0.44] and interaction [F(1,30)=9.21; p≤0.01] factors.
Figure 3.
Olfactory discrimination index (DI) obtained from 24 h of REMSD and 24 h of rebound. A. Social odor. B. Non-social odor (lemon). The bars represent the mean ± standard error of the mean, n=10 per group, *P≤0.05, **P≤0.01, ***P≤0.001 compared to the sham baseline. Two-way ANOVA followed by the Bonferroni test.
Regarding the non-social ODT (Figure 3B), it was observed a significant increase in the DI of the rotenone group (p<0.05) compared to the sham group in the baseline. In addition, both REMSD groups showed an increment (p<0.05) in the DI when compared to the sham baseline group as demonstrated by the lesion [F(2,20)=2.09; p=0.18], REMSD [F(2,20)=1.29; p=0.30] and interaction [F(1,30)=1.2; p=0.32] factors.
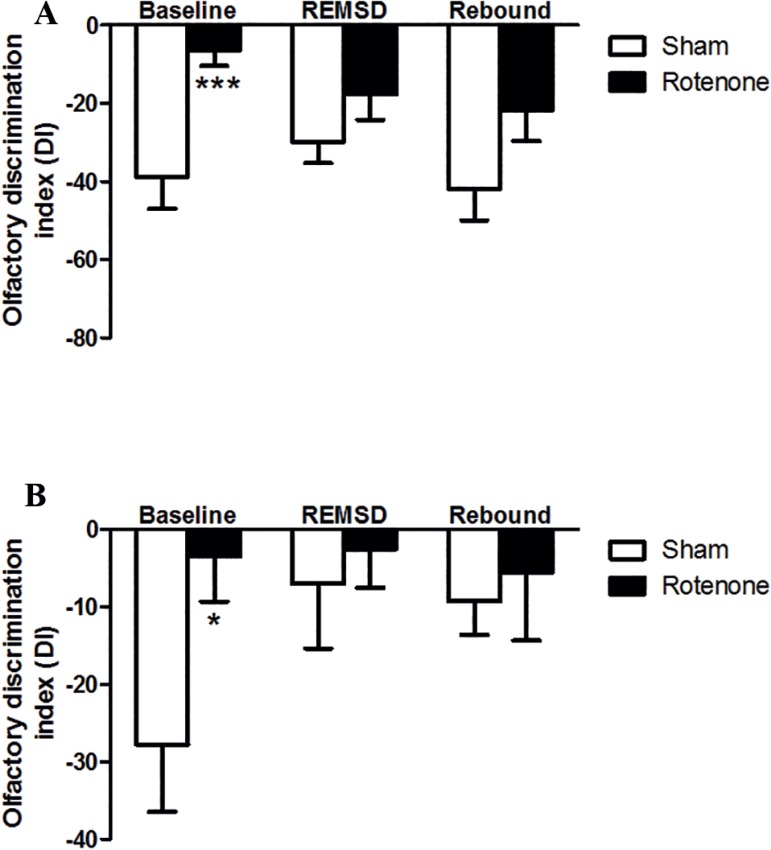
Regarding the 48 h of REMSD exposure (Figure 4) the rotenone group exhibited a significant increase (p<0.001) in the DI, compared to the sham group in the baseline. However, we did not detect significant differences between the groups tested concerning the discrimination of a social odor following 48 h of REMSD and its respective rebound period (Figure 4A), as revealed by the lesion [F(2,52)=9.44; p=0.003], REMSD [F(2,52)=0.22; p=0.8] and interaction [F(1,52)=1.03; p=0.36] factors. The analysis of the ODT for the non-social odor (Figure 4B) showed the occurrence of a significant increment (p<0.05) in the DI for the rotenone in comparison to the sham control group in the baseline. Analogously, it was not observed significant differences between the groups after the 48 h period of REMSD and its respective rebound according to the lesion [F(2,52)=3.56; p=0.05], REMSD [F(2,52)=1.32; p=0.27] and interaction [F(1,52)=1.42; p=0.25] factors.
Figure 4.
Olfactory discrimination index (DI) obtained from 48 h of REMSD and 48 h of rebound. A. Social odor. B. Non-social odor (lemon). The bars represent the mean ± standard error of the mean, n=10 per group, *P≤0.05, **P≤0.01, ***P≤0.001 compared to the sham baseline. Two-way ANOVA followed by the Bonferroni test.
