Abstract
Background: Lipid metabolites may partially explain the inverse association between the Mediterranean diet (MedDiet) and cardiovascular disease (CVD).
Objective: We evaluated the associations between 1) lipid species and the risk of CVD (myocardial infarction, stroke, or cardiovascular death); 2) a MedDiet intervention [supplemented with extra virgin olive oil (EVOO) or nuts] and 1-y changes in these molecules; and 3) 1-y changes in lipid species and subsequent CVD.
Design: With the use of a case-cohort design, we profiled 202 lipid species at baseline and after 1 y of intervention in the PREDIMED (PREvención con DIeta MEDiterránea) trial in 983 participants [230 cases and a random subcohort of 790 participants (37 overlapping cases)].
Results: Baseline concentrations of cholesterol esters (CEs) were inversely associated with CVD. A shorter chain length and higher saturation of some lipids were directly associated with CVD. After adjusting for multiple testing, direct associations remained significant for 20 lipids, and inverse associations remained significant for 6 lipids. When lipid species were weighted by the number of carbon atoms and double bonds, the strongest inverse association was found for CEs [HR: 0.39 (95% CI: 0.22, 0.68)] between extreme quintiles (P-trend = 0.002). Participants in the MedDiet + EVOO and MedDiet + nut groups experienced significant (P < 0.05) 1-y changes in 20 and 17 lipids, respectively, compared with the control group. Of these changes, only those in CE(20:3) in the MedDiet + nuts group remained significant after correcting for multiple testing. None of the 1-y changes was significantly associated with CVD risk after correcting for multiple comparisons.
Conclusions: Although the MedDiet interventions induced some significant 1-y changes in the lipidome, they were not significantly associated with subsequent CVD risk. Lipid metabolites with a longer acyl chain and higher number of double bonds at baseline were significantly and inversely associated with the risk of CVD.
Keywords: Mediterranean diet, PREDIMED, lipids, metabolomics, randomized trial, cardiovascular disease
See corresponding article on page 965.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide, and the number of deaths worldwide attributable to CVD is expected to rise from 16.7 million in 2002 to 23.3 million in 2030 (1). Thus, preventive strategies to tackle this epidemic are of paramount importance.
For decades (2), the Mediterranean diet (MedDiet) has been linked to a reduced risk of CVD. The PREDIMED (PREvención con DIeta MEDiterránea) trial, a large randomized trial designed to assess the effect of a MedDiet supplemented either with extra virgin olive oil (EVOO) or mixed nuts compared with a control group (advised to follow a low-fat diet), found a 30% relative reduction compared with the control group in the risk of CVD for both intervention groups (3).
Several mechanisms have been suggested to underlie the observed benefits of the MedDiet on CVD. The MedDiet decreases inflammatory markers beyond other control diets according to a meta-analysis of randomized trials (4) and increases nitric oxide production (5). In addition, the MedDiet has been suggested to reduce LDL oxidation, decrease LDL atherogenicity, and improve several characteristics of HDL particles (6–8). It further exerts beneficial transcriptomic effects on inflammation and foam cell formation-related genes (4, 9). However, the biological mechanisms underpinning the beneficial effects of the MedDiet for the primary prevention of CVD are not yet fully understood. Recent advances in metabolomic techniques allow for a high-throughput assessment of several small-molecule metabolites involved in different biological pathways, offering a metabolic profile related to biological status (10, 11).
PREDIMED showed a beneficial effect of a holistic dietary pattern on the primary prevention of CVD. This dietary pattern is characterized by a high consumption of healthy fats that stem largely from EVOO and tree nuts. This high fat content may influence plasma lipid profiles. Therefore, PREDIMED offers a unique opportunity for assessing the association between the plasma lipidomic profile (or its changes during the nutritional intervention) and subsequent CVD. In this study, we aimed to 1) describe the association of the baseline lipid metabolic profile of the PREDIMED participants with the risk of CVD, 2) assess the effect of the dietary intervention of the PREDIMED trial on lipid metabolite changes, and 3) depict the association between 1-y changes in the lipidomic profile and subsequent risk of CVD.
METHODS
This study was conducted within PREDIMED (3, 12). Briefly, PREDIMED was a multicenter randomized controlled field trial designed to assess the effect of the MedDiet on the primary prevention of CVD. Participants were recruited from 2003 to 2009, and the cohort included a total of 7447 men and women aged 55–80 y and 60–80 y, respectively, with no prior CVD but at high CVD risk because of the presence of either type 2 diabetes or ≥3 of the following classic CVD risk factors: current smoking, overweight or obesity, high LDL cholesterol, low HDL cholesterol, family history of early coronary artery disease, or hypertension.
Participants were randomly allocated in a 1:1:1 ratio to a MedDiet supplemented with EVOO, a MedDiet supplemented with nuts, or a control group (advised to follow a low-fat diet and to reduce all types of fat). Supplemental Table 1 shows the dietary recommendations to the participants in the different allocation arms. Participants in the 2 MedDiet groups received individual and group dietary educational sessions by a trained dietitian at the baseline visit and quarterly thereafter. Participants in the control group also received dietary education at the baseline visit. During the first 3 y of the trial, participants in the control group received an annual leaflet explaining the low-fat diet. The protocol was amended in October 2006. Thereafter, participants in the control group received a dietary intervention that promoted a low-fat diet with the same intensity and frequency as the 2 MedDiet groups.
Compliance was assessed by monitoring the adherence to the MedDiet annually with a 14-item MedDiet screener (13) and by measuring biomarkers of key foods in the MedDiet in random samples of participants, specifically EVOO (hydroxytyrosol in urine) and walnuts [α-linolenic acid (18:3n–3) in plasma] (3). The trial was stopped because of an early benefit on the basis of endpoints documented through 1 December 2010 after a median intervention time and follow-up of 4.8 y.
We used a case-cohort design for this study and included information from 794 randomly selected participants (∼10% of the original study sample) at baseline and all 233 incident cases of CVD with available plasma samples (55 of the total 288 incident CVD cases in PREDIMED did not have available plasma samples). We excluded 5 participants because of unavailable lipid information and 2 participants in the initial quality check. Thus, we included 983 participants in our analysis: 230 incident CVD cases and 790 participants in the subcohort (including 37 overlapping cases). Furthermore, 907 participants (777 participants in the subcohort and 160 cases, including 30 overlapping cases) had available plasma samples after 1 y of intervention and were included in the lipidomic change analyses. At baseline, participants completed an extensive questionnaire that included information on sociodemographic characteristics and medical conditions, the Minnesota Leisure-Time Physical Activity Questionnaire (14), and a 14-item screener on their adherence to the traditional MedDiet (13).
