Abstract
The role of the progesterone receptor (PR) in the regulation of sexual dimorphism in bone has yet to be determined. Here we utilized genetic fate mapping and western blotting to demonstrate age-dependent PR expression in the mouse femoral metaphysis and diaphysis. To define sex-dependent and osteoblast stage-specific effects of PR on bone acquisition, we selectively deleted PR at different stages of osteoblast differentiation. We found that when Prx1-Cre mice were crossed with PR floxed mice to generate a MSC conditional KO model (Prx1; PRcKO), the mutant mice developed greater trabecular bone volume with higher mineral apposition rate and bone formation. This may be explained by increased number of MSCs and greater osteogenic potential, particularly in males. Age-related trabecular bone loss was similar between the Prx1; PRcKO mice and their WT littermates in both sexes. Hormone deficiency during the period of rapid bone growth induced rapid trabecular bone loss in both the WT and the Prx1; PRcKO mice in both sexes. No differences in trabecular bone mass was observed when PR was deleted in mature osteoblasts using Bglap- Cre. Also, there were no differences in cortical bone mass in all three PRcKO mice. In conclusion, PR inactivation in early osteoprogenitor cells but not in mature osteoblasts influenced trabecular bone accrual in a sex dependent manner. PR deletion in osteoblast lineage cells did not affect cortical bone mass.
Keywords: bone accrual, progesterone receptor, mesenchymal stromal cells, osteoblasts, osteocytes, conditional gene knockout
Introduction
It is known that males achieve higher peak bone mass (PBM) and greater bone size than females. Females experience accelerated bone loss during menopause and aging, primarily due to declining estrogen levels, which places females at higher risk of developing osteopenia and osteoporosis (1). Sex hormones (sex steroids/gonadal steroids) are important mediators of sexual dimorphism and are synthesized from cholesterol in the gonads and adrenal glands. The biosynthesis of these hormones are closely related, which makes it difficult to study individual hormone contribution to bone homeostasis (2).
Sex hormones act on target cells by binding to members of the nuclear hormone receptor superfamily (3). To investigate how estrogen and androgen regulate both bone homeostasis and sexual dimorphism in peak bone mass acquisition, their corresponding receptors have been manipulated (4,5). Although sex hormone receptor knockout models do not necessarily recapitulate the phenotypes of sex hormone deficiency, the functions of estrogen receptors (ERs) and androgen receptors (ARs) on bone homeostasis have been extensively studied using animal models with germ-line or tissue-specific knockouts (6–11). These genetically modified mouse models have provided crucial information regarding the complex functions of sex hormone receptors on bone homeostasis. For example, during male skeletal development, estrogen stimulates periosteal expansion via ERα while androgen acts on the osteoblasts and osteocytes via the AR to increase trabecular bone formation. However, in females, estrogen inhibits periosteal apposition, endocortical bone resorption, and chondrocyte remodeling via ERα resulting in earlier epiphyseal closer and attenuation of bone expansion (1,5).
The effects of another important sex hormone, progesterone, on bone homeostasis remains to be elucidated. Most existing studies utilized either progesterone agonists or antagonists to evaluate their effects on bone mass in either preclinical or clinical settings and had results that were partially due several confounding factors. Treatments with either a progesterone agonist or antagonist may change the levels of other bone active hormones, including estrogen, androgens, and follicle-stimulating hormone (FSH) (12,13). Our research group and others have reported a high-bone-mass phenotype in progesterone receptor (PR) germ-line knockout (PRKO) mice (14,15). Compared to their wild-type (WT) littermates, female PRKO mice had higher bone formation while male PRKO mice had lower bone resorption. PR isoforms are known to be differentially expressed in a wide range of tissues and their presence or relative proportion may explain the PR’s sexual dimorphic roles in different tissues (16–18). Based on the reports that the PR expressed in the osteoblastic cells as measured in vitro (19–21), we hypothesized that the PR may directly participate in the regulation of bone formation and mass acquisition.
To study the PR’s role in bone development and homeostasis in both sexes, we conditionally knocked out the PR gene (PRcKO) using three bone-specific drivers: Prx1-Cre that targets mesenchymal stem cells in developing limb bud (22), Dmp1-Cre that targets osteocytes and a subset of mesenchymal lineage cells (23–25), and the osteocalcin-Cre (Bglap-Cre) that mainly targets the mature osteoblasts (26–28).
Materials and Methods
Mouse lines
The PR-flox mice were obtained from Dr. John Lydon at Baylor College of Medicine. A targeting vector designed to replace part of exon 2 of the PR gene with a selectable marker was used to create a strain of mice carrying a conditional null PR allele (29). Prx1-Cre, Bglap-Cre, Dmp1-Cre, Col1a1(2.3kb)-GFP, PR-Cre, mT/mG, and Ai14D transgenic mice were from the Jackson Laboratory. The Ai14 conditional allele (Rosa-CAG-LSL-tdTomato-WPRE:deltaNeo) has a loxP-flanked STOP cassette to prevent transcription of the downstream red fluorescent protein variant (tdTomato). When bred to mice that expresse Cre recombinase, the resulting offspring would have the STOP cassette deleted in the Cre-expressing tissue(s), resulting in expression of tdTomato (red fluorescence)(30). The PR-Cre mice (B6.129S(Cg)-Pgrtm1.1(cre)Shah/AndJ) harbor an internal ribosome entry site (IRES) and Cre recombinase gene downstream of the progesterone receptor transcriptional stop codon. As such, Cre expression is driven by the endogenous PR promoter/enhancer elements(31). A separate set of Prx1; PRcKO mice from either female or male were ovariectomized (OVX) or orchiectomized (ORX) at two months of age and euthanized at three months of age. A microCT scan was performed before surgeries and repeated prior to euthanasia. A minimum of n = 5 mice was used of each genotype for both sexes for all experiments.
PCR-based strategies were used to genotype mouse genomic DNA. All animal work was done in compliance with the guiding principles of the UC Davis’s “Care and Use of Animals”. Mice were housed in the animal facility under closely controlled environmental conditions (12-hour light/dark cycle, room temperature 22°C), and fed ad libidum (food and water). The Institutional Animal Care and Use Committee of the University of California Davis approved the animal protocol.
MicroCT measurements
Right femurs were scanned and analyzed using VivaCT 40 (Scanco Medical, Bassersdorf, Switzerland) with a voxel resolution of 10 μm in all three spatial dimensions and a mono-energetic (70 Kev) X-ray source. We evaluated 200 slices approximately 2 mm from the distal end of the growth plate. The slices, which covered a total metaphysis tissue volume of 2–3.5 mm3 for each scan were used to obtain the trabecular bone volume/total volume (BV/TV) ratio (14) and trabecular microarchitecture values(32). The same scanning algorithm was used to scan the middle femur shaft and 50 slices were used to obtain cortical bone volume (Ct.BV).
