Abstract
Background: Emerging evidence suggests novel roles for bacterially derived vitamin K forms known as menaquinones in health and disease, which may be attributable in part to anti-inflammatory effects. However, the relevance of menaquinones produced by gut bacteria to vitamin K requirements and inflammation is undetermined.
Objective: This study aimed to quantify fecal menaquinone concentrations and identify associations between fecal menaquinone concentrations and serum vitamin K concentrations, gut microbiota composition, and inflammation.
Design: Fecal and serum menaquinone concentrations, fecal microbiota composition, and plasma and fecal cytokine concentrations were measured in 80 men and postmenopausal women (48 men, 32 women, age 40–65 y) enrolled in a randomized, parallel-arm, provided-food trial. After consuming a run-in diet for 2 wk, participants were randomly assigned to consume a whole grain–rich (WG) or a refined grain–based (RG) diet for 6 wk. Outcomes were measured at weeks 2 and 8.
Results: The median total daily excretion of menaquinones in feces was 850 nmol/d but was highly variable (range: 64–5358 nmol/d). The total median (IQR) fecal concentrations of menaquinones decreased in the WG diet compared with the RG diet [−6.8 nmol/g (13.0 nmol/g) dry weight for WG compared with 1.8 nmol/g (12.3 nmol/g) dry weight for RG; P < 0.01)]. However, interindividual variability in fecal menaquinone concentrations partitioned individuals into 2 distinct groups based on interindividual differences in concentrations of different menaquinone forms rather than the diet group or the time point. The relative abundances of several gut bacteria taxa, Bacteroides and Prevotella in particular, differed between these groups, and 42% of identified genera were associated with ≥1 menaquinone form. Menaquinones were not detected in serum, and neither fecal concentrations of individual menaquinones nor the menaquinone group was associated with any marker of inflammation.
Conclusion: Menaquinone concentrations in the human gut appear highly variable and are associated with gut microbiota composition. However, the health implications remain unclear. This trial was registered at clinicaltrials.gov as NCT01902394.
Keywords: vitamin K, microbiome, metabolomics, menaquinones, phylloquinone, whole grain
INTRODUCTION
Emerging evidence suggests novel roles for vitamin K in health and disease beyond the vitamin’s canonical function in hemostasis (1–5). Underpinning mechanisms have been attributed both to diverse actions of extrahepatic vitamin K-dependent proteins (6, 7) and to pathways that are independent of vitamin K’s enzyme cofactor activity (2, 4, 5, 8). Of particular interest is the growing literature of in vitro (9–13), animal (12, 14), and epidemiologic (15–17) studies suggesting anti-inflammatory effects of vitamin K. Collectively, these discoveries have contributed to increased interest in defining an optimal dietary vitamin K requirement (1). However, one impediment to developing these recommendations is an incomplete understanding of the relevance of vitamin K produced by bacteria in the human gut to health.
K vitamins are a family of fat-soluble vitamers that share a napthoquinone ring structure but differ in the length and saturation of an attached lipophilic side chain (8). Phylloquinone (vitamin K-1) is synthesized by plants (8), is the predominant dietary vitamin K form, and is the form on which current dietary recommendations are based (18). Menaquinone (MKn, with “n” representing the number of isoprenoid units in the side chain) 4 differs from phylloquinone in that it has an unsaturated side chain, is endogenously synthesized by mammals via the conversion of phylloquinone or menadione (19), and is found in some animal-based food products (20). Additional menaquinone forms contain unsaturated side chains varying in length from 5 (MK5) to 13 (MK13) repeating isoprenoid units and are synthesized by bacteria (21–23). These bacterially derived menaquinones are thought to be less abundant than phylloquinone in most diets, although some fermented food products (20), dairy products (24), and meats (25) are sources. All known vitamin K vitamers share bioactivity as enzyme cofactors of the γ-glutamate carboxylase enzyme responsible for activating multiple vitamin K-dependent proteins (6, 7). However, bioavailability and bioactivity appear to differ across vitamers, with evidence supporting superior bioavailability (26–29), greater bioactivity, and possibly unique functions of some bacterially derived menaquinone forms relative to phylloquinone (30–32). These observations suggest that even low amounts of gut bacteria–derived menaquinones could meaningfully affect health.
Despite longstanding knowledge that gut bacteria produce menaquinones (21), relatively few studies have attempted to quantify the menaquinone content of the human gut (33–35), identify factors contributing to variability in gut menaquinone content, or elucidate to what extent menaquinones synthesized by gut bacteria affect human health. To begin addressing these knowledge gaps, our group recently used culture-independent 16S ribosomal DNA sequencing to demonstrate that variability in human fecal menaquinone content is associated with relatively few genera within the gut microbiota and that diet-mediated alterations in gut microbiota composition may provide a feasible approach for altering gut menaquinone content (36). However, limitations to that effort included the inability to quantify fecal menaquinone concentrations, assess dietary vitamin K intake, or measure vitamin K status. This study aimed to both replicate and extend those findings by examining relations between fecal menaquinone content and serum vitamin K concentrations, gut microbiota composition, and inflammation in healthy adults participating in a provided-food study.
METHODS
The study was conducted at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University between May 2012 and September 2014, with approval by the Tufts Medical Center/Tufts University Health Sciences Institutional Review Board. All participants gave written informed consent before participating and received a stipend. The study was registered on clinicaltrials.gov as NCT01902394. The outcomes presented herein were secondary outcomes included in a randomized trial designed to determine the effects of substituting whole grains for refined grains in the diet on immune function, health biomarkers and energy metabolism, results of which have been previously reported (37, 38).
Participants
Men and postmenopausal women, all nonsmokers and 40–65 y old with a BMI (in kg/m2) between 20 and 35 were recruited from the Boston, Massachusetts area with the use of direct mailing, social media, and posted advertisements. Enrolled participants were weight-stable, reported consuming a low-fiber diet (men: <7 g/1000 kcal, women: <8 g/1000 kcal) for ≥2 wk before enrollment, abstained from probiotic or prebiotic supplements for ≥2 wk before enrollment, and abstained from all nutritional supplements other than calcium and vitamin D for ≥1 mo of enrollment. Exclusion criteria included oral antibiotic use within 3 mo of enrollment; consuming >2 alcoholic beverages/d; abnormal liver and kidney function tests; fasting blood glucose ≥125 mg/dL; diabetes; gastrointestinal disease; autoimmune disease; cancer; regular use of medications affecting energy metabolism, glycemia, appetite, or bowel habits; and use of immunosuppressants, proton pump inhibitors, H2-blockers, or prescribed nonsteroidal anti-inflammatory medications. Participants with hypertension or a medical history of cardiovascular, liver, or renal diseases were permitted if the condition was controlled with medication.