The institutional review boards of all the recruitment centers approved the overall PREDIMED design, and the institutional review boards of the University of Navarra, the Broad Institute of MIT and Harvard, and the Harvard T.H. Chan School of Public Health approved the case-cohort subproject. All participants gave written informed consent.
Participants underwent a blood withdrawal after an overnight fast by trained and certified nurses at baseline and after 1 y of intervention. Samples were processed by the study personnel according to the study protocol. EDTA plasma samples were coded and kept refrigerated until they were stored at −80°C in freezers. Randomly ordered paired samples (baseline and 1 y of follow-up) were selected and shipped on dry ice to the Broad Institute for metabolomics analysis.
Metabolomic analyses of plasma
Plasma polar and nonpolar lipids were profiled with the use of a Nexera ×2 U-HPLC system (Shimadzu Scientific Instruments) coupled to an Exactive Plus orbitrap mass spectrometer (Thermo Fisher Scientific). Lipids were extracted from plasma (10 μL) with the use of 190 μL isopropanol containing 1,2-didodecanoyl-sn-glycero-3-phosphocholine as an internal standard (Avanti Polar Lipids). After centrifugation (10 min; 9000 × g; ambient temperature), supernatants (10 μL) were injected directly onto a 100 × 2.1-mm ACQUITY BEH C8 column (1.7 μm) (Waters). The column was eluted isocratically at a flow rate of 450 μL/min for 1 min at 80% mobile-phase A [10 mmol ammonium acetate:methanol:acetic acid/L (95:5:0.1, by vol)], followed by a linear gradient to 80% mobile-phase B [methanol:acetic acid (99.9:0.1 vol:vol)] over 2 min, a linear gradient to 100% mobile-phase B over 7 min, and then 3 min at 100% mobile-phase B. Mass spectrometry analyses were carried out with the use of electrospray ionization in the positive-ion mode with the use of full-scan analysis over m/z 200–1100 at 70,000× resolution and a 3-Hz data acquisition rate. Additional mass spectrometry settings were as follows: ion spray voltage, 3.0 kV; capillary temperature, 300°C; probe heater temperature, 300°C; sheath gas, 50 arbitrary units; auxiliary gas, 15 arbitrary units; and S-lens radiofrequency level, 60%. Raw data were processed with the use of Progenesis QI version 1.0.5165.27075 (NonLinear Dynamics) for feature alignment, nontargeted signal detection, and signal integration. Targeted processing of a subset of lipids was conducted with the use of TraceFinder version 3.2 (Thermo Fisher Scientific). Lipids were denoted by headgroup and total acyl carbon and double-bond content. A full list of measured metabolites, Human Metabolome Database identification numbers, and m/z values is shown in Supplemental Table 2.
Endpoint ascertainment
The primary endpoint of PREDIMED was a composite outcome of nonfatal acute myocardial infarction, nonfatal stroke, and cardiovascular death. Doctors at each recruitment center, blinded with respect to the allocation group, reviewed all the participants’ medical charts annually to assess any incident CVD outcome. Other sources of information such as consultation of the National Death Index were used to ascertain incident cases (3). The anonymized information was then sent from the recruitment center to a blinded central event ascertainment committee that adjudicated the events.
Statistical analyses
Baseline characteristics of the participants in the subcohort and of the participants who experienced a primary outcome (cases) were described as means and SDs for quantitative traits and percentages for qualitative traits.
Baseline individual lipid values were normalized and scaled in multiples of 1 SD with Blom’s rank-based inverse normal transformation (15). Changes in the lipid values were calculated, and the resulting difference was normalized and scaled in multiples of 1 SD with Blom’s inverse normal transformation.
First, we calculated the correlation coefficients between all the individual lipid species. Second, in the subcohort, we estimated the effect of the intervention on the 1-y changes in the lipid values of the 2 intervention groups compared with the control group with multivariable linear regression models adjusted for the baseline lipid value. To correct for multiple testing, we used the procedure described by Benjamini and Yekutieli (16).
Third, we assessed the effect of the intervention (3 categories) on the changes after 1 y in lipid values with linear regression models with the use of the change in the lipid values as the dependent variable and with no constant to assess the lipid changes within each allocation arm among participants in the subcohort. These models were adjusted for the baseline lipid concentrations.
Fourth, we assessed whether each MedDiet intervention had an effect on the lipidomic profile of the participants after 1 y by assessing whether changes in the mean number of carbon atoms per acyl chain or the mean number of double bonds per acyl chain in each lipid species were associated with each of the 2 interventions compared with the control group. As a preliminary step, we ran linear regression models with 1-y changes in each lipid signals as dependent variables and the allocation group as the independent term and adjusted the model for baseline lipid concentrations. We obtained the β coefficients for both intervention groups and their corresponding SEs for each of the 202 assessed lipids. For lipids with ≥1 acyl chain, we attained the mean number of carbon atoms and double bonds per acyl chain. We then ran weighted linear regression models in which we assessed whether the mean number of carbon atoms or number of double bonds of the acyl chain were independent predictors for the β coefficients of the aforementioned regression models. As weights in this regression, we used the inverse of the variance of the β coefficient obtained in the aforementioned regression model. We presented the results graphically and separately for both intervention groups. We then ran the weighted linear regression models in which we assessed whether the mean number of carbon atoms or mean number of double bonds of the acyl chain were independent predictors for the β coefficients of the aforementioned regression models separately for those lipid species with information on ≥5 lipids. The obtained P values were penalized for multiple comparisons with the procedure described by Benjamini and Yekutieli (16) because we assessed 10 lipid families.
Fifth, we calculated HRs and 95% CIs for incident CVD per 1 SD in baseline individual lipid concentrations with weighted Cox regression models taking into account the case-cohort design with Barlow weights (17). We adjusted for age, sex, smoking status, BMI, family history of early coronary artery disease, physical activity, and educational status and stratified by allocation arm in all models. The HRs for individual lipids and their P values were grouped according to lipid species and plotted in a 2-dimensional graph defined by the number of carbon atoms (x axis) and the number of double bonds (y axis) in the acyl chain. Lipids with the same number of carbon atoms and double bonds were slightly pulled apart horizontally to visualize both results.
