Abstract
Background: Vitamin B-12 (cobalamin) deficiency may produce severe neurologic and hematologic manifestations. Approximately 20–25% of circulating cobalamin binds to transcobalamin 2 (TCN2), which is referred to as active vitamin B-12. The G allele of the TCN2 c.776G>C (rs1801198) polymorphism has been associated with a lower plasma concentration of holotranscobalamin. However, genotype association studies on rs1801198 have led to conflicting results regarding its influence on one-carbon metabolism (OCM) markers or its association with pathologic conditions.
Objective: We assessed the association of rs1801198 genotypes with OCM marker concentrations and primary risks of congenital abnormalities, cancer, and Alzheimer disease.
Design: We conducted a systematic review of the literature that was published from January 1966 to February 2017 and included all studies that assessed the association between rs1801198 and OCM markers or a pathologic condition.
Results: Thirty-four studies met the inclusion criteria. Subjects with the rs1801198 GG genotype had significantly lower concentrations of holotranscobalamin [standardized mean difference (SMD): −0.445 (95% CI: −0.673, −0.217; P < 0.001); I2 = 48.16% (95% CI: 0.00%, 78.10%; P = 0.07)] and higher concentrations of homocysteine (European descent only) [SMD: 0.070 (95% CI: 0.020, 0.120; P = 0.01); I2 = 0.00% (95% CI: 0.00%, 49.59%; P = 0.73)] than did subjects with the rs1801198 CC genotype. The meta-analysis on the association between rs1801198 and methylmalonic acid (MMA) lacked statistical power. No significant difference was observed regarding cobalamin, folate, and red blood cell folate. No significant association was observed between rs1801198 and primary risks of congenital abnormalities, cancer, or Alzheimer disease.
Conclusions: Meta-analysis results indicate an influence of rs1801198 on holotranscobalamin and homocysteine concentrations in European-descent subjects. In addition, well-designed and -powered studies should be conducted for assessing the association between rs1801198 and MMA and clinical manifestations that are linked to a decreased availability of cobalamin. This review was registered at www.crd.york.ac.uk/prospero as CRD42017058504.
Keywords: cobalamin, c.776G>C, genetic association studies, holotranscobalamin, homocysteine, meta-analysis, one-carbon metabolism, rs1801198, transcobalamin, transcobalamin II
INTRODUCTION
Vitamin B-12 (cobalamin) serves as a cofactor for methionine synthase [5-methyltetrahydrofolate-homocysteine methyltransferase (MTR; gene identifier: 4548)] and l-methylmalonyl-CoA mutase (gene identifier: 4594) and is necessary for the transformation of methyltetrahydrofolate to tetrahydrofolate for DNA synthesis and for fatty acid metabolism (1, 2). Methionine synthase requires methylcobalamin for the remethylation of methionine from homocysteine, and methylmalonyl-CoA mutase uses adenosylcobalamin to convert methylmalonyl-CoA to succinyl-CoA (3). During digestion, cobalamin is released from food, travels from the stomach to the duodenum, and is bound to haptocorrin (2). In the duodenum, cobalamin is released from haptocorrin by pancreatic proteases and binds to intrinsic factor until it reaches the distal ileum where a specific receptor recognizes the intrinsic factor–cobalamin complex on ileal enterocytes, called cubilin amnionless (2, 4). After basal membrane crossing, cobalamin is bound to transcobalamin 2 (TCN2), which is a 43-kDa protein (TCN2, gene identifier: 6948), which is essential for the transport of cobalamin into all cells in the body (4).
In a randomized, placebo-controlled study that examined the effect of oral cobalamin treatment on fluctuations in plasma total cobalamin and its binding proteins TCN2 and haptocorrin, holotranscobalamin was shown to be an early marker of changes in cobalamin homeostasis with an 82% increase of its concentration after oral cobalamin treatment (5). In the plasma, cobalamin is bound to TCN2 and haptocorrins (6). Serum vitamin B-12 assays measure the sum of haptocorrin-bound and TCN2-bound cobalamin. Approximately 20–25% of circulating cobalamin binds to TCN2 (holotranscobalamin) and, thereby, is available for the cells of the body for meeting the metabolic demand (2, 3, 7). For this reason, holotranscobalamin is also referred to as active vitamin B-12 (7). The lack of TCN2 results in severe megaloblastic anemia in infancy despite normal concentrations of serum cobalamin (8, 9).
Vitamin B-12 deficiency is a common but serious condition (10). Results from the third NHANES from the US population have reported a prevalence of 3.2% of low serum vitamin B-12 concentrations (<148 pmol/L) for all adults, which reaches 4.4% in individuals aged >50 y (11). In 1993, Li et al. (12) first described a variation on TCN2 codon 259 on the basis of the sequencing of 2 complementary DNA clones encoding full-length human TCN2. We reported that this variant was a TCN2 polymorphism (rs1801198; NM_000355.2:c.776G>C; NP_000346.2:p.Arg259Pro) that influences the cellular expression and plasma concentration of TCN2 and the availability of cobalamin (13, 14), thus suggesting that there is a potential influence of rs1801198 on the concentration of one-carbon metabolism (OCM) markers and primary risk of disease. Since the previous reports, several studies have assessed the association between the TCN2 rs1801198 and OCM marker concentrations or primary risk of disease such as congenital abnormalities or cancer with mixed results that have been reported, which may be explained by differences in the study design and power, genetic background of the studied populations, and nutritional status (15).
Therefore, we performed a systematic review of the literature and meta-analysis of studies that have evaluated the potential association between rs1801198 and physiologic or pathologic traits. The aims of this meta-analysis were as follows: 1) to compared OCM marker concentrations between subjects with GG and CC genotypes for rs1801198 and 2) to evaluate primary risks of congenital abnormalities, cancer, and Alzheimer disease in association with rs1801198.
METHODS
Electronic search query
A literature search was conducted of MEDLINE-indexed literature with the use of the PubMed (www.pubmed.gov) search engine from the National Center for Biotechnology Information (January 1966 to February 2017) and the following full electronic search strategy: rs1801198[All Fields] OR ((P259R[All Fields] OR Pro259Arg[All Fields]) AND (TCN2[All Fields] OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR (“transcobalamin”[All Fields] AND “ii”[All Fields]) OR “transcobalamin ii”[All Fields]) OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR “transcobalamin”[All Fields]))) OR ((776C[All Fields] OR 776G[All Fields] OR 776[All Fields] OR 777[All Fields]) AND (TCN2[All Fields] OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR (“transcobalamin”[All Fields] AND “ii”[All Fields]) OR “transcobalamin ii”[All Fields]) OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR “transcobalamin”[All Fields]))) OR (259[All Fields] OR 232[All Fields] OR 255[All Fields]) AND (TCN2[All Fields] OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR (“transcobalamin”[All Fields] AND “ii”[All Fields]) OR “transcobalamin ii”[All Fields]) OR (“transcobalamins”[MeSH Terms] OR “transcobalamins”[All Fields] OR “transcobalamin”[All Fields])). Additional articles were retrieved from primary search references. The EndNote ×7.7.1 software tool was used for reference management (16). The protocol for this systematic review was registered at www.crd.york.ac.uk/prospero as CRD42017058504. The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (17).
Eligibility criteria
A study was considered to be eligible for the systematic review if it assessed the potential association between rs1801198 and a biological trait that is related to OCM or a pathologic condition. Both case-control candidate-gene and genome-wide–association study approaches were eligible for the systematic review. No language restrictions were applied.
Inclusion and exclusion criteria
Studies were included in the meta-analysis if they reported ≥1 outcome measure according to rs1801198 genotypes (CC, CG, and GG). Studies were excluded from the meta-analysis for the following reasons: 1) review paper; 2) letter to the editor with insufficient data for tabulation on OCM markers; 3) no OCM marker data according to rs1801198 genotype available; 4) no data on rs1801198 or no genotype counts for rs1801198; 5) the number of subjects in each rs1801198 genotype subgroup was not reported; and 6) the data were reported in a previously published study (see Supplemental Table 1 for detailed exclusion grounds for articles that were excluded from the systematic review).
Data extraction
The following data were extracted, when available, on the basis of a predefined protocol with the use of Microsoft Excel 2016 software (Microsoft Corp.): first author, year, country, population ethnicity, study design, congenital abnormality disorder, and type of cancer. In the case of a binary trait (congenital abnormality, cancer, or Alzheimer disease) the following additional criteria were extracted: numbers of subjects with CC, CG, and GG genotypes of rs1801198 in cases and controls. In regard to continuous variables, the following items were extracted: OCM marker, number of subjects, mean, SD, 95% CI, median, IQR, and range (minimum to maximum) for each 1 of the 3 genotypes of rs1801198 (CC, CG, and GG). Four investigators (AO, JL, PF-T, and J-LG) independently reviewed the titles and abstracts of all citations that were identified by the literature search. Eligible articles were reviewed in triplicate in an independent manner by 3 investigators (AO, JL, and PF-T). Any disagreement in the data extraction was resolved by consensus. To calculate the pooled effect size for OCM-marker comparisons across genotypes, descriptive data that were reported as the median and range and, if available, the IQR were converted to means ± SDs according to the validated method described by Wan et al. (18).
Quality assessment
All eligible studies were assessed for their quality with the use of the validated Strengthening the Reporting of Genetic Association Studies (STREGA) Reporting Recommendations framework (19). A quantitative STREGA score was calculated for each study and was based on a list of 19 items (Supplemental Tables 2–4). Concomitantly, a post hoc power calculation and adequate sample-size estimation were performed for each OCM biomarker on the basis of data from studies that were included in the meta-analysis according to the method described by Julious (20) (Supplemental Tables 5 and 6). Regarding binary outcomes, the study power and adequate sample size were calculated on the basis of allele frequencies of the rs1801198 in the European (non-Finnish) population from the Exome Aggregation Consortium (http://exac.broadinstitute.org), an α risk of 0.05, a genotype relative risk of 2.0, and a disease prevalence of 10%, with the assumption of both allelic and additive models according to the algorithm of Skol et al. (21) (Supplemental Table 7).
