Abstract
In endemic countries more than 20% of tuberculosis (TB) cases are in infants and children. Current animal models study TB during adulthood but animal models for infant TB are scarce. Here we propose that minipigs can be used as an animal model to study adult, adolescent and infant TB including natural transmission. In these studies, two-month old minipigs (representing infant age in humans) and six-month old minipigs (representing adolescence in humans) were infected via the aerosol route with hyper-virulent clinical strain W-Beijing Mycobacterium tuberculosis (Mtb) HN878 and were monitored for 11 or 36 weeks post-challenge, respectively. In the same studies, infected and unchallenged animals were housed together. Viable bacteria were recovered from pulmonary and thoracic lymph nodes from both - infected and their initially unchallenged natural contacts. Bacillary load, gross lesions and histopathology revealed similarities to the spectrum of disease observed in human TB. The study did not reach terminal end point, thus it was not possible to annotate definitive clinical symptoms of active TB. The results demonstrated that minipigs are experimental hosts of Mtb HN878, and the pathology developed in their lungs resembles pathological findings described in human TB. Importantly, within communities of Mtb infected minipigs natural transmission occurs.
Keywords: minipigs, natural transmission, Mycobacterium tuberculosis, HN878, aerosol
Introduction
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), kills or debilitates more people between 15-59 years of age than any other disease in the world 1. Drug susceptible TB is curable, but nowadays cases of multiple and extensively drug resistant TB are on the rise 2. Few bacilli transmitted through aerosol droplets from an actively infected individual are sufficient to cause infection 3. The disease often affects the lungs, however, only 5-15% of infected individuals progress to active TB 2. Studies in infants and children with TB are few even though this age group accounts for more than 20% of TB cases in high burden countries 4. In this article we show evidence that minipigs provide benefits in the study of human adult, adolescent and infant TB.
Current animal models of TB have numerous limitations. Except for non-human primates (NHP), no other animal model of TB offers the spectrum observed in human disease 5, 6. Rodent animal models are affordable and frequently used but data derived from these models does not directly translate into human TB applications 6. The anatomy, genetics, and physiology of pigs resembles that of humans 7 and functional similarities in organs make pig-to-primate organ transplantation possible 8. Minipigs are already used to study Alzheimer's disease, cystic fibrosis and diabetes 9. Minipigs have large litters (5-8 piglets) and develop human sized organs at six months of age. Aside from the primate and mouse immune system, the pig immune system is the third best characterized. The pig genome has been sequenced 10; this included extensive analysis of the pig immunome structure and function revealing greater similarities between humans and pigs than between humans and mice 11. The main differences between the pig and human immune system are the inversion of lymph nodes (LNs), two types of Peyer's Patches, and the mechanism of passive immunity from sow to piglets 7; newborn passive immunity is only possible through colostrum and milk. Thus, newborn minipigs can also be used to study interactions with pathogens in a fully naïve immune system 7, 12. Fortunately, there is a wide range of swine resources and immune reagents available, supporting the potential this model can offer in TB immunology research 13-15.
The use of pigs in TB research is not an unusual idea. Studies of experimental swine infection with M. bovis or BCG are reported 16, 17. In 2010, minipigs were also challenged via intrapleural injection with Mtb strain H37Rv and monitored for a period of 20 weeks 18. These elegant studies highlighted encapsulation of granulomas as a key factor of latent Mtb infection in swine and suggested that the encapsulation process is directed by the interlobular septae. Interestingly, granuloma encapsulation is reported in humans 19, cattle, goats 20 and swine TB 18 but is lacking in rodent or non-human primates TB models 20.
These studies continued developing the pig as animal model to study human TB. Our approach here mimicked Mtb infection in humans by challenging animals via the aerosol route with the hyper-virulent clinical W- Beijing Mtb strain HN878 21. Our overarching goal is to study the TB immune response and vaccine efficacy in neonatal piglets living in a community where natural transmission and infection is possible. We believe this approach can provide a more relevant animal model to evaluate vaccine efficacy because in endemic countries households with infants and children- are continually exposed to Mtb. Herein, we demonstrate that adolescent and piglet minipigs are susceptible to infection with the hyper-virulent Beijing Mtb strain HN878 and that the pathological course of infection resembles that seen in human TB.
Methods
Animals
Ten six-month old (group 1) and ten one-month old (group 2) minipigs were purchased from Sinclair-BioResources, MO. The vendor raises all animals in TB and brucellosis-free farms as well as free from other common swine diseases. The Institutional Animal Care and Use Committee of Colorado State University (CSU) approved the studies. CSU veterinary-residents monitored animals daily for any clinical symptoms. Each group of animals was socially housed in ABSL-III rooms (72±6°F, 30-70% humidity, 12:12 light cycle) for the duration of the experiment and were allowed to acclimate for at least two weeks prior to challenge. Animals had ad-libitum water access and were fed a locally sourced feed (Panepinto, CO). In group 1, body temperatures were recorded daily with weekly weights whereas in group 2 the same were recorded monthly. Two animals from each group remained unchallenged. The two unchallenged pigs in groups 1 and 2 remained in a separate building and were co-housed with the challenged animals three months (group 1) and three days (group 2) post-challenge. Timelines for each study are shown Table S1.