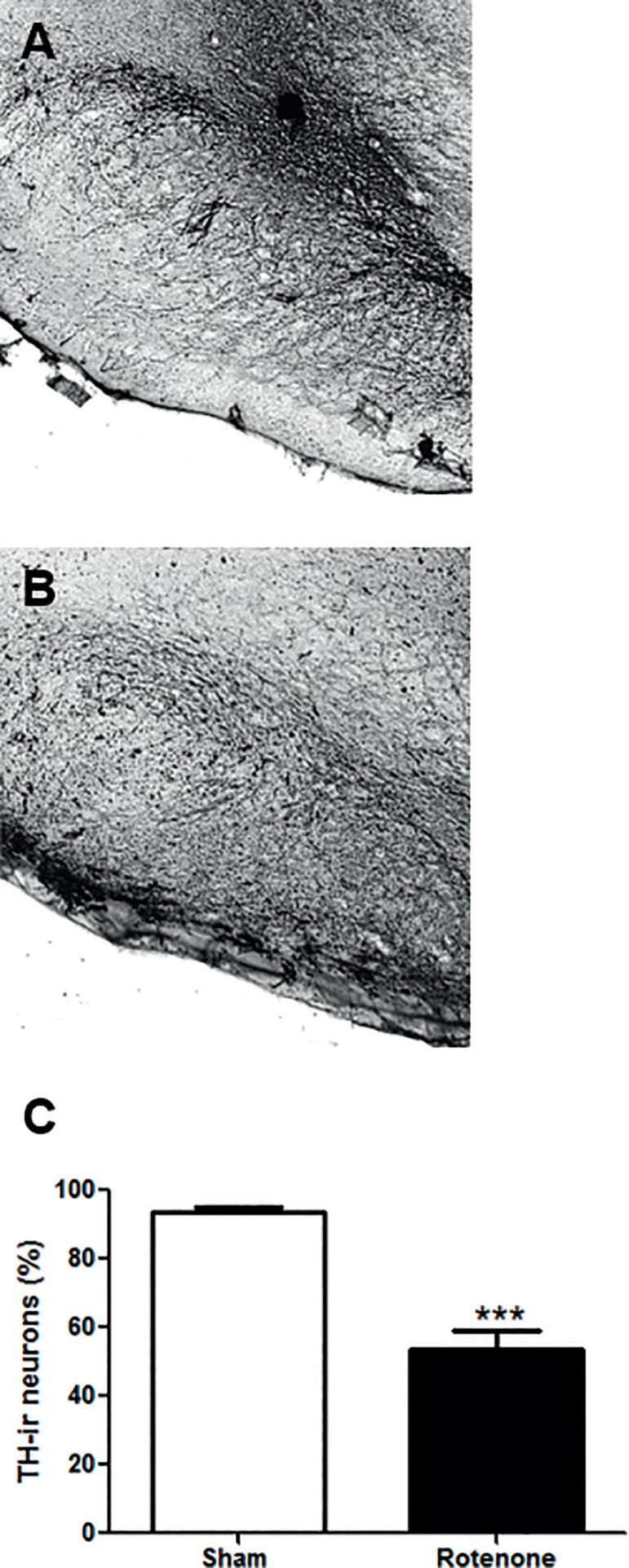
To access the extension of the neuronal lesion inflicted by rotenone we determined the density of TH-ir neurons within the SNpc, since this region is highly populated by such neurons. In fact, we observed a significant reduction of about 40% (p<0.0001; t=7.92 df=25) in the TH-ir neurons density in comparison to the sham group (Figure 5).
Figure 5.
Representative immunohistochemistry labeling of SNpc TH-ir neurons. A. Sham group. B. Rotenone group. C. Percentage of TH-ir neurons density in relation to sham group. The bars represent the mean ± standard error of the mean, n=5 per group. ***P≤0.001. Two-tailed t Test.
Pearson's correlation coefficients (Table 1) revealed significant moderate negative correlations (r=-0.7; p=0.006) and (r=-0.7; p=0.003) between the percentage of TH-ir neurons density and the DI obtained for a social odor at the baseline periods of analysis. However, this level of correlation was only observed after 24 h of REMSD (r=-0.6; p=0.02) and not at 48 h of REMSD (r=-0.2; p=0.43) or their respective rebound periods (r=-0.07; p=0.8), (r=0.04; p=0.9).