Outcome measures
The outcome measures were defined a priori. The meta-analysis evaluated the following 4 groups of outcome variables: 1) OCM markers, 2) congenital abnormalities, 3) cancer, and 4) Alzheimer disease. The OCM markers group included holotranscobalamin, vitamin B-12, homocysteine, methylmalonic acid (MMA), folate, and red blood cell (RBC) folates. The congenital abnormalities group included spontaneous abortion, female infertility, neural tube defect, cleft lip, and Down syndrome. The cancer group included colorectal tumors, glioblastoma, primary central nervous system lymphoma, and ovarian cancer.
Data synthesis and analysis
Data were collected and tabulated with the use of Microsoft Office Excel 2016 (Microsoft Corp.). Data were not used for analyses whenever outcomes of interest were not clearly stated. For the comparison of OCM marker concentrations between subjects with CC and GG genotypes for the rs1801198, we used Hedges’ g statistic as a formulation for the standardized mean difference (SMD) together with the 95% CI. The SMD Hedges’ g is the difference between the 2 means divided by the pooled SD with a correction for small sample bias. The heterogeneity statistic was incorporated to calculate the summary SMD under the random-effects model according to the procedure of DerSimonian and Laird (DL) (22). The robustness of results was assessed via sensitivity analyses. The number of studies that were required for reaching statistical significance on the basis of calculated pooled means ± SEs was estimated for each OCM marker with consideration of an α risk at 0.05 and a statistical power of 80% (20) (Supplemental Tables 5 and 6). For binary outcomes (primary risks of congenital abnormalities, cancer, and Alzheimer disease), categorical measures were summarized with the use of counts and percentages. The following 2 genetic models were used for the assessment of the association between rs1801198 and binary outcomes: CC compared with CG/GG and GG compared with CG/CC. The estimated effect measure was the OR with 95% CI. The overall summary from the meta-analysis was calculated by combining all studies, and the meta-analysis was performed with the use of the random-effects model (DL) (22). The calculated summary effect was denoted by the solid diamond at the bottom of the forest plots, the width of which represents the 95% CI. A pooled analysis of studies that have assessed European-ancestry subjects was performed when ≥5 studies were reported for a specific outcome measure. When the P value that was associated with the DL model was between 0.01 and 0.05, the 95% CI of the pooled effect size and its associated P value were calculated with the use of the Hartung-Knapp-Sidik-Jonkman (HKSJ) procedure (23). We assessed significance for heterogeneity using the χ2-based Q statistic and the I2 statistic for the extent of heterogeneity. Heterogeneity was considered significant at P < 0.1 and when I2 was >50% (24). Publication bias was assessed with the use of funnel plots (25). In a sensitivity analysis, the relation between rs1801198 and the concentration of holotranscobalamin according to the STREGA score was evaluated via a metaregression using weighted linear regression. All reported P values were 2 sided with α = 0.05. The meta-analysis was performed with the statistical software packages Comprehensive Meta-Analysis (version 2.2.050; BioStat Software) and MedCalc for Windows (v16.8.4; MedCalc Software).
RESULTS
Literature search results
The search strategy generated 90 citations of which 66 citations appeared to be relevant to the systematic review. Of these 66 studies, 32 studies were excluded on the basis of the selection criteria, which left 34 studies that were eligible for the systematic review a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart is shown in Figure 1). Of the 34 selected studies, 12 studies were related to OCM markers (Table 1) (14, 26–36), 16 studies were related to primary risk of congenital abnormalities (Table 2) (37–52), 5 studies were related to cancer (Table 3) (53–57), and 2 studies were related to Alzheimer disease (Table 4) (30, 58). One study addressed 2 outcomes, namely holotranscobalamin as an OCM marker and Alzheimer disease as a binary outcome (30).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the systematic review. OSM Table S1, Online Supporting Material Supplemental Table 1.
TABLE 1.
Studies that assessed the concentration of one-carbon metabolism markers according to the TCN2 rs1801198 (c.776G>C) polymorphism1
| CC genotype |
CG genotype |
GG genotype |
||||||||
| Reference | STREGA score | Setting | Country (ethnicity) | Sample | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD |
| Holotranscobalamin, pmol/L | ||||||||||
| Miller et al., 2002 (26) | 8 of 19 | Healthy older adults | United States (Caucasians) | Serum | 39 | 150 ± 812 | 63 | 113 ± 56 | 26 | 104 ± 83 |
| Födinger et al., 2003 (27) | 9 of 19 | End-stage renal disease | Austria (Caucasians) | Plasma | 47 | 132.2 ± 71.43 | 51 | 123 ± 56.5 | 22 | 132 ± 37.2 |
| Wans et al., 2003 (28) | 8 of 19 | Healthy older adults | Germany (Caucasians) | NR | 55 | 68 ± 28 | 77 | 67 ± 41 | 27 | 46 ± 21 |
| Zetterberg et al., 2003 (29) | 8 of 19 | Cognitive dysfunction | Sweden (Caucasians) | Plasma | 24 | 100.3 ± 39.63 | 34 | 83.5 ± 38.7 | 20 | 91.5 ± 56.3 |
| McCaddon et al., 2004 (30) | 9 of 19 | Alzheimer disease | United Kingdom (Caucasians) | Serum | 30 | 58 ± 14.73 | 51 | 45 ± 9.8 | 17 | 43.5 ± 9.8 |
| von Castel-Dunwoody et al., 2005 (31) | 11 of 19 | Healthy nonpregnant women | United States (mixed)4 | Serum | 103 | 87 ± 33 | 161 | 82 ± 26 | 80 | 74 ± 37 |
| Garrod et al., 2010 (32) | 9 of 19 | Community-dwelling older adult5 | United States (Latino) | Plasma | 229 | 80 ± 33 | 261 | 77 ± 33 | 64 | 70 ± 27 |
| Vitamin B-12, pmol/L | ||||||||||
| Miller et al., 2002 (26) | 8 of 19 | Healthy older adults | United States (Caucasians) | Serum | 39 | 319.6 ± 130.6 | 63 | 306.3 ± 124 | 26 | 282.7 ± 141 |
| Födinger et al., 2003 (27) | 9 of 19 | End-stage renal disease | Austria (Caucasians) | Plasma | 47 | 259 ± 141.53 | 51 | 253 ± 103 | 22 | 282.7 ± 161.7 |
| Wans et al., 2003 (28) | 8 of 19 | Healthy older adults | Germany (Caucasians) | NR | 55 | 293 ± 99 | 77 | 305 ± 143 | 27 | 289 ± 122 |
| Zetterberg et al., 2003 (29) | 8 of 19 | Cognitive dysfunction | Sweden (Caucasians) | Plasma | 24 | 419.8 ± 249.73 | 34 | 275.3 ± 72.5 | 20 | 335 ± 161.7 |
| McCaddon et al., 2004 (30) | 9 of 19 | Alzheimer disease | United Kingdom (Caucasians) | Serum | 30 | 251.7 ± 36.73 | 51 | 237.5 ± 28.3 | 17 | 307.8 ± 31.5 |
| Winkelmayer et al., 2004 (33) | 8 of 19 | Kidney-allograft recipients | Austria (Caucasians) | Plasma | 204 | 263 ± 197 | 383 | 253 ± 145 | 145 | 254 ± 116 |
| von Castel-Dunwoody et al., 2005 (31) | 11 of 19 | Healthy nonpregnant women | United States (mixed)4 | Plasma | 103 | 327 ± 144 | 161 | 320 ± 136 | 80 | 345 ± 144 |
| Fredriksen et al., 2007 (34) | 13 of 19 | Healthy population6 | Norway (Caucasians) | Serum | 3278 | 331.1 ± 247.33 | 4783 | 328 ± 235.2 | 2020 | 339.8 ± 226.1 |
| Garrod et al., 2010 (32) | 9 of 19 | Community-dwelling older adults5 | United States (Latino) | Plasma | 229 | 316 ± 142 | 261 | 312 ± 121 | 64 | 316 ± 107 |
| Stanisławska-Sachadyn et al., 2010 (35) | 12 of 19 | Male volunteers7 | Northern Ireland (Caucasians) | Serum | 190 | 253.1 ± 112.23 | 276 | 275.4 ± 97.6 | 131 | 281.7 ± 103.1 |
| Sawaengsri et al., 2016 (36) | 11 of 19 | Home-bound elderly participants8 | United States (mixed)9 | Plasma | 79 | 398 ± 30.5 | 61 | 336.5 ± 31.7 | 31 | 317 ± 33.9 |