Study design and diet
Details of the study design have been previously described (37, 38). The study was an 8-wk randomized, single-blind, parallel-arm, provided-food trial. All participants received the same body weight-maintaining diet for the initial 2 wk of the study. Participants were then randomly assigned by the study statistician using a random-number generator and provided a weight-maintaining whole-grain–rich (WG) diet or a weight-maintaining refined-grain–based (RG) control diet for 6 wk. Dietary staff were aware of group assignment, but study investigators, outcome assessors, and data analysts were not until the primary analyses were complete. Participants were not explicitly informed of their group assignment, but the diets differed in appearance. Participants picked up their measured food from the research center 3 times/wk and were instructed to consume all of the provided food and nothing else, to provide documentation about all food eaten, to return any uneaten food so that it could be weighed back and deducted from daily intake, and to maintain habitual physical activity patterns. Study outcomes were assessed during study weeks 2 (the end of the run-in diet) and 8 (the end of the intervention diet).
A 3-d rotating menu was designed for all diets. The run-in diet contained no whole grain and provided 8 g/1000 kcal fiber (Supplemental Table 1). The WG and RG diets were designed to differ in whole-grain [RG: 0 g/d, WG (mean ± SD): 207 ± 39 g/d] and fiber (RG: 8 g/1000 kcal, WG: 16 g/1000 kcal) contents but to be otherwise similar in energy and macronutrient composition (Supplemental Table 1) and the types of provided foods (37, 38). The primary difference between the intervention diets was whether grains were derived from whole-grain or refined-grain sources. The primary whole grain in the WG diet was wheat, although oats and brown rice were also included. Both diets met Dietary Reference Intakes for all vitamins, minerals, and essential fatty acids. Diet composition was analyzed by using the Nutrition Data System for Research v2011 (Nutrition Coordinating Center, University of Minnesota). Diet adherence was assessed by reviewing daily food checklists maintained by the participants, monitoring body weight, and measuring plasma alkylresorcinol homolog concentrations, which are considered biomarkers of whole-grain wheat intake (39).
Serum, fecal, and diet vitamin K analysis
Blood samples were collected by antecubital venipuncture after a ≥12-h overnight fast. For fecal analysis, volunteers collected all stool produced over 72 h using separate preweighed plastic containers for each sample. Sample weights were recorded by study staff before combining and homogenizing all samples. Aliquots were obtained from the 72-h homogenate, freeze-dried to a constant weight, and homogenized into a fine powder by using a mortar and pestle. For chemical analysis of dietary vitamin K content, aliquots were taken from homogenates of each of the 3 daily menus provided within the RG and WG diets.
All sample analyses were performed under yellow light to prevent photo-oxidation and decomposition of vitamin K vitamers. Serum (500 μL), dried fecal samples (40 mg), and diet samples (200 mg) were used for quantification of phylloquinone and MK4 through MK13 by using HPLC-MS (Agilent 12260 HPLC and 6130 Quadrupole mass spectrometer; Agilent Technologies) as previously described (40). Diet MK4 was also measured by reversed-phase HPLC as previously described (41) because of an interfering peak when using the HPLC-mass spectrometry method. Deuterium-labeled phylloquinone was used as an internal standard in all samples, and calibration standards for each vitamer were used to quantify vitamer concentrations (40). Limits of detection were 1 pmol/g for MK10; 5 pmol/g for MK5, MK7, MK8, MK9, MK11, MK12, and MK13; 10 pmol/g for MK6; and 30 pmol/g for phylloquinone and MK4 (40). MK5 and MK8 were not detected in 27% and 12% of fecal samples, respectively, and the limit of detection was imputed for analysis.
Gut microbiota composition
Gut microbiota analysis was previously reported in detail (38). Briefly, fecal samples for gut microbiota composition were delivered to the laboratory on ice within 24 h of production and then frozen. DNA was extracted by using the QIAamp DNA Stool Mini kit (Qiagen), with slight modifications from manufacturer recommendations as previously described (38). Amplicons of the V4 region of the bacterial 16S ribosomal DNA were generated with the appropriate primers (42) and pooled in equimolar amounts. Amplicon pools were then purified for 250 bp paired end sequencing on the Illumina MiSeq platform (Illumina Inc.). Sequencing data were processed by using the Quantitative Insights Into Microbial Ecology package v1.9.0 (43). Reads were clustered at 97% identity and assigned taxonomy by open reference by using the Greengenes v13_5 reference database (44) and USEARCH v6.1 (45).
Blood biochemistries
Blood samples were collected by antecubital venipuncture after a ≥12-h overnight fast. Plasma TNF-α, IL-6, and IL-1β concentrations were analyzed by electrochemiluminescence assays (Meso Scale Discovery) according to manufacturer instructions. Serum glucose was measured by enzyme-coupled kinetic assay and serum insulin by radioimmunoassay (Millipore) and were used to calculate HOMA-IR. Serum triglycerides were measured by enzymatic, calorimetric endpoint assay.
Fecal cytokines
Fecal water cytokine concentrations were measured by using fecal samples delivered to the laboratory within 24 h of production as previously described (38). Fecal TNF-α, IL-6, and IL-17 were measured by high-sensitivity ELISA following manufacturer instructions.
Statistical analysis
Sample size estimates indicated that 40 volunteers/group, assuming 10% attrition, would provide sufficient power at α = 0.05 and power = 0.80 to detect between-group differences in primary study outcomes, which are reported elsewhere (37, 38). Power calculations were not completed for vitamin K measurements because they were secondary study outcomes. Data were assessed quantitatively and graphically for normality and transformed if necessary to meet model assumptions. Statistical analyses were completed by using SPSS v 21.0 (IBM Analytics), R v 3.0.3, and XLSTAT 2016 (Addinsoft). Values are presented as means ± SEMs or medians (IQRs) unless otherwise noted.
ANCOVA was used to examine intervention effects on vitamin K concentrations. In all models, the log10-transformed change score (week 8 − week 2) was entered as the response variable, study group as a between-subjects factor, and log10-transformed pre-intervention vitamer concentration, age, sex, and pre-intervention BMI as covariates. Associations between fecal vitamin K concentrations, dietary intake, and biological outcomes, including serum phylloquinone, HOMA-IR, and markers of inflammation, were assessed by using linear mixed models. Models included log10-transformed dietary intake or the biological outcome as the response variable, subject as a random factor, log10-transformed fecal vitamer concentration as an independent variable, and pre-intervention BMI, age, sex, and time as covariates. Phylloquinone is transported in circulation on triglyceride-rich proteins (46). Therefore, to control for potential confounding from differences in triglyceride concentrations, log10-transformed serum triglyceride concentrations were included in all models in which serum phylloquinone was the dependent variable. P values from each analysis were adjusted by using Bonferroni’s correction.