Table 1.
Pearson's correlations between the percentage of TH-ir neurons density within the SNpc and DI obtained from social and non-social odors.
| Correlations | Periods | ||
|---|---|---|---|
| Baseline | REMSD | Rebound | |
| Social odor | |||
| TH-ir neurons x DI 24 h | r=-0.7 ; P=0.006* | r=-0.6 ; P=0.02* | r=-0.07 ; P=0.8 |
| TH-ir neurons x DI 48 h | r=-0.7; P=0.003 * | r=-0.2 ; P=0.43 | r=0.04 ; P=0.9 |
| Non-social odor | |||
| TH-ir neurons x DI 24 h | r=-0.9 ; P=0.0004* | r=-0.2 ; P=0.64 | r=0.1 ; P=0.8 |
Significant correlations are indicated.
Significant correlations were also detected for the non-social odor exposure at the baseline periods (r=-0.9; p=0.0004) and (r=-0.8; p=0.001). Conversely, this outcome was not observed after 24 (r=-0.2; p=0.64) or 48 h (r=0.007; p=0.9) of REMSD and their respective rebounds (r=0.1; p=0.8) and (r=0.3; p=0.24).
DISCUSSION
Neurotoxic effects of rotenone are typically related to nigrostriatal dopaminergic neurotransmission mimicking PD35,38,45. In the current study, we observed that the occurrence of TH-ir neuronal loss in the SNpc is able to inflict an olfactory impairment for both, social and non-social odors. Moreover, REMSD most likely generated a similar, however, more predominantly deficit in the discrimination of a non-social odor. Of note, this effect was related to a shorter period of REMSD (24 h). In fact, this is the first study, according to our knowledge, that compares the variations of the olfactory performances using different olfactory stimuli, social and non-social odor (lemon), after a rotenone exposure in different periods of REMSD.
Furthermore, the relationship between dopaminergic neurotransmission and REM sleep is a recent theme in the literature, and growing evidence suggests a significant impact of REM sleep disturbances in PD46.
Besides, electrophysiological data indicated that the absence of half of the SNpc TH-ir neurons, in rats, provoked a major impairment in the sleep-wake parameters, predominantly in REM sleep3. Several sleep deprivation protocols show that REM sleep is related to dopaminergic neurotransmission, through the emergence of a robust dopaminergic D2 supersensitivity36,47,48. Further, in DAT-KO mice the selective activation of D2 receptors promoted recovery of REM sleep, suggesting that this receptor is related to REM sleep regulation6. Here we showed that REMSD associated with rotenone nigral lesion did not enhance this inhibitory effect generated by D2 receptors, therefore, not promoting a synergic olfactory impairment. This suggests the occurrence of different intensities of dopaminergic activation engaged by both manipulations.
Corroborating other studies, which also evaluated the participation of the dopaminergic system in the olfactory function24,31,49,50, rotenone alone, appears to consistently reduce the DI in both social and non-social odor (lemon). Even more recently, it has been demonstrated, the existence of a direct dopaminergic projection, from the SNpc to the olfactory bulb, probably influencing olfactory performance, particularly in PD32.
We observed that 24 h of REMSD affected the discrimination of lemon scent, independently of the rotenone lesion. Besides, a significant negative correlation (r=-0.7, p=0.006) is observed at the baseline period, between the percentage of SNpc TH-ir neurons density and DI, for the social odor. This finding indicates that decreased density in the SNpc neurons is associated to decreased olfactory discrimination performance.
A similar correlation (r=-0.6, p=0.02) is also detected after 24 h of REMSD, but not at the respective rebound period (r=-0.07, p=0.8) indicating that the olfactory impairment, for this condition (social odor), may be occurring although limited to a shorter period of REMSD. In addition, strong correlations obtained at the baseline for the non-social odor (lemon) (r=-0.9, p=0.0004 and r=-0.8, p=0.01) suggest a more prominent impairment in the discrimination of this modality of odorants compared to social odors.