Sixth, because the number of carbon atoms and double bonds appeared to be relevant to CVD, for each lipid species we additionally calculated a weighted score for the baseline lipid value weighted by its number of carbon atoms and by the number of double bonds in the acyl chain. Because some lipids had no double bonds, we added a constant (constant = 1) to the number of double bonds:
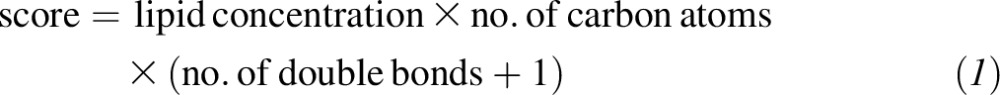 |
We calculated this score for lipid species for which ≥5 molecules were measured and assessed the association of the score for each lipid species with the risk of CVD with the use of weighted Cox models as stated previously and adjusted for age, sex, smoking status, BMI, family history of early coronary artery disease, physical activity, and educational status (17). As sensitivity analyses, we changed some assumptions 1) without adding 1 to the number of double bonds, 2) by using multiple imputation for missing values instead of the half of the lowest lipid-specific detected value, and 3) by adding the lipid metabolites without weighting.
Finally, we modeled the risk of CVD per 1 SD in the 1-y change in individual lipid concentrations with the use of weighted Cox regression models with the same adjustment as the previously described Cox regression models plus an additional adjustment for baseline lipid concentration (17). Again, HRs and their P values were grouped according to lipid species and plotted in a 2-dimensional graph defined by the number of carbon atoms (x axis) and the number of double bonds (y axis) in the acyl chain. Lipids with the same number of carbon atoms and double bonds were slightly pulled apart horizontally to visualize both results. In an additional analysis, we then corrected the associations between the individual baseline lipids (or their 1-y changes) and CVD for multiple testing with the use of the procedure described by Benjamini and Yekutieli (16). All analyses were performed with Stata version 13.1 (StataCorp).
RESULTS
For this analysis, we included 983 participants (230 cases and 790 participants in the subcohort, of whom 37 overlapped) (Supplemental Figure 1). Their baseline characteristics are displayed in Table 1. As expected, the participants included in the subcohort showed similar baseline characteristics and a similar risk to the entire cohort of participants included in PREDIMED. The distribution of the subcohort participants’ characteristics according to their allocation arm is shown in Supplemental Table 3.
TABLE 1.
Baseline participants’ characteristics in the random subcohort and cases1
| Characteristic | Entire PREDIMED cohort | Subcohort2 | Cases |
| n | 7447 | 790 | 230 |
| Women, % | 57.5 | 57.1 | 39.6 |
| Age, y | 67 ± 6 | 67 ± 6 | 70 ± 6 |
| Allocation arm, % | |||
| MedDiet + EVOO | 34.2 | 37.1 | 35.7 |
| MedDiet + nuts | 33.0 | 33.2 | 28.3 |
| Family history of early CAD, % | 22.4 | 24.9 | 19.1 |
| Smoking status, % | |||
| Former | 24.7 | 25.4 | 35.1 |
| Current | 14.1 | 12.3 | 20.0 |
| Dyslipidemia, % | 72.3 | 73.5 | 58.3 |
| Type 2 diabetes, % | 48.5 | 47.1 | 64.8 |
| Hypertension, % | 82.8 | 83.7 | 82.6 |
| Waist circumference, cm | 100 ± 11 | 100 ± 10 | 102 ± 11 |
| BMI, kg/m2 | 30.0 ± 3.8 | 29.8 ± 3.6 | 29.6 ± 3.7 |
| Leisure time physical activity, MET-min/d | 230 ± 239 | 258 ± 258 | 237 ± 238 |
| Education, % | |||
| ≥Secondary school | 22.2 | 23.5 | 19.6 |
| Total energy intake, kcal/d | 2275 ± 606 | 2334 ± 614 | 2366 ± 686 |
| Baseline adherence to the MedDiet3 | 9 ± 2 | 9 ± 2 | 8 ± 2 |
Data are means ± SDs unless otherwise stated. CAD, coronary artery disease; EVOO, extra virgin olive oil; MedDiet, Mediterranean diet; MET-min, metabolic equivalent minutes; PREDIMED, PREvención con DIeta MEDiterránea.
The randomly selected subcohort included 37 cases.
Based on the 14-item screener.
Correlations between the 202 individual lipid metabolites were strongest within each lipid species, but moderate-to-strong correlations (data not shown) were also observed for triacylglycerides with diacylglycerides, phosphatidylcholines, phosphatidylethanolamines, or phosphatidylcholine plasmalogens; phosphatidylcholines with phosphatidylserines or cholesterol esters (CEs); phosphatidylcholine plasmalogens with phosphatidylthanolamine plasmalogens and diacylglycerides; and lysophosphatylcholines with lysophosphatidylethanolamines or phosphatidylethanolamines.
Changes in lipid metabolites in the intervention groups
As shown in Table 2, participants in the MedDiet + EVOO or MedDiet + nuts group showed statistically significant reductions compared with the control group at 1 y in some lipids. Only changes in CE(20:3) in the MedDiet + nuts group compared with the control group remained statistically significant after correcting for multiple comparisons. The full list of 1-y changes in the lipid metabolite concentrations in both intervention groups compared with the control group is shown in Supplemental Table 4. The full list of 1-y changes in the lipid concentrations in each of the 3 arms of the trial is shown in Supplemental Table 5.
TABLE 2.