Exploratory analyses were conducted to identify relations between vitamin K vitamers and between vitamin K vitamer concentrations, gut microbiota composition, and biological outcomes. Relations between fecal vitamin K vitamers were examined by using principal components analysis (PCA) and partitioning around medoids clustering of the Spearman’s correlation matrix constructed from all vitamers measured at both time points in the full cohort. All vitamer concentrations were log10-transformed before analysis to meet model assumptions and to reduce undue influence from outliers. The Silhouette Index (SI) was calculated to measure partitioning around medoids cluster quality and used to determine the optimal number of clusters. An SI score of 0.50–0.75 was considered indicative of moderately strong clustering, and a score ≥0.75 indicative of strong clustering (47). Clusters with SI scores <0.50 were considered weak and were not included in the final results. PCA was completed by using XLSTAT, and clustering was completed by using the R package “cluster.”
Associations between vitamin K clusters identified by partitioning around medoids clustering (see Results) and biological outcomes, including fecal vitamer concentrations, serum phylloquinone, HOMA-IR, and markers of inflammation, were assessed by using linear mixed models. Models included biological outcome as the response variable, subject as a random factor, cluster assignment as an independent variable, and pre-intervention BMI, age, sex, and time as covariates. Log10-transformed serum triglyceride concentrations were included in all models in which serum phylloquinone was the dependent variable. P values for associations between vitamin K clusters and individual vitamers were adjusted by using Bonferroni’s correction.
Procrustes analysis, linear discriminant analysis of effect size (48), and linear mixed models were used to examine associations between fecal vitamin K concentrations and gut microbiota composition. Procrustes analysis superimposes and assesses congruence between ordinations of multiple data matrices by rotating the ordinations to maximal similarity. Herein, Procrustes analysis was used to compare the PCA of fecal vitamin K concentrations with the principal coordinates analysis of gut microbiota composition. Weighted UniFrac distances, which have been reported elsewhere (38), were used for the gut microbiota principal coordinates analysis. Linear discriminant analysis of effect size was used to identify taxa that differed in relative abundance between vitamin K clusters identified by partitioning around medoids clustering (see Results). Operational taxonomic units (OTUs) not present in >10% of samples (n = 142) were removed before analysis to reduce both the influence of uncommon OTUs on results and noise in the visualizations. To further examine associations between individual genera and individual vitamers, the Spearman correlation was used to identify genera significantly associated (P < 0.05) with ≥1 vitamer at both study time points. Relations between those genera and individual vitamers were then assessed by using linear mixed models, which included the log10-transformed vitamer concentration as the dependent variable, arcsine-square root–transformed genus relative abundance as the independent variable, subject as a random factor, and time as a covariate. When an OTU could not be classified to the genus level, the next highest level of classification was used (e.g., family, order). False discovery rate for linear discriminant analysis of effect size analysis and genus-vitamin K associations was controlled by using the Benjamini-Hochberg correction. Benjamini-Hochberg–adjusted P values are presented as Q values. Analyses included all study completers except one individual in the RG diet group who did not provide a fecal sample during the week 8 collection. Statistical significance was set at P ≤ 0.05 or Q ≤ 0.05.
RESULTS
Of 103 enrolled participants, 90 completed the run-in phase and were randomly assigned, 81 completed the study, and 80 provided samples for analyses reported herein (Table 1, Supplemental Figure 1). Dietary compliance and primary study outcomes have been previously reported (37, 38) and are briefly summarized in the following paragraph.
TABLE 1.
Study participant characteristics and estimated vitamin K intake based on chemical analysis of provided diets1
| Refined-grain diet group | Whole-grain diet group | |
| Sex, n, M/F | 24/15 | 24/17 |
| Race, n | ||
| Caucasian | 20 | 23 |
| Black | 9 | 9 |
| Hispanic | 2 | 2 |
| Asian | 3 | 6 |
| Other/not reported | 5 | 1 |
| Age, y | 54 ± 5 | 55 ± 6 |
| Weight, kg | 75.3 ± 12.1 | 74.7 ± 12.4 |
| BMI, kg/m2 | 25.8 ± 3.3 | 25.7 ± 3.9 |
| Phylloquinone, nmol/d | 401 ± 89 | 514 ± 87a |
| MK4, nmol/d | 130 ± 29 | 99 ± 18a |
| MK9, nmol/d | 165 ± 37 | 279 ± 48a |
| MK12, nmol/d | 21 ± 5 | 24 ± 4a |
| MK13, nmol/d | 5 ± 1 | 14 ± 3a |
| MK5–MK8, MK10–MK11, nmol/d | ND | ND |
Values are means ± SDs unless otherwise indicated. aP < 0.001, independent samples t test. MK, menaquinone; ND, not detected.
Plasma alkylresorcinol concentrations increased in WG diet group but did not change in RG diet group (time-by-diet interaction, P < 0.001), and body weight was maintained in both groups (main effect of time, P = 0.13; time-by-diet interaction, P = 0.27) indicating adherence to the study diets (37). Compared with the RG diet, the WG diet was associated with an increase in fecal mass [ΔWG (week 8 — week 2) compared with ΔRG (week 8 — week 2) difference: 69 ± 13 g wet weight/d; P < 0.001], fecal energy content (ΔWG compared with ΔRG difference: 96 ± 18 kcal/d; P < 0.001), and fecal acetate concentrations (ΔWG compared with ΔRG difference: 2.1 ± 1.4 mmol/L; P = 0.02) (37, 38). The relative abundance of Lachnospira increased (ΔWG compared with ΔRG difference: 1.04% ± 0.33% relative abundance), and the relative abundance of Enterobacteriaceae decreased (ΔWG compared with ΔRG difference: −0.07% ± 0.11% relative abundance) in the WG diet group compared with the RG diet group (false discovery rate-adjusted P = 0.25) (38). Changes in fecal anaerobic (WG: −2.2 ± 1.0 CFU × 109 compared with RG: 8.1 ± 9.0 CFU × 109; P = 0.31) and aerobic (WG: −3.7 ± 3.2 CFU × 107 compared with RG: −7.3 ± 6.3 CFU × 107; P = 0.32) bacteria cell counts, and fecal pH (WG: −0.03 ± 0.06 compared with RG: 0.01 ± 0.05; P = 0.31) did not differ between groups (38). Likewise, changes in salivary IgA (ΔWG compared with ΔRG difference: −18 ± 28 μg/mL; P = 0.65), and stool IgA (ΔWG compared with ΔRG difference: −0.1 ± 14.8 mg/mL; P = 0.92) concentrations did not differ between groups (38). No between-group differences in delayed-type hypersensitivity to Tetanus (P = 0.94), Candida (P = 0.51), or Trichophyton (P = 0.93) were observed (38). Further, no between-group differences in changes in T cell proliferation in response to mitogens, white blood cell count differential, or lymphocyte phenotype were observed with the exception that the change in the percentage of total terminal effector memory T cells was higher in the WG diet group than in the RG diet group (ΔWG compared with ΔRG difference: 2.7% ± 2.3%; P = 0.03) (38). Changes in plasma TNF-α (ΔWG compared with ΔRG difference: −0.4 ± 0.4 pg/mL; P = 0.48), IL-6 (ΔWG compared with ΔRG difference: −0.5 ± 0.5 pg/mL; P = 0.32), and IL-1β (ΔWG compared with ΔRG difference: 0.05 ± 0.5 pg/mL; P = 0.55), and fecal water TNF-α (ΔWG compared with ΔRG difference: −0.5 ± 0.6 pg/mL; P = 0.82), IL-6 (ΔWG compared with ΔRG difference: −0.1 ± 0.2 pg/mL; P = 0.89), and IL-17 (ΔWG compared with ΔRG difference: −1.3 ± 1.1 pg/mL; P = 0.88) concentrations did not differ between groups (38). The change in LPS-stimulated production of TNF-α (ΔWG compared with ΔRG difference: 2131 ± 1399 pg/mL; P = 0.04), but not other cytokines, was greater in the WG diet group than in the RG diet group (38). No between-group differences in the changes in the ex vivo production of cytokines after stimulation with anti-CD3 and anti-CD28 were observed, and changes in natural killer cell activity did not differ between groups (38).
