Abstract
Background: Consumption of coffee, one of the most popular beverages around the world, has been associated with a lower risk of cardiovascular and all-cause mortality in population-based studies. However, little is known about these associations in patient populations.
Objective: This prospective study aimed to examine the consumption of caffeinated and decaffeinated coffee in relation to cardiovascular disease (CVD) mortality, ischemic heart disease (IHD) mortality, and all-cause mortality in patients with a prior myocardial infarction (MI).
Design: We included 4365 Dutch patients from the Alpha Omega Cohort who were aged 60–80 y (21% female) and had experienced an MI <10 y before study enrollment. At baseline (2002–2006), dietary data including coffee consumption over the past month was collected with a 203-item validated food-frequency questionnaire. Causes of death were monitored until 1 January 2013. HRs for mortality in categories of coffee consumption were obtained from multivariable Cox proportional hazard models, adjusting for lifestyle and dietary factors.
Results: Most patients (96%) drank coffee, and the median total coffee intake was 375 mL/d (∼3 cups/d). During a median follow-up of 7.1 y, a total of 945 deaths occurred, including 396 CVD-related and 266 IHD-related deaths. Coffee consumption was inversely associated with CVD mortality, with HRs of 0.69 (95% CI: 0.54, 0.89) for >2–4 cups/d and 0.72 (0.55, 0.95) for >4 cups/d, compared with 0–2 cups/d. Corresponding HRs were 0.77 (95% CI: 0.57, 1.05) and 0.68 (95% CI: 0.48, 0.95) for IHD mortality and 0.84 (95% CI: 0.71, 1.00) and 0.82 (95% CI: 0.68, 0.98) for all-cause mortality, respectively. Similar associations were found for decaffeinated coffee and for coffee with additives.
Conclusion: Drinking coffee, either caffeinated or decaffeinated, may lower the risk of CVD and IHD mortality in patients with a prior MI. This study was registered at clinicaltrials.gov as NCT03192410.
Keywords: coffee, myocardial infarction patients, cardiovascular disease, ischemic heart disease, mortality, prospective cohort study
INTRODUCTION
Coffee is among the most widely consumed beverages around the world. Current evidence suggests that coffee (mostly filtered) could protect against cardiovascular disease (CVD), ischemic heart disease (IHD), or all-cause mortality in the general population (1–5). A recent meta-analysis by Grosso et al. (1) of 31 observational studies involving 1.6 million subjects showed a >15% lower risk of CVD, IHD, and all-cause mortality for individuals who consume 4 cups coffee/d, with no further risk reduction at higher intakes. In healthy US men and women aged 55–74 y, an ∼25% lower risk of heart disease mortality was found for individuals who consumed ≥2 cups coffee/d (for both caffeinated and decaffeinated coffee) (2).
Data on coffee consumption in relation to mortality risk in patient populations are limited. Two prospective studies in patients who had experienced an acute myocardial infarction (MI) showed no consistent associations between caffeinated coffee consumption and post-MI mortality (6) or total coffee consumption and incident CVD (7) during ∼4 y of follow-up. However, daily intake of ≥3 cups of filtered, caffeinated coffee (compared with 0–1 cups) was related to an ∼40–50% lower risk of all-cause mortality during 7–10 y of follow-up in 1369 Stockholm Heart Epidemiology Program patients who had experienced an MI, with a similar trend for cardiac mortality (8).
The inconsistent findings for coffee consumption and mortality risk in patient populations deserve further research. We assessed the long-term associations of caffeinated and decaffeinated coffee consumption with CVD, IHD, and all-cause mortality in the Alpha Omega Cohort of stable Dutch patients aged 60–80 y who had experienced an MI.
METHODS
Patients and study design
The Alpha Omega Cohort originates from the Alpha Omega Trial, a 3-y intervention study of n–3 (ω-3) fatty acids, which is described in detail elsewhere (9, 10). The cohort consists of 4837 men and women aged 60–80 y with a clinically diagnosed MI ≤10 y before enrollment (2002–2006). The trial was completed in 2009, after which the cohort was followed up for cause-specific mortality. This study excluded patients with missing dietary data (n = 453) or with implausible high or low energy intakes (<800 or >8000 kcal/d for men and <600 or >6000 kcal/d for women; n = 19). A total of 4365 patients remained for the analysis (Supplemental Figure 1).
Assessment of diet and coffee consumption
Dietary data were collected by an extended and adapted version of a biomarker-validated food-frequency questionnaire (FFQ) (11). This semiquantitative 203-item FFQ included questions on the frequency, amount, type, and preparation method of foods consumed in the previous month. Trained dietitians checked the questionnaires when returned and additional information was obtained if items were unclear or missing. Food consumption data were converted into energy and nutrient intakes using the 2006 Dutch food composition database (12) and items were clustered in 24 food groups as described previously (13).
The FFQ included questions on different coffee preparations, including caffeinated coffee, decaffeinated coffee, instant coffee, and specialty coffees such as cappuccino and Viennese blend. Patients could indicate the amount of coffee per cup (125 mL), glass (150 mL), or mug (187.5 mL). The use of additives in coffee was also assessed, including sugar, types of milk, cream, and coffee creamer. No information on the use of artificial sweeteners was obtained. Total coffee consumption was calculated as the sum of all coffee preparations consumed. The intake of caffeinated and decaffeinated coffee was also computed. Information on brewing methods was not available, but most coffee in the Netherlands is paper filtered (14).