Studies have shown that the preference for certain odors can be an important motivational factor51,52. In fact, social odors allow the transmission of some level of information between individuals. This characteristic can be exemplified by the presence of compounds with this type of odorant15, such as (methylthio)methanethiol, present in the mice urine53. Indeed, a highly complex mixture of volatile and non-volatile molecules15 forms the mixed composition of this type of social odor.
Thus, this processing seems to be more intricate, requiring the participation of both the main olfactory pathway and the vomeronasal system15,16, which interact functionally and anatomically, to perform this function54. Whereas, odors destitute of social components are processed exclusively by the main olfactory pathway16.
At this point a note of caution should be added. Despite the differences in olfaction between humans (microsmatic) and rats (macrosmatic), several studies observed declines in olfactory performances during a short period of sleep deprivation in both humans20,21,55 and rats17,18. Therefore, more studies are needed to determine if these findings, obtained from animal models, could be extrapolated for a human condition.
Interestingly, we detected a strong association between the density of nigral TH-ir neurons and the olfactory discrimination capacity for both odorant stimuli, reinforcing the role of the nigro-olfactory projections for odors processing mechanisms. In addition, REMSD and nigrostriatal lesion can induce dopaminergic D2 supersensitivity3,36,48,56. The activation of this class of dopamine receptors modulates also the gamma-aminobutyric acid receptors (GABAA) of the mitral/tufted cells, facilitating GABAergic neurotransmission from TH-ir periglomerular neurons57. This fact should lead to an increase in the inhibition of the mitral/tufted cells57. Furthermore, when the pre-synaptic D2 receptors is selectively blocked, the release of glutamate is increased, thus producing an increase in DA and GABA levels through higher activation of dopaminergic juxtaglomerular neurons7,58.
CONCLUSIONS
In conclusion, the present data provide novel evidence concerning the participation of the dopaminergic system mainly in the olfactory discrimination of a non-social stimulus, that is, the main olfactory pathway. Rotenone promoted a remarkable olfactory impairment in both types of odors with a notable modulation induced by 24 h of REMSD of the later.
The statistical correlations strongly suggest the occurrence of an association between the density of nigral TH-ir neurons and the olfactory discrimination capacity for both odorant stimuli. Specifically, the occurrences of manipulations that decrease these neurons tend to elicit reductions in the olfactory discrimination ability.
The DA modulation may have important roles in synaptic plasticity in the bulb, since there is a direct projection from the SNpc to the olfactory bulb32 and also DA levels are increased during the odor learning process59. This phenomenon also affects other areas such as the striatum and SNpc. Therefore, these changes may have potential impact in the preclinical abnormalities found in PD patients.
Acknowledgements
This paper was supported by CAPES and the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq - Brasil Grants Casadinho/Procad # 552226/2011-4 and Universal # 473861/2012-7 to MMSL. MMSL is recipient of CNPq fellowship.
Footnotes
Conflict of interests
The authors have declared that no conflict of interests exists.
REFERENCES
- 1.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998 Oct 08;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339(16):1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 3.Lima MM, Andersen ML, Reksidler AB, Vital MA, Tufik S. The role of the substantia nigra pars compacta in regulating sleep patterns in rats. PloS One. 2007;2(6):e513. doi: 10.1371/journal.pone.0000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, et al. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Experimental Neurol. 2009;219(2):574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Lima MM, Andersen ML, Reksidler AB, Silva A, Zager A, Zanata SM, et al. Blockage of dopaminergic D(2) receptors produces decrease of REM but not of slow wave sleep in rats after REM sleep deprivation. Behav Brain Res. 2008;188(2):406–411. doi: 10.1016/j.bbr.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26(41):10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 8.Siderowf A, Jennings D, Eberly S, Oakes D, Hawkins KA, Ascherio A, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27(93):406–412. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, et al. Loss of olfaction in de novo and treated Parkinson's disease: possible implications for early diagnosis. Mov Disord. 2001;16(1):41–46. doi: 10.1002/1531-8257(200101)16:1<41::aid-mds1017>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004;56(2):173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 11.Saifee T, Lees AJ, Silveira-Moriyama L. Olfactory function in Parkinson's disease in ON versus OFF states. J Neurol Neurosurg Psychiatry. 2010;81(11):1293–1295. doi: 10.1136/jnnp.2009.182022. [DOI] [PubMed] [Google Scholar]