Lipid changes after 1 y of intervention in the 2 intervention groups of the PREDIMED trial compared with the control group among subcohort participants1
| MedDiet + EVOO (n = 293) compared with control (n = 235) |
MedDiet + nuts (n = 262) compared with control (n = 235) |
|||
| Lipid | Mean (95% CI) | P value | Mean (95% CI) | P value |
| Lysophosphatidylethanolamine (22:6) | 0.15 (0.00, 0.31) | 0.048 | — | — |
| Phosphatidylcholines | ||||
| 36:4b | −0.21 (−0.37, −0.04) | 0.014 | −0.19 (−0.36, −0.02) | 0.027 |
| 38:4 | −0.18 (−0.35, −0.02) | 0.027 | −0.18 (−0.35, −0.01) | 0.037 |
| 38:3 | — | — | −0.23 (−0.39, −0.07) | 0.004 |
| 38:2 | — | — | −0.17 (−0.33, −0.02) | 0.031 |
| 40:6 | — | — | −0.28 (−0.44, −0.12) | 0.001 |
| Phosphatidylcholine plasmalogens | ||||
| 34:3 | — | — | 0.18 (0.02, 0.34) | 0.032 |
| 34:2 | 0.20 (0.03, 0.36) | 0.021 | — | — |
| Phosphatidylethanolamines | ||||
| 34:0 | — | — | −0.17 (−0.33, −0.01) | 0.033 |
| 38:5 | −0.18 (−0.34, −0.02) | 0.027 | — | — |
| 38:4 | −0.18 (−0.34, −0.03) | 0.022 | — | — |
| Phosphatidylethanolamine plasmalogens | ||||
| 36:5 | −0.23 (−0.38, −0.08) | 0.003 | — | — |
| 36:1 | 0.18 (0.02, 0.34) | 0.031 | — | — |
| 38:5 | −0.18 (−0.34, −0.03) | 0.018 | — | — |
| Phosphatidylserine (40:6) | — | — | −0.23 (−0.39, −0.07) | 0.004 |
| Phosphatidylserine plasmalogen (36:3) | — | — | −0.18 (−0.35, −0.01) | 0.041 |
| Ceramide (24:1) | 0.25 (0.09, 0.41) | 0.002 | — | — |
| Sphingomyelines | ||||
| 18:1 | 0.18 (0.01, 0.34) | 0.038 | — | — |
| 18:0 | 0.18 (0.02, 0.34) | 0.032 | — | — |
| 24:1 | 0.24 (0.08, 0.40) | 0.003 | — | — |
| Cholesterol esters | ||||
| 14:0 | — | — | −0.19 (−0.35, −0.03) | 0.023 |
| 16:1 | −0.22 (−0.38, −0.05) | 0.010 | −0.27 (−0.44, −0.10) | 0.002 |
| 20:3 | — | — | −0.37 (−0.53, −0.21) | <0.0012 |
| Diacylglycerols | ||||
| 32:0 | −0.18 (−0.34, −0.02) | 0.028 | — | — |
| 36:4 | — | — | 0.23 (0.07, 0.39) | 0.006 |
| Triacylglycerols | ||||
| 42:0 | −0.16 (−0.31, −0.01) | 0.040 | — | — |
| 44:0 | −0.17 (−0.33, −0.01) | 0.033 | — | — |
| 46:0 | −0.18 (−0.34, −0.03) | 0.024 | — | — |
| 48:0 | −0.18 (−0.34, −0.02) | 0.025 | — | — |
| 50:0 | −0.16 (−0.32, −0.01) | 0.043 | — | — |
| 52:6 | — | — | 0.16 (0.01, 0.32) | 0.042 |
| 52:5 | — | — | 0.20 (0.05, 0.36) | 0.011 |
| 52:4 | — | — | 0.19 (0.03, 0.35) | 0.020 |
| 54:5 | — | — | 0.19 (0.03, 0.35) | 0.019 |
All data were obtained from multivariable linear regression models and adjusted for baseline lipid values, with 1-y changes in the lipid values as the dependent variable and the allocation groups as independent terms. The data represent liquid chromatography-mass spectrometry peak areas that were transformed by Blom’s inverse normal transformation. P values were unadjusted for multiple comparisons. EVOO, extra virgin olive oil; MedDiet, Mediterranean diet; PREDIMED, PREvención con DIeta MEDiterránea.
This difference remained statistically significant after adjusting for multiple comparisons.
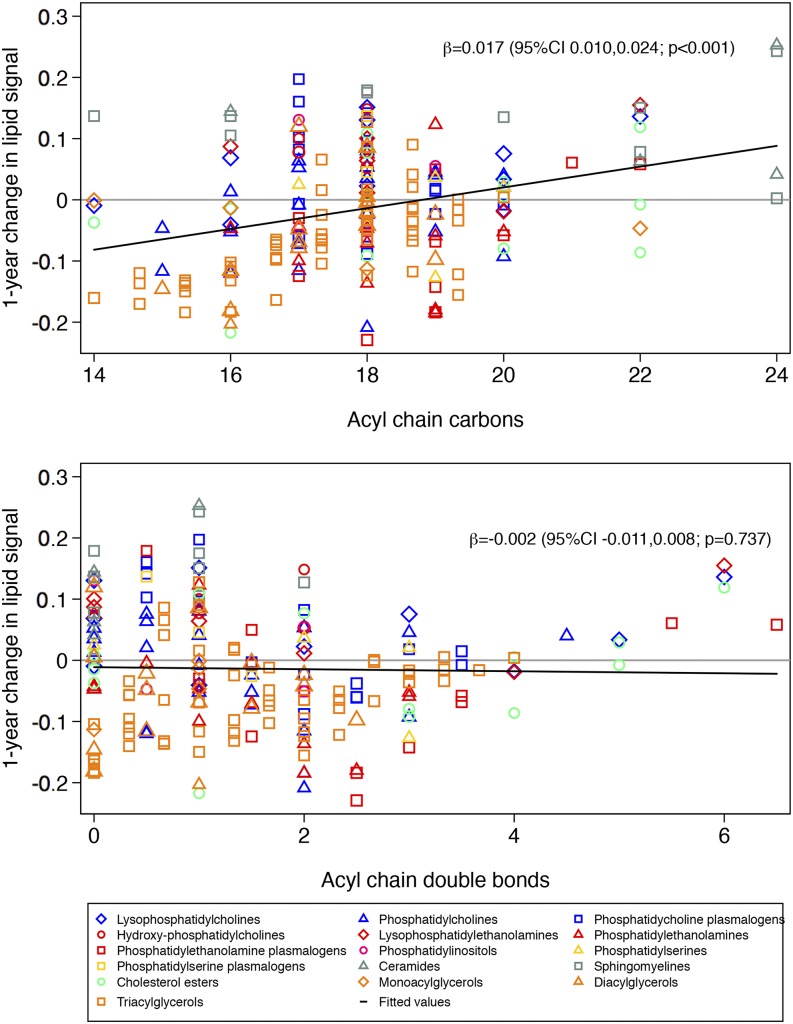
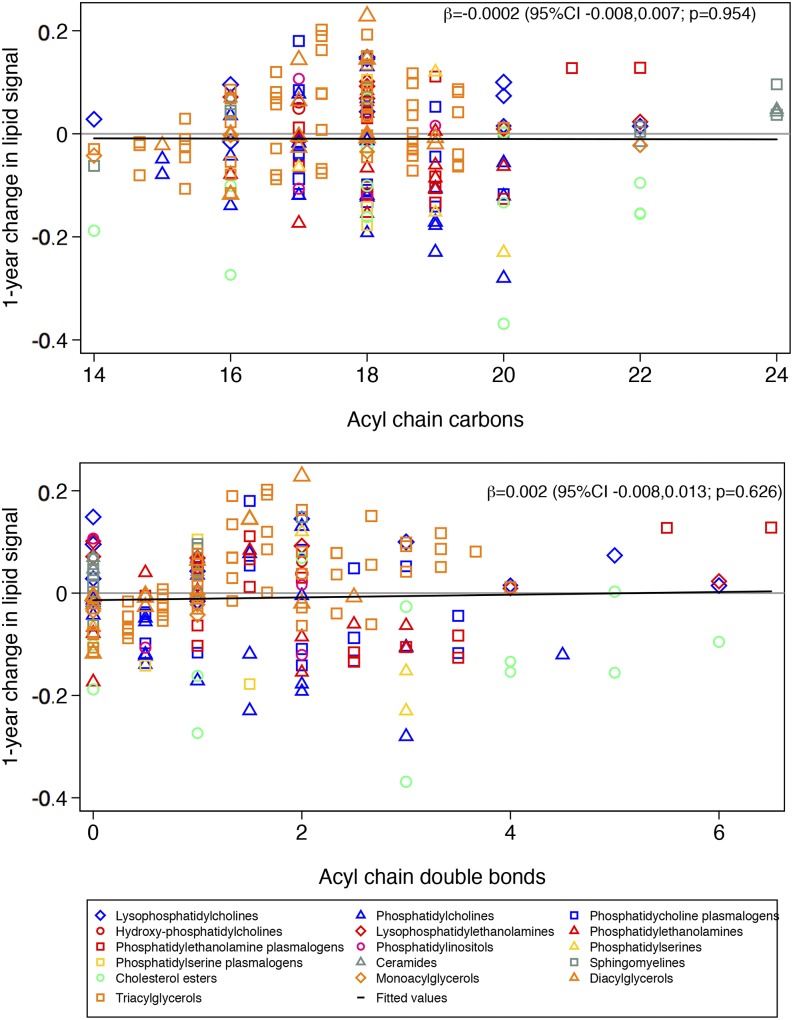