Bone marrow stromal cell (BMSC) isolation and cell culture
The condyles of the proximal and distal femurs were removed and the bone marrow was flushed out. The cell suspension was filtered through a 70μm filter, and plated into a dish. BMSCs were differentiated into osteoblasts in osteogenic media (changed every two days) containing 50 μg/ml ascorbic acid and 10 mM β-glycerol phosphate (βGP) (Life Technologies, Carlsbad, CA, USA). RNA extraction was performed on days 0, 1, 2 3 and 6 to monitor PR expression.
Flow cytometry
BMSCs were isolated from the mid femurs, and cultured for a week before flow cytometric analysis was performed following the protocol instructions from the manufacturer. The mouse mesenchymal stromal cell multi-color flow kit (R&D Systems, Minneapolis, MN, USA) was used to determine the percentage of MSC-like cells.
Histology, quantitative RT-PCR and western blot
The bones from the mT/mG or Ai14D reporter mice were collected, fixed in 4% formaldehyde with 10% sucrose (W/V) at 4°C overnight, and embedded in Optimal Cutting Temperature (O.C.T.) medium (Thermo Fisher, Waltham, MA, USA). Cryosections were prepared using Cryofilm (Section-Lab, Hiroshima, Japan) and observed with a Keyence BZ-X700 all-in-one Fluorescence Microscope (Itasca, IL, USA) and analyzed using Bioquant (Bioquant Image Analysis Corporation. Nashville, TN, USA).
RNA was isolated from cells or bone using a modified two-step purification protocol employing homogenization (PRO250 Homogenizer, 10 mm × 105 mm generator, PRO Scientific IN, Oxford CT, USA) in Trizol (Invitrogen, Carlsbad, CA), followed by purification over a Qiagen RNeasy column (Qiagen, Valencia, CA, USA). Primer set for RT-PCR were purchased from SABiosciences (Frederick, MD, USA).
Protein was extracted from the epiphyses, diaphyses or the ovary from WT or PRcKo mice. A BCA assay (Thermo Scientific, Waltham, MA, USA) was performed to quantify the protein concentration. Western blot analysis of PR levels in various tissues was performed using anti-PR that detected both A&B isoforms (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Bone strength measurements
Each femur was loaded to failure along its long axis using an MTS 831 electro-servo-hydraulic testing system (MTS Systems Corp., Eden Prairie, MN, USA) at a displacement rate of 0.01 mm/s with a 90 N load cell. Sample loads and displacements were continuously recorded throughout each test. Maximum load was determined from the load-displacement curve and the work to fracture was calculated from the area under the load-displacement curve (14,33–35).
Statistical analysis
The results are expressed as mean ± SEM for bone structure, bone turnover and bone strength variables. Two-way ANOVA was used to account for genotype and sex. If significant differences were observed, then a Sidak’s multiple comparisons test was used to assess pairwise comparisons. A value of p < 0.05 was considered statistically significant. Data were analyzed using the GraphPad Prism 7 software package (La Jolla, CA, USA).
Results
PR expression in bone
To characterize the pattern of PR expression in bone, we generated a reporter mouse strain PR-Cre; Ai14D, in which the Cre was driven by the endogenous PR promoter and this activated the tdTomato expression. This mouse strain was crossed with Col1a1-GFP mice, in which osteoblasts and osteocytes express GPF driven by rat Col1a1 promoter (2.3kb). This generated PR-Cre; Ai14D; Col1a1-GFP mice where PR-expressing cells and their descendants expressed red fluorescent tdTomato and osteoblasts/osteocytes expressed GFP. Osteoblasts that expressed PR during their differentiation expressed both GFP and tdTomato (yellow overlay). We collected the right distal femurs from PR-Cre; Ai14D/Col1-GFP mice between three and eight weeks of age and performed cryosectioning. Red fluorescence, corresponding to PR expression, was observed at the bone surface as well as within some areas of the growth-plate cartilage beginning at 3 weeks of age. At 8 weeks of age, red and the amount and intensity of the red fluorescence was observed at the growth plate, endosteal bone surface and within bone marrow (Fig.1A–B). We found that at three weeks of age, 4 ± 1% tdTomato (PR)-expressing cells co-expressed Col1-GFP; and by eight weeks of age, 63 ± 9% of tdTomato (PR)-expressing cells co-expressed Col1-GFP at the primary spongiosa bone surfaces (Fig.1B). The expression of PR protein in the osteoblast-lineage cells was confirmed by anti-PR immunofluorescence on the distal femur from an 8-week-old Col1-GFP mouse (Figure 1C).
Figure 1. Characterization of PR expression in vivo and in vitro.
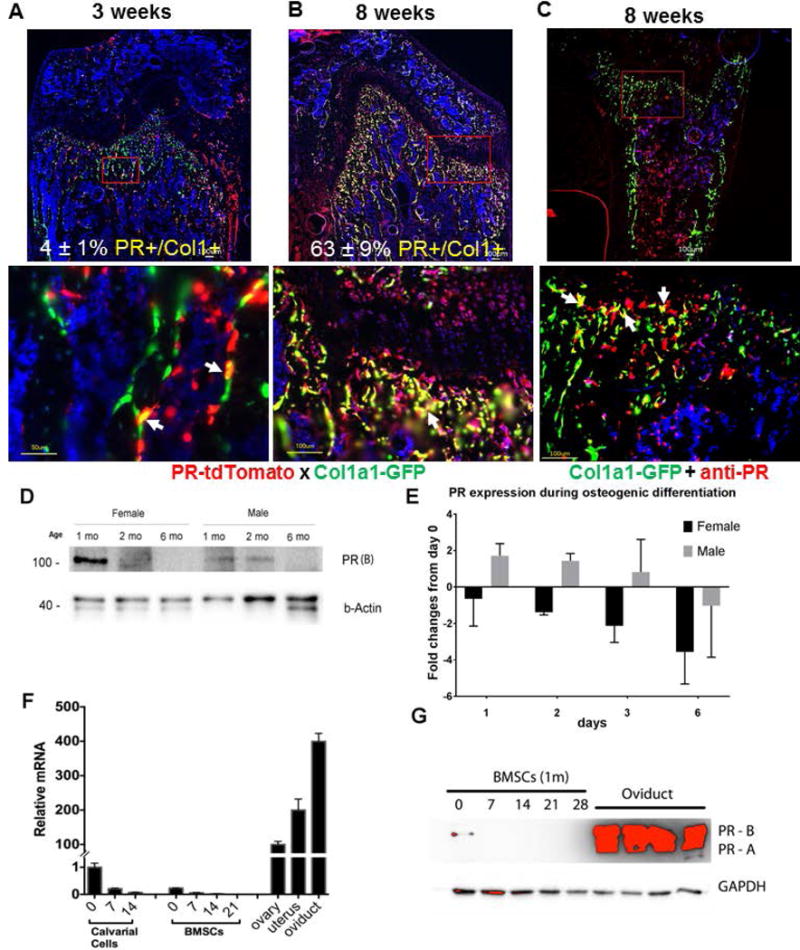
PR-Cre mice were crossed with Ai14D, red fluorescent protein tdTomato expression is activated following the stop codon removal by Cre, and crossed with Col1a1-GFP, where the green fluorescent GFP is driven by 2.3 kb rat procollagen, type 1, alpha 1(Col1a1) promoter, which is known to be active in mature osteoblasts. Representative fluorescent images of distal femur from 5 (A) or 8-week-old (B) male PR-Cre; Ai14D; Col1a1-GFP mice. Green fluorescence indicates mature osteoblast, while red fluorescence indicates PR expression. The rectangle region was magnified in to show the merged fluorescence pictures. Cell nuclei were stained blue with DAPI. (C), Anti-PR staining were performed on the Col1a1-GFP mouse, 8 weeks of age. White arrows illustrated co-localizations of PR and Col1a1-GFP+ cells. (D). PR protein level in bone decreases with age. Protein was extracted from distal femurs of 1-, 2-, and 6-month-old male and female mice, PR protein and internal control protein β-actin were detected with corresponding antibodies. Only isoform PR-B (118 kDa) was detected. (E). Bone marrow stromal cells were differentiated into osteoblasts with osteogenic media. qRT-PCR was performed to detect PR expression at 1, 3 and 6 days. Result was presented as fold-changes from day 0. (F). Calvarial cells or bone marrow stromal cells were differentiated with osteogenic medium for indicated time. RNA was extracted for PR real-time qPCR controlled with internal β-actin mRNA level. Ovary, uterus and oviduct tissues from a 3-month-old female mouse were used as positive controls. (G). Bone marrow stromal cells were differentiated in osteogenic medium for indicated time. Total cell lysate was prepared for PR western blot. Oviduct protein was used as positive control and GAPDH was used as internal control.
