Abstract
Background: Previously, we showed that vegetable oil is necessary for carotenoid absorption from salad vegetables. Research is needed to better define the dose effect and its interindividual variation for carotenoids and fat-soluble vitamins.
Objective: The objective was to model the dose-response relation between the amount of soybean oil in salad dressing and the absorption of 1) carotenoids, phylloquinone, and tocopherols in salad vegetables and 2) retinyl palmitate formed from the provitamin A carotenoids.
Design: Women (n = 12) each consumed 5 vegetable salads with salad dressings containing 0, 2, 4, 8, or 32 g soybean oil. Blood was collected at selected time points. The outcome variables were the chylomicron carotenoid and fat-soluble vitamin area under the curve (AUC) and maximum content in the plasma chylomicron fraction (Cmax). The individual-specific and group-average dose-response relations were investigated by fitting linear mixed-effects random coefficient models.
Results: Across the entire 0–32-g range, soybean oil was linearly related to the chylomicron AUC and Cmax values for α-carotene, lycopene, phylloquinone, and retinyl palmitate. Across 0–8 g of soybean oil, there was a linear increase in the chylomicron AUC and Cmax values for β-carotene. Across a more limited 0–4-g range of soybean oil, there were minor linear increases in the chylomicron AUC for lutein and α- and total tocopherol. Absorption of all carotenoids and fat-soluble vitamins was highest with 32 g oil (P < 0.002). For 32 g oil, the interindividual rank order of the chylomicron AUCs was consistent across the carotenoids and fat-soluble vitamins (P < 0.0001).
Conclusions: Within the linear range, the average absorption of carotenoids and fat-soluble vitamins could be largely predicted by the soybean oil effect. However, the effect varied widely, and some individuals showed a negligible response. There was a global soybean oil effect such that those who absorbed more of one carotenoid and fat-soluble vitamin also tended to absorb more of the others. This trial was registered at clinicaltrials.gov as NCT02867488.
Keywords: bioavailability, carotenoid, concordance, lipid, triglyceride, vitamin A, vitamin E, vitamin K
See corresponding editorial on page 969.
INTRODUCTION
Only 8.9% of US adults consume 2–3-cup equivalents of vegetables/d as recommended for sedentary adults depending on their age and sex (1, 2). Physically active adults should consume more (2). A national diet characterized by low vegetable consumption is a public health concern given the associated negative health outcomes, including increased risk of cardiovascular disease and probable increased overall risk of cancer (3). In view of their low calorie and high fiber contents, the epidemiologic evidence for an obesity-preventing role of vegetables is surprisingly inconclusive (4, 5). Recent research has uncovered a probable explanation, which is the extent to which vegetables are consumed in forms that add calories and fat (5, 6). Previously, we and others showed that added lipid in the form of avocado or oil in salad dressing is needed to absorb β-carotene and other fat-soluble carotenoids in salad vegetables (7–10). Efforts to encourage Americans to consume vegetables with fewer added fat calories will require better definition of the dose-response relation between added vegetable oil and the health benefit in promoting the absorption of carotenoids and also fat-soluble vitamins. Salads are major contributors to vegetable and nutrient intakes in the United States (11, 12). Lettuce and vegetable salads are second only to potatoes among the most commonly consumed vegetables and vegetable products (13). In a nationally representative consumer survey, 59% of respondents reported that they eat salads, including leaf salads, as meals for lunch or dinner ≥1 time/wk (14). US salad consumers tend to have better intakes and higher serum concentrations of nutrients, including folic acid, vitamin C, carotenoids, and vitamin E, which suggests good bioavailability (11). However, the plasma carotenoid concentrations in raw food adherents, which are characterized by high consumption of raw fruits and vegetables, were predicted primarily by their consumption of added fat and oil (15). Thus, the availability of lipid appears to restrict the benefit derived from carotenoids in raw vegetables even when they are consumed in abundance.
In view of the low consumption of vegetables in the United States, there is a particular need to optimize their nutritional benefits. The objectives of this study were to use statistical modeling to characterize the relation between the amount of soybean oil in salad dressing and the absorption of 1) carotenoids, phylloquinone (vitamin K-1), and tocopherols in salad vegetables and 2) retinyl palmitate and vitamin A formed from the provitamin A carotenoids, α- and β-carotene, in salad vegetables. Soybean oil is the predominant salad and cooking oil used by the US food industry (16).
METHODS
Subjects
Twelve healthy, nonsmoking women 19–39 y of age were enrolled in the study from June to September 2009 at the Iowa State University Nutrition and Wellness Research Center. The mean ± SD age of the study participants was 24.0 ± 5.9 y; the mean ± SD BMI (in kg/m2) was 23.60 ± 3.43. The women were recruited from the university community and screened by interview with the use of a standardized questionnaire that addressed health and lifestyle factors (Supplemental Figure 1). They also completed a SCOFF questionnaire, which is a screening tool to detect eating disorders (17). At the time of the interview, the women’s body weights and heights were measured. Those who met the eligibility criteria for the study underwent additional health screening in the form of a complete blood count, blood biochemistry profile, and plasma lipid panel. Inclusion criteria included excellent health as indicated by health history and blood indexes, including normolipidemia, and BMI <30. The exclusion criteria were as previously described (18). Also excluded were those who had ≥2 positive responses on the SCOFF questionnaire. Informed consent was obtained from all participants. All procedures involving human subjects were approved by the Iowa State University Institutional Review Board.
Dietary protocols
During each of the 5 study periods, the participants completed the following protocol: on days 1–3, the participants were given a list of good food sources of carotenoids, phylloquinone, retinoids, and tocopherols and instructed to avoid those foods. On day 4, the participants consumed a standardized, weighed diet of conventional foods, which had low contents of carotenoids and fat-soluble vitamins. The breakfast and dinner were consumed under supervision. The lunch and afternoon snacks were carried out by the participants and consumed outside the research center. The carotenoid and fat-soluble vitamin contents in the diet calculated with the use of Nutritionist Pro software (Axxya Systems) were 10.8 μg β-carotene, 4.3 μg β-cryptoxanthin, 97.6 μg lutein (plus zeaxanthin), 0.7 mg α-tocopherol, 54.7 μg retinol activity equivalents vitamin A, and 20.0 μg vitamin K. On day 5, the participants consumed the test salad in the morning after an overnight fast. The participants first ingested 240 mL bottled water and then waited 15 min before eating the test salad. The test salad was completely consumed with an additional 240 mL bottled water within a period of 30 min. The only other foods consumed were a low-fat (1.25 g) snack eaten after the 3.5-h blood sample collection and a low-fat (3 g) lunch eaten after the 5-h blood sample collection. These foods contained only trace amounts of carotenoids and fat-soluble vitamins.
Test salads
Each participant consumed 5 test salads that had equivalent vegetable composition but were consumed with salad dressings that contained different amounts of soybean oil (0, 2, 4, 8, or 32 g). Participants were randomly assigned to 1 of 10 salad dressing sequences as specified by a balanced Williams Latin square design (19). The remaining 2 participants were randomly assigned to 1 of the 10 salad dressing sequences and its inverse. The consumption of each test salad was separated by a washout period of ≥2 wk. Each test salad contained 48 g spinach (Spinach; Dole Food Company), 48 g romaine (Hearts of Romaine; Fresh Express), 66 g shredded carrots (Shredded Carrots; Dole Food Company), and 85 g cherry tomatoes (NatureSweet) (7). The romaine lettuce and spinach leaves were sorted to select leaves of uniform dark green maturity, which affects the concentrations of carotenoids, tocopherols, and phylloquinone (20).