Aerosol challenge
The hyper-virulent W-Beijing Mtb strain HN878 was obtained from CSU repository bank. Inocula of 1×104 and 1×103 CFU/ml (group 1) and of 1×103 and 1×102 CFU/ml (group 2) were used to test a high and low dose challenge (HD and LD). The inoculum aerosol was created using a ViosH air compressor (PARI Respiratory Equipment, Inc. Midlothian, VA) and LC SprintH nebulizer (mass mean diameter 3.5mm) as reported previously 22. Minipigs received the inoculum (3-5ml and 2 ml of inoculum for group 1 and 2, respectively) while under general anesthesia using xylazine hydrochloride (1.5mg/kg) and ketamine hydrochloride (15mg/kg). Minipigs were monitored after challenge regularly during the first day.
Tuberculin skin test
Tuberculin Purified Protein Derivative (PPD, Mantoux) Tubersol® (Sanofi Pasteur Limited, Canada) was administered intradermally at a dose of 5TU per 0.1ml as recommended for human use. PPD was administered at 24 and 11 weeks post-Mtb challenge in group 1 and 2, respectively.
Dexamethasone Treatment
Starting at 7 months post-Mtb challenge, a subgroup of pigs from group 1 were treated with oral Dexamethasone (DEX: Par-Pharmaceutical Companies) at 0.5mg/kg/day 23. DEX was mixed with food and fed to five pigs (Table 1) for 60 consecutive days until sacrifice.
Table 1.
Summary of gross pathological lesions observed in HN878 challenged (top) and unchallenged (lower) animals in groups 1/young adult animals; group 2/piglets at time of necropsy.
| ID | Dose | Lungs | Lymph Nodes (LN) | Other organs | |
|---|---|---|---|---|---|
| GROUP 1; young adults | 43 | HD | Small lesions | Small lesions | lesions in spleen |
| 59 | HD* | Calcified lesions near bronchial tree Apical lobe hardened with lesions |
Small lesions | No abnormalities | |
| 46 | HD* | Small lesions near bronchial tree | No abnormalities | No abnormalities | |
| 76 | HD | Small lesions | Small lesions | Lesions in spleen | |
| 80 | LD | Small lesions | Calcified lesion in mediastinal LN | No abnormalities | |
| 42 | LD | -One major calcified lesion -Several small lesions disseminated |
Calcified lesion in mediastinal LN | No abnormalities | |
| 67 | LD* | Several small lesions | Calcified lesions | No abnormalities | |
| 58 | LD | -Disseminated numerous lesions -Calcified lesions near bronchial tree -Apical lobe hardened with lesions |
Calcified lesions in submandibular LN | No abnormalities | |
| GROUP 2; piglets | 72 | HD | Small lesions | Small lesions | No abnormalities |
| 78 | HD | Small lesions | Small lesions | No abnormalities | |
| 79 | HD | Small lesions | Small lesions | No abnormalities | |
| 88 | HD | Several small lesions disseminated | Small lesions | No abnormalities | |
| 68 | LD | Small lesions | Small lesions | No abnormalities | |
| 92 | LD | Small lesions | Small lesions | No abnormalities | |
| 93 | LD | Small lesions | Small lesions | No abnormalities | |
| 94 | LD | Small lesions One caseous lesion |
Small lesions | No abnormalities | |
| UNCHALLENGED PIGs | |||||
| GROUP 1; Adult pigs | 68 | none* | Small lesions | No abnormalities | No abnormalities |
| 74 | none* | Small lesions | Small lesions in submandibular LN | No abnormalities | |
| GROUP 2; piglets | 73 | none | Small lesions | No abnormalities | No abnormalities |
| 80 | none | Small lesions | Small lesions | No abnormalities | |
animals receiving Dexamethasone 0.5mg/kg/day sixty days prior to necropsy. Unchallenged adult pigs (group 1) and unchallenged piglets (group 2) co-housed with Mtb HN878 challenged pigs at time of necropsy.
Post-mortem examination
After general anesthesia, administered as described above, animals were euthanized by intracardiac overdose of pentobarbital (80-100mg/kg). Animals in-group 1 were sacrificed at 16 (n=2) and 36 weeks (n=8) post-challenge. For group 2 all pigs were euthanized at 11 weeks post-challenge (Table S1). As a pilot study, these end points were determined due to limited housing space and financial resources. At necropsy, the spleens, liver and cervical LN were examined; the thoracic cavity was opened and macroscopic lesions in the lungs and LNs were identified by observation and palpation. Gross lesions in lungs and LNs were recorded using a Nikon camera. Thereafter samples (approximately 0.5g) were extirpated and their weight recorded prior to processing for further analysis. In some animals there were numerous and varied types of gross lesions; an attempt was made to collect representative samples for every scenario (hard/soft, large/small, uninvolved/involved tissue).