| Homocysteine, μmol/L | ||||||||||
| Namour et al., 2001 (14) | 8 of 19 | Healthy older adults | France (Caucasians) | Plasma | 55 | 10.1 ± 0.4 | 76 | 11.7 ± 0.4 | 28 | 10.2 ± 0.8 |
| Miller et al., 2002 (26) | 8 of 19 | Healthy older adults | United States (Caucasians) | NR | 39 | 10.3 ± 2.6 | 63 | 10.7 ± 2.4 | 26 | 11.2 ± 2.8 |
| Födinger et al., 2003 (27) | 9 of 19 | End-stage renal disease | Austria Caucasians | Plasma | 47 | 25.1 ± 6.63 | 51 | 27.7 ± 7.8 | 22 | 26.9 ± 15.6 |
| Wans et al., 2003 (28) | 8 of 19 | Healthy older adults | Germany (Caucasians) | NR | 55 | 12.3 ± 4 | 77 | 12.2 ± 3.5 | 27 | 12.8 ± 3.4 |
| Zetterberg et al., 2003 (29) | 8 of 19 | Cognitive dysfunction | Sweden Caucasians | Plasma | 24 | 16 ± 6.43 | 34 | 16.3 ± 9 | 20 | 19 ± 6.3 |
| Winkelmayer et al., 2004 (33) | 8 of 19 | Kidney-allograft recipients | Austria Caucasians | Plasma | 204 | 16.8 ± 7.4 | 383 | 17 ± 9.8 | 145 | 17.8 ± 7.4 |
| von Castel-Dunwoody et al., 2005 (31) | 11 of 19 | Healthy nonpregnant women | United States (mixed)4 | Plasma | 103 | 6.2 ± 1 | 161 | 6.5 ± 1 | 80 | 6.3 ± 1 |
| Fredriksen et al., 2007 (34) | 13 of 19 | Healthy population6 | Norway (Caucasians) | Plasma | 3278 | 10.8 ± 4.43 | 4783 | 10.7 ± 4.2 | 2020 | 11 ± 4 |
| Garrod et al., 2010 (32) | 9 of 19 | Community-dwelling older adults5 | United States (Latino) | Plasma | 229 | 10.8 ± 9.1 | 261 | 10.2 ± 3.3 | 64 | 9.7 ± 3.3 |
| Stanisławska-Sachadyn et al., 2010 (35) | 12 of 19 | Male volunteers7 | Northern Ireland (Caucasians) | NR | 195 | 7.3 ± 2.33 | 286 | 7.1 ± 1.9 | 132 | 7.2 ± 2 |
| Sawaengsri et al., 2016 (36) | 11 of 19 | Home-bound elderly participants8 | United States (mixed)9 | Plasma | 79 | 9.9 ± 1.1 | 61 | 10.1 ± 1.1 | 31 | 10.7 ± 1.1 |
| Methylmalonic acid, nmol/L | ||||||||||
| Miller et al., 2002 (26) | 8 of 19 | Healthy older adults | United States (Caucasians) | NR | 39 | 208 ± 96 | 63 | 206 ± 80 | 26 | 264 ± 138 |
| Wans et al., 2003 (28) | 8 of 19 | Healthy older adults | Germany (Caucasians) | NR | 55 | 375.5 ± 274.93 | 77 | 315.5 ± 217.1 | 27 | 243 ± 107.4 |
| Fredriksen et al., 2007 (34) | 13 of 19 | Healthy population6 | Norway (Caucasians) | Plasma | 3278 | 177 ± 8.83 | 4783 | 181 ± 8.8 | 2020 | 187 ± 8 |
| Garrod et al., 2010 (32) | 9 of 19 | Community-dwelling older adults5 | United States (Latino) | Plasma | 229 | 286 ± 841 | 261 | 204 ± 182 | 64 | 207 ± 134 |
| Folate, nmol/L | ||||||||||
| Födinger et al., 2003 (27) | 9 of 19 | End-stage renal disease | Austria (Caucasians) | Plasma | 47 | 16.9 ± 7.73 | 51 | 16 ± 7.3 | 22 | 17.7 ± 6 |
| Wans et al., 2003 (28) | 8 of 19 | Healthy older adults | Germany (Caucasians) | NR | 55 | 19.1 ± 93 | 77 | 37.2 ± 29.7 | 27 | 23.7 ± 14.9 |
| Winkelmayer et al., 2004 (33) | 8 of 19 | Kidney-allograft recipients | Austria (Caucasians) | Plasma | 204 | 15.2 ± 17.2 | 383 | 14.8 ± 11.5 | 145 | 14.5 ± 6.9 |
| Fredriksen et al., 2007 (34) | 13 of 19 | Healthy population6 | Norway (Caucasians) | Serum | 3278 | 17.2 ± 13.33 | 4783 | 17.3 ± 12.7 | 2020 | 16.7 ± 12.3 |
| Sawaengsri et al., 2016 (36) | 11 of 19 | Home-bound elderly participants8 | United States (mixed)9 | Plasma | 79 | 31 ± 18.8 | 61 | 31.5 ± 21.1 | 31 | 34.4 ± 20.2 |
| Folate, red blood cells, nmol/L | ||||||||||
| Miller et al., 2002 (26) | 8 of 19 | Healthy older adults | United States (Caucasians) | Whole blood | 39 | 822.6 ± 219.8 | 63 | 856.6 ± 240.2 | 26 | 901.9 ± 240.2 |
| Födinger et al., 2003 (27) | 9 of 19 | End-stage renal disease | Austria (Caucasians) | Whole blood | 47 | 1345.7 ± 589.63 | 51 | 1162 ± 427.2 | 22 | 1272.3 ± 368.5 |
| Zetterberg et al., 2003 (29) | 8 of 19 | Cognitive dysfunction | Sweden (Caucasians) | Whole blood | 24 | 209.5 ± 61.23 | 34 | 277 ± 164 | 20 | 366.8 ± 250.9 |
| Garrod et al., 2010 (32) | 9 of 19 | Community-dwelling older adults5 | United States (Latino) | Whole blood | 229 | 1144.3 ± 346.7 | 261 | 1162.5 ± 362.6 | 64 | 1153.4 ± 358 |
NR, not reported; STREGA, Strengthening the Reporting of Genetic Association Studies; TCN2, transcobalamin 2.
Concentration is expressed as pg/mL.
Value was calculated on the basis of the median and IQR or the median and range according to the validated method described by Wan et al. (18).
White: 81%; African: 9%; Hispanic: 4%; Asian: 2%; other: 4%.
Sacramento Area Latino Study on Aging.
Norwegian Colorectal Cancer Prevention study.
Belfast-based industrial workforce.
Nutrition, Aging, and Memory in Elders study.
Non-Hispanic white: 61.4%; non-Hispanic African: 33.9%; Asian: 1.8%; Native American or Alaskan Native: 1.8%; Hispanic: 1.2%.
TABLE 2.
Studies that assessed the risk of congenital abnormalities according to the TCN2 rs1801198 (c.776G>C) polymorphism1
| Cases, n |
Controls, n |
||||||||
| Reference | STREGA score | Congenital abnormality | Country (ethnicity) | Study design | Population | Total | CC/CG/GG | Total | CC/CG/GG |
| Zetterberg et al., 2002 (37) | 9 of 19 | Spontaneous abortion | Crete (Caucasians) | Case-control | Children | 77 | 7/51/19 | 115 | 37/57/21 |
| Parle-McDermott et al., 2005 (38) | 12 of 19 | Spontaneous abortion | Ireland (Caucasians) | Case-control | Mother | 123 | 33/61/29 | 611 | 184/306/121 |
| Kim et al., 2014 (39) | 12 of 19 | Spontaneous abortion | Korea (East Asians) | Case-control | Mother | 378 | 88/200/90 | 207 | 55/111/41 |
| Altmäe et al., 2010 (40) | 14 of 19 | Female infertility | Sweden (Caucasians) | Case-control | Mother | 62 | 20/29/13 | 389 | 124/184/81 |
| Afman et al., 2002 (41) | 9 of 19 | Neural tube defect | Netherlands (Caucasians) | Case-control | Mother | 42 | 11/25/6 | 73 | 22/36/15 |
| Pietrzyk and Bik-Multanowski, 2003 (42) | 9 of 19 | Neural tube defect | Poland (Caucasians) | Case-control | Mother | 103 | 30/44/29 | 100 | 24/61/15 |
| Candito et al., 2008 (43) | 11 of 19 | Neural tube defect | France (Caucasians) | Case-control | Mother | 77 | 26/33/18 | 61 | 26/23/12 |
| Godbole et al., 2011 (44) | 13 of 19 | Neural tube defect | India (South Asians) | Case-control | Mother | 262 | 33/120/109 | 255 | 34/109/112 |
| Godbole et al., 2011 (44)2 | 13 of 19 | Neural tube defect | India (South Asians) | Case-control | Children | 255 | 34/109/112 | 668 | 105/309/254 |
| Martinelli et al., 2006 (45) | 11 of 19 | Cleft lip | Italy (Caucasians) | Case-control | Children | 218 | 85/110/23 | 289 | 89/150/50 |
| Jin et al., 2015 (46) | 12 of 19 | Cleft lip | China (East Asians) | Case-control | Children | 429 | 76/215/138 | 461 | 75/231/155 |
| Waltrick-Zambuzzi et al., 2015 (47) | 11 of 19 | Cleft lip | Brazil (admixed Americans) | Case-control | Children | 359 | 139/160/60 | 440 | 179/199/62 |
| Biselli et al., 2008 (48) | 9 of 19 | Down syndrome | Brazil (admixed Americans) | Case-control | Mother | 67 | 32/24/11 | 113 | 48/54/11 |
| Fintelman-Rodrigues et al., 2009 (49) | 12 of 19 | Down syndrome | Brazil (admixed Americans) | Case-control | Mother | 114 | 46/51/17 | 110 | 39/48/23 |
| Zampieri et al., 2012 (50) | 9 of 19 | Down syndrome | Brazil (admixed Americans) | Case-control | Mother | 105 | 42/46/17 | 185 | 75/93/17 |
| Liao et al., 2014 (51) | 10 of 19 | Down syndrome | China (East Asians) | Case-control | Mother | 76 | 14/42/20 | 108 | 28/43/37 |
| Mills et al., 2005 (52) | 12 of 19 | Omphalocele | USA (mixed)3 | Case-control | Children | 24 | 6/13/5 | 47 | 9/28/10 |
STREGA, Strengthening the Reporting of Genetic Association Studies; TCN2, transcobalamin 2.
DNA extracted from fetal tissue.
Non-Hispanic Caucasians: 48%; non-Hispanic Africans: 28%; Hispanic 20%; other 4%.
TABLE 3.