The salad dressings were supplied by Unilever R&D Vlaardingen. The phylloquinone and tocopherol constituents had been stripped from the soybean oil at Unilever with the use of a short-path distillation procedure. The salad dressings were prepared by combining an aqueous base formulation with increasing amounts of the stripped soybean oil to create salad dressings containing 0, 2, 4, 8, or 32 g soybean oil per 60-g serving. The ingredients in the aqueous base formulation were as follows (grams per kilogram): ultrapure water (897.125), sugar (60), salt (6), spirit vinegar (35), citric acid monohydrate (1.8), and EDTA Dissolvine (Akzo Nobel Functional Chemicals) (0.075). To prepare the different salad dressings, the oil was substituted wt:wt for the water in the aqueous base formulation.
HPLC-electrochemical detection analyses of chylomicron fractions
Blood samples (10 mL) were collected from a forearm vein as previously described (18) and transferred to evacuated blood collection tubes containing dipotassium EDTA. A blood sample was collected at baseline, which was after a 12-h overnight fast. Additional blood samples were collected 2, 3.5, 5, 7, and 9.5 h after the test salad was consumed. Blood samples were immediately placed on ice, protected from light, and centrifuged to separate plasma. The chylomicron fraction was isolated from each plasma sample with the use of cumulative rate ultracentrifugation (18). The plasma chylomicron fractions were stored at −70°C until analyzed.
The carotenoids and fat-soluble vitamins were extracted from each plasma chylomicron fraction as previously described (18). The extracts were dried under vacuum and reconstituted in 60 μL methanol: methyl-tert-butyl ether (MTBE) (1:1, by vol). A 25-μL aliquot was injected into the HPLC-electrochemical detection (ECD) system. The 5 μm C30 Carotenoid Column (4.6 × 250 mm; Waters Corp) was eluted by a gradient; the proportions of methanol:MTBE:aqueous ammonium acetate (1.0 M, pH 4.6) in solvent A and solvent B were 95:3:2 and 25:73:2 (vol:vol), respectively. The following gradient was used: 0–40 min, linear gradient from 0% to 75% solvent B; 40–60 min, linear gradient to 100% solvent B. The flow rate was 1.0 mL/min. A CoulArray system (ESA) consisting of two 582 solvent delivery modules, 542 autosampler set at 4°C, 16-channel 5600 CoulArray coulometric array electrochemical detector, and thermal organizer set at 33°C was operated with the use of CoulArray software version 3.10. The following cell potentials in consecutive order were applied to 10 detector channels: 100, 300, 450, 550, 600, 750, 800, −1000, 200, and 500 mV. The dominant channels were 300, 450, and 750 mV for tocopherols, carotenoids, and retinyl palmitate, respectively. A reductive potential (−1000 mV) followed by an oxidative potential (200 mV) on an upstream channel was applied for analysis of phylloquinone. Calibration curves were generated for each analyte with the use of commercially available standards. The analyst (YZ) was blinded regarding the participants’ randomly assigned orders of the salad dressing treatments.
Carotenoid and fat-soluble vitamin analyses of the salad vegetables
Representative samples of the salad vegetables from each of the 5 study periods were stored at −70°C. The carotenoid and tocopherol concentrations in the vegetables were analyzed in duplicate with the use of a modification of the method of Granado et al. (21). The dried extract was reconstituted with 2 mL MTBE:methanol (1:1, vol:vol). A 25-μL aliquot was injected into the HPLC-ECD system. The phylloquinone in the salad vegetables was extracted according to a modification of the method of Koivu et al. (22). The hexane extract was evaporated to dryness under vacuum, and the dried extract was reconstituted with 200 μL MTBE:methanol (1:1, vol:vol). A 25-μL aliquot was injected into the HPLC-ECD system. Additional details regarding these extraction protocols are provided in Supplemental Methods.
HPLC-ECD analyses of the soybean oil
The concentrations of α-, δ-, and γ-tocopherols in the stripped soybean oil used to prepare the salad dressings were analyzed with the use of the rapid saponification protocol of Granado et al. (21). The reconstituted extract was filtered with the use of a 0.2-μm nylon syringe filter (Corning Inc.), and 100 μL was injected into the HPLC-ECD system. The lutein and phylloquinone contents in the stripped soybean oil were analyzed with the use of the method of Puspitasari-Nienaber et al. (23). Briefly, 40 mg oil was dissolved in 2.5 mL methanol:MTBE (1:1, vol:vol) and filtered through a 0.2-μm nylon filter. A 25-μL aliquot was then injected directly into the HPLC-ECD system.
Data analyses
For the plasma chylomicron data, the outcome variables were the 0–9.5-h total AUC values for the carotenoids and fat-soluble vitamins. Plasma chylomicron total AUC values were calculated by the trapezoidal method with the use of carotenoid and fat-soluble vitamin content (in the chylomicron fraction present in 1 L plasma) as the y axis and time (hours) as the x axis. Total AUC values included the area both above and below the baseline (0 h) value. In addition, we determined maximum content in the plasma chylomicron fraction (Cmax). The postprandial content of retinyl palmitate in the plasma chylomicron fraction was used as an indicator of the content of vitamin A, which was formed from the provitamin A carotenoids in the salad vegetables (18, 24).
Statistical modeling
The dose-response relation between the grams of soybean oil in the salad dressing and the absorption of individual carotenoid and fat-soluble vitamins in the salad vegetables (with the use of plasma chylomicron AUC and Cmax values as indicators of absorption) was modeled with the use of the following linear mixed-effects random coefficient model (25):
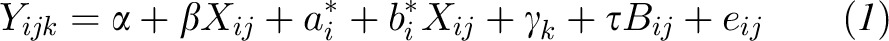 |
where Yijk is the AUC or Cmax value for the ith subject, jth amount of soybean oil in the salad dressing, and kth study period; Xij is a continuous covariate for the jth amount of soybean oil for the ith subject; γk is the period effect for the kth study period; τ is the regression coefficient for Bij; Bij is the baseline chylomicron carotenoid and fat-soluble vitamin content for the ith subject and jth amount of soybean oil; and i = 1,…,12 subjects, j = 1,…,5 amounts of soybean oil, and k = 1,…,5 study periods. According to this model, the average true trajectory of change in AUC or Cmax with increasing amount of soybean oil is defined by fixed effects α (the intercept that represents the average true AUC or Cmax value when soybean oil is 0 g), β (the slope coefficient that represents the average true change in AUC or Cmax value when soybean oil increases), γk (the average true period effect for the kth study period), and τ (the regression coefficient that represents the average true contribution of baseline chylomicron carotenoid and fat-soluble vitamin content to the AUC or Cmax value). Thus, the group-averaged (mean) fixed effects part of the model is
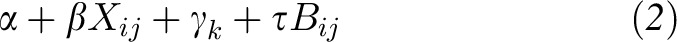 |
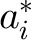 and
and 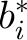 represent deviations of the intercepts and slopes of the individual subjects from their respective population averages (25). eij is the unexplained residual error associated with the measurement of subject i at soybean oil amount j. Thus, the subject-specific random-effects part of the model is
represent deviations of the intercepts and slopes of the individual subjects from their respective population averages (25). eij is the unexplained residual error associated with the measurement of subject i at soybean oil amount j. Thus, the subject-specific random-effects part of the model is
 |
The advantage of the linear mixed-effects random coefficient model is that it predicts both subject-specific and group-average trajectories of change in AUC and Cmax values with increasing amounts of soybean oil. By incorporating random effects, the group-average prediction model may be generalized to apply to future unknown subjects.
For lutein, the 0–4-g soybean oil dose response showed no evidence of subject-specific variation in the slopes. Therefore, a linear mixed-effects random intercept model was used to model the dose-response relation
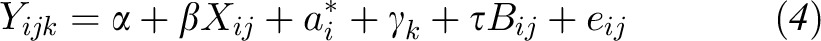 |
Based on our previous studies (7, 18), we used a Cohen’s d effect size of 1.0 (large effect size) (26) as a basis for power calculations. Our sample size of 12 subjects would detect a soybean oil slope effect (β) size of 1.0 in a 2-sided 5% test with 90% power. All random coefficient models shown were linear as indicated by P > 0.10 in the ANOVA lack-of-fit test.