When we considered the lipidome as a whole, there was not a clear pattern in changes in lipid signals according to acyl chain length and the number of double bonds. The change in the lipid signal was significantly higher with a higher mean acyl chain carbon number in the MedDiet + EVOO group than the control group (Figure 1). This means that lipids with a longer mean acyl chain carbon number showed greater increases than lipids with a shorter mean acyl chain carbon number in the MedDiet + EVOO group compared with the control group. In contrast, no significant changes in the lipid signal were observed in the MedDiet + nuts group compared with the control group (Figure 2) for mean acyl chain length or saturation. When we repeated the analyses separately for 10 lipid species and after accounting for multiple comparisons, the only associations that remained significant were a greater increase in triacylglycerols with longer acyl chains in the MedDiet + EVOO group than in the control group and a greater increase in triacylglycerols with more double bonds in the MedDiet + nuts group than in the control group (Supplemental Figures 2–21).
FIGURE 1.
One-year changes in each lipid signal according to lipid carbon number and double-bond content for participants in the Mediterranean diet group supplemented with extra virgin olive oil (n = 293) compared with the control group (n = 235). Results from linear regression models were weighted by the inverse of the variance.
FIGURE 2.
One-year changes in each lipid signal according to lipid carbon number and double-bond content for participants in the Mediterranean diet group supplemented with nuts (n = 262) compared with the control group (n = 235). Results from linear regression models were weighted by the inverse of the variance.
Risk of CVD by baseline lipid metabolite by acyl chain carbon number and double-bond number
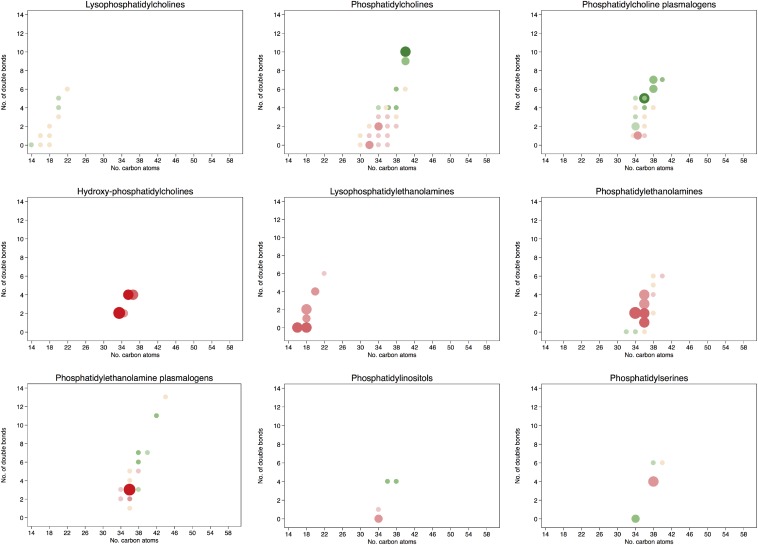
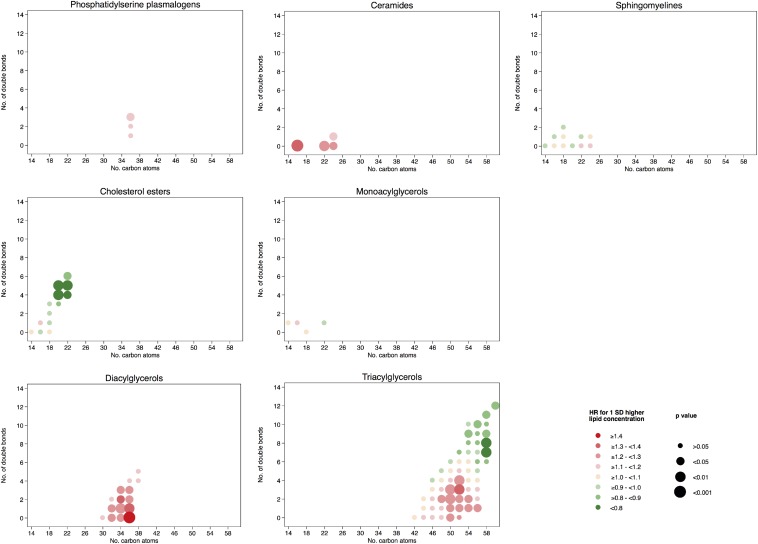
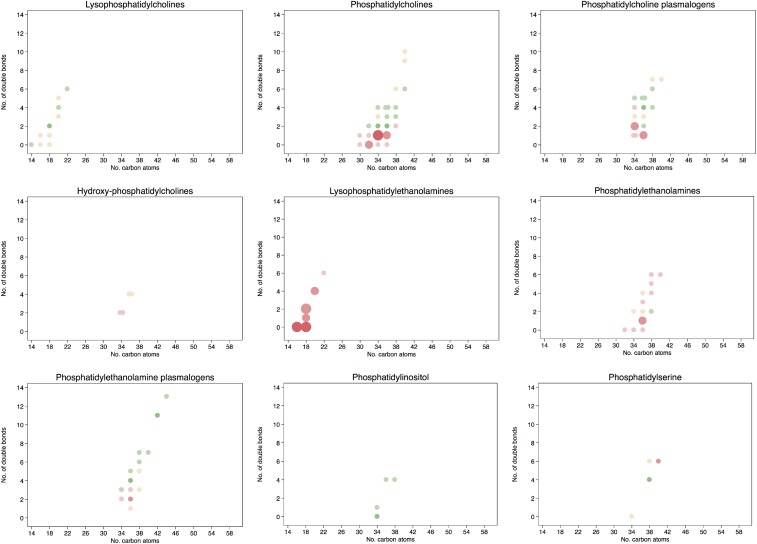
The associations between individual baseline lipid concentrations and the risk of CVD are shown graphically in Figure 3 according to the number of carbon atoms in the acyl chain and to the number of double bonds. All specific comparisons can be found in Supplemental Table 6. A higher risk of CVD for those lipids with a lower number of carbon atoms in the acyl chain or with fewer double bonds was observed for phosphatidylcholines, phosphatidylethanolamine plasmalogens, lysophosphatidylehtanolamines, phosphatidylinositols, diacylglycerols, and triacylglycerols. On the other hand, a lower risk of CVD was found for those lipids with an increasing number of carbon atoms and more double bonds, including phosphatidylcholines, phosphatidylcholine plasmalogens, CEs, and triacylglycerols. Baseline concentrations of CEs with 20 or 22 carbon atoms in their acyl chain and 4, 5, or 6 double bonds showed inverse associations with CVD. On the contrary, higher baseline concentrations of hydroxyphosphatidylcholines, ceramides and, to a lesser extent, phosphatidylethanolamines with a medium-to-long acyl chain and phoshpatidylserine plasmalogens were associated with a higher risk of CVD.