To characterize PR expression with age, the distal femoral total protein from male and female mice was collected for western blot analysis of PR protein levels at one, two or six months of age. The bone tissue primary expressed PR-B and the expression of PR-B declined rapidly with age, in both male and female mice (Fig.1D). To confirm our in vivo osteoblast whether PR expression, we collected bone marrow stromal cells (BMSCs) from two-month-old mice, and differentiated the cells into osteoblasts. RNA was collected at days 0, 1, 2, 3 and 6 days during the differentiation and used for RT-PCR analysis. We found that in females, PR expression started to decrease rapidly as soon as the BMSCs started differentiating into osteoblasts; while in males, PR expression was sustained early on (days 1–3) and then reduced as the BMSCs were further matured into osteoblasts (Fig.1E). We next isolated osteoblast-like populations from the calvarium and bone marrow, differentiated the cells into osteoblasts with osteogenic medium in vitro, and isolated RNA at different time points. PR (A+B) mRNA levels in primary bone stromal cells obtained from both the calvarium and bone marrow were considerably lower than that of cells from female reproductive tissues (p < 0.05) (Fig.1F). The PR mRNA levels were highest at day 0, of the culture and declined rapidly as the cells differentiated into osteoblasts (Fig.1F). We confirmed the PR protein expression in BMSCs by western blot (Fig.1G). These results suggested that the PR gene was expressed in osteoblastic lineage, especially in osteoblast precursors.
PR conditional knockout in early osteoprogenitor cells, marked by Prx1, resulted in high bone formation and bone mass at the trabecular bone site with sex difference
The next experiment included crossing Prx1-Cre with PR-flox/flox mice to conditionally inactivate the PR gene (PRcKO) in MSCs (Fig.2A). Prx1-Cre was active in both chondrogenic and osteoblastic cells in long bones, which is confirmed by Prx1; mT/mG reporter model (Fig.2B). The femoral protein was collected from wild type, heterozygous (PR-flox/+), or homozygous (PR-flox/flox) Prx1; PRcKO mice. Western blot analysis confirmed the successful removal of PR from the bone tissue in Prx1; PRcKO mice. PR expression was detected at the epiphyseal area and was undetectable at the femoral shafts (Fig.2C). Serum progesterone levels were significantly lower in male mice at 6 months of age as compared to the females, but it was not different between mutant Prx1; PRcKO mice as compared to their WT controls for both sexes at 2 or 6 months of age (Fig. 2D). At two months of age, the percentage of mesenchymal stromal cell-like cell population (CD105+CD29+Sca1+CD45−) in bone marrow stromal cells was ~40% higher in the male Prx1; PRcKO homozygotes compared to their WT controls (Fig. 2E).
Figure 2. Construction of Prx1; PRcKO conditional knockout mouse model.
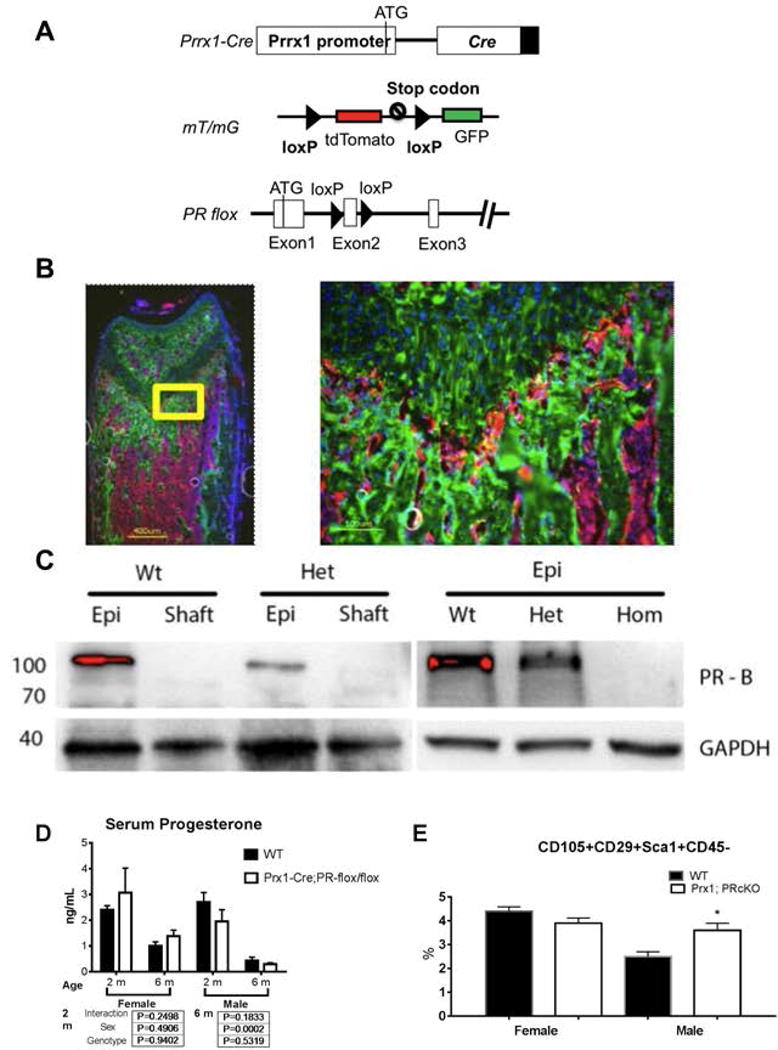
(A). A diagram shows Prx1-Cre, mT/mG, and PR-flox cassettes. (B). A representative fluorescent image of distal femur from 5-week-old Prx1-Cre; mT/mG mice. Green fluorescence indicates Cre activity in both osteoblasts and chondrocytes, while red fluorescence indicates all other cells without Cre activity. The rectangle region was magnified to show the detailed structure in growth plate region. Cell nuclei were stained blue with DAPI. (C). Protein was extracted from epiphysis (Epi) or femoral middle shaft (Shaft) of wild-type (WT), heterozygous ((PR-flox/+; Het), or homozygous (PR-flox/flox; Hom) Prx1; PRcKO 1-month-old male mice. PR protein and internal control protein GAPDH were detected with corresponding antibodies. Only isoform PR-B (118 kDa) was detected. (D). Serum level of progesterone measured at two and four months of age WT and Prx1; PRcKO animals. (E). Bone marrow stromal cells were collected from 2-month-old wild type or mutant Prx1; PRcKO mice and were cultured for a week before flow cytometric analyses of MSC markers.