Concordance
Kendall’s coefficient of concordance (Kendall’s W) was used to evaluate whether there was significant agreement across the individual carotenoids and fat-soluble vitamins in the rank order of the 12 participants in regard to their chylomicron AUC values after consuming the highest amount of soybean oil, 32 g. The value of W ranges between 0 and 1, with increasing values reflecting increasing degrees of agreement in the rank orders (27).
RESULTS
Participant compliance
Each of the 12 participants completed each of the 5 periods of the study. There were only 2 deviations from the study protocol. One participant took a single dose of drospirenone and ethinyl estradiol and a second participant took a daily dose of norgestimate and ethinyl estradiol during one of the 2-wk washout periods. These steroid hormones could potentially increase hepatic clearance of chylomicron remnants (28). The statistical analyses presented here included all data for all participants.
Carotenoids and fat-soluble vitamins in the salads
The carotenoid and fat-soluble vitamin contents in the salad vegetables across the 5 study periods are shown in Table 1. The stripped soybean oil that was used to prepare the salad dressings contained low residual amounts of tocopherols and no detectable carotenoids or phylloquinone. The analyzed mean ± SD α-, δ-, and γ-tocopherol concentrations in the stripped soybean oil were 3.75 ± 0.12 μg/g (8.71 ± 0.28 nmol/g), 7.24 ± 0.13 μg/g (17.98 ± 0.32 nmol/g), and 12.65 ± 0.40 μg/g (30.36 ± 0.96 nmol/g), respectively. The initial total tocopherol concentration in the soybean oil before short-path distillation when analyzed by Unilever R&D was 545 μg/g. Thus, the tocopherol content was reduced by 96%.
TABLE 1.
Carotenoid and fat-soluble vitamin contents in the vegetable salads from each of the 5 study periods1
| Vegetable | Weight, g | α-Carotene, mg | β-Carotene, mg | Lutein, mg | Lycopene, mg | Phylloquinone, mg | α-Tocopherol, mg | δ-Tocopherol, mg | γ-Tocopherol, mg |
| Carrot, grated | 66 | 6.688 ± 0.581 | 7.094 ± 0.505 | 0.304 ± 0.017 | 0 | 0.005 ± 0.000 | 0.536 ± 0.039 | 0 | 0 |
| Lettuce, romaine | 48 | 0 | 1.127 ± 0.063 | 1.441 ± 0.070 | 0 | 0.085 ± 0.003 | 0.298 ± 0.021 | 0.011 ± 0.001 | 0.182 ± 0.025 |
| Spinach, leaf | 48 | 0 | 2.177 ± 0.031 | 3.925 ± 0.065 | 0 | 0.120 ± 0.003 | 1.237 ± 0.165 | 0 | 0.112 ± 0.019 |
| Tomato, cherry | 85 | 0 | 1.142 ± 0.080 | 0.287 ± 0.008 | 4.485 ± 0.400 | 0.006 ± 0.000 | 1.340 ± 0.086 | 0 | 0.422 ± 0.018 |
| Total | 247 | 6.688 ± 0.581 | 11.540 ± 0.500 | 5.957 ± 0.051 | 4.485 ± 0.400 | 0.216 ± 0.004 | 3.409 ± 0.214 | 0.011 ± 0.001 | 0.716 ± 0.040 |
Values are means ± SEMs. n = 5. A representative sample of each vegetable from each of 5 study periods was analyzed in duplicate.
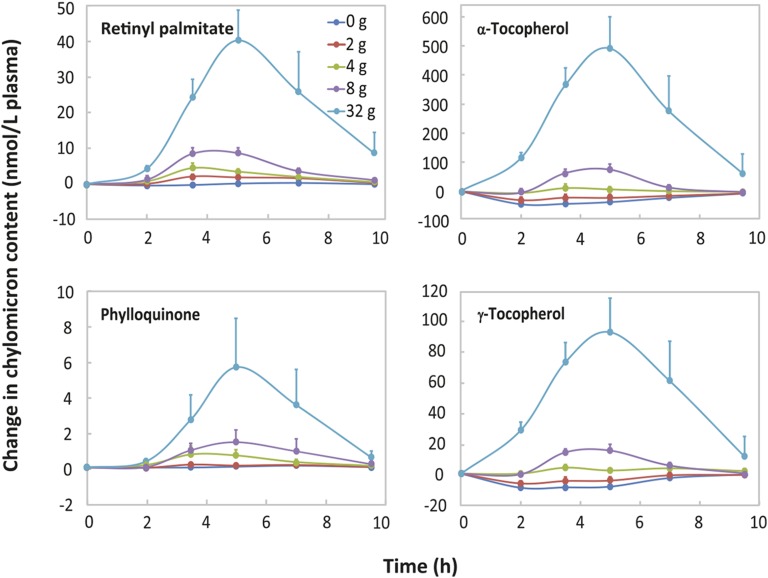
The absorption of δ-tocopherol was used as an indicator of the potential contribution of the residual α- and γ- tocopherol in the stripped soybean oil to their quantified total absorption from the salads. δ-Tocopherol was a suitable indicator because 1) δ-tocopherol was not detected in the salad vegetables with the exception of trace amounts (11 ± 1 μg or 27.3 ± 2.5 nmol) in the romaine lettuce (Table 1), and 2) there is no selectivity in the chylomicron-mediated absorption of tocopherol vitamers (29, 30). To be conservative, the small δ-tocopherol contribution from the romaine lettuce was ignored and the measured total absorption of δ-tocopherol from the salads was assumed to reflect solely the residual amounts in the stripped soybean oil. The calculations were based on the mean increment in the chylomicron δ-tocopherol AUC values, over the 0–32-g range of soybean oil, which was 105 nmol · h/L plasma (Supplemental Table 1). If normalized for the ratio of the residual amounts of α- and δ-tocopherol in the stripped soybean oil (8.71 nmol/g, α-tocopherol:17.98 nmol/g, δ-tocopherol or 0.48:1), the expected contribution of the residual α-tocopherol in the oil to the 0–32-g soybean oil mean increment in the plasma chylomicron α-tocopherol AUC was only 50 nmol · h/L plasma (0.48 × 105 nmol ∙ h/L plasma). This contribution represented <3% of the actual 0–32-g soybean oil mean increment in the chylomicron α-tocopherol AUC, which was 1939 nmol · h/L plasma (Supplemental Table 1). This minor α-tocopherol contribution from the oil was ignored. If normalized for the ratio of the residual γ- and δ-tocopherol amounts in the stripped soybean oil (30.36 nmol/g, γ-tocopherol:17.98 nmol/g, δ-tocopherol or 1.69:1), the corresponding contribution of the residual γ-tocopherol in the oil to the 0–32-g soybean oil mean increment in the plasma chylomicron γ-tocopherol AUC was 177 nmol · h/L plasma (1.69 × 105 nmol · h/L plasma). This contribution from the stripped oil represented 46% of the actual 0–32-g soybean oil mean increment in the chylomicron γ-tocopherol AUC, which was 388 nmol · h/L plasma (Supplemental Table 1). Due to the potential substantive contribution from the residual γ-tocopherol in the stripped soybean oil, unless otherwise specified, the dose-response for γ-tocopherol was analyzed in terms of the chylomicron total (α- plus γ-) tocopherol AUC values. The combined mean contribution of the residual α- and γ-tocopherol in the stripped oil, 227 nmol·h/L plasma (50 nmol · h/L plasma for α-tocopherol plus 177 nmol∙h/L plasma for γ-tocopherol), accounted for <10% of the 0–32-g soybean oil mean increment in the plasma chylomicron total (α- plus γ-) tocopherol AUC, which was 2332 nmol·h/L (Supplemental Table 1).