FIGURE 3.
HRs for 1-SD higher baseline lipid concentration and the composite cardiovascular disease endpoint. Lipid species were inverse normally transformed, and HRs were calculated from weighted Cox models adjusted for age, sex, smoking status, BMI, family history of early coronary artery disease, physical activity, and educational status and stratified by the allocation arm in the trial (17).
After adjusting for multiple testing, the associations remained statistically significant for the phosphatidylethanolamines 34:2, 36:2, and 36:1; lysophosphatidylethanolamines 16:0, 18:2, and 18:0; phosphatidylethanolamine plasmalogen 36:3; phosphatidylserine 38:4; ceramides 16:0 and 22:0; hydroxyphosphatidylcholines [M + Na+] 34:2 and 36:4; hydroxyphosphatidylcholine 36:4; diacylglycerols 34:2, 34:1, 36:1, and 36:0; and triacylglycerols 50:3, 50:2, and 52:3. All were directly associated with the risk of CVD; however, after adjusting for multiple testing, an inverse significant association was observed for the phosphatidylcholine 40:10, phosphatidylcholine plasmalogen 36:5a; CE(20:5), CE(20:4), and CE(22:5); and triacylglycerol 58:8.
Risk of CVD by baseline lipid species weighted by acyl chain carbon number and double-bond number
To test our hypotheses that the length of the acyl chain and number of double bonds were key elements that accounted for the association between lipid species and CVD, we weighted the metabolites by these 2 parameters. Results of the associations between the baseline concentrations of the different lipid species—with individual lipids weighted according to their number of double bonds and to the number of carbon atoms in each lipid species—and the future risk of CVD are displayed in Supplemental Table 7. Higher (weighted) concentrations of lysophosphatidylethanolamines [HR: 2.43 (95% CI: 1.41, 4.19); P-trend = 0.003] and diacylglycerols [HR: 1.71 (95% CI: 1.02, 2.87); P-trend = 0.016] were significantly associated with a higher risk of CVD. Contrarily, higher concentrations of CEs [HR: 0.39 (95% CI: 0.22, 0.68); P-trend = 0.002] weighted by their number of double bonds and carbon atoms were significantly associated with a lower risk of CVD. When we changed the definition of the score with the use of different assumptions, the only noticeable changes were that the linear trend for the association between baseline phosphatidylethanolamines and CVD became statistically significant [HR: 1.62 (95% CI: 0.98, 2.67); P-trend = 0.030] and that the association of the CE score was attenuated [HR: 0.65 (95% CI: 0.37, 1.13); P-trend = 0.041], both with the unweighted approach.
Risk of CVD by changes in lipid metabolite by acyl chain carbon number and double-bond number
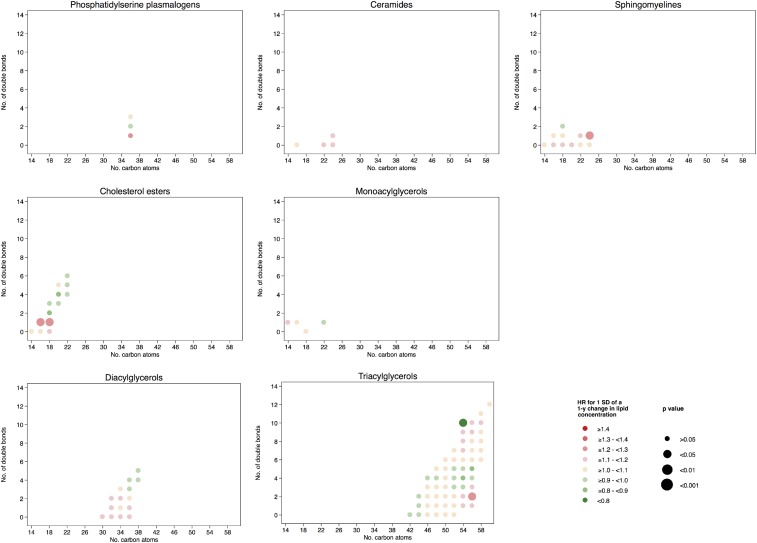
Figure 4 shows the associations between 1-y changes (per 1 SD) in individual lipid concentrations and the risk of CVD according to the number of carbon atoms in the acyl chain and to the number of double bonds. The specific estimates of the HRs for all comparisons can be found in Supplemental Table 8. A higher risk of CVD for those lipids with fewer carbon atoms in the acyl chain and fewer double bonds were observed for phosphatydylcholines, phosphatidylcholine plasmalogens, and CEs. However, these associations were of smaller magnitude and mainly absent for changes in these metabolite values as opposed to the associations observed with their baseline concentrations. In addition, after adjusting for multiple testing, none of these associations remained statistically significant. Therefore, we did not further assess associations of 1-y changes in lipids with CVD according to lipid species or build weighted scores for these changes.