Intervention effects on serum and fecal vitamin K
The measured phylloquinone, MK9, MK12, and MK13 contents of the WG diet were greater than those in the RG diet, resulting in a modestly higher measured phylloquinone (WG compared with RG: 232 ± 40 μg/d compared with 181 ± 40 μg/d), MK9 (WG compared with RG: 219 ± 38 μg/d compared with 130 ± 29 μg/d), MK12 (WG compared with RG: 24 ± 4 μg/d compared with 21 ± 5 μg/d), and MK13 (WG compared with RG: 15 ± 3 μg/d compared with 5 ± 1 μg/d) intake in the WG diet group compared with the RG diet group (P < 0.001 for all) (Table 1). In contrast, the measured MK4 intake was modestly lower in the WG diet group (45 ± 8 μg/d) than in the RG diet group (59 ± 13 μg/d, P < 0.001). No additional menaquinones were detected in either diet.
Changes in serum phylloquinone concentrations did not differ between the groups, and no menaquinones were detected in any serum sample at either time point (Table 2). Fecal MK4, MK7, MK8, MK10, MK11, and total MK5–MK13 concentrations decreased in the WG diet group compared with the RG diet group (Table 2, Supplemental Table 2). However, when fecal vitamer concentrations were expressed as total daily excretion in feces, only between-group differences in MK13 were statistically significant after Bonferroni’s correction (Table 2, Supplemental Table 2) because of the increase in daily fecal mass in the WG diet group compared with the RG diet group. Serum phylloquinone was positively associated with daily phylloquinone excretion in feces (β ± SE: 0.2 ± 0.1, P < 0.001), but not with daily excretion of any menaquinones. Dietary intakes of phylloquinone (β ± SE: 0.8 ± 0.3, P = 0.03), MK12 (β ± SE: 1.2 ± 0.4, P = 0.004), and MK13 (β ± SE: 0.7 ± 0.05, P < 0.001), but not MK9 (β ± SE: 0.4 ± 0.2, P = 0.21), were associated with daily excretion of those vitamers in feces.
TABLE 2.
Serum and fecal vitamin K before and after consuming a refined grain–based or whole grain–rich diet for 6 wk1
| Refined grain (n = 39) | Whole grain (n = 40 or 41)2 | ||||
| Week 2 | ΔWeek 2–8 | Week 2 | ΔWeek 2–8 | P3 | |
| Serum, nmol/L | |||||
| Phylloquinone | 1.0 (0.6) | 0.02 (0.8) | 1.2 (0.7) | −0.1 (0.7) | 0.96 |
| MK4–MK13 | <LOD | <LOD | <LOD | <LOD | — |
| Fecal, nmol/g dry weight | |||||
| Phylloquinone | 2.7 (0.9) | 1.7 (3.3) | 2.6 (1.9) | 0.6 (2.2) | 0.04 |
| MK4 | 1.4 (1.6) | 0.8 (1.0) | 1.3 (1.9) | 0.04 (1.0) | <0.001 |
| MK5 | 0.3 (0.4) | 0 (0.1) | 0.2 (0.4) | −0.02 (0.1) | 0.02 |
| MK6 | 1.0 (1.9) | 0.04 (0.4) | 1.0 (1.3) | −0.1 (0.4) | 0.02 |
| MK7 | 0.9 (0.8) | 0.2 (0.6) | 0.8 (0.7) | −0.1 (0.3) | 0.002 |
| MK8 | 0.3 (0.3) | −0.01 (0.3) | 0.3 (0.3) | −0.1 (0.2) | 0.002 |
| MK9 | 1.6 (1.6) | −0.03 (0.5) | 1.5 (1.7) | −0.4 (0.9) | 0.005 |
| MK10 | 8.0 (8.5) | 0.4 (3.1) | 8.0 (6.1) | −2.7 (4.1) | <0.001 |
| MK11 | 7.4 (12.6) | 0.6 (3.0) | 5.7 (6.7) | −1.5 (2.7) | <0.001 |
| MK12 | 0.8 (20.2) | 0.03 (1.2) | 0.5(17.0) | −0.03 (0.7) | 0.09 |
| MK13 | 0.4 (31.6) | 0.0 (1.7) | 0.3 (24.9) | 0.05 (1.9) | 0.02 |
| Total MK5–MK13 | 34.5 (59.9) | 1.8 (12.3) | 25.3 (39.8) | −6.8 (13.0) | <0.001 |
| Fecal, nmol/d | |||||
| Phylloquinone | 63 (54) | 11 (95) | 76 (77) | 68 (123) | 0.07 |
| MK4 | 33 (44) | 8 (51) | 33 (48) | 15 (36) | 0.53 |
| MK5 | 5 (12) | 0 (5) | 6 (9) | 0 (6) | 0.94 |
| MK6 | 27 (41) | −4 (30) | 22 (43) | 4 (28) | 0.13 |
| MK7 | 25 (26) | 0 (21) | 20 (19) | 8 (27) | 0.25 |
| MK8 | 6 (8) | 0 (8) | 7 (7) | 0 (7) | 0.11 |
| MK9 | 43 (58) | −6 (36) | 43 (51) | 0 (43) | 0.27 |
| MK10 | 194 (205) | 0 (163) | 247 (281) | 15 (198) | 0.69 |
| MK11 | 207 (259) | −9 (127) | 166 (200) | 18 (184) | 0.38 |
| MK12 | 17 (430) | 0 (76) | 13 (354) | 5 (145) | 0.03 |
| MK13 | 21 (607) | 0 (110) | 10 (570) | 8 (144) | <0.001 |
| Total MK5–MK13 | 909 (1161) | −85 (710) | 780 (1167) | 77 (863) | 0.48 |
Values are medians (IQRs). No statistically significant differences at week 2. See also Supplemental Table 2. LOD, limit of detection; MK, menaquinone.