There were no differences in either bone mass or volume in the mid-shaft of the femur measured by microCT, femoral length or mechanical properties assessed by 3-point-bending tests of the femurs (data on file). Peak trabecular bone volume at the distal femur was achieved by 2 months of age in both sexes and decreased with age thereafter for both sex, which was independent of genotype (Fig.3). The BV/TV of the distal femur was 51.5% and 85% higher in female and male mutant mice at 2 months of age and about 40% higher than WT in both sexes at 6 months of age as compared to their wild-type littermates (Fig.3). The high trabecular bone observed resulted from an increase in bone volume (BV) and trabecular thickness (Tb. Th), but not total volume (TV) (Fig.3).
Figure 3. Prx1; PRcKO mice developed high bone mass at the distal femurs in both sexes.
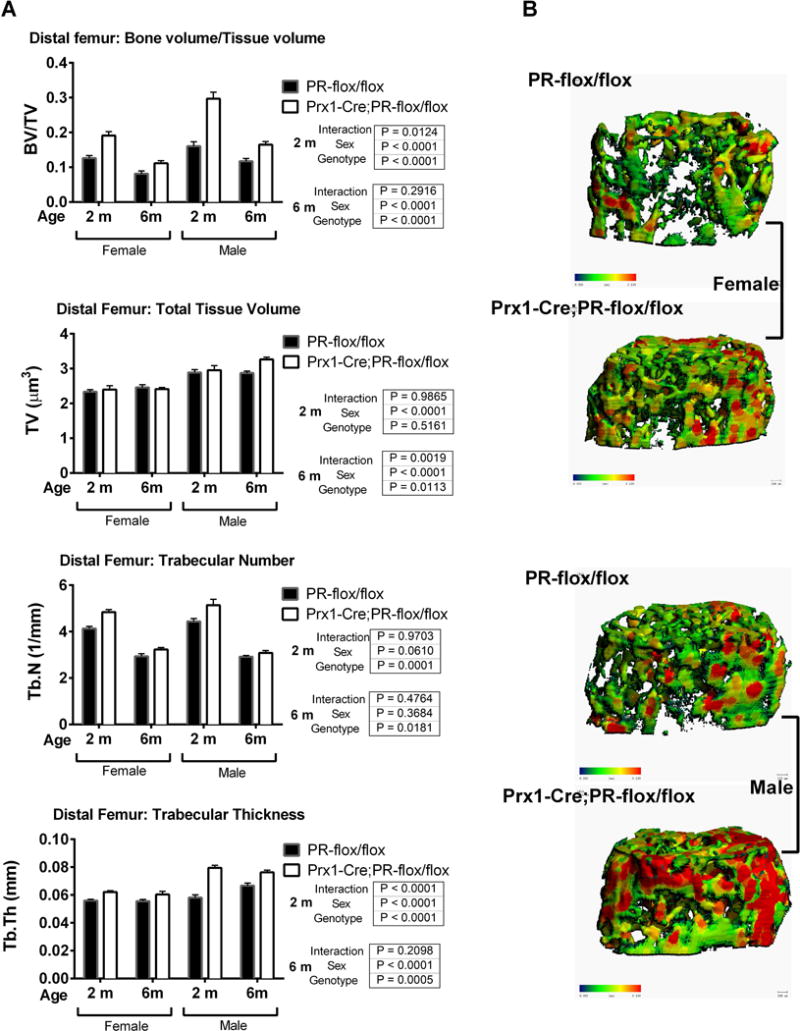
(A). Distal femurs from 2- and 6-month-old female and male Prx1; PRcKO mice were scanned with microCT. Bone volume (BV), total volume (TV), BV/TV, trabecular number (Tb. N), and trabecular thickness (Tb. Th) were compared between wild type and mutant mice. (B). Three-dimensional images were reconstructed for trabeculae at the distal femurs of 2-month-old mice.
Dynamic histomorphometric analysis of bone formation in 2-month-old Prx1; PRcKO mice revealed significantly higher mineral apposition rate and surface-based bone formation rate in the trabecular bone surface with a lower osteoclast surface in the female mutant mice as compared to their WT littermates. The male 2-month-old Prx1; PRcKO mice had higher mineral apposition rate and bone formation rate as compared to their WT littermates. At six months of age, bone formation and resorption parameters were not significantly differing between the Prx1; PRcKO mice in the females. The 6-month-old male Prx1; PRcKO mice had higher serum osteocalcin (Supplementary Fig. 1A), mineral apposition rate and bone formation rate as compared to their WT littermates (Fig. 4 A–C). Osteoclast surface and activity, as measured by serum CTX1 level (Supplementary Fig. 1A), were similar between the WT and mutant mice (Fig. 4C) These data suggest that the high trabecular bone mass phenotype in Prx1; PRcKO mice resulted from an uncoupling of bone turnover, favoring bone formation.
Figure 4. Prx1; PRcKO mice displayed high bone formation in both sexes.