Cause-specific mortality
Information on vital status and causes of death from May 2002 until January 2013 was obtained from the Dutch National Mortality Registry (Statistics Netherlands). One patient was lost to follow-up and censored after 2.9 y. Fatal events were coded according to the International Classification of Diseases, 10th Revision (15), combining primary and secondary causes of death. CVD mortality included IHD (codes I20–I25), cardiac arrest (I46), heart failure (I50), stroke (I60–I69), and sudden death, undefined (R96). IHD mortality included codes I20–I25, I46, and R96.
Other measurements
At baseline, data were collected on medical history, medication use, and demographic, anthropometric, and lifestyle factors, as described in detail elsewhere (9, 10). BMI (in kg/m2) was calculated. Obesity was defined as a BMI ≥30. Medication was coded according to the Anatomical Therapeutic Chemical Classification System (16) as follows: C02, C03, C07, C08, and C09 for antihypertensive drugs; C10 for lipid-modifying drugs; and A10 for antidiabetic drugs. Trained research nurses measured blood pressure twice with an automated device (HEM-711; Omron) and the mean was taken. Nonfasting blood was collected. Standard assays and an automated analyzer (Hitachi 912; Roche Diagnostics) were used to determine serum lipids and plasma glucose. The intra- and interassay CVs for analytic variation were 0.9% and 1.8% for glucose and 0.8% and 1.7% for total cholesterol, respectively (17).
Diabetes mellitus was considered present in the case of a self-reported physician diagnosis, use of antidiabetic medication, or elevated plasma glucose (≥7.0 mmol/L if fasted >4 h or ≥11.1 mmol/L if nonfasted). Smoking status was assessed in 4 categories (current; former, quit >10 y before study enrollment; former, quit ≤10 y before study enrollment; or never). Consumption of alcoholic beverages was assessed by the FFQ from which ethanol intake was computed, which was categorized as 0, >0–≤10, >10–≤20, or >20 g/d. Physical activity was assessed by the Physical Activity Scale for the Elderly (18) and patients were categorized as having no or light activity only [≤3 metabolic equivalents (METs)], moderate or vigorous activity [>3 METs] 1–5 d/wk, or moderate or vigorous activity [>3 METs] ≥5 d/wk. The highest level of education attained was categorized as primary (or less), lower secondary, higher secondary or lower tertiary, or higher tertiary. Self-rated health was assessed by the question “How do you rate your overall health at this moment?” using a 5-point scale (poor, moderate, good, very good, or excellent).
Statistical analysis
Patients were categorized according to their baseline total coffee consumption as follows: 0–2 cups/d (0–250 mL/d), >2–4 cups/d (>250–500 mL/d), or >4 cups/d (>500 mL/d). Follow-up data on coffee consumption were available for approximately half of the patients (n = 2095), showing that 63% were in the same category and 31% were in the nearest adjacent category after a median follow-up of 41 mo.
Baseline characteristics in coffee categories are presented as unadjusted means ± SDs for normally distributed variables, medians with IQRs for skewed variables, and percentages for categorical variables. Cox proportional hazard models were used to examine the associations of coffee consumption in categories with CVD mortality, IHD mortality, and all-cause mortality. The proportionality of hazards assumption was checked by log-minus-log plots and was met. Survival time (in years) was defined as the period between the date of inclusion and the date of death, censoring date, or end of follow-up (1 January 2013), whichever came first. HRs with 95% CIs were computed in coffee categories, using the lower category (0–2 cups/d) as the reference. For fatal stroke, the number of events was limited and HRs for coffee consumption were estimated on a continuous scale (in cups per day) to retain statistical power.
HRs for coffee consumption were adjusted for age, sex, and type of intervention during the initial Alpha Omega Trial phase (“treatment code” for types of n–3 FAs or placebo) (model 1). Model 2 also included prevalent diabetes (yes or no), BMI (continuous), physical activity (3 categories), educational level (4 categories), smoking status (4 categories), and alcohol use (4 categories). Model 3 also included dietary factors such as intake of total energy (kilocalories per day), black or green tea (milliliters per day), whole grains (grams per day), red and processed meats (grams per day), dairy (grams per day), vegetables and fruits (grams per day), chocolate (grams per day), sugar-sweetened beverages including sweetened fruit juices (milliliters per day), plant oils (grams per day), legumes (grams per day), and nuts and seeds (grams per day). There were missing data for BMI (n = 6), physical activity (n = 25), educational level (n = 24), and smoking status (n = 1). Missing data were imputed by the sex-specific medians to retain these covariables in the multivariable models.
The P value for linear trend across HRs was computed by entering median values within the coffee categories (for total coffee: 125, 375, and 750 mL/d) in the Cox model. Coffee was also analyzed continuously (full model) for potential threshold or nonlinear relations using restricted cubic spline (RCS) analyses, with knots at the 5th, 50th, and 95th percentiles.
HRs for CVD, IHD, and all-cause mortality were obtained separately for caffeinated coffee (0–2, >2–4, and >4 cups/d) and decaffeinated coffee consumption (0–2 and >2 cups/d), using the full model. In the analysis of caffeinated coffee, an additional adjustment was made for decaffeinated coffee (as a continuous variable) and vice versa.