Bacterial load determination
Samples (0.5 grams placed in 3ml sterile-tubes containing 1 ml PBS and 5×3.2mm sterile-stainless-beads) were homogenized (4min/8000rpm) using the Next-Advance-Bullet-Blender (Averill-Park, NY). Bacterial loads in samples were determined by plating serial dilutions of the homogenates on nutrient Middlebrook-7H11 agar-plates. A total of 15 samples were plated for each animal. The number of colony forming units (CFU) that appeared on the agar-plates after incubation at 37°C for 3-5 weeks were used to determine the bacterial load per gram of tissue sample.
Speciation of bacteria
Colonies appearing on agar plates were further verified through DNA extraction using Trizol® reagent (Thermo-Fisher), following the manufacturer's instructions. Extracted DNA was evaluated by PCR with specific primers for Mtb and non-tuberculous mycobacteria (NTM) rpoB 24 [Table S2]. PCR products were analyzed by electrophoresis in 2% agarose-gel followed by sequencing of the 210bp band to confirm they were Mtb 25.
Histology
Tissue samples from each animal were fixed in 4% paraformaldehyde. Sections were stained with hematoxylineosin (H & E) or acid-fast Auramine-Rhodamine (AFB). H&E staining was analyzed using the Aperio-Digital Scanner (Leica-Biosystems) and Image-Scope software. The AFB stained sections were examined using Zeiss LSM 510 confocal microscope and Zen 2009 software.
Statistical analysis
The viable CFU counts were converted to CFU per gram of tissue, which were then evaluated using Student's t-test. Differences were considered significant at the 95% level of confidence.
Results
The main aims for these studies were to determine if miniature pigs are hosts to the hyper-virulent W-Beijing Mtb strain HN878 and to annotate the progression of disease upon aerosol infection. Two age groups of female animals representing adolescence and infant age in humans were used. Six-month old (n=8) and two-month old minipigs (n=8) were infected with aerosols of Mtb strain HN878 and monitored as noted in Table S1. In each group of animals two doses of inoculum were tested. In-group 1, each pig was nebulized with approximately 3-5×104 CFU (HD; n=4) or 3-5×103 CFU (LD; n=4) of Mtb HN878, respectively. Each pig in-group 2 was nebulized with approximately 338 (HD; n=4) and 25 CFU (LD; n=4). Based on previous studies we estimated that 10% of the nebulized bacteria were deposited in the lungs 26. Therefore an estimate of actual dose deposited in the lungs of these animals is 50-500 CFU for group 1 and 2-33 CFU for group 2. Two unchallenged pigs were united with the challenged animals 3 months (group 1) or three days (group 2) after bacterial aerosol exposure. Group 1 animals remained under the same ABSL3 conditions for 9 months, or 2 months in group 2 pigs. PPD skin test was administered in the neck of all group 1 pigs, 24 weeks post-challenge, and all of the animals developed a large induration (>10mm) indicative of a positive result. In-group 2, PPD was administered at 11 weeks post-challenge, two days before euthanasia and read during necropsy. In this group, only one pig had a positive induration reaction to PPD.
Clinical symptoms
It is well described that Mtb is transmitted via aerosol when patients cough or sneeze, common symptoms associated with TB. Consequently, a main reason pigs were chosen to model TB in our study is their ability to cough and sneeze. Weight loss and fevers are also common symptoms of active TB and thus all pigs in-group 1 were monitored for clinical symptoms for a total of nine months and, except for some incidental coughing and sneezing (explained below), no changes were observed.
To evaluate the status of infection two pigs were euthanized (ID# 43 and 76) at 16 weeks post-challenge. These animals presented small lesions compatible with TB in the lungs, LNs, and spleen (Table 1) indicating successful infection of the animals under study. The remaining pigs were continuously monitored for weight loss, fever, coughing and sneezing. At 28 weeks post-challenge minipigs did not show clinical symptoms. In an attempt to speed up progression to active TB disease animals were treated with dexamenthasone (DEX). DEX is known to cause immunosuppression 23. Thus at 28 weeks post-challenge five animals in group 1, randomly selected from the HD, LD groups and unchallenged pigs, were subjected to oral DEX treatment for the last 60 days prior to necropsy. Animals tolerated the treatment with DEX but none of the animals appeared to develop any overt clinical symptoms suggestive of active TB but incidental coughing and sneezing was reported prior to necropsies. Animals in group 2 did not receive DEX treatment and did not develop any clinical symptoms suggestive of active TB during the 11 weeks period these animals were monitored.