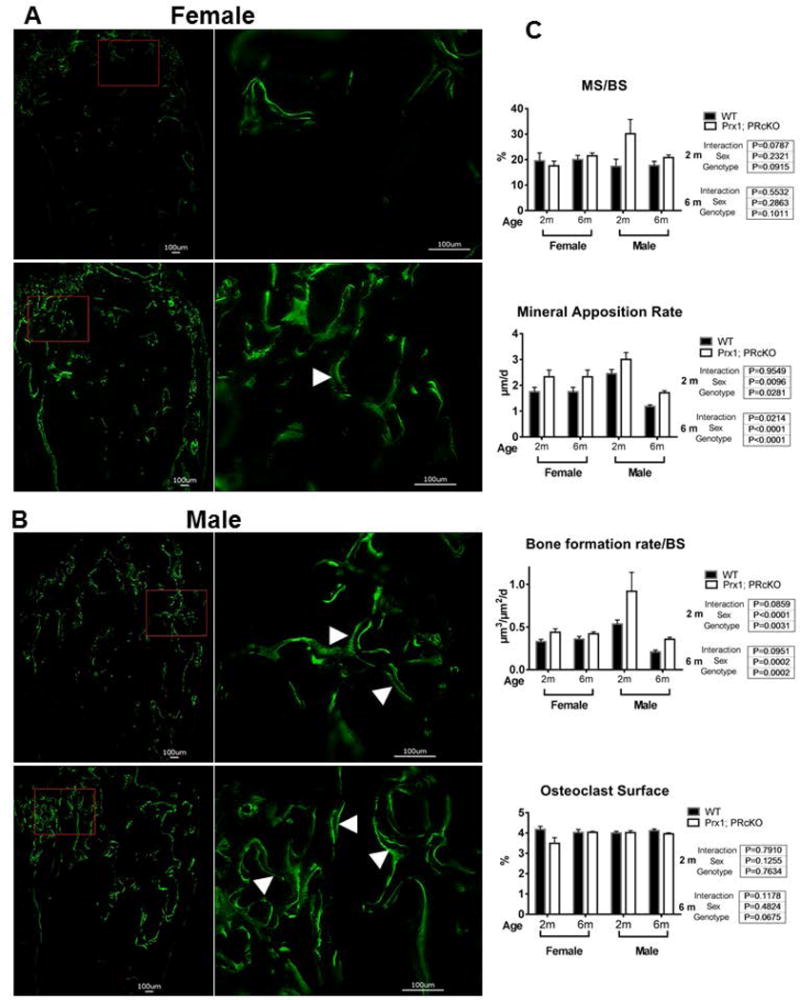
Mice were given two fluorescents labeling at −7 and −1 day(s) before sacrifice. Dynamic bone histomorphometry was performed at the distal femurs from wild type and Prx1; PRcKO mice of both sexes at 2 or 6 months of age. Representative images of double calcein labeling (white arrowheads) at the trabeculae obtained from 6-month-old female (A) and male (B) WT or Prx1; PRcKO mice. (C). Quantitative measurements for mineralizing surface (MS/BS), mineral apposition rate, bone formation rate and osteoclast surface at the distal femoral metaphysis.
Cortical bone mass was similar between WT and Prx1; PRcKO mice in both sexes. Bone formation rate was similar between the Prx1; PRcKO mice at the endocortical and periosteal bone surfaces for both female and male mice (Supplementary Fig. 1B).
To evaluate if the high trabecular bone phenotype resulting from targeted deletion of PR in the entire mesenchymal lineage was ligand dependent, we performed OVX or ORX on the 2-month-old WT and Prx1; PRcKO mice. One month after gonadectomy, the rate of trabecular bone loss at the distal femur due to aging or gonadal hormone deficiency were similar between the WT and Prx1; PRcKO mice for both the female (Fig. 5A) and the male (Fig. 5B). There was an approximately 5% cortical bone gain from two months to three months of age, which was similar between sexes, and was independent of genotype and hormone status (Fig. 5C).
Figure 5. Prx1; PRcKO mice had similar rate of trabecular bone loss following gonadectomy.
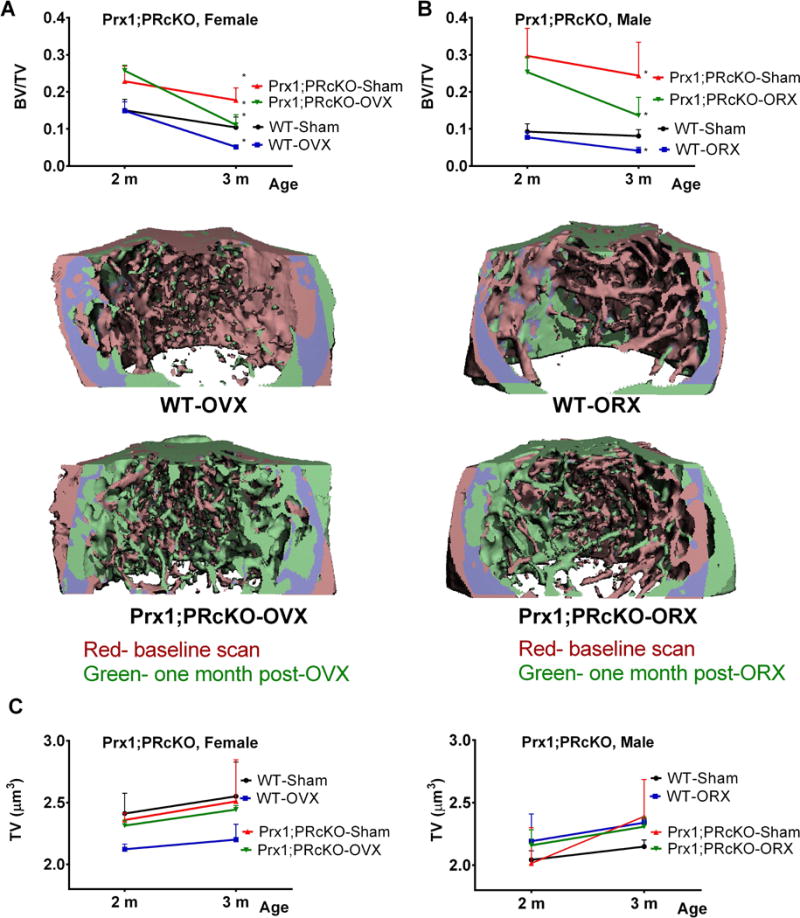
Two-month-old mice were sham-operated, ovariectomized (OVX) or orchiectomized (ORX) and euthanized 4 weeks later (n=5–10/group). Repeated in vivo microCT was performed before surgeries and prior to euthanization. Trabecular bone volume was obtained from the distal femurs when at 2 months (baseline) and 3 months (one-month post-surgeries) in female (A) and male (B mice. Three-dimensional image registrations were reconstructed for baseline (red) and one-month post-surgery (green), with overlapping regions colored purple. (C), Cortical bone volume was measured at the middle femurs when at 2 months (baseline) and 3 months (one-month post-surgeries) in both female and male mice.
PR conditional knockout in osteocytes, marked by Dmp1, resulted in moderate increased in bone formation and bone mass at the trabecular bone
Using a Dmp1-Cre; mT/mG reporter model, most the Dmp1+ cells were confined to the trabecular and endocortical bone surfaces that may include osteoblasts, osteoclasts and osteocytes (Fig. 6A). These results confirm other reports that Dmp1 marked a broader cell population than just the “osteocytes” (25,36,37). Distal femur trabecular BV/TV and middle femur cortical bone volume differed between the sexes in both the WT and mutant mice but were not different between the genotypes at 2 and 4 months of age, (Fig. 6B). Dynamic histomorphometric analyses on 2- and 4-month-old Dmp1; PRcKO mice revealed that mineralized surface and bone formation rate at the trabecular bone surface were not different by genotype (Fig. 6C). Osteoclast surface also did not differ between the genotypes for both female and male mice (data on file). Compared to their WT controls, the femoral maximum load and work-to-failure were higher in the 2-month-old female Dmp1; PRcKO mice and the maximum load was higher in the male Dmp1; PRcKO mice at 4 months of age (Fig. 6D). These data suggested that for the Dmp1; PRcKO mutant mice developed a modest bone mass phenotype.
Figure 6. Dmp1 expression in bone and surface-based histomorphometric analysis of Dmp1; PRcKO mice.