Additional analyses by type of coffee were performed using the full model. The analysis for caffeinated coffee consumption was repeated after the exclusion of decaffeinated coffee drinkers, and the analysis for decaffeinated coffee was repeated after the exclusion of caffeinated coffee drinkers. The analyses were also repeated with further classification at low levels of total coffee intake (0–1, >1–2, >2–4, and >4 cups/d) and at high levels of total coffee intake (0–2, >2–4, >4–6, and >6 cups/d). The analyses for total coffee were performed separately for users and nonusers of additives (any additives, sugar, or milk, cream, or coffee creamer). Non-coffee drinkers (n = 156) were included in the reference groups of all analyses.
Analyses of total coffee consumption in relation to CVD, IHD, and all-cause mortality were stratified by sex, obesity (absent or present), smoking status (never, former, or current), diabetes (absent or present), self-rated health (poor to moderate or good to excellent), and time since last MI (≤2 y or >2 y before study enrollment). To examine whether associations could be affected by reverse causation bias, we conducted a sensitivity analysis excluding the first 2 y of follow-up, during which 155 deaths occurred.
Two-sided P values <0.05 indicated statistical significance. SAS software (version 9.3; SAS Institute Inc.) was used for all analyses.
RESULTS
The mean age of the cohort was 69.0 ± 5.6 y and 21% of participants were women. Patients had their last MI ∼4 y ago and 20% had diabetes. Most patients (96%) drank coffee and consumed caffeinated coffee (70%), decaffeinated coffee (11%), or both (15%). The median total coffee intake was 375 mL/d (∼3 cups); 4% of patients drank no coffee, 10% drank >0–1 cup/d, and 10% drank >6 cups/d. Table 1 shows baseline characteristics in categories of total coffee consumption. Patients with higher coffee intake were slightly younger and more often were men, current smokers, and alcohol drinkers. They had a lower intake of black or green tea and sugar-sweetened beverages and a higher intake of total energy, whole grains, red or processed meats, dairy, plant oils, chocolate, and nuts or seeds.
TABLE 1.
Baseline characteristics of 4365 patients in the Alpha Omega Cohort, by categories of total coffee consumption1
| Total coffee consumption, cups/d | ||||
| Variable | 0–2 (n = 849)2 | >2–4 (n = 1935) | >4 (n = 1581) | P value3 |
| Age, y | 70.1 ± 5.6 | 69.5 ± 5.5 | 67.8 ± 5.4 | <0.001 |
| Female sex | 239 (28) | 359 (19) | 335 (21) | <0.001 |
| BMI,4 kg/m2 | 27.8 ± 4.0 | 27.7 ± 3.7 | 27.8 ± 3.8 | 0.34 |
| Obese | 213 (25) | 444 (23) | 376 (24) | 0.46 |
| Highest level of education attained5 | 0.22 | |||
| Primary | 172 (20) | 378 (20) | 331 (21) | |
| Lower secondary | 284 (34) | 722 (38) | 551 (35) | |
| Higher secondary or lower tertiary | 290 (34) | 577 (30) | 501 (32) | |
| Higher tertiary | 98 (12) | 247 (13) | 190 (12) | |
| Smoking status6 | <0.001 | |||
| Never | 193 (23) | 329 (17) | 200 (13) | |
| Former, quit >10 y ago | 157 (19) | 350 (18) | 259 (16) | |
| Former, quit ≤10 y ago | 403 (48) | 980 (51) | 781 (49) | |
| Current | 96 (11) | 276 (14) | 341 (22) | |
| Physical activity7 | 0.038 | |||
| Low | 364 (43) | 781 (41) | 639 (41) | |
| Intermediate | 288 (34) | 735 (38) | 611 (39) | |
| High | 189 (23) | 409 (21) | 324 (21) | |
| Alcohol intake, g/d | <0.001 | |||
| 0 | 247 (29) | 341 (18) | 297 (19) | |
| >0–10 | 312 (37) | 788 (41) | 620 (39) | |
| >10–20 | 146 (17) | 364 (19) | 254 (16) | |
| >20 | 144 (17) | 442 (23) | 410 (26) | |
| Self-rated health8 | <0.001 | |||
| Very good or excellent | 74 (9) | 242 (13) | 193 (12) | |
| Good | 529 (63) | 1277 (66) | 1022 (65) | |
| Moderate or poor | 241 (29) | 409 (21) | 360 (23) | |
| Time since last MI,9 y | 3.5 (1.6–6.1) | 3.7 (1.7–6.4) | 3.7 (1.7–6.3) | 0.59 |
| Prevalent diabetes mellitus10 | 189 (22) | 379 (20) | 316 (20) | 0.26 |
| Plasma glucose,11 mmol/L | 6.2 ± 2.2 | 6.2 ± 2.1 | 6.2 ± 2.0 | 0.91 |