FIGURE 4.
HRs for 1 SD of a 1-y change in lipid concentration and the composite cardiovascular disease endpoint. Lipid species were inverse normally transformed, and HRs were calculated from weighted Cox models adjusted for age, sex, smoking status, BMI, family history of early coronary artery disease, physical activity, educational status, and baseline lipid concentration and stratified by the allocation arm in the trial (17).
DISCUSSION
To our knowledge, this is the first study to assess the association between lipid metabolomics data and the subsequent risk of CVD in the context of a dietary intervention trial. These data suggest that 1) the baseline lipid metabolic profile was associated with the risk of CVD in PREDIMED; 2) the intervention, which assessed the effect of the MedDiet supplemented either with EVOO or nuts, induced some modest changes in the lipid profile of the participants, especially for MedDiet + nuts; and 3) 1-y changes in the metabolic profile were not statistically associated with the subsequent risk of CVD.
We observed that baseline triacylglycerols, phosphatidylcholines, and lysophosphatidylethanolamines were differentially associated with CVD depending on the structure of the acyl chains such that the shorter the chain and the higher the saturation in the chain, the higher the risk of CVD. This pattern is consistent with other studies. Stegemann et al. (18) observed that triacylglycerols with a low carbon number and a lower number of double bonds were predictive of CVD. Analogously, this triacylglycerol pattern was previously associated with a larger BMI, waist circumference, and HOMA-IR in a cross-sectional study in the Framingham Offspring Cohort (19). This same pattern was observed for triacylglycerol and phosphatidylcholine associations with the risk of diabetes in the Framingham Heart Study (20). The detrimental effect of saturation in triacylglycerols may be attributable to the higher atherogenic potential of saturated fats.
For CEs, we observed that the longer and the more unsaturated the acyl chain, the lower the risk of CVD. This finding is consistent with a reported lower diabetes risk, although this latter association disappeared after multivariable adjustment (20).
The observation that the longer and more unsaturated the acyl chain in phosphatidylcholines, the lower the associated CVD risk, is also consistent with the inverse association of carbon number and double-bond content and the risk of diabetes observed by Rhee et al. (20). On the other hand, in the Bruneck study, no clear association was observed for phosphatidylcholines and CVD (18). Phosphatidylcholines are the most abundant membrane lipids in mammals (21). If phosphatidylcholines with shorter chains and more highly saturated acyl chains are available, this could confer less fluidity to the cell membranes. SFA intake is known to be associated with adverse blood lipid profiles (increased LDL) and the induction of insulin resistance (22). Therefore, it is likely that a lower number of double bonds in plasma lipids may be associated with a higher risk of CVD events. On the other hand, long-chain fatty acids with many double bonds (i.e., long-chain PUFAs), mainly ω-3 PUFAs, are associated with reduced triglyceride concentrations, reduced myocardial oxygen demands, and beneficial changes in endothelial function (23–25). These physiologic benefits, together with a reduced heart rate (and lower heart rate variability) and decreased blood pressure observed in association with long-chain ω-3 fatty acids, support the biologic plausibility for a protective effect of long-chain PUFAs on cardiovascular clinical events. In addition, in substitution models, the intake of SFAs with a lower number of carbon atoms (16:0) are known to exert more detrimental effects on lipids and CVD than those with a higher number of carbon atoms (18:0) (26). Long-chain PUFAs are also precursors to bioactive lipid metabolites, including specialized proresolving mediators and cytochrome P450–generated monoepoxides (27, 28). Long-chain PUFAs are also assumed to have membrane-stabilizing actions in the context of ischemia-induced ventricular fibrillation (29).
We also observed that higher baseline concentrations of diacylglycerols, ceramides, hydroxyphosphatidylcholines, and medium-chain phosphatidylethanolamines were associated with an increased risk of CVD. A specific analysis on the association between ceramides and CVD has been previously reported (30). Diacylglycerols that showed stronger associations with the risk of CVD were consistent with the increasing saturation as those triacylglycerols that also showed a direct association with the risk of CVD. On the other hand, sphingolipids such as ceramides have been associated with insulin resistance and vascular dysfunction, thus suggesting a potential mechanism to explain their association with CVD (31). In addition, hydroxyphosphatidylcholines can be formed as adducts under conditions of oxidative stress and inflammation, or both, and may be components of oxidized LDL, thus increasing the risk of CVD. Finally, Stegemann et al. (18) observed a higher risk of CVD for the ratio of phosphatidylethanolamines to phosphatidylcholines, which is consistent with our finding of a higher risk with higher concentrations of phosphatidylethanolamines.
It could be thought that our results mainly support an inverse association of long-chain PUFAs, primarily of the ω-3 type, with CVD, and this is already well known. However, the omic approach in conjunction with a randomized intervention trial adds novel findings because 1) we were able to assess the role of lipids jointly classified according to these 2 features, namely the length of the acyl chain and number of double bonds; 2) we assessed these associations not only for baseline lipids but also for their changes after 1 y of dietary intervention with the use of repeated measurements of the lipidome; and 3) we provide a systematic analysis of the effect of the dietary intervention on the whole lipidome. To our knowledge, these 3 approaches to assess the role of the lipidome on the risk of CVD have not been reported previously.
We did not find an association between induced changes in the lipidome by the intervention conducted in PREDIMED and subsequent CVD. These results suggest that the effect of the intervention may be caused by other factors (e.g., bioactive polyphenols with anti-inflammatory properties) (32).
Our study has several strengths. First, this study was built on a successful dietary intervention trial that demonstrated beneficial effects of MedDiet interventions for the primary prevention of CVD. Second, we conducted repeated measurements of lipid metabolites at baseline and 1 y of intervention, enabling us to examine the effects of the interventions on changes in lipids.
We also acknowledge some limitations of this study. First, although we observed changes in the lipidome between intervention arms, we did not see appreciable changes within each intervention arm after 1 y of intervention, although the lipid composition of the intervention diets differed. Several studies have observed short-term changes in the metabolome induced by a dietary intervention, but these changes were nevertheless toned down after 6 mo of follow-up (33, 34). Second, PREDIMED mostly consisted of European whites, and ∼50% had diabetes, which may limit the generalizability of our results. Third, we observed mainly nonsignificant changes in metabolite concentrations induced by the intervention and no associations between changes in the metabolite concentrations and future risk of CVD. It is possible that 1-y changes in metabolites were too small to detect subtle mediational effects. It is also possible that mechanisms other than lipidomic changes may have accounted for the observed benefit on clinical hard events. Fourth, some of our analyses were based on observational data. Despite having adjusted for the main potential confounders, residual confounding may still have played a role in the observed associations. Fifth, the specific benefit of the MedDiet might have been confounded because during the first period of the trial the comparator group received less attention than during the second period. However, the diet for the control group was precisely defined from the very beginning of the trial, and we did not change the definition or the targets of the control group—we only modified the intensity and frequency of contacts with the dietitians.