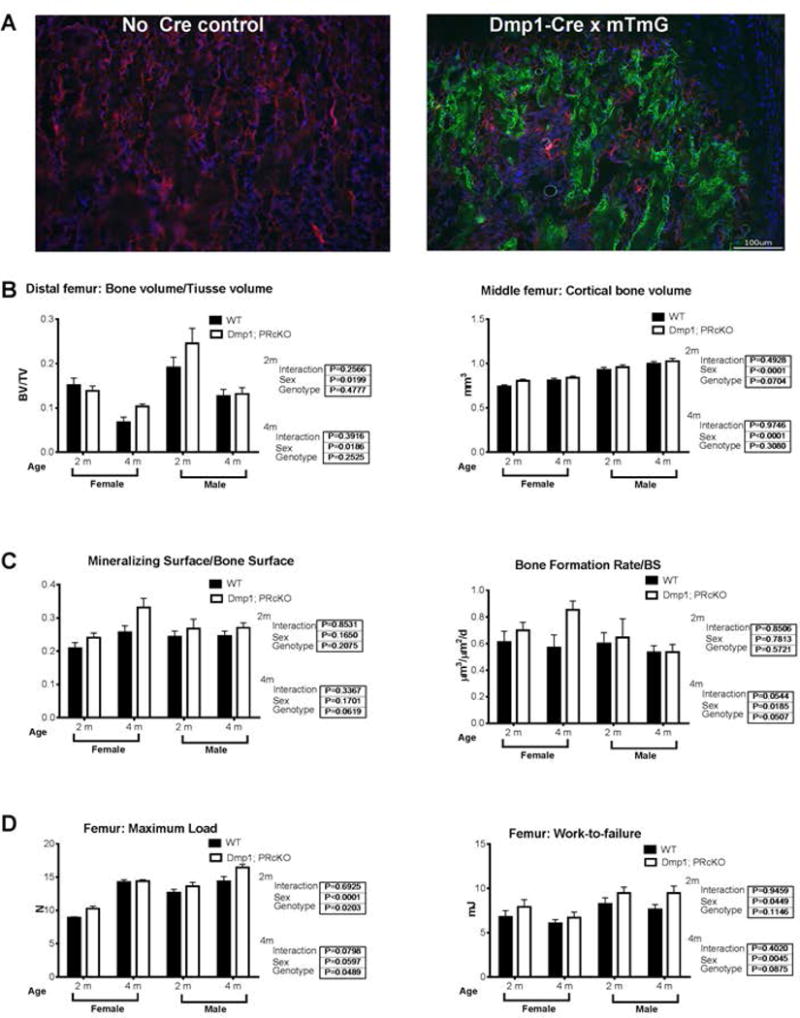
(A). A representative fluorescent image of distal femur from 4-week-old Dmp1-Cre; mT/mG mice. Green fluorescence indicated Cre activity was being observed at the trabecular bone surface. (B). Trabecular bone volume/total volume (BV/TV) was obtained from the distal femurs and cortical bone volume was obtained at the middle femurs from 2- and 4-month-old female and male Dmp1; PRcKO mice by microCT scans. (C). Surface-based bone formation was measured at the distal femurs. (D). Maximum load and work-to-failure were measured at the femurs.
PR conditional knockouts in osteoblasts, marked by Bglap (Osteocalcin), did not affect bone phenotype and bone turnover in both sexes
When PR was selectively deleted in mature osteoblasts and a small population of chondrocytes using the Bglap-Cre (Osteocalcin-Cre) driver (supplementary Fig. 2A), no differences in bone mass at the distal femurs (supplementary Fig. 2B), or biochemical assessment of bone formation (osteocalcin) or bone resorption (CTX-1) was observed at 6 months of age (supplementary Fig. 2C). PR deletion in the mature osteoblasts did not result in a gain in either bone mass or bone turnover.
Discussion
We found that mice lacking PR in the mesenchymal lineage represented by Prx1+cells, displayed significantly higher bone formation that corresponded to higher trabecular bone volume detected during the periods of rapid bone growth and maintenance in both sexes, especially in the male mutants. Age-related trabecular bone loss was similar between the Prx1; PRcKO and their WT littermates in both sexes. However, hormone deficiency during the rapid growing period induced similar rate of trabecular bone loss in the Prx1; PRcKO mice in both sexes, suggesting that the ligand-dependent role of PR signaling in the MSCs is directed at skeleton accrual. Additionally, Dmp1-Cre targeted a broader cell population than osteocytes, and it is activated in early osteoblast lineage cells, in bone marrow cells and in tissue not related to bone (36,37). Selective deletion of PR in Dmp1+ cells resulted in a modest higher trabecular bone mass and bone strength. Mice lacking the PR in mature osteoblasts did not have altered trabecular bone mass or turnover. Targeted PR deletion in osteoblast lineage cells did not affect cortical bone mass.
PR expression levels in bone were approximately 100-fold lower than other PR-dominant tissues such as the ovary and uterus. We used mouse genetic fate-mapping approaches to demonstrate that the PRs were expressed primarily by cells at the epiphyseal and metaphyseal regions at 1–2 months of age. This was the same area where most of the Prx1 and Col1 positive cells were found. Bone PR expression in the bone reached its peak at 1 month of age in both sexes and decreased rapidly such that it became undetectable by 6 months. The highest expression of PR was in the un-differentiated stromal cells obtained from bone marrow or from the calvarium, and was much lower or not present as the stromal cells differentiated into either osteoblasts or chondrocytes. Utilizing genetically engineered mouse models that targeted the PR, we have demonstrated that inactivation of PR either globally or in osteoprogenitor specific cells, significantly enhanced bone mass accrual in the trabecular bone compartment. Based on the pattern and timing of PR expression in osteoprogenitor cells, our data support the idea that an intervention targeting PR regulation would be most effective if it were initiated during the period of skeletal maturation.