| Serum LDL cholesterol,12 mmol/L | 2.6 ± 0.9 | 2.6 ± 0.8 | 2.6 ± 0.8 | 0.43 |
| Systolic blood pressure,13 mm Hg | 142.4 ± 22.6 | 142.5 ± 21.4 | 140.9 ± 21.2 | 0.057 |
| Diastolic blood pressure,13 mm Hg | 79.9 ± 11.2 | 80.3 ± 11.2 | 80.3 ± 11.0 | 0.54 |
| Use of antihypertensive drugs | 769 (91) | 1752 (91) | 1398 (88) | 0.083 |
| Use of lipid-modifying drugs | 723 (85) | 1664 (86) | 1398 (88) | 0.036 |
| Dietary factors | ||||
| Total coffee intake, mL/d | 125 (45–146) | 375 (375–375) | 750 (563–767) | <0.001 |
| Caffeinated coffee drinker | 595 (70) | 1702 (88) | 1428 (90) | <0.001 |
| Caffeinated coffee intake, mL/d | 89 (0–125) | 375 (375–375) | 563 (563–750) | <0.001 |
| Decaffeinated coffee drinker14 | 199 (23) | 483 (25) | 473 (30) | <0.001 |
| Black or green tea drinker | 706 (83) | 1512 (78) | 1085 (69) | <0.001 |
| Black or green tea intake, mL/d | 188 (67–450) | 150 (21–375) | 125 (0–375) | <0.001 |
| Total energy intake, kcal/d | 1757 ± 517 | 1899 ± 515 | 1975 ± 503 | <0.001 |
| Whole grain intake, g/d | 103 (88–158) | 110 (88–159) | 120 (88–161) | 0.005 |
| Red or processed meat intake, g/d | 52 (28–80) | 67 (41–92) | 68 (42–94) | <0.001 |
| Dairy intake, g/d | 271 (172–426) | 298 (192–422) | 311 (200–437) | 0.001 |
| Vegetable or fruit intake, g/d | 210 (129–379) | 212 (142–369) | 211 (136–373) | 0.75 |
| Plant oil intake, g/d | 24 (14–37) | 27 (16–40) | 29 (17–42) | <0.001 |
| Sugar-sweetened beverage intake, mL/d | 146 (52–245) | 129 (52–209) | 108 (41–202) | 0.001 |
| Chocolate intake, g/d | 3.9 (0.0–9.1) | 4.5 (0.9–11.5) | 4.5 (0.9–12.5) | 0.003 |
| Nut or seed intake, g/d | 0.8 (0.0–2.8) | 1.8 (0.0–2.8) | 1.8 (0.0–2.8) | 0.001 |
| Legume intake, g/d | 8.7 (4.4–15.0) | 8.7 (4.5–14.6) | 9.0 (4.6–14.9) | 0.58 |
Values are means ± SDs for normally distributed variables, medians (IQRs) for skewed variables, or n (%) for categorical variables. MI, myocardial infarction.
Includes 156 non-coffee drinkers.
P values for differences between coffee categories were obtained from ANOVA (normally distributed continuous variables), the Kruskal-Wallis test (skewed variables), or the chi-square test (categorical variables).
Missing data for 6 patients. Obesity was defined as a BMI ≥30.
Missing data for 24 patients.
Missing data for 1 patient.
Missing data for 25 patients. Low indicates no or light activity only (<3 metabolic equivalents), intermediate indicates moderate or vigorous activity (≥3 metabolic equivalents) 1–5 d/wk, and high indicates moderate or vigorous activity ≥5 d/wk.
Missing data for 18 patients.
Missing data for 38 patients. MI was based on a verified clinical diagnosis <10 y before study enrollment.
Defined as a self-reported physician diagnosis, use of antidiabetic medication, or elevated plasma glucose (≥7.0 mmol/L if fasted >4 h or ≥11.1 mmol/L if nonfasted).
Missing data for 86 patients. To convert the values for plasma glucose to milligrams per deciliter, divide by 0.056.
Missing data for 309 patients. To convert the values for serum cholesterol to milligrams per deciliter, divide by 0.026.
Missing data for 6 patients. Mean of 2 nonfasting office blood pressure measurements.
Median decaffeinated coffee intake of 0 mL/d in all coffee categories.
There were 945 deaths, including 396 from CVD, 266 from IHD, and 71 from stroke. HRs for CVD mortality, IHD mortality, and all-cause mortality by categories of total coffee consumption are presented in Table 2, using 0–2 cups/d as the reference. Coffee was inversely associated with CVD mortality in the fully adjusted model, with HRs of 0.69 (95% CI: 0.54, 0.89) for >2–4 cups/d and 0.72 (95% CI: 0.55, 0.95) for >4 cups/d. Corresponding HRs were 0.77 (95% CI: 0.57, 1.05) and 0.68 (95% CI: 0.48, 0.95) for IHD mortality and 0.84 (95% CI: 0.71, 1.00) and 0.82 (95% CI: 0.68, 0.98) for all-cause mortality. The multivariable HR for stroke mortality was 1.06 (95% CI: 0.96, 1.16) per cup per day (data not in table).
TABLE 2.