Evaluation of gross lesions
Gross pathological examination of animals in group 1 at necropsy revealed TB-compatible lesions in all pigs; as summarized in Table 1, the number and severity of lesions varied notably between animals. Lesions were mostly found in the apical lobes and near the bronchial tree of the lungs; similar lesions were found in the mediastinal LNs some of which contained caseous necrosis. Figure 1A-C shows representative images of the broad spectrum of gross pathology observed at necropsy in group 1 animals. In Figure 1A numerous disseminated lesions were present in the apical lobe of pig # 59 whereas Figure 1B and 1C shows a left lobe with only a few, 2-3 lesions, respectively. Regardless of lesion burden, both calcified and soft lesions were present and could be found in all animals. Furthermore two pigs, 58 and 59 infected with a HD and LD, respectively, were found to have severe pathology in comparison to the rest of the animals. Both of these pigs had disseminated lesions throughout the lung lobes (Figure 2A); both animals had their apical lobes and mediastinal LNs consolidated by numerous calcified lesions and lesions containing caseous matter (Figure 2B-F). By palpation, enlargement was noted in other thoracic and submandibular LNs of some animals, however, these organs were not included to be compared with healthy animals. No abnormalities were seen in other organs except for two animals (ID# 43 and 76) that showed small extra pulmonary lesions in spleen. After evaluation of gross lesions we concluded that, upon aerogenic infection of adolescent pigs with Mtb HN878, marked heterogeneity of pulmonary and LN lesions was present; lesion severity did not correlate with HD or LD. Interestingly, DEX treatment had no noticeable effect on gross pathology.
Figure 1. Gross pathology.
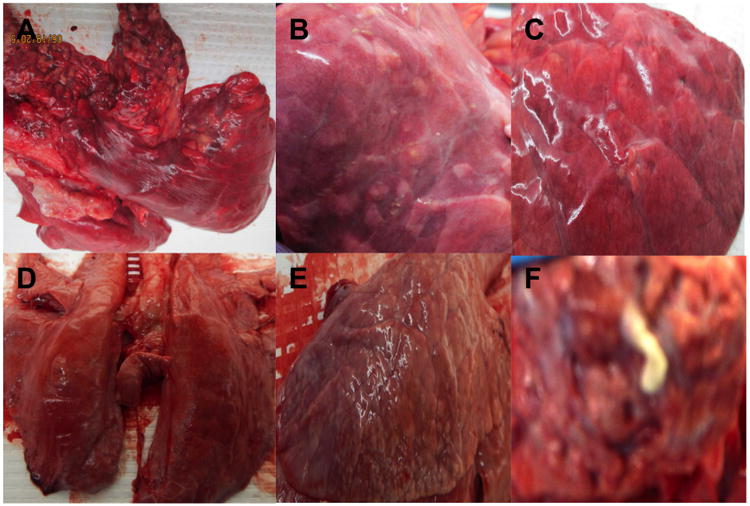
Representative gross lesions found in the lungs of pigs from group 1 (A, B, C) and group 2 (D, E, F) demonstrating heterogeneity of TB-compatible lesions. (A) numerous soft and calcified disseminated lesions in apical lobe of pig 59; (B and E) few disseminated soft lesions in one lobe of pig 67; (C-D) very few lesions of pig 76; (F) caseous matter from a lesion collected from pig 94 in group 2 (BSL3 image out of focus but only one available).
Figure 2. Severe gross pathology.
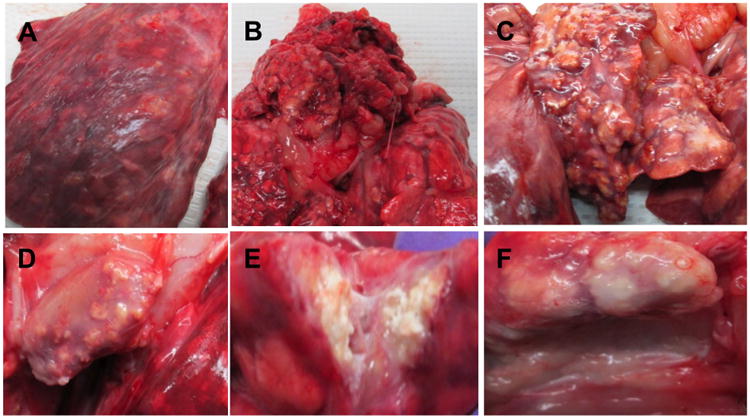
Photographs of lungs and lymph nodes from pigs 58 and 59 (group 1) challenged with a high dose and low dose aerosol of Mtb strain HN878, respectively. (A) Numerous disseminated lesions in left lobe; (B) lesions in apical lung lobe; (C) numerous lesions many of which were calcified in mediastinal lymph node; (D) cross-section of lymph node showing several lesions; (E) cross-section of calcified lesion containing caseous matter; (F) lesions near bronchial tree.