Studies that assessed risk of cancer according to the TCN2 rs1801198 (c.776G>C) polymorphism1
| Cases, n |
Controls, n |
|||||||
| Reference | STREGA score | Type of cancer | Country (ethnicity) | Study design | Total | CC/CG/GG | Total | CC/CG/GG |
| Hazra et al., 2007 (53) | 14 of 19 | Colorectal adenoma | United States (Caucasians) | Case-control | 522 | 150/273/99 | 522 | 182/248/92 |
| Koushik et al., 2006 (54) | 14 of 19 | Colorectal cancer | United States (Caucasians) | Case-control | 349 | 119/168/62 | 796 | 249/392/155 |
| Semmler et al., 2006 (55) | 12 of 19 | Glioblastoma multiforme | Germany (Caucasians) | Case-control | 328 | 92/151/85 | 400 | 110/180/110 |
| Kurzwelly et al., 2010 (56) | 11 of 19 | PCNSL | Germany (Caucasians) | Case-control | 185 | 61/76/48 | 212 | 57/100/55 |
| Pawlik et al., 2012 (57) | 12 of 19 | Ovarian cancer | Poland (Caucasians) | Case-control | 134 | 50/58/26 | 160 | 48/77/35 |
PCNSL, primary central nervous system lymphoma; STREGA, Strengthening the Reporting of Genetic Association Studies; TCN2, transcobalamin 2.
TABLE 4.
Studies that assessed risk of Alzheimer disease according to the TCN2 rs1801198 (c.776G>C) polymorphism1
| Cases, n |
Controls, n |
||||||
| Reference | STREGA score | Country (ethnicity) | Study design | Total | CC/CG/GG | Total | CC/CG/GG |
| McCaddon et al., 2004 (30)2 | 12 of 19 | United Kingdom (Caucasians) | Case-control | 65 | 16/34/15 | 71 | 26/36/9 |
| McCaddon et al., 2004 (30)3 | 12 of 19 | United Kingdom (Caucasians) | Case-control | 94 | 32/45/17 | 107 | 42/50/15 |
| Cascalheira et al., 2015 (58) | 11 of 19 | Portugal (Caucasians) | Case-control | 26 | 11/8/7 | 28 | 4/17/7 |
STREGA, Strengthening the Reporting of Genetic Association; TCN2, transcobalamin 2.
Clinical series.
Histopathologic series.
Association between rs1801198 and concentration of OCM markers
Holotranscobalamin
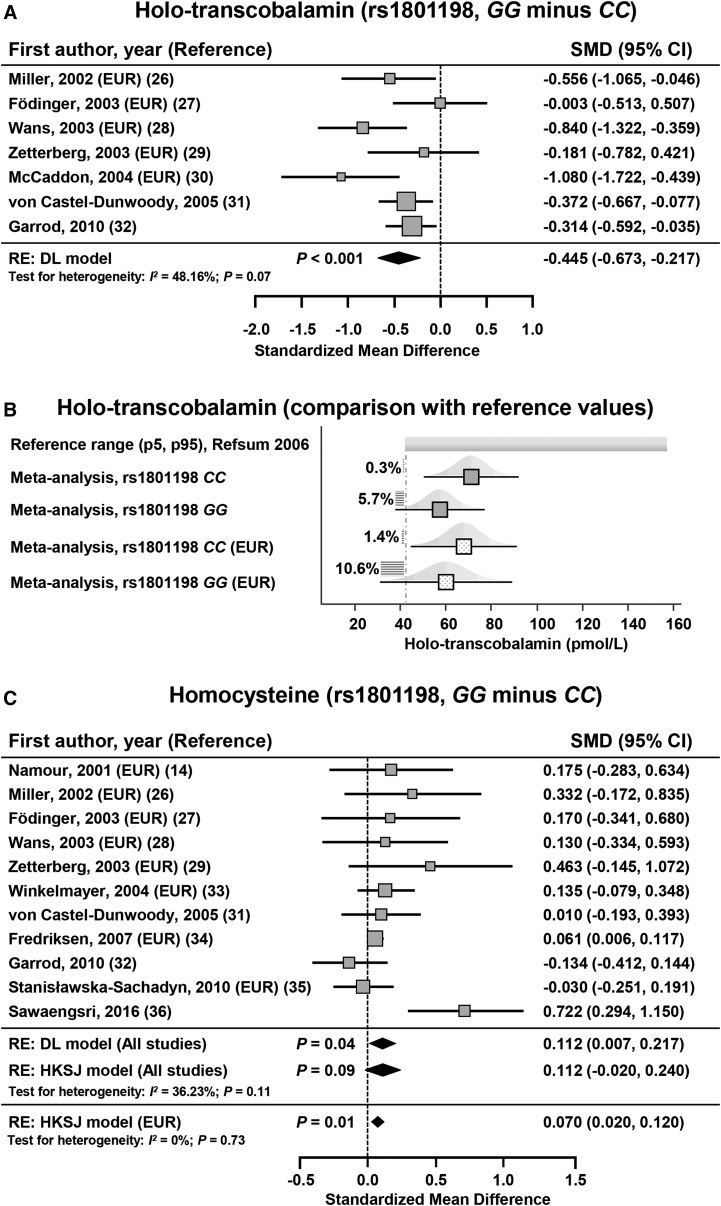
Seven studies on 1481 subjects assessed the concentration of holotranscobalamin according to rs1801198 (26–32). Of these studies, 6 studies used a radioimmunoassay method (26–28, 30–32), and 1 study used an ELISA method (29), for quantifying holotranscobalamin. With the use of a random-effects model, the holotranscobalamin concentration was significantly lower in subjects with the GG genotype than in those with the CC genotype with an SMD ± SE of −0.445 ± 0.116 [95% CI: −0.673, −0.217; P < 0.001 (DL)] and an I2 of 48.16% (95% CI: 0.00%, 78.10%; P = 0.07) (Figure 2A, Table 5). The Funnel plot showed a low risk of bias (Supplemental Figure 1). Results were similar when the pooled effect size was calculated on the 5 studies (26–30) that have assessed European-ancestry subjects (SMD ± SE: −0.524 ± 0.194; 95% CI: −0.906, −0.141; P = 0.007 (DL); I2 = 61.02%; 95% CI: 0.00%, 85.37%; P = 0.04). The pooled mean concentration of holotranscobalamin was 19% lower in subjects with the GG genotype (57.486 pmol/L; 95% CI: 37.900, 77.072 pmol/L) than in those with the CC genotype (71.135 pmol/L; 95% CI: 50.274, 91.997 pmol/L) (Figure 2B, Supplemental Table 5). According to holotranscobalamin reference values that were reported by Refsum et al. (59), subjects with the GG genotype were at risk of exhibiting holotranscobalamin concentrations below the reference range (Figure 2B). Indeed, the probability of presenting a holotranscobalamin concentration <42 pmol/L ranged from 0.3% in a subject who harbored an rs1801198 CC genotype to 10.6% in a European-ancestry subject who harbored an rs1801198 GG genotype (Figure 2B). Furthermore, we calculated the pooled effect size after excluding the study by Zetterberg et al. (29), which used an ELISA method and showed similar results with an SMD of −0.479 [95% CI: −0.731, −0.228; P < 0.001 (DL)] and an I2 of 54.16% (95% CI: 0.00%, 81.64%; P = 0.05). The number of studies that were needed to reach significance on the basis of calculated pooled means was 9, thereby corroborating the robustness of the meta-analysis (Supplemental Tables 5 and 6). In a meta-regression analysis, the STREGA score did not significantly influence the SMD for the comparison of holotranscobalamin concentrations between subjects with an rs1801198 GG or CC genotype (meta-regression slope: 0.05; 95% CI: −0.08, 0.18; P = 0.46).
FIGURE 2.
SMD for holotranscobalamin between subjects with the GG and CC genotypes for rs1801198 (A). The calculated summary effects are denoted by the solid diamonds at the bottom of the forest plots, the widths of which represent the 95% CIs. (B) Pooled mean concentrations (95% CI) of holotranscobalamin in subjects with an rs1801198 CC or GG genotype. Pooled mean concentrations are also reported in European-ancestry subjects (EUR). The horizontal hatchings and corresponding percentages represent the probability of exhibiting a holotranscobalamin concentration less than the threshold of 42 pmol/L. (C) SMD for homocysteine between subjects with GG and CC genotypes for the rs1801198. When the P value that is associated with the DL model was between 0.01 and 0.05, the 95% CI of the pooled effect size and its associated P value were calculated with the use of the HKSJ (23). For each study, the gray box and the horizontal line denote the central value of the effect size and its 95% CI, respectively. DL, DerSimonian and Laird procedure; EUR, study that included European-descent subjects; HKSJ, Hartung-Knapp-Sidik-Jonkman procedure; p5, 5th percentile; p95, 95th percentile; RE, random-effects model; Refsum, reference 59; SMD, standardized mean difference.
TABLE 5.
Pooled effect size for the difference of concentration of OCM markers between GG and CC rs1801198 genotypes1
| OCM marker | Studies, n | GG, n | CC, n | Total, n | SMD ± SE (95% CI of SMD)2 | P2 | I2 (95% CI),3 % | P4 |
| Holotranscobalamin | 7 | 256 | 527 | 783 | −0.445 ± 0.116 (−0.673, −0.217) | <0.001 | 48.16 (0.00, 78.10) | 0.07 |
| EUR | 5 | 112 | 195 | 307 | −0.524 ± 0.194 (−0.906, −0.141) | 0.007 | 61.02 (0.00, 85.37) | 0.04 |
| Vitamin B-12 | 11 | 2583 | 4278 | 6861 | −0.099 ± 0.144 (−0.381, 0.183) | 0.49 | 91.67 (87.09, 94.62) | <0.0001 |
| EUR | 8 | 2408 | 3867 | 6275 | 0.103 ± 0.106 (−0.105, 0.311) | 0.33 | 75.78 (51.45, 87.91) | 0.0002 |
| Homocysteine | 11 | 2595 | 4308 | 6903 | 0.112 ± 0.0585 (−0.020, 0.240)5 | 0.095 | 36.23 (0.00, 68.66) | 0.11 |
| EUR | 8 | 2420 | 3897 | 6317 | 0.070 ± 0.0215 (0.020, 0.120)5 | 0.015 | 0.00 (0.00, 49.59) | 0.73 |
| Folates | 5 | 2245 | 3663 | 5908 | −0.003 ± 0.052 (−0.106, 0.099) | 0.948 | 17.67 (0.00, 83.88) | 0.30 |
| EUR | 4 | 2214 | 3584 | 5798 | −0.011 ± 0.057 (−0.124, 0.101) | 0.846 | 22.73 (0.00–90.02) | 0.27 |
| Methylmalonic acid6 | 4 | 2137 | 3601 | 5738 | 0.255 ± 0.458 (−0.643, 1.153) | 0.58 | 97.70 (96.09, 98.65) | <0.0001 |
| RBC folates6 | 4 | 132 | 339 | 471 | 0.230 ± 0.191 (−0.145, 0.605) | 0.23 | 63.23 (0.00, 87.60) | 0.04 |
EUR, European-ancestry subjects; OCM, one-carbon metabolism; RBC, red blood cell; SMD, standard mean difference.