- 12.Ansari KA, Johnson A. Olfactory function in patients with Parkinson's disease. J Chronic Dis. 1975;28(9):493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 13.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 14.Double KL, Rowe DB, Hayes M, Chan DK, Blackie J, Corbett A, et al. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol. 2003;60(4):545–549. doi: 10.1001/archneur.60.4.545. [DOI] [PubMed] [Google Scholar]
- 15.Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg LM, Allen TA, Ly D, Fortin NJ. Recognition memory for social and non-social odors: differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem. 2012;97(1):7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Greiner RS, Moriguchi T, Slotnick BM, Hutton A, Salem N. Olfactory discrimination deficits in n-3 fatty acid-deficient rats. Physiol Behav. 2001;72(3):379–385. doi: 10.1016/s0031-9384(00)00437-6. [DOI] [PubMed] [Google Scholar]
- 18.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26(37):9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantini ML, Postuma RB, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull. 2006;70(4-6):386–390. doi: 10.1016/j.brainresbull.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Killgore WD, McBride SA. Odor identification accuracy declines following 24 h of sleep deprivation. J Sleep Res. 2006;15(2):111–116. doi: 10.1111/j.1365-2869.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 21.Killgore WD, Killgore DB, Grugle NL, Balkin TJ. Odor identification ability predicts executive function deficits following sleep deprivation. Int J Neurosci. 2010;120(5):328–334. doi: 10.3109/00207450903389396. [DOI] [PubMed] [Google Scholar]
- 22.Killgore WD, Killgore DB, McBride SA, Kaminori GH, Balkin TJ. Odor identification ability predicts changes in symptoms of psychopathology following 56 h of sleep deprivation. J Sens Stud. 2008;23(1):35–51. [Google Scholar]
- 23.Wong KK, Muller ML, Kuwabara H, Studenski SA, Bohnen NI. Olfactory loss and nigrostriatal dopaminergic denervation in the elderly. Neurosci Lett. 2010;484(3):163–167. doi: 10.1016/j.neulet.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Mundiñano IC, Caballero MC, Ordóñez C, Hernandez M, DiCaudo C, Marcilla I, et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122(1):61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- 25.Escanilla O, Yuhas C, Marzan D, Linster C. Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav Neurosci. 2009;123(4):828–833. doi: 10.1037/a0015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borgmann-Winter KE, Wang HY, Ray R, Willis BR, Moberg PJ, Rawson NE, et al. Altered G Protein Coupling in Olfactory Neuroepithelial Cells From Patients With Schizophrenia. Schizophr Bull. 2016;42(2):377–385. doi: 10.1093/schbul/sbv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson's disease. Mov Disord. 2004;19(6):687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 28.Doty RL, Risser JM. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology (Berl) 1989;98(3):310–315. doi: 10.1007/BF00451680. [DOI] [PubMed] [Google Scholar]
- 29.Koster NL, Norman AB, Richtand NM, Nickell WT, Puche AC, Pixley SK, et al. Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol. 1999;411(4):666–673. doi: 10.1002/(sici)1096-9861(19990906)411:4<666::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, et al. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;67(11):1072–1083. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues LS, Targa AD, Noseda AC, Aurich MF, Da Cunha C, Lima MM. Olfactory impairment in the rotenone model of Parkinson's disease is associated with bulbar dopaminergic D2 activity after REM sleep deprivation. Front Cell Neurosci. 2014;8:383–383. doi: 10.3389/fncel.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Höglinger GU, Alvarez-Fischer D, Arias-Carrión O, Djufri M, Windolph A, Keber U, et al. A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015;130(3):333–348. doi: 10.1007/s00401-015-1451-y. [DOI] [PubMed] [Google Scholar]
- 33.Alam M, Schmidt WJ. L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res. 2004;153(2):439–446. doi: 10.1016/j.bbr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, et al. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23(34):10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dos Santos AC, Castro MA, Jose EA, Delattre AM, Dombrowski PA, Da Cunha C, et al. REM sleep deprivation generates cognitive and neurochemical disruptions in the intranigral rotenone model of Parkinson's disease. J Neurosci Res. 2013;91(11):1508–1516. doi: 10.1002/jnr.23258. [DOI] [PubMed] [Google Scholar]