Soybean oil–mediated effects on the postprandial plasma chylomicron response
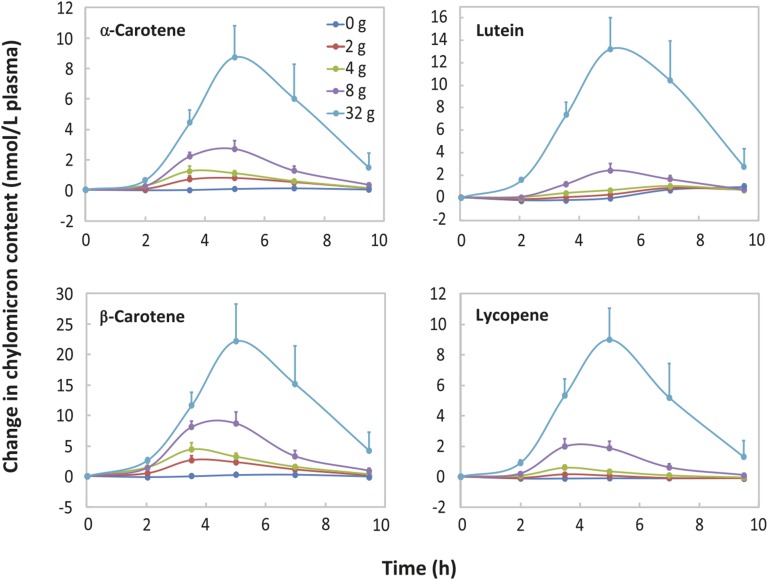
An HPLC-ECD chromatogram of the carotenoids and fat-soluble vitamins in a postprandial chylomicron fraction is shown in Supplemental Figure 2. Simultaneous analysis of carotenoids, retinyl palmitate, tocopherols, and phylloquinone revealed their remarkably similar absorption kinetics (Figures 1 and 2). The exceptions were the α- and γ-tocopherol chylomicron response curves for the lowest doses of soybean oil, 0 and 2 g (Figure 2). After consuming the test salad with 0 or 2 g of oil, the α- and γ-tocopherol contents in the chylomicron fraction initially fell below their baseline (0 h) contents in the chylomicron fraction. (Please see Supplemental Methods and Supplemental Results for the statistical analyses.) When the salads were consumed with the salad dressing containing 0 g soybean oil, there was negligible absorption of all carotenoids and fat-soluble vitamins (Figures 1 and 2). In the case of each carotenoid and fat-soluble vitamin, the highest plasma chylomicron AUC value occurred when the salad vegetables were consumed with salad dressing containing 32 g soybean oil (P < 0.002; Supplemental Table 1). (Please see Supplemental Methods and Supplemental Results for the statistical analyses).
FIGURE 1.
Mean ± SEM postprandial carotenoid contents in the plasma chylomicron fraction in young women. In a Williams Latin square design, each subject (n = 12) consumed each of the indicated amounts of soybean oil in salad dressing with fresh vegetable salads.
FIGURE 2.
Mean ± SEM postprandial fat-soluble vitamin contents in the plasma chylomicron fraction in young women. In a Williams Latin square design, each subject (n = 12) consumed each of the indicated amounts of soybean oil in salad dressing with fresh vegetable salads.
Modeling the dose-response relation between soybean oil and carotenoid and fat-soluble vitamin absorption
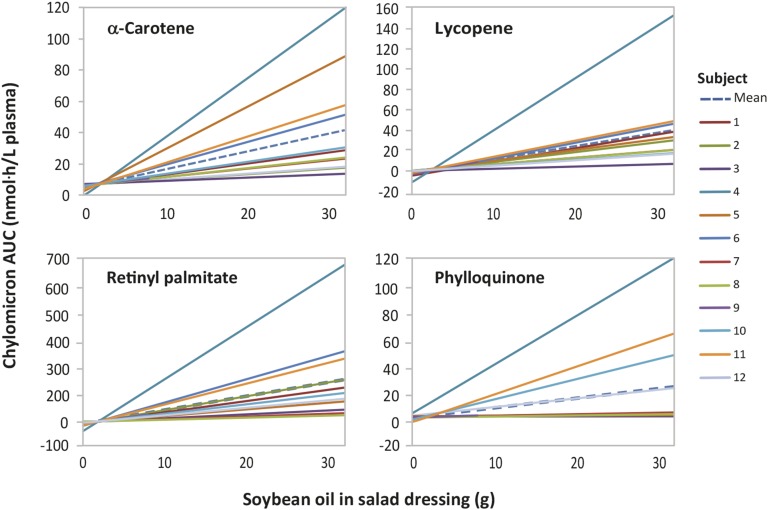
Across the entire 0–32-g range, the amount of soybean oil in the salad dressing was a significant linear predictor of the intestinal absorption, as indicated by chylomicron AUC and Cmax values, of α-carotene, lycopene, and retinyl palmitate (Tables 2 and 3, Figure 3). A nonsignificant lack-of-fit test indicated that a linear model also fit the increase in phylloquinone absorption across the entire 0–32-g range of soybean oil. The group slope variable, β (soybean oil effect), for phylloquinone approached but did not achieve significance (P = 0.057) due to the wide variability in response among the subjects (Tables 2 and 3).
TABLE 2.
Linear mixed-effects random coefficient models for predicting plasma chylomicron AUC 0–9.5-h values for carotenoids and fat-soluble vitamins after consuming a vegetable salad as a function of soybean oil (grams) in salad dressing1
| Carotenoid/fat-soluble vitamin | Intercept (α), (nmol · h/L plasma) · g−1 | Slope (β), (nmol·h/L plasma) · g−1 | P-soybean-oil effect (β) |
| Soybean oil intake range (0–32 g) | |||
| α-Carotene | 2.471 | 1.164 ± 0.326 | 0.0044 |
| Lycopene | −0.005 | 1.288 ± 0.371 | 0.0052 |
| Retinyl palmitate | 2.583 | 5.786 ± 1.681 | 0.0055 |
| Phylloquinone | 1.244 | 0.708 ± 0.333 | 0.0568 |
| Soybean oil intake range (0–8 g) | |||
| β-Carotene | 3.509 | 4.625 ± 0.881 | 0.0003 |
| Soybean oil intake range (0–4 g) | |||
| Lutein | 5.390 | 0.525 ± 0.099 | < 0.0001 |
| α-Tocopherol | 312.1 | 34.1 ± 8.0 | 0.0018 |
| Total tocopherol | 369.2 | 44.6 ± 11.0 | 0.0023 |
Values are means ± SEs; n = 12 healthy women who each consumed 5 equivalent salads. The salads with salad dressings containing 0, 2, 4, 8, or 32 g phylloquinone- and tocopherol-stripped soybean oil were consumed in random order separated by ≥2 wk. Linear mixed-effects random coefficient models were used to predict the dose-response relation between grams of soybean oil in salad dressing and the absorption of carotenoids and fat-soluble vitamins in salad vegetables with the use of the plasma chylomicron total AUC values as indicators of absorption. The model includes intercept (the average true AUC value when soybean oil intake is 0), slope (the average true change in AUC value when soybean oil intake increases), study period effects, and contribution of baseline plasma chylomicron carotenoid or fat-soluble vitamin content to the plasma chylomicron AUC value. Study period and baseline effects were recentered on their respective means; thus, the group intercept α and slope β (soybean oil effect) coefficients shown are those predicted for an average woman. All random coefficient models shown were linear as indicated by P > 0.20 in the ANOVA lack-of-fit test.
TABLE 3.