All young pigs in group 2 were euthanized 11 weeks post-challenge. Gross pathological examination of the lungs, LNs, and spleen did not reveal as many lesions as in group 1 (Table 1). Small and few lesions were observed in the lungs and LNs of all animals. Representative gross pathology images are shown in Figure 2D-F. Pig 88 infected with a HD aerosol had small and disseminated lesions through its lung lobes (Figure 2E) whereas pig 94 infected with a LD aerosol had different pathology consisting of a caseous lesion (Figure 2F). None of the piglets infected with Mtb HN878 demonstrated signs of disseminated infection outside the pulmonary cavity. As in group 1, after evaluation of gross lesions, we concluded that, upon aerogenic infection of 2 month old piglets with the Mtb HN878 strain, there was marked heterogeneity of pulmonary and LN lesions, dissemination of infection in the lungs and LN, and the severity of the lesions did not correlate with dose of the inoculum. Therefore, we concluded that when comparing the severity of lesions in animals euthanized at 2, 4 and 9 months post-Mtb infection pathology worsened as infection progressed in time.
Natural Transmission
Transmission of Mtb bacilli between TB patients is poorly understood and there is lack of animal models available to study this phenomenon. In group 1 unchallenged and challenged minipigs were co-housed in the same room separated by a physical barrier that allowed them to touch their noses and mouth but they did not share food or water. The two unchallenged animals co-housed with challenged animals demonstrated lesions at necropsy (Table 1); lesions in the lung were small and none were calcified. Both unchallenged pigs showed enlarged LNs. The submandibular LN of pig 74 (but not pig 68) contained small lesions. All other organs appeared healthy in unchallenged animals. Samples from lesions of these animals demonstrated positive bacteria growth on agar cultures (Figure 3A).
Figure 3. Bacterial burden.
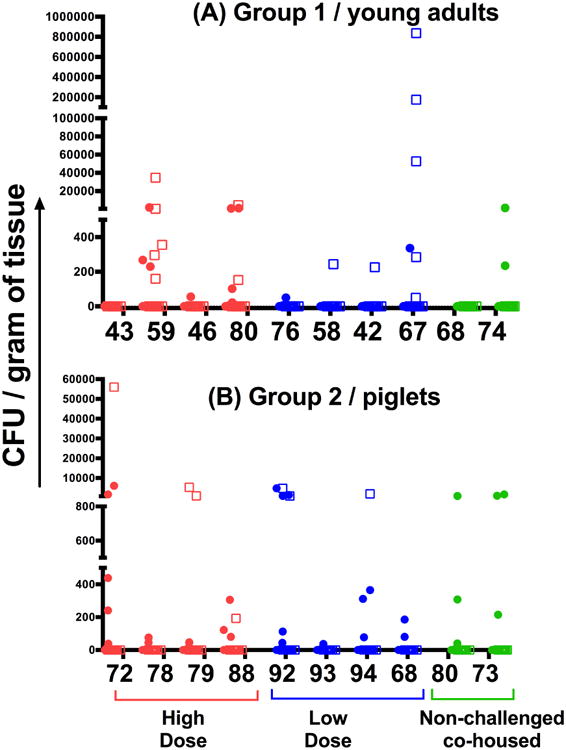
Bacterial load in lung (circles) and lymph node (squares) samples. Tissue bacterial load from lesions from group 1 (top graph) and group 2 (lower graph) challenged with a high dose (HD; red) and low dose (LD; blue) aerosol of Mtb strain HN878, and from pigs left unchallenged (green) but co-housed with the challenged animals. Data is presented as the number of colony forming units (CFU) per gram of tissue obtained after plating serial dilutions of the sample homogenate onto 7H11 agar plates and incubating for 3-4 weeks at 37°C.
In group 2, unchallenged and challenged piglets were housed in the same room without physical barriers and they shared food and water. Unchallenged animals in group 2 also demonstrated lesions and positive bacteria growth on agar culture (Figure 3B). Furthermore, salivary gland sections obtained from pig 80 demonstrated presence of positive AFB (data not shown). Altogether, we conclude that in both groups of animals, natural bacilli transmission and successful infection occurred when unchallenged animals were co-housed with challenged animals.
Bacterial burden
In group 1 the bacteria load obtained in samples from the same animal varied between 0 – 1×106 CFU per gram of tissue (Figure 3A). Similar differences in levels of bacterial load varied between samples from different animals. Furthermore there was no statistical difference in bacterial load between samples collected from animals infected with HD or LD. Comparative analysis of colony counts of tissue samples from group 2 also revealed no statistical difference between the bacterial burden obtained from animals receiving HD or LD inoculum (Figure 2B). Further, not all samples produced viable bacilli whereas some lesions contained as many as 104 CFU per gram of tissue.
Pigs are known to host other mycobacteria species such as NTMs 27, thus CFU were collected and processed by PCR. The speciation of selected colonies was performed by differential PCR using specific primers Mtb and NTMs 27. All colonies tested demonstrated that bacteria isolated from lesions in challenged and unchallenged animals belonged to the Mtb species; none of them appeared to be positive for NTMs (data not shown).