Random-effect model according to the DerSimonian and Laird procedure (22).
I2 is the percentage of the observed total variation across studies that was due to real heterogeneity rather than to chance (24).
Significance for heterogeneity was assessed with the use of the χ2-based Q statistic (24).
When the P value that was associated with the DerSimonian and Laird model was between 0.01 and 0.05, the 95% CI of the pooled effect size and its associated P value were calculated using the Hartung-Knapp-Sidik-Jonkman procedure (23).
No analysis on EUR was performed because of the low number of studies.
Homocysteine
Eleven studies in 13,139 subjects assessed the concentration of homocysteine according to rs1801198 (14, 26–29, 31–36). When all studies were included in the meta-analysis, the homocysteine concentration was not significantly higher in subjects with the GG genotype than in those with a CC genotype with an SMD ± SE of 0.112 ± 0.058 [95% CI: −0.020, 0.240; P = 0.09 (HKSJ)] and an I2 of 36.23% (95% CI: 0.00%, 68.66%; P = 0.11) (Figure 2C, Table 5). The funnel plot showed risk of bias that was related to the study by Sawaengsri et al. (36) (Supplemental Figure 1). When the meta-analysis included only the 8 studies in European-ancestry subjects (14, 26–29, 33–35), homocysteine was significantly higher in subjects who harbored the GG genotype than in those with the CC genotype with an SMD ± SE of 0.070 ± 0.021 [95% CI: 0.020, 0.120; P = 0.01 (HKSJ)] and no heterogeneity (I2 = 0.00%; 95% CI: 0.00%, 49.59%; P = 0.73) (Supplemental Figure 1, Figure 2C, Table 5).
Other OCM markers
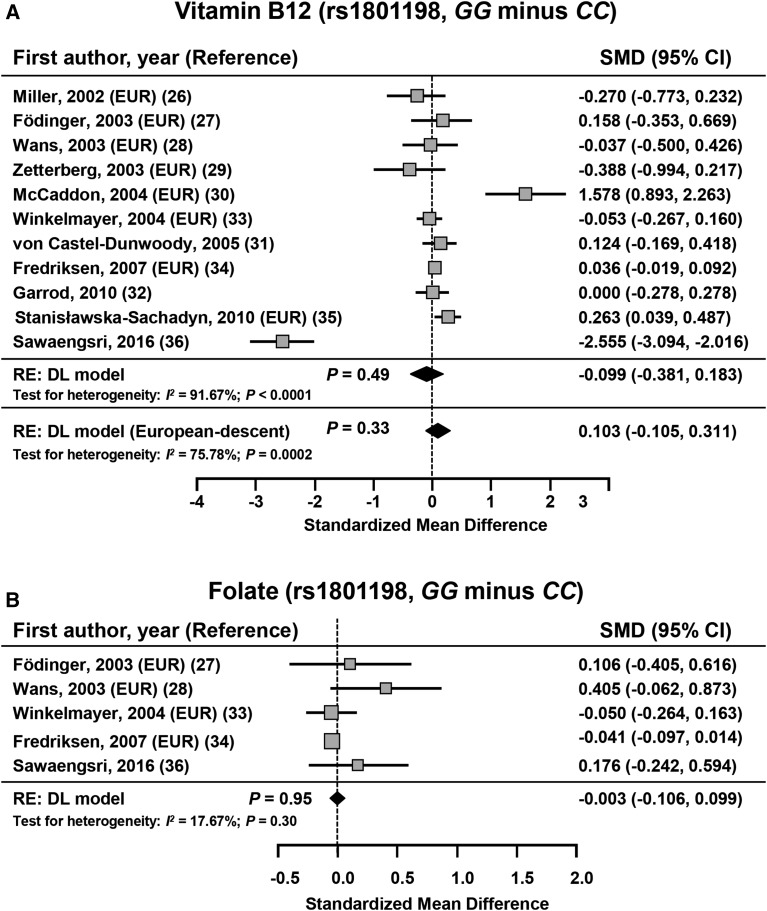
No significant difference was shown between subjects with the rs1801198 CC genotype and those with the GG genotype regarding concentrations of vitamin B-12 (n = 13,062), MMA (n = 10,922), and folates (n = 11,263) or RBC folates (n = 880) (Figure 3, Table 5). On the basis of calculated pooled means ± SEs, the number of studies that were required for reaching significance for vitamin B-12, MMA, folates, and RBC folates were 30, 11, 447, and 84, respectively. Thus, with only 4 studies included, the meta-analysis on the association between rs1801198 and MMA lacked statistical power. The Funnel plot showed risk of bias that was related to studies that were reported by Sawaengsri et al. (36) and McCaddon et al. (30) in relation to the vitamin B-12 meta-analysis (Supplemental Figure 1).
FIGURE 3.
SMD in vitamin B-12 (A) and folate (B) between subjects with GG and CC genotypes for the rs1801198. The calculated summary effects are denoted by the solid diamonds at the bottom of the forest plots, the widths of which represent the 95% CIs. For each study, the gray box and the horizontal line denote the central value of the effect size and its 95% CI, respectively. DL, DerSimonian and Laird procedure; EUR, study that included European-descent subjects; RE, random-effects model; SMD, standardized mean difference.
Association between rs1801198 and primary risk of congenital abnormalities
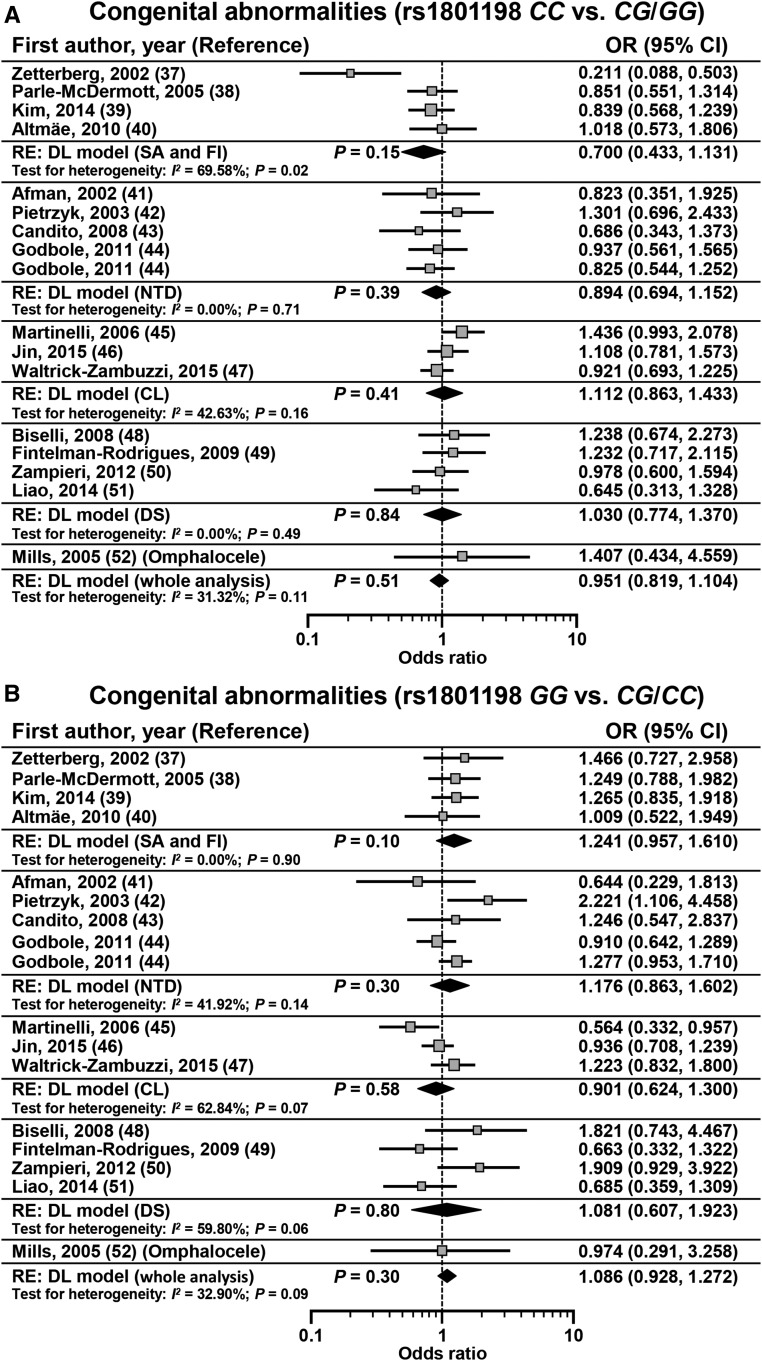
Sixteen studies in 7003 subjects (cases: n = 2771; controls: n = 4232) assessed the association between rs1801198 and primary risk of congenital abnormalities (37–52). No significant association was observed between the rs1801198 CC genotype and primary risk of congenital abnormalities [OR: 0.951; 95% CI: 0.819, 1.104; P = 0.51 (DL); I2 = 31.32%; 95% CI: 0.00%, 61.75%; P = 0.11]. Similarly, no significant association was observed between the rs1801198 GG genotype and primary risk of congenital abnormalities [OR: 1.086; 95% CI: 0.928, 1.272; P = 0.30 (DL); I2 = 32.90%; 95% CI: 0.00%, 62.60%; P = 0.09] (Figure 4, Table 6). A subgroup analysis according to the congenital abnormality subtype did not show a significant association when taking into account the CC compared with CG/GG or GG compared with CG/CC models (Table 6). The Funnel plot showed risk of bias related to the study by Zetterberg et al. (37) (Supplemental Figure 1).