Stool weight was unavailable for 1 subject.
ANCOVA, main effect of the group adjusted for week 2 vitamer concentration, age, sex, and pre-intervention BMI. Serum phylloquinone also adjusted for change in serum triglycerides. Dependent variables are the change in vitamer concentration. All vitamers were log10-transformed for analysis. P ≤ 0.004 is statistically significant after Bonferroni’s adjustment.
Covariance between fecal vitamin K vitamers partitioned individuals into menaquinotypes
Principal components analysis indicated that 74% of the variability in fecal vitamin K vitamer concentrations was explained by 3 principal components that were not influenced by study group or time point (Figure 1A). Rather, MK5–MK6 and MK11–MK13 concentrations were the strongest determinants of variability along the first component, MK9 and MK10 along the second component, and phylloquinone and MK4 along the third component.
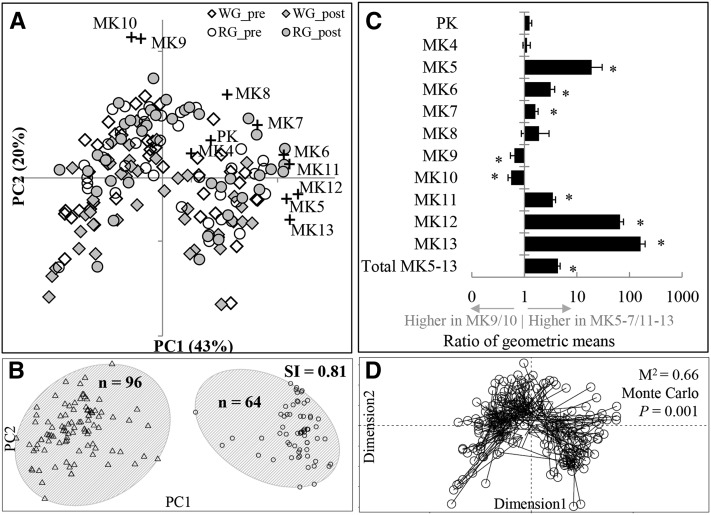
FIGURE 1.
Covariance between fecal vitamin K vitamers partitions individuals into distinct clusters, or menaquinotypes, which are associated with gut microbiota composition. (A) PC analysis of fecal vitamin K vitamer concentrations measured before and after consuming a WG diet or an RG diet for 6 wk (n = 80). Data points (solid shapes) represent the fecal vitamin K vitamer composition of an individual. Spatial locations of the + symbols indicate the relative contribution of each vitamer to the variance explained by the first and second principal components, with greater distance from the (x- and y-axes) origin, indicating a larger contribution of that vitamer to variance in fecal vitamin K content. (B) Partitioning around medoids analysis of fecal vitamin K vitamer concentrations (n = 80). Menaquinotypes are indicated by gray ellipses, triangles are MK9–MK10-enriched samples, and circles are MK5–MK7/MK11–MK13-enriched samples. Both pre- and postintervention samples from each individual are represented in the plot. (C) Differences in fecal vitamin K vitamer concentrations between menaquinotypes (n = 80). Bars are the ratio of geometric means ± SEs of the ratio calculated from the β (95% CI) obtained from linear mixed models, which included the log10-vitamer as the response variable, subject as a random factor, menaquinotype as the independent variable, and pre-intervention BMI, age, sex, and time as covariates. *Bonferroni-adjusted P ≤ 0.01. (D) Procrustes analysis of fecal vitamin K PC analysis (open circles) and principal coordinates analysis of fecal microbiota composition based on weighted UniFrac distances of operational taxonomic unit data (arrowheads) (n = 77). Procrustes rotation rotates ordinations to maximal similarity. Vectors connect microbiota composition with vitamer profiles of the same individual for each time point. Longer vectors indicate greater intraindividual dissimilarity. Monte Carlo P values represent 1000 permutations. MK, menaquinone; PC, principal component; PK, phylloquinone; post, postintervention; pre, pre-intervention; RG, refined-grain based; SI, Silhouette Index; WG, whole-grain rich.
Partitioning around medoid analysis indicated that individuals were optimally partitioned into 2 distinct clusters, herein described as menaquinotypes, on the basis of the variability in fecal vitamin K content (Figure 1B). This was supported by SIs, indicating strong clustering with 2 clusters (SI = 0.81) and weak-to-moderate clustering with >2 clusters (SI = 0.48). Two clusters also provided the highest SI (SI = 0.51) when Euclidean distances were used in the analysis. Study group (P = 0.52), race (P = 0.63), sex (P = 0.19), age (P = 0.48), pre-intervention BMI (P = 0.28), and dietary menaquinone intakes (P ≥ 0.32) did not differ between menaquinotypes, and menaquinotype membership was not affected by the intervention because no individual changed cluster membership. Rather, fecal concentrations of several menaquinones discriminated menaquinotypes. The highest membership menaquinotype was enriched in MK9 and MK10, whereas the other was enriched in MK5, MK6, MK7, MK11, MK12, and MK13 (Figure 1C). The ratio of the geometric mean of total fecal MK5–MK13 concentrations (expressed as nmol/g dry weight) of the MK5–MK7/MK11–MK13-enriched menaquinotype to that of the MK9–MK10-enriched menaquinotype was 4.4 (95% CI: 3.6, 5.3; Figure 1C). As a result, daily fecal MK5–MK13 excretion in feces was substantially higher in the MK5–MK7/MK11–MK13-enriched menaquinotype [week 2: 1670 nmol/d (1244 nmol/d), week 8: 2433 nmol/d (1886 nmol/d)] than in the MK9–MK10-enriched menaquinotype [week 2: 533 nmol/d (511 nmol/d), week 8: 471 nmol/d (455 nmol/d), P < 0.001 for both time points] (Supplemental Figure 2).
Log10-total fecal bacterial cell counts demonstrated a modest positive correlation with log10-total fecal MK5–MK13 concentrations (β ± SE: 0.04 ± 0.02, P = 0.04) but not with total daily MK5–13 excretion in feces (P = 0.49). Fecal bacterial cell counts also demonstrated a trend (P = 0.06) toward being higher in the MK5–MK7/MK11–MK13-enriched menaquinotype than in the MK9–MK10-enriched menaquinotype (Table 3). No marker of inflammation measured in feces or plasma was associated with menaquinotype membership. Likewise, fecal concentrations (expressed as nmol/g dry weight) and total daily excretion in feces (expressed as nmol/d) of individual vitamers were not associated with any marker of inflammation measured in feces or plasma (data not shown).