Histopathology
Histopathological analysis confirmed the TB-compatible lesions. By H&E staining, many samples from uninvolved lung tissue demonstrated low or high thickening of the lung parenchyma (Figure 4A-B), while others (Figure 4C) showed typical tuberculous granulomas formed by cells resembling lymphocytes and macrophages surrounding a core of foamy macrophages and neutrophils. Other lesions obtained from lungs (Figure 4 E-G and I) and LNs (Figure 4 D, F, H) demonstrated different levels of progression in the formation of necrotic and caseating granulomas containing calcified centers surrounded by lymphoid cells and fibrosis. Many lesions in the LNs, and less frequently in the lungs, appeared as multifocal necrotic and caseating lesions. Fibrosis was also noted in most lesions. The histopathology of animals from group 2 (Figure 4) revealed fewer granulomas than animals from group 1 but lesions demonstrated similar spectrum of lesions. Other samples from the same group demonstrated extensive to little thickening of the parenchyma (Figure 4J –K).
Figure 4. Histopathology.
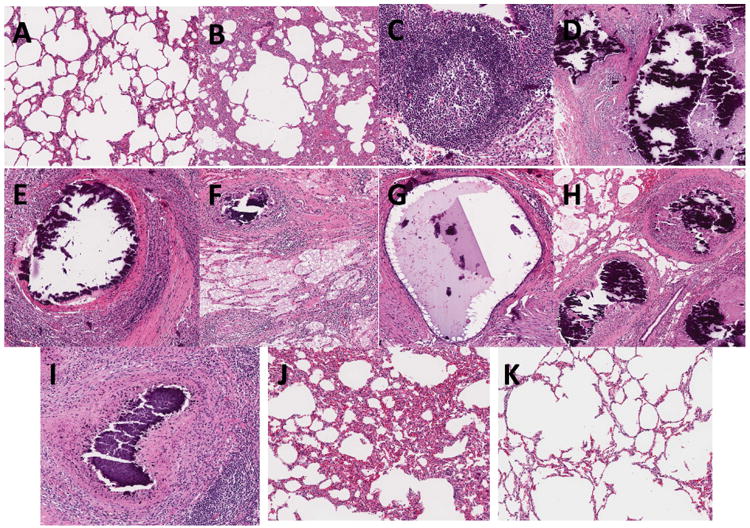
Representative H&E stained tissue sections of pigs in group 1 (AH) and group 2 (I-K) at 36 and 11 weeks post-challenge of aerosol Mtb strain HN878, respectively. (A-B; J-K) Lung sections from samples obtained in uninvolved areas of the lungs showing low (A,K) or high (B and J) thickening of lung parenchyma; (C) TB compatible granulomas showing sheets of lymphocytes, macrophages, foamy cells and neutrophils; (D-I) TB compatible necrotic granulomas with calcified center, lymphocytes, rim of foamy cells and surrounding fibrosis in the mediastinal lymph node (D, F, H) and lung tissue (E-G and I); (D-H) multifocal necrotic granulomas in lymph nodes.
AFB were also present in lesions, including necrotic granulomas but sometimes were also found outside the granulomas. A representative lung sample of pig 58 stained with AFB, demonstrates a granuloma with a necrotic center (Figure5 A-E); AFB surround the necrotic center along with macrophages, further surrounded by a lymphocytic rim and fibrosis. Figure 5B-E shows intracellular AFB in foamy cells located at the rim of the necrotic granuloma. Many macrophages at the necrotic rim had foamy morphology and at times appeared also as multinucleated cells (Figure 5D). AFB was not found in samples analyzed from group 2.
Figure 5. Detection of acid fast positive bacilli.
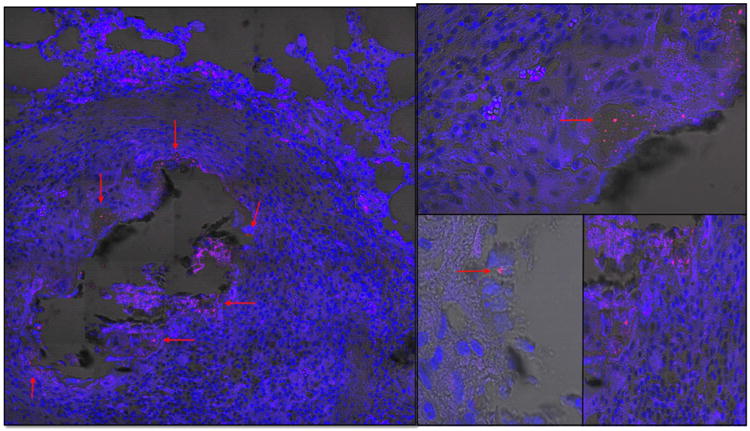
Representative images showing acid fast positive bacilli in lungs of pigs 36 weeks post aerosol challenge with Mtb HN878. (A) Granuloma of lung with necrotic center showing positive staining (red arrows) in the rim of the core of the granuloma. Center of granuloma contains acid fast positive staining associated with cell debris or free; (B, C, E) high magnification of foamy cells at the rim of granuloma and positive for intracellular acid fast staining; (D) multinucleated cells found at the rim of the granuloma core.