FIGURE 4.
Effect sizes of studies that assessed primary risk of congenital abnormalities according to rs1801198 that were determined with the use of a CC compared with CG/GG genetic model (A) or GG compared with CG/CC genetic model (B). The calculated summary effects are denoted by the solid diamonds at the bottom of the forest plots, the widths of which represent the 95% CIs. For each study, the gray box and the horizontal line denote the central value of the effect size and its 95% CI, respectively. CL, cleft lip; DL, DerSimonian and Laird procedure; DS, Down syndrome; FI, female infertility; NTD, neural tube defects; RE, random-effects model; SA, spontaneous abortion.
TABLE 6.
Pooled effect size for the association between rs1801198 and primary risk of congenital abnormalities
| Congenital abnormality | Cases, n | Controls, n | OR (95% CI)1 | P1 | I2 (95% CI),2 % | P3 |
| CC/CG + GG | ||||||
| Spontaneous abortion and female infertility | 148/640 | 400/1322 | 0.700 (0.433, 1.131) | 0.15 | 69.58 (12.40, 89.44) | 0.02 |
| Neural tube defects | 134/739 | 211/1157 | 0.894 (0.694, 1.152) | 0.39 | 0.00 (0.00, 63.65) | 0.71 |
| Cleft lip | 300/1006 | 343/1190 | 1.112 (0.863, 1.433) | 0.41 | 42.63 (0.00, 82.70) | 0.16 |
| Down syndrome | 134/362 | 190/516 | 1.030 (0.774, 1.370) | 0.84 | 0.00 (0.00, 84.05) | 0.49 |
| Whole analysis4 | 722/2771 | 1153/4232 | 0.951 (0.819, 1.104) | 0.51 | 31.32 (0.00, 61.75) | 0.11 |
| GG/CG + CC | ||||||
| Spontaneous abortion and female infertility | 151/640 | 264/1322 | 1.241 (0.957, 1.610) | 0.10 | 0.00 (0.00, 36.13) | 0.90 |
| Neural tube defects | 274/739 | 408/1157 | 1.176 (0.863, 1.602) | 0.30 | 41.92 (0.00, 78.62) | 0.14 |
| Cleft lip | 221/1006 | 267/1190 | 0.901 (0.624, 1.300) | 0.58 | 62.84 (0.00, 89.38) | 0.07 |
| Down syndrome | 65/362 | 88/516 | 1.081 (0.607, 1.923) | 0.80 | 59.80 (0.00, 86.59) | 0.06 |
| Whole analysis4 | 716/2771 | 1037/4232 | 1.086 (0.928, 1.272) | 0.30 | 32.90 (0.00, 62.60) | 0.09 |
Random-effect model according to the DerSimonian and Laird procedure (22).
I2 is the percentage of observed total variation across studies that was due to real heterogeneity rather than to chance (24).
Significance for heterogeneity was assessed with the use of the χ2-based Q statistic (24).
Study by Mills et al. (52) that assessed the association with the primary risk of omphalocele was included in the whole analysis but not in subgroup analyses because of a lack of other studies on omphalocele.
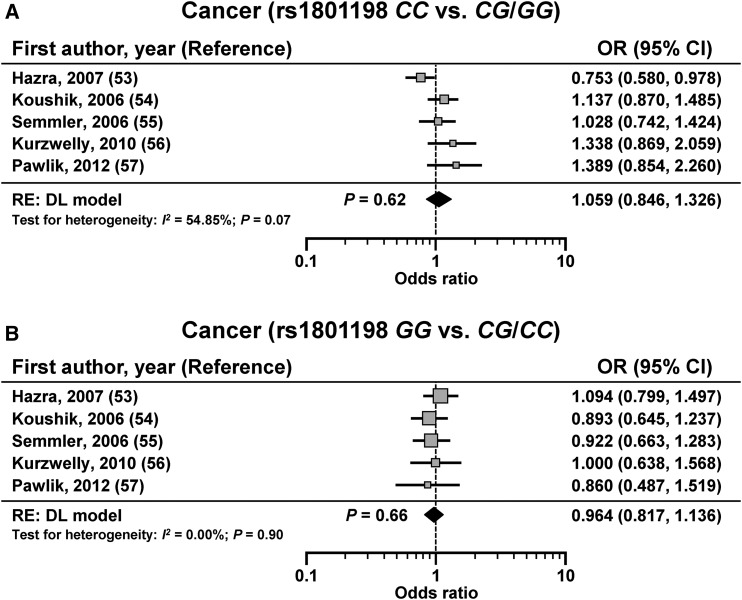
Association between rs1801198 and primary risk of cancer
Five studies in 3608 subjects (cases: n = 1518; controls: n = 2090) assessed the association between rs1801198 and primary risk of cancer (53–57) and showed no significant association using both the CC compared with CG/GG model [OR: 1.059; 95% CI: 0.846, 1.326; P = 0.62 (DL); I2 = 54.85%; 95% CI: 0.00%, 83.34%; P = 0.07] or the GG compared with CG/CC model [OR: 0.964; 95% CI: 0.817, 1.136; P = 0.66 (DL); I2 = 0.00%; 95% CI: 0.00%, 27.65%; P = 0.90] (Figure 5). The Funnel plot showed low risk of bias (Supplemental Figure 1).
FIGURE 5.
Effect size for studies that have assessed the primary risk of cancer according to rs1801198 that were determined with the use of a CC compared with CG/GG genetic model (A) or GG compared with CG/CC genetic model (B). The calculated summary effects are denoted by the solid diamonds at the bottom of the forest plots, the widths of which represent the 95% CIs. For each study, the gray box and the horizontal line denote the central value of the effect size and its 95% CI, respectively. DL, DerSimonian and Laird procedure; RE, random-effects model.
Association between rs1801198 and primary risk of Alzheimer disease
Two studies in 391 subjects (cases: n = 185; controls: n = 206) assessed the association between rs1801198 and primary risk of Alzheimer disease (30, 58) and showed no significant association using both the CC compared with CG/GG model [OR: 1.058; 95% CI: 0.428, 2.616; P = 0.12 (DL); I2 = 72.36%; 95% CI: 6.71%, 91.81%; P = 0.03] or the GG compared with CG/CC model [OR: 1.502; 95% CI: 0.889, 2.539; P = 0.13 (DL); I2 = 0.00%; 95% CI: 0.00%, 91.53%; P = 0.67].
DISCUSSION
This meta-analysis established that subjects with the rs1801198 GG genotype had significantly lower concentrations of holotranscobalamin with a moderate-to-high effect size and higher concentrations of homocysteine (European descent only) than did subjects with the rs1801198 CC genotype. These results potentially support the relevance of holotranscobalamin as an active vitamin B-12 status marker because no significant effect was shown on cobalamin concentrations. No significant difference was observed regarding concentrations of MMA, folate, and RBC folate. Furthermore, no significant association was observed between rs1801198 and primary risks of congenital abnormalities, cancer, or Alzheimer disease.
In patients who presented with a suspicion of cobalamin deficiency, the clinical picture represents the most important factor because, to our knowledge, no single gold-standard test defines cobalamin status with a high degree of accuracy (60). Guidelines for the diagnosis and treatment of cobalamin disorders from the British Committee for Standards in Haematology state that, although serum cobalamin currently remains the first-line test, holotranscobalamin is potentially useful as a first-line test in conjunction with homocysteine and MMA (60). The lack of a diagnostic gold standard limits the ability to weigh the diagnostic accuracies of holotranscobalamin and MMA for a confident diagnosis of cobalamin deficiency or subclinical cobalamin deficiency (61). In this context, combining cobalamin or holotranscobalamin with an MMA assessment has been suggested as a valuable approach (62, 63).
The present meta-analysis revealed a significant effect of rs1801198 on holotranscobalamin, but it failed to show a significant association of this genetic polymorphism with MMA concentrations. These data are consistent with the previous demonstration that rs1801198 influences the cellular expression and plasma concentration of TCN2 (13, 14). MMA is a metabolic marker of cobalamin deficiency, which does not directly reflect the concentration of holotranscobalamin (3); rs1801198 might be associated with an increase of MMA only in subjects with subnormal or borderline serum vitamin B-12 concentrations. These subjects represent a very-small subset of the populations who were included in our meta-analysis. From a statistical point of view, the lack of an association between rs1801198 and the MMA concentration could be considered cautiously, taken into account the low number of included studies, increased risk of type II error, and high risk of heterogeneity (I2 = 97.7%). Indeed, on the basis of calculated pooled means, the number of studies that were required to reach significance for MMA was 11. As a general comment regarding the lack of an association between rs1801198 and the MMA concentration, it could be assume that the “Absence of evidence is not evidence of absence” (64) and that additional well-powered studies should assess this association.
The present meta-analysis did not report a significant association between rs1801198 and primary risk of congenital abnormalities including spontaneous abortion, female infertility, neural tube defect, cleft lip, omphalocele, and Down syndrome. No significant association was shown when subgroup analyses were performed according to congenital abnormality subtype. Furthermore, visual inspection of the funnel plots did not show the asymmetry that is typically associated with publication bias. Further high-quality, well-designed genetic-association studies are warranted to clarify the potential involvement of genetic variants of TCN2 in the determinism of primary risk of congenital abnormalities.