TABLE 3.
Associations between menaquinotypes and serum vitamin K, markers of gut microbiota density and activity, and markers of inflammation1
| n | β ± SE2 | P | |
| Log10 serum phylloquinone, nmol/L | 80 | 0.01 ± 0.04 | 0.84 |
| Log10 HOMA-IR | 79 | 0.04 ± 0.04 | 0.37 |
| Fecal markers | |||
| pH | 80 | 0.03 ± 0.05 | 0.56 |
| Log10 total bacteria, CFU/g | 80 | 0.28 ± 0.15 | 0.06 |
| Log10 IL-6, pg/mL | 75 | 0.33 ± 0.18 | 0.07 |
| Log10 TNF-α, pg/mL | 63 | −0.13 ± 0.17 | 0.46 |
| Log10 IL-17, pg/mL | 76 | −0.02 ± 0.11 | 0.87 |
| Plasma markers | |||
| Log10 IL-6, pg/mL | 79 | 0.06 ± 0.10 | 0.55 |
| Log10 TNF-α, pg/mL | 79 | 0.07 ± 0.08 | 0.39 |
| Log10 IL-1β, pg/mL | 79 | 0.10 ± 0.13 | 0.44 |
CFU, colony-forming unit; MK, menaquinone.
Linear mixed model in which the outcome in the first column was entered as the dependent variable; subject as a random factor; menaquinotype as the independent variable; and age, pre-intervention BMI, sex, and time as covariates. Log10-triglyceride values were also entered as a covariate in the serum phylloquinone model. The MK9–MK10-enriched menaquinotype is the reference group.
Interrelationships between fecal vitamin K content and gut microbiota composition
There were 5341 unique OTUs, which classified into 10 bacterial phyla, 69 genera, and 49 additional phylotypes that could not be classified to the genus level, that were detected in fecal samples (38). Procrustes analysis indicated congruence between the first 3 principal coordinates extracted from the PCA of fecal vitamin K content and the principal coordinates analysis of gut microbiota composition (M2 = 0.66, Monte Carlo P = 0.001; Figure 1D), indicating that variability in fecal vitamin K vitamer concentrations was associated with variability in gut microbiota composition.
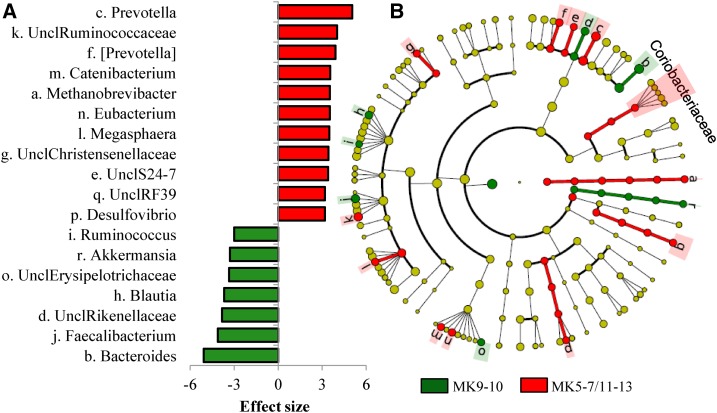
Linear discriminant analysis of effect size identified 98 features (features are taxonomic assignments ranging from the OTU level to the kingdom level) with an effect size of >3.0, which differed in relative abundance between menaquinotypes (Figure 2, Supplemental Table 3). At the genus level, effect size estimation indicated that Bacteroides (more abundant in the MK9–MK10-enriched menaquinotype) and Prevotella (more abundant in the MK5–MK7/MK11–M13-enriched menaquinotype) were the most influential genera in discriminating menaquinotypes (effect size > 5.0). In univariate analyses, a total of 73 taxa were statistically significantly correlated with ≥1 vitamer at both time points (P < 0.05), of which 52 remained statistically significant (Q < 0.05) after linear mixed-model analysis and false-discovery rate adjustment (Supplemental Table 4).
FIGURE 2.
Multiple taxa differentiate menaquinotypes. Linear discriminant analysis of effect size (48) for taxa differing in relative abundance between menaquinotypes before and after the diet intervention (n = 77). (A) Significantly different genera. Bars are effect sizes. Rings within the cladogram (B) correspond to different taxonomic ranks from phyla (innermost ring) to genus (outermost ring). Colored circles represent individual taxa and are sized in proportion to relative abundance. (A and B) Green shading and negative effect sizes represent higher abundance in the MK9–MK 10-enriched menaquinotype, and red shading and positive effect sizes represent the higher abundance in the MK5–MK7/MK11–MK13-enriched menaquinotype (effect size ≥3.0, P ≤ 0.01, Q < 0.05). Lowercase letters in the cladogram are genera that are differentially abundant between menaquinotypes (see panel A for legend). For clarity, discriminant features at higher levels of taxonomy (family to kingdom) are not listed but are indicated by the green and red circles. Both yellow coloring and no shading indicate no difference in relative abundance. Brackets around taxa indicate predicted taxonomy. MK, menaquinone; Uncl, unable to classify to genus level.
DISCUSSION
The primary findings of this study suggest that menaquinones are abundant in the human gut and that within the gut the concentrations of different menaquinone forms demonstrate substantial intra- and interindividual variability, which is associated with variability in gut microbiota composition. However, the health implications of these observations remain unclear because menaquinones were not detected in circulation and associations between fecal menaquinones and markers of inflammation were not observed.
Vitamin K is one of the few vitamins for which an Estimated Average Requirement does not exist. Current recommended Adequate Intakes for vitamin K as defined by the US Institute of Medicine are 90–120 μg/d (200–266 nmol/d) and based on US median intake of phylloquinone (18). The recommendations do not consider diet-derived menaquinones, and the contribution of gut bacteria-derived menaquinones to the Estimated Average Requirement is unknown (1, 18). In the present study, the measured median daily MK5–MK13 excretion in feces equated to >3 times the current US Adequate Intake for phylloquinone. This finding is consistent with and extends those of several studies limited by sample size and analytic methods incapable of measuring the full variability in gut menaquinone content (33–35) by demonstrating substantial intra- and interindividual variability in the concentrations of different menaquinone forms in the gut. The variability was characterized by covariance between several menaquinone forms (i.e., MK9 and MK10 covaried in an inverse manner with MK5, MK6, MK11, MK12, and MK13) which discriminated individuals into 2 distinct groups, or menaquinotypes. This observation replicates our group’s previous findings in a cohort of Chinese adults (36) and suggests that this pattern of variability in gut menaquinones is generalizable and reproducible across different geographical regions and population groups. Understanding the source of this variability may have important implications for elucidating vitamin K requirements, given the substantial differences in total daily menaquinone excretion between menaquinotypes and that different menaquinone forms appear to vary in bioavailability, bioactivity, and possibly health effects (26–32).