Discussion
This pilot study sought to determine whether adolescent and piglet minipigs are susceptible hosts to the clinical and highly virulent Mtb strain HN878 and if natural transmission is possible within a community of minipigs. Post-mortem examination revealed both groups of pigs demonstrated TB-compatible lesions in the lungs and LNs with heterogeneity in bacterial burden and lesion pathology. The pigs in the adolescent group were monitored (for nine months) until adulthood and some animals showed severe pathology with lesions varying in size, dissemination throughout lungs and LNs, and calcification necrosis in many lesions. Some lesions demonstrated AFB. Some granulomas were found to have necrotic centers with pronounced fibrosis, a common feature found in human TB but not reproduced in some TB animal models 28, 29, whereas other granulomas did not develop necrotic centers. While at least two minipigs demonstrated severe pulmonary pathology, other animals in the same group presented only mild pathology but with variable levels of bacterial burden within lesions. The younger pigs were monitored for almost 3 months and had smaller sized lesions with no calcification present. Lesions in this group were more homogeneous overall, however, with variable levels of bacterial burden within lesions. Caseous matter was found within some lesions of lung and LNs in both groups of animals. Overall lesions appeared to cover the spectrum of granuloma types previously reported for TB in humans, NHP, guinea pigs and mice 6, 30.
At the time of necropsy, it was not possible to obtain a definitive clinical picture as found with active TB in humans. The latter suggest that the course of infection in most animals progressed into a latent TB infection (LTBI), similar to a previous study 18. However despite lack of obvious clinical symptoms within the community of minipigs there were also active cases of TB capable of infecting unchallenged animals. Future studies will differentiate LTBI from active TB cases within minipig TB community.
As expected, an aerosol infection with the clinical and hyper-virulent Mtb strain HN878 developed into severe pathology in the lungs and LNs of some pigs 21. Interestingly, some lesions obtained from animals contained very high bacterial burden whereas other lesions obtained from the same animal did not demonstrate bacterial growth. Similar observations have been found in human and NHP TB 31. In this regard, Mtb infection in minipigs resembles NHP and human TB disease. It has been previously suggested 18 that encapsulation of some granulomas –as observed in pigs, humans, cattle and goats- limits bacteria growth within granuloma. In this study, it remains to be determined if low bacterial burden containing granulomas were also encapsulated.
Importantly, this study demonstrated transmission of bacilli and infection in unchallenged animals suggesting active cases of TB in the community. In both groups of pigs, the unchallenged animals revealed small TB-compatible lesions at time of necropsy with viable growth of Mtb bacilli in agar plates. Natural transmission may have occurred through possible aerosol droplets or sharing of oral-nasal fluids. From the severe pathology observed at necropsy in at least two pigs, occasional signs of cough and sneeze and AFB in salivary glands it could be inferred these communities of pigs were actively shedding bacilli into the environment. Further these communities of pigs infected with Mtb could have progressed to a more prominent active TB stage of disease if maintained in the study until terminal end point. Thus, the results shown in this pilot study suggest that minipigs provide a suitable animal model to study TB infection, immunity and natural transmission.
Supplementary Material
Acknowledgments
We want to thank many people for their help and contributions during the development of these studies, particularly, Ms. Jennifer Arab and Andrea Sanchez Hidalgo for their continuous technical support. We acknowledge and thank undergraduate and graduate students who assisted with sampling, cataloging and staining assays: Tanya Gonzalez, Camron Pearce, Isabella Mazariegos, Stephen Rickett. We thank Dr. J. Ayers and veterinary residents (Drs. J. Kopanke, E. Lee, K. Knapek, E. McWhorter, S. Fisher), as well as animal care staff (M. Adams, S. Masters, A. Waller) at Colorado State University, for daily care and monitoring of the animals and to the Bowen lab for additional help with animal handling and care.
Financial Support: This work was supported by the National Institute of Health, National Institute Allergy and Infectious Diseases under award # AI111168
Footnotes
Competing interest: Authors declare no conflict of interest with research presented in this manuscript.