We did not report a significant association between rs1801198 and primary risk of cancer. Results from randomized trials led to mixed results regarding the relation between folic acid, vitamin B-6, and vitamin B-12 treatment and cancer risk modification (65–67). In the Women’s Antioxidant and Folic Acid Cardiovascular Study in 5442 US female health professionals with preexisting cardiovascular disease or ≥3 coronary risk factors who were treated for 7.3 y, a combined folic acid, vitamin B-6, and vitamin B-12 treatment had no significant effect on overall risk of total invasive cancer or breast cancer (67). Consistently, a double-blind randomized controlled trial of 12,064 survivors of myocardial infarction in secondary care hospitals in the United Kingdom between 1998 and 2008 showed that folic acid and vitamin B-12 supplementation was not associated with adverse effects on cancer incidence (65). However, a combined analysis and extended follow-up of participants from 2 randomized, double-blind, placebo-controlled, clinical trials (the Norwegian Vitamin Trial and the Western Norway B Vitamin Intervention Trial) in a total of 6837 patients with ischemic heart disease who were treated with B vitamins or a placebo between 1998 and 2005 showed an association with increased cancer outcomes (HR: 1.21; 95% CI: 1.03, 1.41; P = 0.02) and cancer-related mortality (HR: 1.38; 95% CI: 1.07, 1.79; P = 0.01) in Norway, where there is no folic acid fortification of foods (66). The disparity in these results could be related to the effective metabolic availability of vitamin B-12 through the holotranscobalamin concentration. Future trials should take into account the holotranscobalamin concentration in addition to the cobalamin concentration.
Our meta-analysis has several strengths. First, the literature search was comprehensive, and to our knowledge, we identified all relevant studies that have assessed potential genetic associations with the TCN2 rs1801198 variant. Second, the study quality and post hoc study power were systematically evaluated to assess the robustness and quality of each pooled effect size. Third, when appropriate, meta-analyses regarding the association between rs1801198 and OCM markers were systematically performed before and after the exclusion of subjects with non-European ancestry. Fourth, we used a random-effects model, which provided a more conservative result with wider CIs, and systematically assessed publication bias. Fifth, the present meta-analysis assessed the pooled effect size from all genetic association studies on OCM-related biologic traits and pathologic conditions leading to an overview of the physiology-pathology spectrum that is related to TCN2.
We acknowledge several limitations in this meta-analysis. The potential for statistical heterogeneity is always present when combining case-control studies, which is the reason why we used a random-effects model in the analysis that provides conservative quantitative results. Second, the meta-analysis of studies that have assessed the association between rs1801198 and MMA, folate, and RBC folate included <5 studies with consequent risk of type II error. Third, meta-analyses that have assessed the association of rs1801198 with primary risk of pathologic conditions included genetic-association studies with relatively low quality and, thus, should be interpreted with caution. Fourth, the present meta-analysis did not assess genetic association studies on rs1801198 according to folate status, and notably, those dealing with pathologic conditions (15). Future genetic association studies should address this issue.
Taken together, the results of our analyses show that the TCN2 polymorphism rs1801198 significantly influences the concentration of holotranscobalamin. Because holotranscobalamin represents the active part of vitamin B-12, it is, therefore, reasonable to state that this polymorphism may affect diseases that are related to vitamin B-12 deficiency. However, to our surprise, we have not shown any significant association of the polymorphism rs1801198 with any of the 2 main groups of diseases (i.e., congenital malformations and cancer) that have been studied in the literature. Few studies have focused on neurologic pathologies, which are nevertheless pathologies that should deserve the most critical attention. In contrast, none of the studies have considered a specific analysis in subjects with a low vitamin B-12 concentration. This subset of the population is probably that for which the polymorphism rs1801198 can have an influence, because we can expect an additive effect when the holotranscobalamin concentration becomes a limiting factor for vitamin B-12 bioavailability.
In conclusion, this meta-analysis shows an influence of rs1801198 on holotranscobalamin and homocysteine concentrations in European-descent subjects. Further well-designed and powered studies should be conducted for assessing the association between rs1801198 and MMA and clinical manifestations that are linked to a decreased availability of cobalamin, particularly the neurologic manifestations in subjects with low holotranscobalamin.
Acknowledgments
The authors’ responsibilities were as follows—AO: performed the literature review, data extraction, data synthesis, and statistical analysis, drafted and revised the manuscript, and analyzed and interpreted the data; JL and PF-T: performed the literature review and data extraction, drafted and revised the manuscript, and analyzed and interpreted the data; FN: drafted and revised the manuscript and interpreted the data; J-LG: created the study concept, performed the literature review, drafted and revised the manuscript, and analyzed and interpreted the data; and all authors: read and approved the final draft of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DL, DerSimonian and Laird; HKSJ, Hartung-Knapp-Sidik-Jonkman; MMA, methylmalonic acid; OCM, one-carbon metabolism; RBC, red blood cell; SMD, standardized mean difference; STREGA, Strengthening the Reporting of Genetic Association Studies; TCN2, transcobalamin 2.
REFERENCES
- 1.Guéant JL, Caillerez-Fofou M, Battaglia-Hsu S, Alberto JM, Freund JN, Dulluc I, Adjalla C, Maury F, Merle C, Nicolas JP, et al. . Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase. Biochimie 2013;95:1033–40. [DOI] [PubMed] [Google Scholar]
- 2.Stabler SP. Vitamin B12 deficiency. N Engl J Med 2013;368:2041–2. [DOI] [PubMed] [Google Scholar]
- 3.Harrington DJ. Laboratory assessment of vitamin B12 status. J Clin Pathol 2017;70:168–73. [DOI] [PubMed] [Google Scholar]
- 4.Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management, expert consult premium edition-enhanced online features. Philadelphia: Elsevier Health Sciences; 2010. [Google Scholar]
- 5.Nexo E, Hvas AM, Bleie O, Refsum H, Fedosov SN, Vollset SE, Schneede J, Nordrehaug JE, Ueland PM, Nygard OK. Holo-transcobalamin is an early marker of changes in cobalamin homeostasis. A randomized placebo-controlled study. Clin Chem 2002;48:1768–71. [PubMed] [Google Scholar]
- 6.McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. Philadelphia: Elsevier Health Sciences; 2016. [Google Scholar]
- 7.Nexo E, Hoffmann-Lucke E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr 2011;94:359S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yildirim ZK, Nexo E, Rupar T, Buyukavci M. Seven patients with transcobalamin deficiency diagnosed between 2010 and 2014: a single-center experience. J Pediatr Hematol Oncol 2017;39:38–41. [DOI] [PubMed] [Google Scholar]
- 9.Trakadis YJ, Alfares A, Bodamer OA, Buyukavci M, Christodoulou J, Connor P, Glamuzina E, Gonzalez-Fernandez F, Bibi H, Echenne B, et al. . Update on transcobalamin deficiency: clinical presentation, treatment and outcome. J Inherit Metab Dis 2014;37:461–73. [DOI] [PubMed] [Google Scholar]
- 10.Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ 2014;349:g5226. [DOI] [PubMed] [Google Scholar]
- 11.Evatt ML, Terry PD, Ziegler TR, Oakley GP. Association between vitamin B12-containing supplement consumption and prevalence of biochemically defined B12 deficiency in adults in NHANES III (third national health and nutrition examination survey). Public Health Nutr 2010;13:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Seetharam S, Lindemans J, Alpers DH, Arwert F, Seetharam B. Isolation and sequence analysis of variant forms of human transcobalamin II. Biochim Biophys Acta 1993;1172:21–30. [DOI] [PubMed] [Google Scholar]
- 13.Namour F, Guy M, Aimone-Gastin I, de Nonancourt M, Mrabet N, Gueant JL. Isoelectrofocusing phenotype and relative concentration of transcobalamin II isoproteins related to the codon 259 Arg/Pro polymorphism. Biochem Biophys Res Commun 1998;251:769–74. [DOI] [PubMed] [Google Scholar]
- 14.Namour F, Olivier J, Abdelmouttaleb I, Adjalla C, Debard R, Salvat C, Gueant J. Transcobalamin codon 259 polymorphism in HT-29 and Caco-2 cells and in Caucasians: relation to transcobalamin and homocysteine concentration in blood. Blood 2001;97:1092–8. [DOI] [PubMed] [Google Scholar]
- 15.Green R. Peripheral neuropathy risk and a transcobalamin polymorphism: connecting the dots between excessive folate intake and disease susceptibility. Am J Clin Nutr 2016;104:1495–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuters T. EndNote X7. Philadelphia: Thomson Reuters; 2013. [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, et al. . STrengthening the REporting of Genetic Association studies (STREGA): an extension of the STROBE Statement. Ann Intern Med 2009;150:206–15. [DOI] [PubMed] [Google Scholar]
- 20.Julious SA. Sample sizes for clinical trials with normal data. Stat Med 2004;23:1921–86. [DOI] [PubMed] [Google Scholar]
- 21.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet 2006;38:209–13. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 23.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R. Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood 2002;100:718–20. [DOI] [PubMed] [Google Scholar]