Sexual dimorphism in the regulation of PR has been reported in the brain (38). PR expression is found in the prenatal medial preoptic nucleus, with males have significantly higher levels of PR-immunoreactivity than females from approximately embryonic day 19 through at least postnatal day (P) 28, suggesting that PR may play a role in sex differences (39). In the global progesterone receptor knockout (PRKO) mice, males exhibited reduced mount frequencies compared to WT mice. The heterozygous male PRKO mice had a reduced response to testosterone with a profound loss in many measures of sexual behavior following castration (40). We have reported that compared to their controls, 2-month-old female PRKO mice developed 300% higher bone mass whereas male PRKO mice only had 50% increased bone mass (14). In the current study, deletion of PR in mesenchymal progenitor cells achieved approximately 50% higher trabecular bone mass that peaked at 2 months of age, which was associated with higher surface-based bone formation, and did not change bone resorption. These differences were less profound in the females than males. PR conditional knockout (PRcKO) in the mesenchymal progenitor cells induced 85% higher trabecular bone mass compared to WT in 2-month-old male mice. At 6 months of age, the trabecular bone mass was more than 40% higher than their WT. Mechanistically, when compared to WT, the male Prx1; PRcKO mice had increased bone formation during skeletal growth, which correlated with a higher percentage of mesenchymal stromal cells within the bone marrow, greater osteoblast activity measured by serum osteocalcin, and an increased mineral apposition rate. These differences were sustained at 6 months of age. These data indicate that the PR indeed plays an important role in the male skeleton acquisition. Circulating progesterone is generally lower in males compared to females and decreases with age. We did not observe a change in the serum progesterone levels between Prx1; PRcKO mice and their WT littermates at 2 or 6 months of age. Peak bone mass was achieved at two months of age in both sexes. The rate of age-dependent trabecular bone loss was similar between the WT and the Prx1; PRcKO mice. Interestingly, the change in trabecular bone mass with hormone deficiency was similar between the Prx1; PRcKO mice and their WTs. However, repeated in vivo microCT scans with image registration, determined that while there was rapid bone loss resulting from hormonal deficiency was predominantly from the pre-existing trabecular bone (red color) in both the WT and the Prx1; PRcKO mice. In the Prx1; PRcKO mice, while the pre-exiting trabeculae was lost following gonadal hormone deficiency, new bone formation (green color) was observed arising from the endocortical bone surface in the Prx1; PRcKO mice of both sexes, resulting in higher net bone mass than the WT gonadal deficiency mice. These data are consistent with other reports on PR regulation of sexual dimorphism in sexual behaviors and suggest novel mechanisms underlying the critical role of PR in the sexual dimorphism of bone mass regulation.
We found bone size and femur length were similar between Prx1-Cre; PRcKO mice and WT controls. There were no differences in bone mass, architecture measurements, or surface-based bone formation in the cortical bone of femurs from Prx1-Cre; PRcKO, or Bglap-Cre; PRcKO mice. Also, there were no differences in cortical bone strength between any of these PRcKO mouse lines and their WT mice (data on file). Our cortical bone results may be due to the absence of PR expression in the diaphysis. Interestingly, cortical bone apposition and bone volume is reported to be independent of AR and ERβ signaling in bone cells (41–45). In fact, cortical bone accrual has been shown to be regulated by ERα signaling in the osteoprogenitor cells in the bone marrow and at the periosteum was estrogen-independent (46,47). Our findings and those reported by other investigators suggest that hormonal receptors may not contribute significantly to cortical bone growth and maintenance in the mouse strains studied.
The primary focus of this report was to evaluate the osteoblast stage specific effects of PR regulation on post-natal bone development. It is beyond our scope to compare and to confirm the specificity of these Cre mouse lines. For example, it was reported that Col1a1 promoter is likely the most specific osteoblast driver (48,49). However, Col1a1-Cre might not penetrate as well as Bglap-Cre in the mouse skeleton (50) as Bglap-Cre is active in the skeleton as early as embryonic day 14.5 (51). Moreover, Dmp1-Cre labels broader cell populations than osteocytes (25). The complexity and specificity of these Cres may confound our results and is a shortcoming of the technique. Nevertheless, PR expression may be controlled by systemic hormones, whose serum levels begin to increase when the mice are weaned at three weeks of age. Female mice usually become sexually mature at 6 weeks after birth and males at 8 weeks (52). PR expression is low in bone until about three weeks of age, which would eliminate some of the concerns for PR interactions with these Cres before the mice are weaned.
We found that the lack of nuclear PR in mesenchymal stromal cells permitted higher trabecular bone mass acquisition. However, the effects of PR on trabecular bone seemed to be opposed to those of AR, in that global AR deletion reduced trabecular bone gain (43) through regulation of the mature osteoblasts (53,54) or osteocytes (41,42). Like the PR, ERα in osteoblast progenitor cells influences trabecular bone mass (47). However, the lack of PR signaling in osteoprogenitor cells had an opposite effect to ERα knockout in Prx1+ cells, which was reported to have reduced trabecular bone formation and bone mass compared to the wild-type (47). It is not clear if ERα signaling in osteoblasts and osteocytes can alter trabecular bone (27,28,55). Deletion of ERβ in Prx1+ cells resulted in an increase in trabecular but not cortical bone mass of female mice (45). We have not yet dissected out the downstream pathways of PR in osteoprogenitor cells at this stage. Based on some studies from cancer cell models, with the absence of ligand-engagement, PR may bind genomic sites and target a repressive complex to silence a subset of hormone-inducible genes (56). Therefore, upon PR conditional deletion in bone-forming cells, those physiologically silenced hormone-inducible genes might be re/deactivated accordingly. For this set of hormone-inducible genes, PR conditional knockout may have similar effects as simply adding progesterone receptor agonists, which in turn will release PR from the repressive complex and activate the downstream genes. Alternatively, the removal of PR nuclear signaling may potentiate the cellular response to progesterone’s membrane signaling and observe activation of signal transduction pathways such as activating the RANKL-RANK pathway and the intracellular Mitogen-activated protein kinase (MAPK) pathway (57–60). The MAPK pathway is the key extra-nuclear signaling pathway for steroid regulation of cell proliferation and survival in various cell types including mesenchymal derived cells, osteoblasts and osteocytes (61–63). On the other hand, PR ligands can interact with ERα and direct ERα chromatin-binding events (64), which may have further compromised our interpretation. Taken together, our finding on PR signaling in osteoblast lineage cells adds to the current understanding of the distinct roles of PR signaling in bone-forming cells during trabecular bone acquisition in both sexes.
In conclusion, selective inhibition of PR in the osteoprogenitor cells, but not in the mature osteoblasts, resulted in higher trabecular bone mass and bone formation, with greater effects being observed in males than in females. The presence of PR expression in cells of mesenchymal lineage may inhibit the maturation of osteoprogenitors during the phase of rapid bone acquisition. Inactivating PR signaling in both the early osteoprogenitor cells and in the mature osteoblasts did not influence cortical bone, which may be due to the lack of PR expression in the diaphysis. Our results support the concept that selective nuclear PR inhibition in osteoprogenitor cells during skeleton development may be beneficial for individuals who are at high risk for developing osteoporosis, warranting further investigation into the development of agents that can inhibit PR in osteoprogenitor cells and augment peak bone mass.
Supplementary Material
Supplementary Figure 1. Systemic bone turnover and cortical bone formation in the Prx1; PRcKO mice.
(A). Serum osteocalcin, and CTX-1 were analyzed in 2- and 6-month-old Prx1-Cre; PRcKO mice. (B). Mineralized surface was measured at the endocortical and periosteal bone surface of the middle femurs in intact WT and Prx1; PRcKO mice at 2 and 6 months of age.
Supplementary Figure 2. Characterization of Bglap; PRcKO mice.
(A). A representative image of Bglap-Cre; mT/mG distal femur shows that Bglap-Cre is active primarily in osteoblasts. (B). MicroCT analysis was performed on 2- and 6-month-old Bglap; PRcKO mice. Serum osteocalcin (C) and CTX-1 (D) were analyzed in 6-month-old Bglap; PRcKO mice.
Acknowledgments
These studies were supported by SCOR: NIH/NIAMS 1P50AR063043 and NIH/NIAMS R01AR061366 (WY), Endowment for Aging (NEL), and R01AR043052 (NEL). We thank the SCOR External Advisory Board Member Dr. Mark Johnson, and the Internal Advisory Board Members Dr. Robert Nissenson and Dr. Edward Hsiao for their consultation, technical support, and editorial assistance.