Appendix A. Supplementary data. Supplementary data related to this article is available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Weekly Epidemiological Record. 2004 [Google Scholar]
- 2.WHO. Global Tuberculosis Report. 2015 [Google Scholar]
- 3.Cruz-Knight W, Blake-Gumbs L. Tuberculosis: an overview. Primary care. 2013;40:743–756. doi: 10.1016/j.pop.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Moyo S, Verver S, Mahomed H, Hawkridge A, Kibel M, Hatherill M, Tameris M, Geldenhuys H, Hanekom W, Hussey G. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14:149–154. [PubMed] [Google Scholar]
- 5.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. Journal of Medical Primatology. 2012;41:191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunological reviews. 2015;264:60–73. doi: 10.1111/imr.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends in microbiology. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotranspl antation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 9.Aigner B, Renner S, Kessler B, Klymiuk N, Kurome M, Wunsch A, Wolf E. Transgenic pigs as models for translational biomedical research. Journal of molecular medicine (Berlin, Germany) 2010;88:653–664. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- 10.Bambery RK. International Swine Genome Sequencing Consortium. 2016 [Google Scholar]
- 11.Dawson HD, Loveland JE, Pascal G, Gilbert JG, Uenishi H, Mann KM, Sang Y, Zhang J, Carvalho-Silva D, Hunt T, Hardy M, Hu Z, Zhao SH, Anselmo A, Shinkai H, Chen C, Badaoui B, Berman D, Amid C, Kay M, Lloyd D, Snow C, Morozumi T, Cheng RP, Bystrom M, Kapetanovic R, Schwartz JC, Kataria R, Astley M, Fritz E, Steward C, Thomas M, Wilming L, Toki D, Archibald AL, Bed'Hom B, Beraldi D, Huang TH, Ait-Ali T, Blecha F, Botti S, Freeman TC, Giuffra E, Hume DA, Lunney JK, Murtaugh MP, Reecy JM, Harrow JL, Rogel-Gaillard C, Tuggle CK. Structural and functional annotation of the porcine immunome. BMC genomics. 2013;14:332. doi: 10.1186/1471-2164-14-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Developmental and comparative immunology. 2009;33:384–393. doi: 10.1016/j.dci.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Summerfield A. Special issue on porcine immunology: An introduction from the guest editor. 2009;33:265–266. doi: 10.1016/j.dci.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Lunney JK. Advances in swine biomedical model genomics. International journal of biological sciences. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuggle CK, Bearson SM, Uthe JJ, Huang TH, Couture OP, Wang YF, Kuhar D, Lunney JK, Honavar V. Methods for transcriptomic analyses of the porcine host immune response: application to Salmonella infection using microarrays. Veterinary immunology and immunopathol ogy. 2010;138:280–291. doi: 10.1016/j.vetimm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolin CA, Whipple DL, Khanna KV, Risdahl JM, Peterson PK, Molitor TW. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. The Journal of infectious diseases. 1997;176:1559–1566. doi: 10.1086/514155. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Choi K, Olin MR, Cho SN, Molitor TW. Gammadelta T cells in immunity induced by Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infection and immunity. 2004;72:1504–1511. doi: 10.1128/IAI.72.3.1504-1511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil O, Diaz I, Vilaplana C, Tapia G, Diaz J, Fort M, Caceres N, Pinto S, Cayla J, Corner L, Domingo M, Cardona PJ. Granuloma encapsulation is a key factor for containing tuberculosis infection in minipigs. PloS one. 2010;5:e10030. doi: 10.1371/journal.pone.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canetti G. Exogenous reinfection and pulmonary tuberculosis a study of the pathology. Tubercle. 1950;31:224–233. doi: 10.1016/s0041-3879(50)80092-2. [DOI] [PubMed] [Google Scholar]
- 20.Cardona PJ. The key role of exudative lesions and their encapsulation: lessons learned from the pathology of human pulmonary tuberculosis. Frontiers in microbiology. 2015;6:612. doi: 10.3389/fmicb.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Juarrero M, Bosco-Lauth A, Podell B, Soffler C, Brooks E, Izzo A, Sanchez-Campillo J, Bowen R. Experimental aerosol Mycobacterium bovis model of infection in goats. Tuberculosis (Edinburgh, Scotland) 2013;93:558–564. doi: 10.1016/j.tube.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 23.van Mierlo GJ, Frieke Kuper C, de Zeeuw-Brower ML, Schijf MA, Bruijntjes JP, Otto M, Ganderup NC, H PA. A Sub Acute Immunotoxicity Study in Göttingen Minipigs® with the Immunosuppressive Compounds Cyclosporin A and Dexamethasone. Clinical & Experimental Pharmacology. 2013;2013 doi: 10.4172/2161-1459.S4-006. [DOI] [Google Scholar]
- 24.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NTN, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. Rapid Detection of Mycobacterium tuberculosis and Rifampin Resistance by Use of On-Demand, Near-Patient Technology*†‡. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/jcm.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HY, Kim H, Kim S, Kim DK, Cho SN, Lee H. Performance of a real-time PCR assay for the rapid identification of Mycobacterium species. Journal of microbiology (Seoul, Korea) 2015;53:38–46. doi: 10.1007/s12275-015-4495-8. [DOI] [PubMed] [Google Scholar]
- 26.Soffler C, Bosco-Lauth AM, Aboellail TA, Marolf AJ, Bowen RA. Development and characterization of a caprine aerosol infection model of melioidosis. PloS one. 2012;7:e43207. doi: 10.1371/journal.pone.0043207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoen CO, Lobue PA, Enarson DA, Kaneene JB, de Kantor IN. Tuberculosis: a re-emerging disease in animals and humans. Veterinaria italiana. 2009;45:135–181. [PubMed] [Google Scholar]
- 28.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature immunology. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, Lenaerts AJ. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2012;56:3181–3195. doi: 10.1128/aac.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Seminars in immunology. 2014;26:601–609. doi: 10.1016/j.smim.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, Sacchettini J, Fortune SM, Flynn JL. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nature medicine. 2014;20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