- 27.Födinger M, Veitl M, Skoupy S, Wojcik J, Röhrer C, Hagen W, Puttinger H, Hauser AC, Vychytil A, Sunder-Plassmann G. Effect of TCN2 776C>G on vitamin B12 cellular availability in end-stage renal disease patients. Kidney Int 2003;64:1095–100. [DOI] [PubMed] [Google Scholar]
- 28.Wans S, Schüttler K, Jakubiczka S, Müller A, Luley C, Dierkes J. Analysis of the transcobalamin II 776C>G (259P>R) single nucleotide polymorphism by denaturing HPLC in healthy elderly: associations with cobalamin, homocysteine and holo-transcobalamin II. Clin Chem Lab Med 2003;41:1532–6. [DOI] [PubMed] [Google Scholar]
- 29.Zetterberg H, Nexo E, Regland B, Minthon L, Boson R, Palmer M, Rymo L, Blennow K. The transcobalamin (TC) codon 259 genetic polymorphism influences holo-TC concentration in cerebrospinal fluid from patients with Alzheimer disease. Clin Chem 2003;49:1195–8. [DOI] [PubMed] [Google Scholar]
- 30.McCaddon A, Blennow K, Hudson P, Hughes A, Barber J, Gray R, Davies G, Williams JH, Duguid J, Lloyd A, et al. . Transcobalamin polymorphism and serum holo-transcobalamin in relation to Alzheimer’s disease. Dement Geriatr Cogn Disord 2004;17:215–21. [DOI] [PubMed] [Google Scholar]
- 31.von Castel-Dunwoody KM, Kauwell GP, Shelnutt KP, Vaughn JD, Griffin ER, Maneval DR, Theriaque DW, Bailey LB. Transcobalamin 776C->G polymorphism negatively affects vitamin B-12 metabolism. Am J Clin Nutr 2005;81:1436–41. [DOI] [PubMed] [Google Scholar]
- 32.Garrod MG, Allen LH, Haan MN, Green R, Miller JW. Transcobalamin C776G genotype modifies the association between vitamin B12 and homocysteine in older Hispanics. Eur J Clin Nutr 2010;64:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkelmayer WC, Skoupy S, Eberle C, Fodinger M, Sunder-Plassmann G. Effects of TCN2 776C>G on vitamin B, folate, and total homocysteine levels in kidney transplant patients. Kidney Int 2004;65:1877–81. [DOI] [PubMed] [Google Scholar]
- 34.Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J. Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum Mutat 2007;28:856–65. [DOI] [PubMed] [Google Scholar]
- 35.Stanisławska-Sachadyn A, Woodside JV, Sayers CM, Yarnell JW, Young IS, Evans AE, Mitchell LE, Whitehead AS. The transcobalamin (TCN2) 776C>G polymorphism affects homocysteine concentrations among subjects with low vitamin B(12) status. Eur J Clin Nutr 2010;64:1338–43. [DOI] [PubMed] [Google Scholar]
- 36.Sawaengsri H, Bergethon PR, Qiu WQ, Scott TM, Jacques PF, Selhub J, Paul L. Transcobalamin 776C→G polymorphism is associated with peripheral neuropathy in elderly individuals with high folate intake. Am J Clin Nutr 2016;104:1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zetterberg H, Regland B, Palmer M, Rymo L, Zafiropoulos A, Arvanitis DA, Spandidos DA, Blennow K. The transcobalamin codon 259 polymorphism influences the risk of human spontaneous abortion. Hum Reprod 2002;17:3033–6. [DOI] [PubMed] [Google Scholar]
- 38.Parle-McDermott A, Pangilinan F, Mills JL, Signore CC, Molloy AM, Cotter A, Conley M, Cox C, Kirke PN, Scott JM, et al. . A polymorphism in the MTHFD1 gene increases a mother’s risk of having an unexplained second trimester pregnancy loss. Mol Hum Reprod 2005;11:477–80. [DOI] [PubMed] [Google Scholar]
- 39.Kim HS, Lee BE, Jeon YJ, Rah H, Lee WS, Shin JE, Choi DH, Kim NK. Transcobalamin II TCN2 67A>G and TCN2 776C>G) and transcobalamin II receptor (TCblR 1104C>T) polymorphisms in Korean patients with idiopathic recurrent spontaneous abortion. Am J Reprod Immunol 2014;72:337–46. [DOI] [PubMed] [Google Scholar]
- 40.Altmäe S, Stavreus-Evers A, Ruiz JR, Laanpere M, Syvänen T, Yngve A, Salumets A, Nilsson TK. Variations in folate pathway genes are associated with unexplained female infertility. Fertil Steril 2010;94:130–7. [DOI] [PubMed] [Google Scholar]
- 41.Afman LA, Lievers KJ, van der Put NM, Trijbels FJ, Blom HJ. Single nucleotide polymorphisms in the transcobalamin gene: relationship with transcobalamin concentrations and risk for neural tube defects. Eur J Hum Genet 2002;10:433–8. [DOI] [PubMed] [Google Scholar]
- 42.Pietrzyk JJ, Bik-Multanowski M. 776C>G polymorphism of the transcobalamin II gene as a risk factor for spina bifida. Mol Genet Metab 2003;80:364. [DOI] [PubMed] [Google Scholar]
- 43.Candito M, Rivet R, Herbeth B, Boisson C, Rudigoz RC, Luton D, Journel H, Oury JF, Roux F, Saura R, et al. . Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: a multicenter case-control study. Am J Med Genet A 2008;146A:1128–33. [DOI] [PubMed] [Google Scholar]
- 44.Godbole K, Gayathri P, Ghule S, Sasirekha BV, Kanitkar-Damle A, Memane N, Suresh S, Sheth J, Chandak GR, Yajnik CS. Maternal one-carbon metabolism, MTHFR and TCN2 genotypes and neural tube defects in India. Birth Defects Res A Clin Mol Teratol 2011;91:848–56. [DOI] [PubMed] [Google Scholar]
- 45.Martinelli M, Scapoli L, Palmieri A, Pezzetti F, Baciliero U, Padula E, Carinci P, Morselli PG, Carinci F. Study of four genes belonging to the folate pathway: transcobalamin 2 is involved in the onset of non-syndromic cleft lip with or without cleft palate. Hum Mutat 2006;27:294. [DOI] [PubMed] [Google Scholar]
- 46.Jin LL, Chen EJ, Hou W, Liu XH, Hu Y. The association between folate pathway genes and cleft lip with or without cleft palate in a Chinese population. Biomed Environ Sci 2015;28:136–9. [DOI] [PubMed] [Google Scholar]
- 47.Waltrick-Zambuzzi M, Tannure PN, Vieira TC, Antunes LS, Romano FL, Zambuzzi WF, Granjeiro JM, Kuchler EC. Genetic variants in folate and cobalamin metabolism-related genes in nonsyndromic cleft lip and/or palate. Braz Dent J 2015;26:561–5. [DOI] [PubMed] [Google Scholar]
- 48.Biselli JM, Brumati D, Frigeri VF, Zampieri BL, Goloni-Bertollo EM, Pavarino-Bertelli EC. A80G polymorphism of reduced folate carrier 1 (RFC1) and C776G polymorphism of transcobalamin 2 (TC2) genes in Down’s syndrome etiology. Sao Paulo Med J 2008;126:329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fintelman-Rodrigues N, Correa JC, Santos JM, Pimentel MM, Santos-Reboucas CB. Investigation of CBS, MTR, RFC-1 and TC polymorphisms as maternal risk factors for Down syndrome. Dis Markers 2009;26:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zampieri BL, Biselli JM, Goloni-Bertollo EM, Vannucchi H, Carvalho VM, Cordeiro JA, Pavarino EC. Maternal risk for Down syndrome is modulated by genes involved in folate metabolism. Dis Markers 2012;32:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao YP, Zhang D, Zhou W, Meng FM, Bao MS, Xiang P, Liu CQ. Combined folate gene MTHFD and TC polymorphisms as maternal risk factors for Down syndrome in China. Genet Mol Res 2014;13:1764–73. [DOI] [PubMed] [Google Scholar]
- 52.Mills JL, Druschel CM, Pangilinan F, Pass K, Cox C, Seltzer RR, Conley MR, Brody LC. Folate-related genes and omphalocele. Am J Med Genet A 2005;136:8–11. [DOI] [PubMed] [Google Scholar]
- 53.Hazra A, Wu K, Kraft P, Fuchs CS, Giovannucci EL, Hunter DJ. Twenty-four non-synonymous polymorphisms in the one-carbon metabolic pathway and risk of colorectal adenoma in the Nurses’ Health Study. Carcinogenesis 2007;28:1510–9. [DOI] [PubMed] [Google Scholar]
- 54.Koushik A, Kraft P, Fuchs CS, Hankinson SE, Willett WC, Giovannucci EL, Hunter DJ. Nonsynonymous polymorphisms in genes in the one-carbon metabolism pathway and associations with colorectal cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2408–17. [DOI] [PubMed] [Google Scholar]
- 55.Semmler A, Simon M, Moskau S, Linnebank M. The methionine synthase polymorphism c.2756A>G alters susceptibility to glioblastoma multiforme. Cancer Epidemiol Biomarkers Prev 2006;15:2314–6. [DOI] [PubMed] [Google Scholar]
- 56.Kurzwelly D, Knop S, Guenther M, Loeffler J, Korfel A, Thiel E, Hebart H, Simon M, Weller M, Linnebank M, et al. . Genetic variants of folate and methionine metabolism and PCNSL incidence in a German patient population. J Neurooncol 2010;100:187–92. [DOI] [PubMed] [Google Scholar]
- 57.Pawlik P, Mostowska A, Lianeri M, Sajdak S, Kedzia H, Jagodzinski PP. Folate and choline metabolism gene variants in relation to ovarian cancer risk in the Polish population. Mol Biol Rep 2012;39:5553–60. [DOI] [PubMed] [Google Scholar]
- 58.Cascalheira JF, Goncalves M, Barroso M, Castro R, Palmeira M, Serpa A, Dias-Cabral AC, Domingues FC, Almeida S. Association of the transcobalamin II gene 776C → G polymorphism with Alzheimer’s type dementia: dependence on the 5, 10-methylenetetrahydrofolate reductase 1298A → C polymorphism genotype. Ann Clin Biochem 2015;52:448–55. [DOI] [PubMed] [Google Scholar]
- 59.Refsum H, Johnston C, Guttormsen AB, Nexo E. Holotranscobalamin and total transcobalamin in human plasma: determination, determinants, and reference values in healthy adults. Clin Chem 2006;52:129–37. [DOI] [PubMed] [Google Scholar]
- 60.Devalia V, Hamilton MS, Molloy AM, British Committee for Standards in Haematology . Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 2014;166:496–513. [DOI] [PubMed] [Google Scholar]
- 61.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94:348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fedosov SN, Brito A, Miller JW, Green R, Allen LH. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med 2015;53:1215–25. [DOI] [PubMed] [Google Scholar]
- 63.Fedosov SN. Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: the age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta 2013;422:47–53. [DOI] [PubMed] [Google Scholar]
- 64.Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ 1995;311:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, et al. . Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303:2486–94. [DOI] [PubMed] [Google Scholar]
- 66.Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njolstad I, Refsum H, Nilsen DW, et al. . Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009;302:2119–26. [DOI] [PubMed] [Google Scholar]
- 67.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA 2008;300:2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]