Associations of total coffee consumption with CVD mortality, IHD mortality, and all-cause mortality in 4365 Dutch post-MI patients1
| Total coffee consumption, cups/d | ||||
| 0–2 (n = 849) | >2–4 (n = 1935) | >4 (n = 1581) | P-trend | |
| Person-years | 5840 | 13,937 | 11,402 | |
| Median coffee intake, mL/d | 125 | 375 | 750 | |
| CVD mortality | ||||
| Cases | 107 | 163 | 126 | |
| Model 12 | 1.00 | 0.66 (0.52, 0.84) | 0.73 (0.56, 0.95) | 0.082 |
| Model 23 | 1.00 | 0.66 (0.52, 0.85) | 0.69 (0.53, 0.90) | 0.032 |
| Model 34 | 1.00 | 0.69 (0.54, 0.89) | 0.72 (0.55, 0.95) | 0.073 |
| IHD mortality | ||||
| Cases | 69 | 118 | 79 | |
| Model 12 | 1.00 | 0.74 (0.55, 1.00) | 0.70 (0.50, 0.97) | 0.062 |
| Model 23 | 1.00 | 0.75 (0.55, 1.01) | 0.66 (0.47, 0.92) | 0.023 |
| Model 34 | 1.00 | 0.77 (0.57, 1.05) | 0.68 (0.48, 0.95) | 0.037 |
| All-cause mortality | ||||
| Cases | 220 | 416 | 309 | |
| Model 12 | 1.00 | 0.82 (0.69, 0.96) | 0.86 (0.72, 1.02) | 0.25 |
| Model 23 | 1.00 | 0.82 (0.69, 0.96) | 0.80 (0.67, 0.95) | 0.033 |
| Model 34 | 1.00 | 0.84 (0.71, 1.00) | 0.82 (0.68, 0.98) | 0.070 |
Values are n or HRs (95% CIs) obtained from Cox proportional hazards analysis, using the lowest intake group as the reference, unless otherwise indicated. CVD, cardiovascular disease; IHD, ischemic heart disease; MI, myocardial infarction.
HRs were adjusted for age, sex, and treatment code.
HRs were adjusted as in model 1, with additional adjustment for prevalent diabetes, BMI (in kg/m2), physical activity, educational level, smoking status, and alcohol use. Missing data on BMI, physical activity, educational level, and smoking status were imputed by sex-specific medians.
HRs were adjusted as in model 2, with additional adjustment for intakes of total energy, black or green tea, whole grains, red or processed meats, dairy, vegetables or fruits, chocolate, sugar-sweetened beverages, plant oils, legumes, and nuts or seeds.
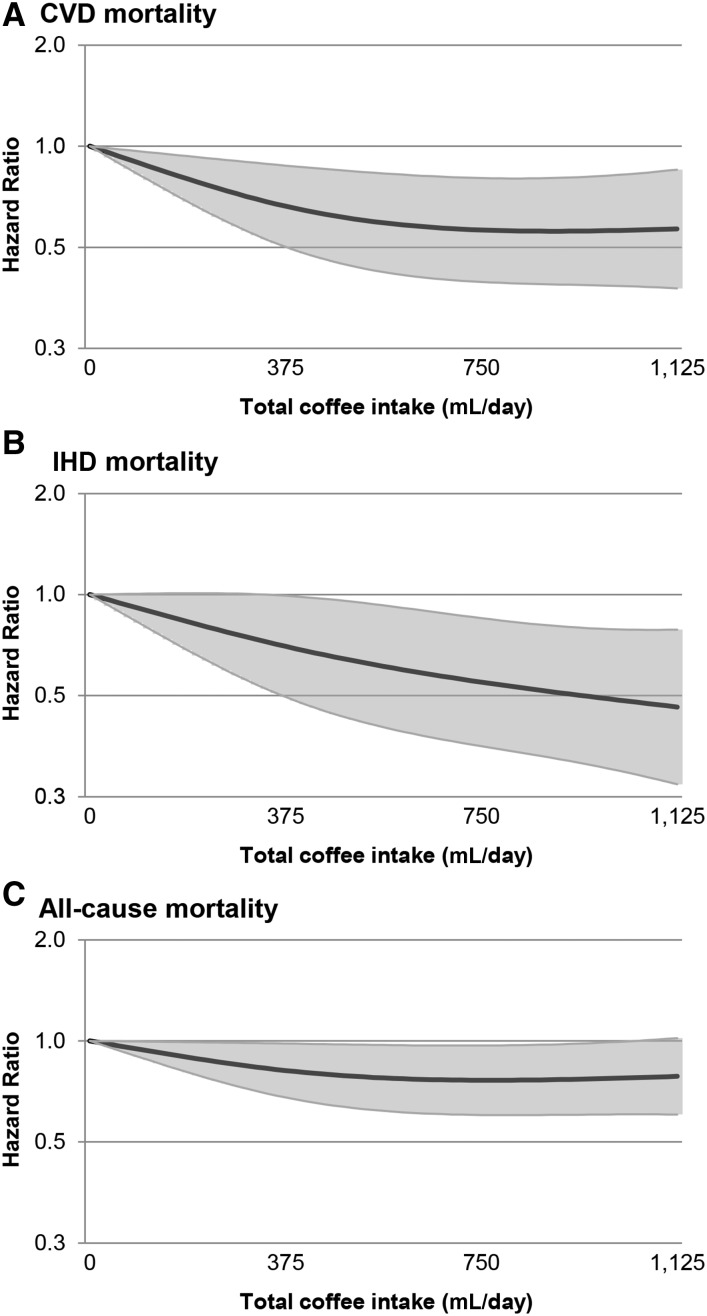
Figure 1 shows the results from the RCS analyses for total coffee consumption and CVD, IHD, and all-cause mortality, using the fully adjusted model. Tests for a nonlinear association were not statistically significant (P = 0.06 for CVD mortality and P > 0.10 for IHD and all-cause mortality), indicating linear dose-response relationships. For CVD and all-cause mortality, RCS analysis suggests there is no further risk reduction for intakes >∼4 cups/d, whereas estimates for IHD risk continued to decrease.