Our findings suggest that dietary menaquinone intake may contribute to variability in fecal menaquinone content but that in this study the primary source of that variability was the gut microbiota. In support, chemical analysis of the provided diets indicated that MK9, MK12, and MK13 were likely the only bacterially derived menaquinones consumed (MK4 is not known to be synthesized by bacteria). Daily intake of those vitamers did exceed the total daily amounts of each that was measured in feces, and MK12 and MK13 intakes were associated with fecal MK12 and MK13. However, multivariate analyses indicated that interindividual variability in fecal menaquinone concentrations exceeded diet-mediated effects on fecal menaquinones and that the predominant determinant of this variability was gut microbiota composition. Consistent with our previous findings (36), Bacteroides and Prevotella appeared to be particularly important determinants of this variability because these genera were found to have the largest effect size when taxonomic differences between menaquinotypes were examined. The observation is also consistent with both genomic (49) and culture-based analyses (34, 50), which have linked MK9–MK10 biosynthesis to Bacteroides species and MK11–MK13 biosynthesis to Prevotella species within the human gut. Interestingly, Prevotella abundance in human fecal samples is inversely associated with Bacteroides abundance (47), and this association may separate individuals into distinct groups based on interindividual variability in gut microbiota community composition commonly known as “enterotypes” (47, 51, 52). Although evidence suggests that the genetic capacity for menaquinone biosynthesis does not differ between enterotypes (51), our findings suggest potentially important differences resulting from the amounts of different menaquinone forms synthesized.
To our knowledge, this study is the first to examine associations between fecal menaquinone concentrations with markers of vitamin K status in healthy adults. Neither fecal menaquinone concentrations nor menaquinotype membership was associated with vitamin K status as measured by serum phylloquinone concentrations, and menaquinones were not detectable in circulation. The inability to detect menaquinones in circulation unless concentrated doses are consumed is consistent with the current evidence base (53) and suggests that few menaquinones synthesized by gut bacteria are absorbed into systemic circulation despite high concentrations in the gut. Although the bioavailability of gut bacteria-derived menaquinones is poorly characterized, most menaquinones synthesized by bacteria are thought to be embedded in bacterial cytoplasmic membranes, which likely reduces bioavailability (21). Bacteria can, however, secrete menaquinones in water-soluble complexes (54) and in lipid-soluble (33) or other forms (55). Further, despite the fact that fecal menaquinone concentrations almost certainly overestimate the quantity of bioavailable bacterially derived menaquinones, these menaquinone forms have been measured in human ileum samples (33), where bile salts could facilitate absorption (8), and in human hepatic tissue (56, 57). Interestingly, the most abundant vitamin K vitamers in the human liver are not the putative-predominant dietary forms phylloquinone and MK4 but rather MK10 and MK11 (56, 57), which were also the most abundant fecal vitamers in our cohort. Although these findings collectively suggest, but do not establish, that menaquinones derived from gut bacteria are absorbed and stored in the human liver, the detection of menaquinones in the diets used in this study in combination with recent evidence that menaquinones may be more abundant in the food supply than previously thought (24, 25) precludes more definitive conclusions and highlights a need for greater characterization of dietary menaquinone content.
Regardless, a requirement for gut bacteria–derived menaquinones to be absorbed from the gut to exert direct biological effects should not be assumed. These vitamers could plausibly act locally in the gut epithelium without entering systemic circulation. Although our findings did not demonstrate associations between fecal menaquinone concentrations and gut inflammation as measured by fecal cytokine concentrations, multiple studies using in vitro and animal models have demonstrated that multiple vitamin K vitamers and their metabolites may reduce inflammation (9–14). Whether associations between fecal menaquinone concentrations and gut inflammation could be detected in populations with higher levels of inflammation or using more sensitive markers of intestinal inflammation warrants further investigation.
Strengths of this study include the controlled diet, accurate quantification of multiple menaquinone forms, assessment of serum menaquinone concentrations, and the use of culture-independent methods to examine gut microbiota composition. Although chemically analyzing the menaquinone content of the provided diets is also a study strength, a limitation to our approach was relying on homogenates of a full day’s menu rather than analyzing individual meals or foods. As such, we cannot dismiss the possibility that small quantities of menaquinones in the diets may have gone undetected. An additional limitation, which may have masked any changes in vitamin K status concomitant to changes in fecal menaquinone concentrations, was the use of serum phylloquinone and menaquinones to assess vitamin K status. Although we hypothesized that fecal menaquinone concentrations may account for some of the previously reported unexplained interindividual variability in serum phylloquinone concentrations (53), serum phylloquinone is not a sensitive indicator of functional vitamin K status (53). Measuring more sensitive indicators of vitamin K status in future studies, especially studies in which dietary vitamin K intake is inadequate, could identify relations between fecal menaquinone content and vitamin K nutriture that are not reflected by serum phylloquinone or menaquinone concentrations.
In summary, menaquinones appear to be abundant in the human gut but demonstrate substantial variability in the concentrations of different menaquinone forms, likely because of variability in gut microbiota composition. As such, dietary manipulation of gut microbiota composition and activity could plausibly provide a viable means of modulating gut menaquinone concentrations. Although associations between fecal menaquinones and markers of inflammation were not detected in this study, studying interactions between diet-mediated effects on menaquinone biosynthesis within the gut microbiota and human health warrant continued investigation in lieu of emerging roles for bacterially derived menaquinones in health and disease. Because MK4, MK9, MK12, or MK13, despite being detected in the provided diets, and MK5–MK13, despite being abundant in fecal samples, were not measured in circulation, these investigations may benefit by initially focusing on localized effects in the gut.
Acknowledgments
We thank Carrie Brown for statistical support.
The authors’ responsibilities were as follows—JPK, JBB, SMV, BG, AK, and HR: conducted the research; AK, PV, and DK: conducted the gut microbiota measurements; JPK and XF: conducted and interpreted the vitamin K measurements; JPK and KB: analyzed the data; JPK: wrote the manuscript; JPK and SLB: had primary responsibility for the final content; and all authors: designed the research and read and approved the final manuscript. SSJ was employed by the General Mills Bell Institute of Health and Nutrition during the study conception and conduct. None of the other authors reported a conflict of interested related to this study. Kerry Ingredients did not contribute financially or intellectually to this research study.
Footnotes
Abbreviations used: MK, menaquinone; OTU, operational taxonomic unit; PCA, principal components analysis; RG, refined-grain based; SI, silhouette index; WG, whole-grain rich.