Linear mixed-effects random coefficient models for predicting plasma chylomicron Cmax values for carotenoids and fat-soluble vitamins after consuming a vegetable salad as a function of soybean oil (grams) in salad dressing1
| Carotenoid/fat-soluble vitamin | Intercept (α), (nmol/L plasma) · g−1 | Slope (β), (nmol/L plasma) · g−1 | P-soybean oil effect (β) |
| Soybean oil intake range (0–32 g) | |||
| α-Carotene | 0.523 | 0.282 ± 0.075 | 0.0031 |
| Lycopene | 0.008 | 0.292 ± 0.068 | 0.0013 |
| Retinyl palmitate | 0.450 | 1.360 ± 0.344 | 0.0023 |
| Phylloquinone | 0.252 | 0.178 ± 0.084 | 0.0593 |
| Soybean oil intake range (0–8 g) | |||
| β-Carotene | 0.725 | 1.198 ± 0.225 | 0.0003 |
| Soybean oil intake range (0–4 g) | |||
| Lutein | 1.438 | 0.012 ± 0.055 | 0.82 |
| α-Tocopherol | 67.8 | 2.8 ± 1.8 | 0.15 |
| Total tocopherol | 82.9 | 3.8 ± 2.3 | 0.13 |
Values are means ± SEs; n = 12 healthy women who each consumed 5 equivalent salads. The salads with salad dressings containing 0, 2, 4, 8, or 32 g phylloquinone- and tocopherol-stripped soybean oil were consumed in random order separated by ≥2 wk. Linear mixed-effects random coefficient models were used to predict the dose-response relation between grams of soybean oil in salad dressing and the absorption of carotenoids and fat-soluble vitamins in salad vegetables with the use of the plasma chylomicron Cmax values as indicators of absorption. The model includes intercept (the average true Cmax value when soybean oil intake is 0), slope (the average true change in Cmax value when soybean oil intake increases), study period effects, and contribution of baseline plasma chylomicron carotenoid or fat-soluble vitamin content to the plasma chylomicron Cmax value. Study period and baseline effects were recentered on their respective means; thus, the group intercept α and slope β (soybean oil effect) coefficients shown are those predicted for an average healthy young woman. All random coefficient models shown were linear as indicated by P > 0.10 in the ANOVA lack-of-fit test. Cmax, maximum content in the plasma chylomicron fraction.
FIGURE 3.
Linear dose-response relation between 0 and 32 g soybean oil in salad dressing and the absorption of α-carotene, lycopene, phylloquinone, and retinyl palmitate from salad vegetables in young women (n = 12). AUC 0–9.5-h values for contents in the plasma chylomicron fraction were used as indicators of absorption. Data were analyzed with the use of a linear mixed-effects random coefficient model. Solid lines show the predicted dose responses for individual subjects. Dotted lines indicate the group-average prediction model. The models shown are linear across the entire 0–32-g range of soybean oil as indicated by P > 0.20 in the ANOVA lack-of-fit tests.
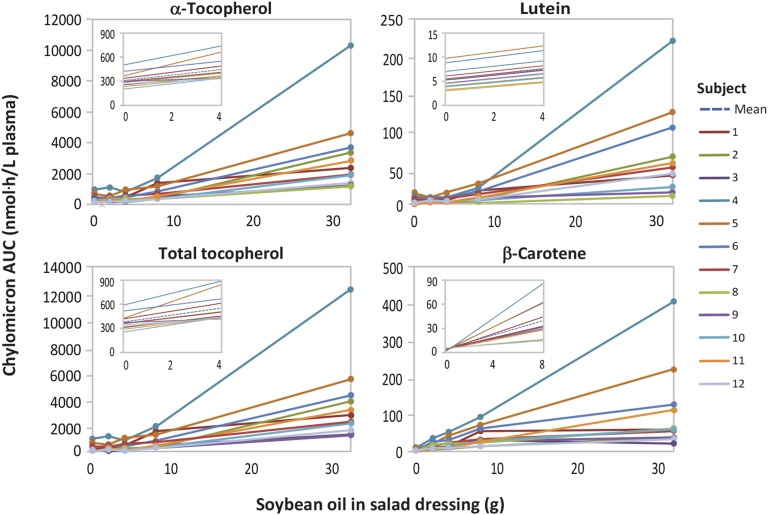
Our data indicate that the amount of coconsumed lipid was a key determinant of the absorptive capacity of the small intestine for dietary β-carotene, a major source of vitamin A. A linear model predicted the absorption of β-carotene across a limited 0–8-g range of soybean oil (Tables 2 and 3). When the amount of soybean oil increased from 8 to 32 g, about half of the subjects showed diminished responsiveness in terms of the increase in their plasma chylomicron β-carotene AUC values (Figure 4). This saturation of the soybean oil effect could reflect the higher content of β-carotene relative to the other carotenoids and fat-soluble vitamins in the salad vegetables (Table 1).
FIGURE 4.
Nonlinear dose-response relation between 0 and 32 g soybean oil in salad dressing and the absorption of α-tocopherol, total tocopherol, β-carotene, and lutein from salad vegetables in healthy young women (n = 12). AUC 0–9.5-h values for contents in the plasma chylomicron fraction were used as indicators of absorption. Solid lines show the AUC data for individual subjects. The 0–32 g soybean oil dose response for these carotenoids and fat-soluble vitamins did not fit a linear mixed-effects random coefficient model as indicated by significant ANOVA lack-of-fit tests, P < 0.04. Insets show the linear dose-response relation for a limited range of added soybean oil when analyzed with the use of a linear mixed-effects random coefficient model. Solid lines show the predicted dose responses for individual subjects. Dotted lines indicate the group-average prediction model. The models shown were linear as indicated by P > 0.10 in the ANOVA lack-of-fit tests.
Over a more limited 0–4-g range, the chylomicron AUC values for lutein, α-tocopherol, and total tocopherol were linearly related to the amount of soybean oil (P < 0.003; Table 2). Across the entire 0–32-g range of soybean oil, the AUC and Cmax responses for lutein and α- and γ-tocopherols were nonlinear as indicated by a nonsignificant lack-of-fit test. Although linear, the 0–4-g soybean oil responses were disproportionately low compared with the responses to the higher amounts of soybean oil (Figure 4). Based on the β values (Table 2), 4 g soybean oil would be predicted to increase the chylomicron AUC for lutein, α-tocopherol, and total tocopherol by a mean of 2, 136, and 180 nmol · h/L plasma, respectively. These chylomicron AUC increments represent only 5–8% of the 0–32-g soybean oil mean increments in the chylomicron AUC for lutein, α-tocopherol, and total tocopherol (42, 1939, and 2332 nmol · h/L plasma, respectively; Supplemental Table 1). Because 2 and 4 g soybean oil produced only subtle gains in lutein, α-tocopherol, and total tocopherol absorption, for these treatments, there was no clear Cmax (Figures 1 and ). As a result, the group mean 0–4-g soybean oil effect, β, was not statistically significant for Cmax (Table 3).