FIGURE 1.
Multivariable-adjusted restricted cubic spline analysis for the continuous association of total coffee consumption with CVD mortality (A), IHD mortality (B), and all-cause mortality (C) in 4365 Dutch post-MI patients. Covariables are listed in the Table 2 footnotes (model 3). The knots located at the 5th, 50th, and 95th percentiles correspond to coffee intakes of 45, 375, and 1125 mL/d, respectively. The y-axes show the predicted HRs for CVD mortality for any value of coffee intake, compared with the reference value set at an intake of 0. The gray areas indicate the 95% CIs. CVD, cardiovascular disease; IHD, ischemic heart disease.
The fully adjusted HRs in categories of caffeinated and decaffeinated coffee separately (Table 3) were essentially similar to those for total coffee consumption. HRs were also of the same magnitude when decaffeinated coffee drinkers were excluded from the analysis of caffeinated coffee (Supplemental Table 1) and vice versa (Supplemental Table 2). Further stratification at higher levels of total coffee intake showed multivariable HRs of 0.68 (95% CI: 0.44, 1.03) for CVD mortality and 0.56 (95% CI: 0.32, 0.97) for IHD mortality for >6 cups/d than 0–2 cups/d (Supplemental Table 3). Further stratification at lower levels of total coffee intake showed multivariable HRs of 0.62 (95% CI: 0.46, 0.83) for CVD mortality and 0.62 (95% CI: 0.43, 0.89) for IHD mortality for >4 cups/d than 0–1 cups/d (Supplemental Table 4).
TABLE 3.
Associations of caffeinated and decaffeinated coffee consumption with CVD mortality, IHD mortality, and all-cause mortality in 4365 Dutch post-MI patients1
| Caffeinated coffee, cups/d | Decaffeinated coffee,2 cups/d | ||||
| Variable | 0–2 (n = 1481)3 | >2–4 (n = 1641) | >4 (n = 1243) | 0–2 (n = 3662)3 | >2 (n = 703) |
| CVD mortality | |||||
| Cases | 151 | 136 | 109 | 346 | 50 |
| Multivariable HR4,5 | 1.00 | 0.71 (0.54, 0.91) | 0.81 (0.62, 1.07) | 1.00 | 0.71 (0.51, 0.97) |
| IHD mortality | |||||
| Cases | 100 | 99 | 67 | 234 | 32 |
| Multivariable HR | 1.00 | 0.75 (0.55, 1.03) | 0.71 (0.50, 1.00) | 1.00 | 0.63 (0.42, 0.94) |
| All-cause mortality | |||||
| Cases | 343 | 347 | 255 | 806 | 139 |
| Multivariable HR | 1.00 | 0.85 (0.72, 1.01) | 0.87 (0.73, 1.05) | 1.00 | 0.88 (0.73, 1.08) |
Values are n or HRs (95% CIs) obtained from Cox proportional hazards analysis, using the lowest intake group as the reference. CVD, cardiovascular disease; IHD, ischemic heart disease; MI, myocardial infarction.
Further stratification to >4 cups/d was not possible because of the relatively low consumption of decaffeinated coffee.
Includes 156 non-coffee drinkers.
Covariables are listed in the Table 2 footnotes (model 3), with additional adjustment for decaffeinated coffee (in the analysis of caffeinated coffee) or caffeinated coffee (in the analysis of decaffeinated coffee).
Missing data on BMI (in kg/m2), physical activity, educational level, and smoking status were imputed by sex-specific medians.
The inverse associations of total coffee intake with CVD mortality, IHD mortality, and all-cause mortality were also present when patients used any additives in coffee (Supplemental Table 5), sugar in coffee (Supplemental Table 6), or milk, cream, or coffee creamer (Supplemental Table 7), but statistical significance was lost for most associations in strata.
Subgroup analysis by sex showed significant inverse associations for total coffee consumption and CVD and IHD mortality in men and weaker, nonsignificant inverse associations in the smaller group of women (Supplemental Table 8). Inverse associations with CVD and IHD mortality were observed in nonobese and obese patients (Supplemental Table 9) and in nonsmokers and smokers (Supplemental Table 10). Significant inverse associations were observed in patients without diabetes, whereas the associations were weak and nonsignificant in the smaller group of patients with diabetes (Supplemental Table 11). The associations tended to be stronger in patients with poor to moderate self-rated health than in patients with good to excellent self-rated health (Supplemental Table 12). Stratification by time since last MI did not materially change the associations (Supplemental Table 13). Results were also similar when the first 2 y of follow-up were excluded from the analysis (Supplemental Table 14).
DISCUSSION
This prospective study in 4365 Dutch patients with a history of myocardial infarction (79% men) showed that the consumption of filtered coffee was associated with a 20–30% lower risk of CVD and IHD mortality. The inverse associations were present both for caffeinated and decaffeinated coffee and for coffee with and without additives.