REFERENCES
- 1.Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr 2013;110:1357–68. [DOI] [PubMed] [Google Scholar]
- 2.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89–110. [DOI] [PubMed] [Google Scholar]
- 3.Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol 2013;9:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferland G. Vitamin K, an emerging nutrient in brain function. Biofactors 2012;38:151–7. [DOI] [PubMed] [Google Scholar]
- 5.Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep 2016;5:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr 2009;90:889–907. [DOI] [PubMed] [Google Scholar]
- 7.Shearer MJ. Vitamin K in parenteral nutrition. Gastroenterology 2009;137:S105–18. [DOI] [PubMed] [Google Scholar]
- 8.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost 2008;100:530–47. [PubMed] [Google Scholar]
- 9.Ozaki I, Zhang H, Mizuta T, Ide Y, Eguchi Y, Yasutake T, Sakamaki T, Pestell RG, Yamamoto K. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor κB activation. Clin Cancer Res 2007;13:2236–45. [DOI] [PubMed] [Google Scholar]
- 10.Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M, Hodges SJ. Interleukin-6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995;7:287–90. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med 2011;27:3–14. [DOI] [PubMed] [Google Scholar]
- 12.Shiraishi E, Iijima H, Shinzaki S, Nakajima S, Inoue T, Hiyama S, Kawai S, Araki M, Yamaguchi T, Hayashi Y, et al. . Vitamin K deficiency leads to exacerbation of murine dextran sulfate sodium-induced colitis. J Gastroenterol 2016;51:346–56. [DOI] [PubMed] [Google Scholar]
- 13.Pan MH, Maresz K, Lee PS, Wu JC, Ho CT, Popko J, Mehta DS, Stohs SJ, Badmaev V. Inhibition of TNF-α IL-1α, and IL-1β by pretreatment of human monocyte-derived macrophages with menaquinone-7 and cell activation with TLR agonists in vitro. J Med Food 2016;19:663–9. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem 2006;70:926–32. [DOI] [PubMed] [Google Scholar]
- 15.Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB Sr., Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, Keaney JF Jr., et al. . Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 2008;167:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea MK, Cushman M, Booth SL, Burke GL, Chen H, Kritchevsky SB. Associations between vitamin K status and haemostatic and inflammatory biomarkers in community-dwelling adults. The Multi-Ethnic Study of Atherosclerosis. Thromb Haemost 2014;112:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea MK, Dallal GE, Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Gundberg CM, Peterson JW, Booth SL. Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr 2008;88:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 2012;3:182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008;283:11270–9. [DOI] [PubMed] [Google Scholar]
- 20.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr 2013;4:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev 1981;45:316–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 1992;16:307–43. [PubMed] [Google Scholar]
- 23.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr 1995;15:399–417. [DOI] [PubMed] [Google Scholar]
- 24.Fu X, Harshman SG, Shen X, Hayowitz DB, Karl JP, Wolfe BE, Booth SL. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr 2017;1:e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SL. Measurement of multiple vitamin K forms in processed and fresh-cut pork products in the U.S. food supply. J Agric Food Chem 2016;64:4531–5. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama Y, Hara K, Matsumoto A, Takahashi S, Tajima T. Comparison of intestinal absorption of vitamin K2 (menaquinone) homologues and their effects on blood coagulation in rats with hypoprothrombinaemia. Biochem Pharmacol 1995;49:1801–7. [DOI] [PubMed] [Google Scholar]
- 27.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007;109:3279–83. [DOI] [PubMed] [Google Scholar]
- 28.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000;30:298–307. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J 2012;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489–93. [DOI] [PubMed] [Google Scholar]
- 31.Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 2009;19:504–10. [DOI] [PubMed] [Google Scholar]
- 32.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 2004;134:3100–5. [DOI] [PubMed] [Google Scholar]
- 33.Conly JM, Stein K. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am J Gastroenterol 1992;87:311–6. [PubMed] [Google Scholar]
- 34.Ramotar K, Conly J, Chubb H, Louie T. Production of menaquinones by intestinal anaerobes. J Infect Dis 1984;150:213–8. [DOI] [PubMed] [Google Scholar]
- 35.Sakano T, Nagaoka T, Morimoto A, Hirauchi K. Measurement of K vitamins in human and animal feces by high-performance liquid chromatography with fluorometric detection. Chem Pharm Bull (Tokyo) 1986;34:4322–6. [DOI] [PubMed] [Google Scholar]
- 36.Karl JP, Fu X, Wang X, Zhao Y, Shen J, Zhang C, Wolfe BE, Saltzman E, Zhao L, Booth SL. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am J Clin Nutr 2015;102:84–93. [DOI] [PubMed] [Google Scholar]
- 37.Karl JP, Meydani M, Barnett JB, Vanegas SM, Goldin B, Kane A, Rasmussen H, Saltzman E, Vangay P, Knights D, et al. . Substituting whole grains for refined grains in a 6-week randomized trial favorably affects energy balance parameters in healthy men and postmenopausal women. Am J Clin Nutr 2017;105:589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonalagadda S, et al. . Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota, and immune and inflammatory markers of healthy adults. Am J Clin Nutr 2017;105:635–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross AB. Present status and perspectives on the use of alkylresorcinols as biomarkers of wholegrain wheat and rye intake. J Nutr Metab 2012;2012:462967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;963:128–33. [DOI] [PubMed] [Google Scholar]
- 41.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 42.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. . Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. . QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 2012;6:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 46.Lamon-Fava S, Sadowski JA, Davidson KW, O’Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr 1998;67:1226–31. [DOI] [PubMed] [Google Scholar]
- 47.Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLOS Comput Biol 2013;9:e1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, Holland TA, Keseler IM, Kothari A, Kubo A, et al. . The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 2014;42:D459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi H, Shibata K, Sakamoto M, Tomita S, Benno Y. Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2007;57:941–6. [DOI] [PubMed] [Google Scholar]
- 51.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. . Enterotypes of the human gut microbiome. Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. . Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 2016;8:E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda H, Doi Y. A vitamin-K2-binding factor secreted from Bacillus subtilis. Eur J Biochem 1990;192:219–24. [DOI] [PubMed] [Google Scholar]
- 55.Rezaïki L, Lamberet G, Derre A, Gruss A, Gaudu P. Lactococcus lactis produces short-chain quinones that cross-feed group B Streptococcus to activate respiration growth. Mol Microbiol 2008;67:947–57. [DOI] [PubMed] [Google Scholar]
- 56.Usui Y, Tanimura H, Nishimura N, Kobayashi N, Okanoue T, Ozawa K. Vitamin K concentrations in the plasma and liver of surgical patients. Am J Clin Nutr 1990;51:846–52. [DOI] [PubMed] [Google Scholar]
- 57.Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr 1996;75:121–7. [DOI] [PubMed] [Google Scholar]