Figure 3 presents the results of the use of the linear mixed-effects random coefficient model to fit the 0–32-g soybean oil data for all 12 participants (regressing predicted chylomicron AUC values for α-carotene, lycopene, phylloquinone, and retinyl palmitate on grams of soybean oil individually by participant). Although in each case there was a strong group-average dose-response relation (Tables 2 and 3), the variability in the subject-specific slopes, 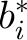 , was strikingly high. The CV ranged from 96% for α-carotene (1.16 ± 1.12 nmol · h/L plasma per gram of soybean oil) to 162% for phylloquinone (0.71 ± 1.15 nmol·h/L plasma per gram of soybean oil). There was similar high variability in the 0–8-g soybean oil data for β-carotene; the CV for
, was strikingly high. The CV ranged from 96% for α-carotene (1.16 ± 1.12 nmol · h/L plasma per gram of soybean oil) to 162% for phylloquinone (0.71 ± 1.15 nmol·h/L plasma per gram of soybean oil). There was similar high variability in the 0–8-g soybean oil data for β-carotene; the CV for 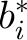 for the subject-specific trajectories of change in the AUC was 113% (3.00 ± 3.41 nmol·h/L plasma per gram of soybean oil) (Figure 4). Several participants (most notably subject 4) showed a dramatic effect of soybean oil in enhancing the absorption of the carotenoids and fat-soluble vitamins from the salad vegetables. Surprisingly, other participants showed almost no response, such that the chylomicron carotenoid and fat-soluble vitamin AUC values for 32 g soybean oil were similar to those for 0 g soybean oil. The nutritional implications are illustrated by a comparison of subjects 3 and 4, who were, respectively, the least and the most responsive to the soybean oil. When modeling the effects of 0–32 g soybean oil on the AUC for lycopene, the
for the subject-specific trajectories of change in the AUC was 113% (3.00 ± 3.41 nmol·h/L plasma per gram of soybean oil) (Figure 4). Several participants (most notably subject 4) showed a dramatic effect of soybean oil in enhancing the absorption of the carotenoids and fat-soluble vitamins from the salad vegetables. Surprisingly, other participants showed almost no response, such that the chylomicron carotenoid and fat-soluble vitamin AUC values for 32 g soybean oil were similar to those for 0 g soybean oil. The nutritional implications are illustrated by a comparison of subjects 3 and 4, who were, respectively, the least and the most responsive to the soybean oil. When modeling the effects of 0–32 g soybean oil on the AUC for lycopene, the 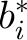 coefficient for participant 3, who was the least responsive, was 0.215 nmol · h/L plasma per gram of soybean oil. In contrast, the
coefficient for participant 3, who was the least responsive, was 0.215 nmol · h/L plasma per gram of soybean oil. In contrast, the 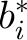 coefficient for participant 4, who was the most responsive, was 5.060 nmol · h/L plasma per gram of soybean oil. Thus, there was a 24-fold difference across the participants in the predicted enhancing effect of the soybean oil on lycopene absorption. The amount of soybean oil needed to achieve a similar nutritional benefit was highly individualized.
coefficient for participant 4, who was the most responsive, was 5.060 nmol · h/L plasma per gram of soybean oil. Thus, there was a 24-fold difference across the participants in the predicted enhancing effect of the soybean oil on lycopene absorption. The amount of soybean oil needed to achieve a similar nutritional benefit was highly individualized.
Within the bounds of the linear response, the random coefficient model indicated that the chylomicron carotenoid and fat-soluble vitamin AUC could be predicted by the intercept (chylomicron AUC for 0 g of soybean oil) and the slope (soybean oil effect). The intercept included these contributors to the chylomicron AUC: 1) the baseline (0 h) chylomicron carotenoid and fat-soluble vitamin content and 2) the absorbed carotenoids and fat-soluble vitamins for 0 g soybean oil. As expected, the baseline chylomicron carotenoid and fat-soluble vitamin contents were low, and there was negligible absorption of carotenoids and fat-soluble vitamins when the salads were consumed with 0 g soybean oil (Figures 1 and 2) (Please see Supplemental Methods and Supplemental Results for the statistical analyses). Therefore, the observed large interindividual differences in carotenoid and fat-soluble vitamin bioavailability (Figures 3 and 4) could be predicted almost entirely by the differences in slope (i.e., the responsiveness to the coconsumed oil or lipid).
Comparing carotenoid and fat-soluble vitamin bioavailability
Given the simultaneous quantification of the carotenoids and fat-soluble vitamins and their similar absorption kinetics, we were able to compare their plasma chylomicron Cmax values for the 32-g soybean oil treatment. To account for the higher baseline (0 h) content of the tocopherols in the plasma chylomicron fraction, for this comparison, baseline correction was applied to the AUC values. The amount of each absorbed carotenoid and fat-soluble vitamin circulating in the plasma chylomicron fraction at Cmax was estimated by multiplying the baseline-adjusted Cmax (nmol/L plasma) by each subject’s plasma volume (0.0427 L/kg body weight) (31, 32). The geometric means for the circulating amounts were 17.5 nmol α-carotene, 41.7 nmol β-carotene, 28.1 nmol lutein, 18.7 nmol lycopene, and 2.7 nmol phylloquinone (Supplemental Table 2). These circulating amounts represented these respective percentages of the amounts consumed in the salad vegetables: 0.14%, 0.19%, 0.27%, 0.22%, and 0.57%. The lower fractional circulating amounts of α- and β-carotene, compared with the nonprovitamin A carotenoids, lutein and lycopene, would be expected due to the partial bioconversion of the former to retinyl esters, including retinyl palmitate. These minute fractional amounts suggest that, even when consumed with 32 g soybean oil, the bioavailability of the carotenoids and phylloquinone was remarkably low. After consuming 32 g tocopherol-stripped soybean oil, the geometric means for the contents of α-tocopherol and total (α- plus γ-) tocopherol in the plasma chylomicron fraction at the baseline-adjusted Cmax were 1154 and 1370 nmol, respectively. These amounts represent ∼14% of the tocopherols in the salad vegetables (Supplemental Table 2). These single measurements at Cmax underrepresent total absorption and exclude 1) carotenoids, phylloquinone, and tocopherols already cleared from the plasma or currently in chylomicron remnants not isolated with the plasma chylomicron fraction and 2) tocopherols secreted by enterocytes in HDL in a secondary, minor pathway (30, 33). Nevertheless, at Cmax, the percentage of the α- and γ-tocopherol in the salad vegetables that was circulating in plasma chylomicrons was ∼50- to 60-fold higher compared with the percentage of the nonprovitamin A carotenoids lutein and lycopene. (These carotenoids were used for comparison because they did not undergo metabolism to vitamin A). A higher fraction of α-tocopherol and total tocopherol was circulating in the plasma chylomicron fraction at Cmax despite the higher content of each carotenoid compared with that of the α- and γ-tocopherol in the test salads (Table 1).
Rank order
When analyzed for the highest amount of soybean oil, 32 g, there was strong agreement across the individual micronutrients in the rank order of the 12 subjects in terms of their plasma chylomicron carotenoid and fat-soluble vitamin AUC values [Kendall’s coefficient of concordance W = 0.65, P value for the test of W = 0: P < 0.0001]. (Retinyl palmitate was not included in this ranking analysis because it was a metabolite of the provitamin A carotenoids). Thus, participants who absorbed higher amounts of one carotenoid and fat-soluble vitamin were also likely to absorb higher amounts of the others. This concordance is consistent with shared mechanisms of absorption.
DISCUSSION
Across the entire 0–32-g range of soybean oil, the average absorption of α-carotene, lycopene, and retinyl palmitate and vitamin A could be largely predicted by the amount of soybean oil added to fresh vegetables of dietary importance to US consumers. There was also a linear dose-response relation for phylloquinone; due to interindividual variability, the soybean oil effect (slope, β) approached but did not reach significance (P = 0.057). The absorption of β-carotene was linear over a limited range of 0–8 g soybean oil, which likely reflected the higher content of β-carotene in the vegetable salads (Table 1). There were minor linear increases in the absorption of lutein, α-tocopherol, and total tocopherol over a more limited range of 0–4 g soybean oil. An important implication is that, within the specified linear bounds, any additional soybean oil would be predicted to increase the absorption of these carotenoids and fat-soluble vitamins.