Our results are in line with a long-term prospective analysis by Mukamal et al. (8), showing an ∼40% lower risk of CVD mortality for >2 cups caffeinated coffee/d compared with 0–1 cups/d in Swedish patients with IHD. No association with post-MI mortality was found in a study in which caffeinated coffee was examined as a possible determinant of MI onset in 1935 patients (6). Silletta et al. (7) performed a multivariable analysis and did not find an association of coffee with incident CVD in a large cohort of Italian patients with IHD. However, the follow-up time was relatively short and espresso and mocha coffees were mainly consumed, which could contain diterpenes (19). These fractions in unfiltered coffee could raise LDL cholesterol (19), which could possibly counteract beneficial effects of other coffee components.
We found similar associations for caffeinated and decaffeinated coffee, making caffeine an unlikely candidate for the observed associations. Apart from caffeine, coffee is rich in chlorogenic acid and other polyphenols that could improve vascular function and insulin sensitivity (20–22). Caffeinated and decaffeinated coffee may lower the risk of diabetes (23), a major risk factor for CVD. Moreover, epidemiologic studies suggest that coffee is associated with beneficial endothelial and inflammation markers (24, 25). The lower mortality risks in our study were also present when common additives were used in coffee, as also reported by others (2). In our study, only 10% of patients had a daily coffee intake of >6 cups. Therefore, we cannot draw meaningful conclusions for coffee and mortality risk for intakes >∼750 mL/d. In our continuous analysis, the lowest risk of mortality occurred at ∼500 mL/d, with no further benefit at higher intakes.
The strength of our study is the unique cohort of post-MI patients, with detailed data on coffee consumption and dietary and lifestyle factors and almost complete follow-up data for cause-specific mortality. Our study also has limitations. The study population was rather homogenous for the amount of coffee consumed, with only 156 non–coffee drinkers. We performed an additional analysis in which we defined 0–1 cups/d (instead of 0–2 cups/d) as the reference. This yielded similar results, suggesting that a benefit may already be achieved at coffee intakes <2 cups/d. The FFQ we used did not allow for separation by brewing method and we cannot draw conclusions on unfiltered coffee and mortality risk. Dutch food consumption surveys have shown that filtered coffee is consumed by 92% of elderly individuals in the Netherlands (14), and we assume that most coffee in our study was paper filtered. We cannot extrapolate our findings to those who drink other types of coffee, to younger and healthy populations, or to patients with a more recent MI.
This study had insufficient power to assess the association of coffee consumption with stroke mortality, but the findings suggested no beneficial effect of coffee on this outcome. In addition, we lacked power to examine effect modification by sex. In our cohort, the inverse association of coffee with mortality risk was more pronounced in men than in women. Lopez-Garcia et al. (26) did not find an association of filtered, caffeinated coffee with CVD and all-cause mortality in women with a history of CVD. It has been suggested that coffee alters estrogen metabolism, which could possibly explain weaker associations in women (27). However, all of our female patients were postmenopausal, and whether the interaction of coffee with estrogen is relevant for CVD mortality risk is unclear. Therefore, our findings in female patients must be confirmed in other cohort studies.
Our patients experienced an MI ∼4 y before inclusion in the study and we cannot exclude the possibility that they altered their coffee-drinking habits. They may have also lowered their coffee intake because of current health complaints or (pre)clinical disease (28). We performed sensitivity analyses in which we excluded patients with poor to moderate self-rated health or an MI in the 2 y before inclusion. We also excluded the first 2 y of follow-up. The results provided no evidence that our risk estimates were biased by recent changes in coffee intake. Drinking coffee is a lifestyle habit, and we therefore adjusted for educational level, BMI, lifestyle factors, and dietary confounders. Coffee was associated with a lower risk of CVD and IHD mortality in our cohort, despite being part of a less healthy lifestyle (e.g., more smoking, more alcohol use, or less physical activity). If residual confounding were still present, it would have biased the risk estimates toward the null, meaning that the actual risk reductions may be larger than we observed.
Our findings suggest that moderate consumption of filtered coffee does not need to be reduced after an MI. We did not study whether acute, high intakes of coffee could trigger CVD events, especially in the absence of tolerance to caffeine (29, 30). In addition, more research is needed to examine coffee consumption in relation to stroke, for which the present study lacked power. In conclusion, our findings in the Alpha Omega Cohort suggest that filtered coffee (either caffeinated or decaffeinated) could contribute to a lower risk of mortality in patients with a prior MI.
Acknowledgments
We thank Eveline Waterham for providing the required datasets of the Alpha Omega Cohort.
The authors’ responsibilities were as follows—LHvD: conducted the research, performed the statistical analysis, interpreted the data, and drafted the initial manuscript; FJMM: performed the statistical analysis, interpreted the data, and wrote and critically reviewed the manuscript; SSS-M: critically reviewed the manuscript; DK: performed data acquisition and critically reviewed the manuscript; JMG: conceived and designed the study, performed data acquisition, and wrote the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CVD, cardiovascular disease; FFQ, food-frequency questionnaire; IHD, ischemic heart disease; MET, metabolic equivalent; MI, myocardial infarction; RCS, restricted cubic spline.