- 36.Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensitivity of dopaminergic receptors in the rat brain? Pharmacology. 1978;16(2):98–105. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- 37.Saravanan KS, Sindhu KM, Mohanakumar KP. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson's disease. Brain Res. 2005;1049(2):147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Moreira CG, Barbiero JK, Ariza D, Dombrowski PA, Sabioni P, Bortolanza M, et al. Behavioral, neurochemical and histological alterations promoted by bilateral intranigral rotenone administration: a new approach for an old neurotoxin. Neurotox Res. 2012;21(3):291–301. doi: 10.1007/s12640-011-9278-3. [DOI] [PubMed] [Google Scholar]
- 39.Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004(1-2):45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Soffié M, Lamberty Y. Scopolamine effects on juvenile conspecific recognition in rats: Possible interaction with olfactory sensitivity. Behav Processes. 1988;17(3):181–190. doi: 10.1016/0376-6357(88)90001-0. [DOI] [PubMed] [Google Scholar]
- 41.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26(6):957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res. 2005;159(2):197–205. doi: 10.1016/j.bbr.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Chioca LR, Antunes VD, Ferro MM, Losso EM, Andreatini R. Anosmia does not impair the anxiolytic-like effect of lavender essential oil inhalation in mice. Life Sci. 2013;92(20-21):971–975. doi: 10.1016/j.lfs.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego: Academic Press; 2005. [Google Scholar]
- 45.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 46.Lima MM. Sleep disturbances in Parkinson's disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev. 2013;17(5):367–375. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology (Berl) 1981;72(3):257–260. doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- 48.Nunes GP, Júnior, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull. 1994;34(5):453–456. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 49.Borghammer P, Knudsen K, Østergaard K, Danielsen EH, Pavese N, Arveschoug A, et al. Combined DaT imaging and olfactory testing for differentiating parkinsonian disorders. Int J Clin Pract. 2014;68(11):1345–1351. doi: 10.1111/ijcp.12445. [DOI] [PubMed] [Google Scholar]
- 50.Hutter JA, Chapman CA. Exposure to cues associated with palatable food reward results in a dopamine D2 receptor-dependent suppression of evoked synaptic responses in the entorhinal cortex. Behav Brain Funct. 2013;9:37–37. doi: 10.1186/1744-9081-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devore S, Lee J, Linster C. Odor preferences shape discrimination learning in rats. Behav Neurosci. 2013;127(4):498–504. doi: 10.1037/a0033329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hackett C, Choi C, O'Brien B, Shin P, Linster C. Odor Memory and Discrimination Covary as a Function of Delay between Encoding and Recall in Rats. Chem Senses. 2015;40(5):315–323. doi: 10.1093/chemse/bjv013. [DOI] [PubMed] [Google Scholar]
- 53.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434(7032):470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Marcos A. On the organization of olfactory and vomeronasal cortices. Progr Neurobiol. 2009;87(1):21–30. doi: 10.1016/j.pneurobio.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Killgore WD, McBride SA, Killgore DB, Balkin TJ, Kamimori GH. Baseline odor identification ability predicts degradation of psychomotor vigilance during 77 hours of sleep deprivation. Int J Neurosci. 2008;118(9):1207–1225. doi: 10.1080/00207450801941368. [DOI] [PubMed] [Google Scholar]
- 56.Lima MM, Andersen ML, Reksidler AB, Ferraz AC, Vital MA, Tufik S. Paradoxical sleep deprivation modulates tyrosine hydroxylase expression in the nigrostriatal pathway and attenuates motor deficits induced by dopaminergic depletion. CNS Neurol Disord Drug Targets. 2012;11(4):359–368. doi: 10.2174/187152712800792839. [DOI] [PubMed] [Google Scholar]
- 57.Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006;172(1):97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 58.O'Connor S, Jacob TJ. Neuropharmacology of the olfactory bulb. Curr Mol Pharmacol. 2008;1(3):181–190. doi: 10.2174/1874467210801030181. [DOI] [PubMed] [Google Scholar]
- 59.Coopersmith R, Weihmuller FB, Kirstein CL, Marshall JF, Leon M. Extracellular dopamine increases in the neonatal olfactory bulb during odor preference training. Brain Res. 1991;564(1):149–153. doi: 10.1016/0006-8993(91)91365-8. [DOI] [PubMed] [Google Scholar]