Compared with the other carotenoids and fat-soluble vitamins, our findings indicate that lutein, α-tocopherol, and total tocopherol bioavailability may benefit more from amounts >4 g soybean oil. Across the 0–32-g range of soybean oil, the dose-response for lutein, α-tocopherol, and total (α- plus γ-) tocopherol was nonlinear because of a disproportionately lower absorption response to 0–4 g compared with 8 or 32 g soybean oil (Figure 4). The xanthophyll carotenoids, lutein and zeaxanthin, and the tocopherols were reported to share 2 pathways for basolateral secretion from the enterocyte: the major triacylglycerol-dependent pathway involving chylomicron assembly and a minor ATP-binding cassette transporter A1 (ABCA1)-mediated pathway with lipid-poor apolipoprotein A1 as the acceptor, leading to small HDL formation (30, 33, 34). In human intestinal cells, both α- and γ-tocopherol were reported to inhibit apo A-I–mediated cholesterol efflux by downregulating ABCA1 via reduced liver X receptor transactivation (35). Subsequently, in human liver cells and macrophages, α- and γ-tocopherol were shown to inhibit expression of the ATP-binding cassette transporters ABCA1 and ABCG1 (36). The silencing of ABCG1 expression in these cells decreased α- and γ-tocopherol efflux to HDL. These findings suggest that the intestinal secretion of α- and γ-tocopherol to HDL in our study may have been inhibited by increased tocopherol uptake in the presence of more oil or lipid. The chylomicron-mediated absorption of tocopherols could then potentially have become disproportionately more important, resulting in the observed nonlinear dose response.
Previously, investigators compared the enhancing effects of 3 different amounts of oil or lipid on the absorption of individual carotenoids and did not observe a linear response. Unlu et al. (8) reported that, compared with control salads (no avocado), carotenoid absorption from salad vegetables was increased to the same extent by 12 or 24 g lipid from avocado. Goltz et al. (9) reported that carotenoid absorption from salad vegetables was similarly enhanced by 3 or 8 g lipid added as canola oil, soybean oil, or butter. When considering all lipid sources, 20 g lipid promoted more absorption than 3 or 8 g. The discrepancy with our findings may be attributed to our use of advanced linear mixed-effects regression modeling. Especially with the incorporation of 5 dose amounts, linear mixed-effects regression modeling has the flexibility to treat soybean oil dose as a continuous variable rather than only estimating discrete mean AUC and Cmax outcomes (37). An added advantage is that the linear regression models may then be used to interpolate the expected change in carotenoid and fat-soluble vitamin absorption due to any amount of soybean oil within the bounds of the linear range.
As highlighted by a recent review (38), there is a need for better understanding of the basis for the huge differences in carotenoid bioavailability among healthy individuals. Although candidate contributors to interindividual variability have been identified, much less is known about their relative importance (38). Linear mixed-effects modeling appropriately characterizes heterogeneity by assessing both individual-specific and group-average dose-response trends. As a result, our study revealed that the large interindividual differences in carotenoid and fat-soluble vitamin bioavailability were largely mediated by the variable responsiveness to the coconsumed lipid. This observation is consistent with recent reports that single nucleotide polymorphisms in 4–7 genes related to the postprandial chylomicron triacylglycerol response were among 25–28 single nucleotide polymorphisms in 12–16 genes associated with β-carotene and lycopene bioavailability from processed tomatoes (39, 40). We also showed the extent to which the reported “nonresponder” carotenoid bioavailability phenotype (41) is defined by nonresponse to lipid. Thus, our findings highlight the importance of personalized guidance relating to the nutritional benefits of added oil or lipid.
By addressing the holistic effects of 32 g added oil or lipid on the absorption of multiple carotenoids and fat-soluble vitamins, we identified phenotypic profiles. Study participants could be stratified according to the consistency of their bioavailability responses across the carotenoids and fat-soluble vitamins. This concordance indicates these carotenoids and fat-soluble vitamins share mechanisms for the enhancing effects of the soybean oil, which could include the following (42): 1) providing an oil phase in which the fat-soluble carotenoids and vitamins could be solubilized or dispersed during emulsification; 2) stimulating biliary secretion and thereby facilitating micelle formation; 3) on hydrolysis, providing fatty acids and monoglycerides, which are needed for the assembly of micelles; 4) providing fatty acids that may modify micellar size and surface electric charge and thereby influence interactions with scavenger receptors SR-BI and CD36 (43); and 5) inducing the formation of chylomicrons. Under controlled conditions, we showed markedly different combined carotenoid and fat-soluble vitamin exposures among healthy individuals consuming the same lipid and vegetables.
The bioavailability of the α- and γ-tocopherol in the salad vegetables was higher than that of the phylloquinone and dramatically higher than that of the carotenoids. There are few human studies of the bioavailability of vitamin E in food sources (44). Recently, the mean fractional absorption of α-tocopherol in deuterium-labeled cooked collard greens consumed with only 1.6 g fat was reported to be ≥24% (45). Among US adults who obtain vitamin E only from foods, 96% have vitamin E intakes below the estimated average requirement (46). Because vitamin E deficiency is not prevalent, there is concern that either the estimated average requirement is too high or α-tocopherol bioavailability in foods is underestimated (45). The relatively high α-tocopherol bioavailability in the salad vegetables suggests their contribution to meeting vitamin E requirements has been underestimated.
The limitations of this study include the following: 1) measurement of carotenoid and fat-soluble vitamin absorption from a single salad, which may not predict changes in carotenoid and fat-soluble vitamin status after repeated salad consumption and 2) unknown applicability of our findings to processed vegetables and other lipids. The strengths include the following: 1) use of linear mixed-effects random coefficient modeling to appropriately characterize interindividual heterogeneity and thereby also minimize bias in the group estimates of the dose-response trajectory, 2) simultaneous high-sensitivity quantification of the chylomicron response curves for 9 carotenoids and fat-soluble vitamins (including δ-tocopherol), and 3) realistic test salads that incorporated the major US dietary sources of carotenoids, phylloquinone (47), and salad and cooking oil (16). In conclusion, our findings indicate that the ability to respond to oil or lipid in the diet is a key factor that determines an individual’s global absorption of carotenoids and fat-soluble vitamins from vegetables with implications for their micronutrient status and preventable disease risk.
Acknowledgments
We thank Cindy Claassen and Diane Gillott for the excellent phlebotomy.
The authors’ responsibilities were as follows—WSW: designed and implemented the study, analyzed the data, and wrote the manuscript; YZ and AC: implemented the study, including the HPLC–ECD analyses; AC: wrote the first draft of the manuscript; PD: completed the statistical analyses, including the linear mixed-effects random coefficient model; FQ: completed the ANCOVA analyses; LMF: designed the study; and all authors: read and approved the final manuscript. FQ was hired by Unilever as a consultant, and LMF was employed by Unilever; neither FQ nor LMF had a role in conducting the study, collecting the data, statistical modeling, or preparing the manuscript. None of the remaining authors reported a conflict of interest related to the study. Unilever R&D had no role in the conduct of the study, data collection, statistical modeling, or manuscript preparation.
Footnotes
Abbreviations used: ABCA1, ATP-binding cassette transporter A1; Cmax, maximum content in the plasma chylomicron fraction; ECD, electrochemical detection; MTBE, methyl-tert-butyl ether.