In conclusion, the MedDiet interventions induced some significant 1-y changes in the lipidome, but 1-y changes were not significantly associated with subsequent CVD risk. Our study suggests that some lipid classes may be related to the risk of CVD. The effect may be different in the different lipid species, although for several lipid species we observed that those lipids with a shorter and more saturated acyl chain were more strongly associated with the risk of CVD. On the contrary, lipid metabolites with a longer acyl chain and a higher number of double bonds were inversely associated with the risk of CVD.
Acknowledgments
We thank Amy Deik for her contributions in acquiring, processing, and performing the quality check for all lipid-profiling data in this manuscript.
The authors’ responsibilities were as follows—ET, FBH, and MAM-G: designed the research; ET, MR-C, CBC, and CR: conducted the research; DC, EG-G, M Fiol, RE, ER, JL, M Fito, FA, LS-M, JS-S, and MAM-G: coordinated the recruitment of the subjects; ET, DDW, MR-C, YZ, LL, and MAM-G: analyzed the data and performed the statistical analysis; ET: wrote the manuscript; FBH and MAM-G: had primary responsibility for the final content; and all authors: read and approved the final manuscript. ER and FBH received grants from the California Walnut Commission. JS-S and MAM-G received grants from the International Nut Council. The remaining authors reported no conflict of interest related to the study.
Footnotes
Abbreviations used: CE, cholesterol ester; CVD, cardiovascular disease; EVOO, extra virgin olive oil; MedDiet, Mediterranean diet; PREDIMED, PREvención con DIeta MEDiterránea.
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol 1986;124:903–15. [DOI] [PubMed] [Google Scholar]
- 3.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 4.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- 5.Medina-Remón A, Tresserra-Rimbau A, Pons A, Tur JA, Martorell M, Ros E, Buil-Cosiales P, Sacanella E, Covas MI, Corella D, et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis 2015;25:60–7. [DOI] [PubMed] [Google Scholar]
- 6.Fitó M, Guxens M, Corella D, Sáez G, Estruch R, de la Torre R, Francés F, Cabezas C, López-Sabater Mdel C, Marrugat J, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–203. [DOI] [PubMed] [Google Scholar]
- 7.Hernáez Á, Castañer O, Elosua R, Pintó X, Estruch R, Salas-Salvadó J, Corella D, Arós F, Serra-Majem L, Fiol M, et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: a randomized controlled trial. Circulation 2017;135:633–43. [DOI] [PubMed] [Google Scholar]
- 8.Hernáez Á, Castañer O, Goday A, Ros E, Pintó X, Estruch R, Salas-Salvadó J, Corella D, Arós F, Serra-Majem L, et al. The Mediterranean diet decreases LDL atherogenicity in high cardiovascular risk individuals: a randomized controlled trial. Mol Nutr Food Res 2017. Apr 3 (Epub ahead of print; DOI: 10.1002/mnfr.201601015). [DOI] [PubMed] [Google Scholar]
- 9.Llorente-Cortés V, Estruch R, Mena MP, Ros E, Martinez-González MA, Fitó M, Lamuela-Raventós RM, Badimon L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010;208:442–50. [DOI] [PubMed] [Google Scholar]
- 10.Mundra PA, Shaw JE, Meikle PJ. Lipidomic analyses in epidemiology. Int J Epidemiol 2016;45:1329–38. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-González MÁ, Ruiz-Canela M, Hruby A, Liang L, Trichopoulou A, Hu FB. Intervention trials with the Mediterranean diet in cardiovascular prevention: understanding potential mechanisms through metabolomic profiling. J Nutr 2016. Mar 9 (Epub ahead of print; DOI: 10.3945/jn.115.219147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz-Gutiérrez V, Lamuela-Raventós RM, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–85. [DOI] [PubMed] [Google Scholar]
- 13.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 2011;141:1140–5. [DOI] [PubMed] [Google Scholar]
- 14.Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. Am J Epidemiol 1994;139:1197–209. [DOI] [PubMed] [Google Scholar]
- 15.Blom G. Statistical estimates and transformed beta-variables. New York: Wiley; 1958. [Google Scholar]
- 16.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–88. [Google Scholar]
- 17.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 18.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014;129:1821–31. [DOI] [PubMed] [Google Scholar]
- 19.Ho JE, Larson MG, Ghorbani A, Cheng S, Chen MH, Keyes M, Rhee EP, Clish CB, Vasan RS, Gerszten RE, et al. Metabolomic profiles of body mass index in the Framingham Heart Study reveal distinct cardiometabolic phenotypes. PLoS One 2016;11:e0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008;9:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koska J, Ozias MK, Deer J, Kurtz J, Salbe AD, Harman SM, Reaven PD. A human model of dietary saturated fatty acid induced insulin resistance. Metabolism 2016;65:1621–8. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Wu JH. (n-3) Fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr 2012;142:614S–25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- 25.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, et al. ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, Hu FB, Sun Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ 2016;355:i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep 2010;62:536–47. [DOI] [PubMed] [Google Scholar]
- 28.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 2010;177:1576–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siscovick DS, Lemaitre RN, M\ozaffarian D. The fish story: a diet-heart hypothesis with clinical implications: n-3 polyunsaturated fatty acids, myocardial vulnerability, and sudden death. Circulation 2003;107:2632–4. [DOI] [PubMed] [Google Scholar]
- 30.Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz-Canela M, Guasch-Ferré M, et al. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial. Circulation 2017;135:2028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 2008;29:381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E. Benefits of the Mediterranean diet: insights from the PREDIMED Study. Prog Cardiovasc Dis 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
- 33.Bondia-Pons I, Martinez JA, de la Iglesia R, Lopez-Legarrea P, Poutanen K, Hanhineva K, Zulet Mde L. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: the Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol Nutr Food Res 2015;59:711–28. [DOI] [PubMed] [Google Scholar]
- 34.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, Seppänen-Laakso T, Poutanen K, Hyötyläinen T, Risérus U, Savolainen MJ, et al. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J Nutr 2016. Mar 9 (Epub ahead of print; DOI: 10.3945/jn.115.220459). [DOI] [PubMed] [Google Scholar]