REFERENCES
- 1.Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martínez-González MA, Giovannucci EL, Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol 2016;31:1191–205. [DOI] [PubMed] [Google Scholar]
- 2.Loftfield E, Freedman ND, Graubard BI, Guertin KA, Black A, Huang WY, Shebl FM, Mayne ST, Sinha R. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol 2015;182:1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ, Wang CL, Chen YM. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol 2009;137:216–25. [DOI] [PubMed] [Google Scholar]
- 4.Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, Tavani A. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol 2013;28:527–39. [DOI] [PubMed] [Google Scholar]
- 5.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014;129:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Caffeinated coffee consumption and mortality after acute myocardial infarction. Am Heart J 2004;147:999–1004. [DOI] [PubMed] [Google Scholar]
- 7.Silletta MG, Marfisi R, Levantesi G, Boccanelli A, Chieffo C, Franzosi M, Geraci E, Maggioni AP, Nicolosi G, Schweiger C. Coffee consumption and risk of cardiovascular events after acute myocardial infarction results from the GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico)-Prevenzione Trial. Circulation 2007;116:2944–51. [DOI] [PubMed] [Google Scholar]
- 8.Mukamal KJ, Hallqvist J, Hammar N, Ljung R, Gémes K, Ahlbom A, Ahnve S, Janszky I. Coffee consumption and mortality after acute myocardial infarction: the Stockholm Heart Epidemiology Program. Am Heart J 2009;157:495–501. [DOI] [PubMed] [Google Scholar]
- 9.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n–3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26. [DOI] [PubMed] [Google Scholar]
- 10.Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Oude Griep LM, Teitsma-Jansen AM, Katan MB, Kromhout D; Alpha Omega Trial Group. Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J 2010;159:539–46.e2. [DOI] [PubMed] [Google Scholar]
- 11.Feunekes GI, Van Staveren WA, De Vries J, Burema J, Hautvast J. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 1993;58:489–96. [DOI] [PubMed] [Google Scholar]
- 12. Dutch Nutrition Center. Dutch Food Composition Table 2006 NEVO-tabel: Nederlands Voedingsstoffenbestand 2006/NEVO Foundation. The Hague (Netherlands); 2006 (in Dutch).
- 13.Sijtsma FP, Soedamah-Muthu SS, de Goede J, Oude Griep LM, Geleijnse JM, Giltay EJ, de Boer MJ, Jacobs DR Jr, Kromhout D. Healthy eating and lower mortality risk in a large cohort of cardiac patients who received state-of-the-art drug treatment. Am J Clin Nutr 2015;102:1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocké MC, Buurma-Rethans E, de Boer E, Wilson-van den Hooven C, Etemad-Ghameslou Z, Drijvers J, van Rossum C. Diet of community-dwelling older adults: Dutch National Food Consumption Survey of Older Adults 2010–2012. RIVM report 050413001. Bilthoven (Netherlands): National Institute for Public Health and the Environment; 2013. [Google Scholar]
- 15.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Geneva (Switzerland): WHO; 2004. [Google Scholar]
- 16.World Health Organization Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical Classification System (ACT). Oslo (Norway): WHO; 2009. [Google Scholar]
- 17.Giltay EJ, Geleijnse JM, Schouten EG, Katan MB, Kromhout D. High stability of markers of cardiovascular risk in blood samples. Clin Chem 2003;49:652–5. [DOI] [PubMed] [Google Scholar]
- 18.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol 1997;50:541–6. [DOI] [PubMed] [Google Scholar]
- 19.Aro A, Tuomilehto J, Kostiainen E, Uusitalo U, Pietinen P. Boiled coffee increases serum low density lipoprotein concentration. Metabolism 1987;36:1027–30. [DOI] [PubMed] [Google Scholar]
- 20.Ochiai R, Jokura H, Suzuki A, Tokimitsu I, Ohishi M, Komai N, Rakugi H, Ogihara T. Green coffee bean extract improves human vasoreactivity. Hypertens Res 2004;27:731–7. [DOI] [PubMed] [Google Scholar]
- 21.Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol Res 2007;55:187–98. [DOI] [PubMed] [Google Scholar]
- 22.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr 2005;81:292S–7S. [DOI] [PubMed] [Google Scholar]
- 23.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care 2014;37:569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr 2006;84:888–93. [DOI] [PubMed] [Google Scholar]
- 25.Williams CJ, Fargnoli JL, Hwang JJ, Van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care 2008;31:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Mukamal KJ, Hu FB, van Dam RM. Coffee consumption and mortality in women with cardiovascular disease. Am J Clin Nutr 2011;94:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisti JS, Hankinson SE, Caporaso NE, Gu F, Tamimi RM, Rosner B, Xu X, Ziegler R, Eliassen AH. Caffeine, coffee, and tea intake and urinary estrogens and estrogen metabolites in premenopausal women. Cancer Epidemiol Biomarkers Prev 2015;24:1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallström P, Mattisson I, Tydén P, Berglund G, Janzon L. Dietary habits after myocardial infarction–results from a cross‐sectional study. J Intern Med 2005;257:329–37. [DOI] [PubMed] [Google Scholar]
- 29.Riksen NP, Rongen GA, Smits P. Acute and long-term cardiovascular effects of coffee: implications for coronary heart disease. Pharmacol Ther 2009;121:185–91. [DOI] [PubMed] [Google Scholar]
- 30.Robertson D, Wade D, Workman R, Woosley RL, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest 1981;67:1111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]