REFERENCES
- 1.Moore LV, Thompson FE. Adults meeting fruit and vegetable intake recommendations – United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:709–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Moore LV, Dodd KW, Thompson FE, Grimm KA, Kim SA, Scanlon KS. Using behavioral risk factor surveillance system data to estimate the percentage of the population meeting U.S. Department of Agriculture Food Patterns fruit and vegetable intake recommendations. Am J Epidemiol 2015;181:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, et al. . Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 2012;51:637–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergnaud AC, Norat T, Romaquera D, Mouw T, May AM, Romieu I, Freisling H, Slimani N, Boutron-Ruault MC, Clavel-Chapelon F, et al. . Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am J Clin Nutr 2012;95:184–93. [DOI] [PubMed] [Google Scholar]
- 5.Rautiainen S, Wang L, Lee I-M, Manson JE, Buring JE, Sesso HD. Higher intake of fruit, but not vegetables or fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of normal BMI at baseline. J Nutr 2015;145:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin BH, Wendt M, Guthrie JF. Impact on energy, sodium and dietary fibre intakes of vegetables prepared at home and away from home in the USA. Public Health Nutr 2013;16:1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 8.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr 2005;135:431–6. [DOI] [PubMed] [Google Scholar]
- 9.Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res 2012;56:866–77. [DOI] [PubMed] [Google Scholar]
- 10.Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, Schwartz SJ. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-β-carotene tomato sauce and from carrots. J Nutr 2014;144:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su LJ, Arab L. Salad and raw vegetable consumption and nutritional status in the adult US population: results from the Third National Health and Nutrition Examination Survey. J Am Diet Assoc 2006;106:1394–404. [DOI] [PubMed] [Google Scholar]
- 12.Kimmons J, Gillespie C, Seymour J, Serdula M, Blanck HM. Fruit and vegetable intake among adolescents and adults in the United States: percentage meeting individualized recommendations. Medscape J Med 2009;11:26. [PMC free article] [PubMed] [Google Scholar]
- 13.Produce for Better Health Foundation. State of the plate: 2015 study on America’s consumption of fruit and vegetables, Produce for Better Health Foundation [Internet]. 2015. [cited 2015 May 7]. Available from: http://pbhfoundation.org/pdfs/about/res/pbh_res/State_of_the_Plate_2015_WEB_Bookmarked.pdf.
- 14. Mintel Group. Fruit and vegetables. London: Mintel Group Ltd.; 2012.
- 15.Garcia AL, Koebnick C, Dagnelie PC, Strassner C, Elmadfa I, Katz N, Leitzmann C, Hoffmann I. Long-term strict raw food diet is associated with favourable plasma β-carotene and low plasma lycopene concentrations in Germans. Br J Nutr 2008;99:1293–300. [DOI] [PubMed] [Google Scholar]
- 16.USDA, Economic Research Service. Oil crops yearbook 2017. Table 37–Salad and cooking oils: fats and oils used in manufacturing, U.S., 1980-2010 [Internet]. [updated 2017 Mar 29; cited 2017 May 22]. Available from: https://www.ers.usda.gov/data-products/oil-crops-yearbook.
- 17.Hill LS, Reid F, Morgan JF, Lacey JH. SCOFF, the development of an eating disorder screening questionnaire. Int J Eat Disord 2010;43:344–51. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Nugroho A, Rocheford T, White WS. Vitamin A equivalence of the β-carotene in β-carotene-biofortified maize porridge consumed by women. Am J Clin Nutr 2010;92:1105–12. [DOI] [PubMed] [Google Scholar]
- 19.Chinchilli VM, Esinhart JD. Design and analysis of intra-subject variability in cross-over experiments. Stat Med 1996;15:1619–34. [DOI] [PubMed] [Google Scholar]
- 20.Lester GE, Makus DJ. Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: effects of cultivar, leaf size, and storage duration. J Agric Food Chem 2010;58:2980–7. [DOI] [PubMed] [Google Scholar]
- 21.Granado F, Olmedilla B, Gil-Martinez E, Blanco I. A fast, reliable and low-cost saponification protocol for analysis of carotenoids in vegetables. J Food Compos Anal 2001;14:479–89. [Google Scholar]
- 22.Koivu TJ, Piironen VI, Henttonen SK, Mattila PH. Determination of phylloquinone in vegetables, fruits, and berries by high-performance liquid chromatography with electrochemical detection. J Agric Food Chem 1997;45:4644–9. [Google Scholar]
- 23.Puspitasari-Nienaber NL, Ferruzzi MG, Schwartz SJ. Simultaneous detection of tocopherols, carotenoids, and chlorophylls in vegetable oils by direct injection C30 RP-HPLC with coulometric electrochemical array detection. J Am Oil Chem Soc 2002;79:633–40. [Google Scholar]
- 24.Berr F, Kern F. Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res 1984;25:805–12. [PubMed] [Google Scholar]
- 25.Singer J, Willett J. Applied longitudinal data analysis. Modelling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc.; 1988. p. 45–8. [Google Scholar]
- 27.Gibbons JD. Nonparametric measures of association. Newbury Park (CA): Sage Publications; 1993. [Google Scholar]
- 28.Berr F, Eckel RH, Kern F. Contraceptive steroids increase hepatic uptake of chylomicron remnants in healthy young women. J Lipid Res 1986;27:645–51. [PubMed] [Google Scholar]
- 29.Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J Lipid Res 1992;33:1171–82. [PubMed] [Google Scholar]
- 30.Nicod N, Parker RS. Vitamin E secretion by Caco-2 monolayers to APOA1, but not to HDL, is vitamer selective. J Nutr 2013;143:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol 1984;247:F632–6. [DOI] [PubMed] [Google Scholar]
- 32.Bruno RS, Leonard SW, Park S, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am J Clin Nutr 2006;83:299–304. [DOI] [PubMed] [Google Scholar]
- 33.Anwar K, Iqbal J, Hussain MM. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J Lipid Res 2007;48:2028–38. [DOI] [PubMed] [Google Scholar]
- 34.Niesor EJ, Chaput E, Mary J-L, Staempfli A, Topp A, Stauffer A, Wang H, Durrwell A. Effect of compounds affecting ABCA1 expression and CETP activity on the HDL pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids 2014;49:1233–43. [DOI] [PubMed] [Google Scholar]
- 35.Landrier JF, Gouranton E, Reboul E, Cardinault N, El Yazidi C, Malezet-Desmoulins C, André M, Nowicki M, Souidi M, Borel P. Vitamin E decreases endogenous cholesterol synthesis and apo-AI-mediated cholesterol secretion in Caco-2 cells. J Nutr Biochem 2010;21:1207–13. [DOI] [PubMed] [Google Scholar]
- 36.Olivier M, Bott GR, Frisdal E, Nowicki M, Plengpanich W, Desmarchelier C, Roi S, Quinn CM, Gelissen I, Jessup W, et al. . ABCG1 is involved in vitamin E efflux. Biochim Biophys Acta 2014;1841:1741–51. [DOI] [PubMed] [Google Scholar]
- 37.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004;61:310–7. [DOI] [PubMed] [Google Scholar]
- 38.Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W, Rühl R, Keijer J, Borel P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res 2017. Feb 27 (Epub ahead of print; DOI: 10.1002/mnfr.201600685). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borel P, Desmarchelier C, Nowicki M, Bott R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med 2015;83:238–44. [DOI] [PubMed] [Google Scholar]
- 40.Borel P, Desmarchelier C, Nowicki M, Bott R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary β-carotene bioavailability in healthy men. J Nutr 2015;145:1740–7. [DOI] [PubMed] [Google Scholar]
- 41.Bowen PE, Garg V, Stacewicz-Sapuntzakis M, Yelton L, Schreiner RS. Variability of serum carotenoids in response to controlled diets containing six servings of fruits and vegetables per day. Ann N Y Acad Sci 1993;691:241–3. [DOI] [PubMed] [Google Scholar]
- 42.Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients 2013;5:3563–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goncalves A, Gontero B, Nowicki M, Margier M, Masset G, Amiot M-J, Reboul E. Micellar lipid composition affects micelle interaction with class B scavenger receptor extracellular loops. J Lipid Res 2015;56:1123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borel P, Preveraud D, Desmarchelier C. Bioavailability of vitamin E in humans: an update. Nutr Rev 2013;71:319–31. [DOI] [PubMed] [Google Scholar]
- 45.Traber MG, Leonard SW, Bobe G, Fu X, Saltzman E, Grusak MA, Booth SL. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr 2015;101:752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey RL, Fulgoni VL III, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 2012;112:657–63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet 2012;112:222–9. [DOI] [PubMed] [Google Scholar]