Footnotes
Disclosure: none for all the authors.
Author contributions:
ZDZ: study conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript AK: collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript. EAY: collection and assembly of data, data analysis, and final approval of manuscript. HLZ: collection and assembly of data, data analysis and interpretation, and final approval of manuscript. JJ: collection and assembly of data, data analysis, and final approval of manuscript. NEL: data analysis and interpretation, and final approval of manuscript. WY: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript.
References
- 1.Seeman E. Periosteal bone formation–a neglected determinant of bone strength. N Engl J Med. 2003 Jul 24;349(4):320–3. doi: 10.1056/NEJMp038101. Epub 2003/07/25. [DOI] [PubMed] [Google Scholar]
- 2.Heffner LJ, Schust DJ. The Reproductive System at a Glance. 3rd. West Sussex, UK: John Wiley and Sons; 2010. p. 16. [Google Scholar]
- 3.Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003 Oct 10;278(41):39261–4. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 4.Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, et al. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010 Mar;25(3):617–26. doi: 10.1359/jbmr.090828. [DOI] [PubMed] [Google Scholar]
- 5.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nature reviews Endocrinology. 2013 Dec;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor beta−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001 Aug;16(8):1388–98. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- 7.Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002 Jul;13(5):195–200. doi: 10.1016/s1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 8.Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV, et al. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res. 2006 Apr;21(4):576–85. doi: 10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001 Nov;171(2):229–36. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 10.Chagin AS, Lindberg MK, Andersson N, Moverare S, Gustafsson JA, Savendahl L, et al. Estrogen receptor-beta inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. J Bone Miner Res. 2004 Jan;19(1):72–7. doi: 10.1359/JBMR.0301203. [DOI] [PubMed] [Google Scholar]
- 11.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13498–503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barengolts EI, Lathon PV, Lindh FG. Progesterone antagonist RU 486 has bone-sparing effects in ovariectomized rats. Bone. 1995 Jul;17(1):21–5. doi: 10.1016/8756-3282(95)00138-4. Epub 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 13.Liu JH, Muse KN. The effects of progestins on bone density and bone metabolism in postmenopausal women: a randomized controlled trial. Am J Obstet Gynecol. 2005 Apr;192(4):1316–23. doi: 10.1016/j.ajog.2004.12.067. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 14.Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, et al. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PloS one. 2010 Jul 01;5(7):e11410. doi: 10.1371/journal.pone.0011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rickard DJ, Iwaniec UT, Evans G, Hefferan TE, Hunter JC, Waters KM, et al. Bone growth and turnover in progesterone receptor knockout mice. Endocrinology. 2008 May;149(5):2383–90. doi: 10.1210/en.2007-1247. Epub 2008/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997 Aug;18(4):502–19. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 17.Scarpin KM, Graham JD, Mote PA, Clarke CL. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nuclear receptor signaling. 2009;7:e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PloS one. 2012;7(4):e35859. doi: 10.1371/journal.pone.0035859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei LL, Leach MW, Miner RS, Demers LM. Evidence for progesterone receptors in human osteoblast-like cells. Biochem Biophys Res Commun. 1993 Sep 15;195(2):525–32. doi: 10.1006/bbrc.1993.2077. [DOI] [PubMed] [Google Scholar]
- 20.MacNamara P, O’Shaughnessy C, Manduca P, Loughrey HC. Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures. Calcif Tissue Int. 1995 Dec;57(6):436–41. doi: 10.1007/BF00301947. [DOI] [PubMed] [Google Scholar]
- 21.Rickard DJ, Waters KM, Ruesink TJ, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, et al. Estrogen receptor isoform-specific induction of progesterone receptors in human osteoblasts. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002 Apr;17(4):580–92. doi: 10.1359/jbmr.2002.17.4.580. [DOI] [PubMed] [Google Scholar]
- 22.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002 Jun;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007 Apr;86(4):320–5. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 24.Komori T. Mouse models for the evaluation of osteocyte functions. J Bone Metab. 2014 Feb;21(1):55–60. doi: 10.11005/jbm.2014.21.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Link DC. Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J Bone Miner Res. 2016 May 30; doi: 10.1002/jbmr.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melville KM, Kelly NH, Surita G, Buchalter DB, Schimenti JC, Main RP, et al. Effects of Deletion of ERalpha in Osteoblast-Lineage Cells on Bone Mass and Adaptation to Mechanical Loading Differ in Female and Male Mice. J Bone Miner Res. 2015 Aug;30(8):1468–80. doi: 10.1002/jbmr.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, et al. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res. 2014 Feb;29(2):370–9. doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 28.Maatta JA, Buki KG, Gu G, Alanne MH, Vaaraniemi J, Liljenback H, et al. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J. 2013 Feb;27(2):478–88. doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, et al. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010 Feb;48(2):106–13. doi: 10.1002/dvg.20586. Epub 2009/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010 Jan;13(1):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013 May 9;153(4):896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010 Jul;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 33.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone Review. 1993 Jul-Aug;14(4):595–608. doi: 10.1016/8756-3282(93)90081-k. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 34.Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010 Feb;25(2):190–9. doi: 10.1359/jbmr.090719. Epub 2009/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, et al. Reversing bone loss by directing mesenchymal stem cells to bone. Stem cells. 2013 Sep;31(9):2003–14. doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J, Shi Y, Karner CM, Lee SY, Lee WC, He G, et al. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development. 2016 Jan 15;143(2):339–47. doi: 10.1242/dev.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim J, Burclaff J, He G, Mills JC, Long F. Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Res. 2017;5:16049. doi: 10.1038/boneres.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002 Oct 30;59(2):105–9. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- 39.Quadros PS, Goldstein AY, De Vries GJ, Wagner CK. Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol. 2002 Oct;14(10):761–7. doi: 10.1046/j.1365-2826.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 40.Phelps SM, Lydon JP, O’Malley BW, Crews D. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav. 1998 Dec;34(3):294–302. doi: 10.1006/hbeh.1998.1485. [DOI] [PubMed] [Google Scholar]
- 41.Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, et al. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012 Dec;27(12):2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 42.Maatta JA, Buki KG, Ivaska KK, Nieminen-Pihala V, Elo TD, Kahkonen T, et al. Inactivation of the androgen receptor in bone-forming cells leads to trabecular bone loss in adult female mice. BoneKEy reports. 2013;2:440. doi: 10.1038/bonekey.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009 Jan;23(1):232–40. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 44.Ucer S, Iyer S, Bartell SM, Martin-Millan M, Han L, Kim HN, et al. The Effects of Androgens on Murine Cortical Bone Do Not Require AR or ERalpha Signaling in Osteoblasts and Osteoclasts. J Bone Miner Res. 2015 Jul;30(7):1138–49. doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicks KM, Fujita K, Fraser D, McGregor U, Drake MT, McGee-Lawrence ME, et al. Deletion of Estrogen Receptor Beta in Osteoprogenitor Cells Increases Trabecular but Not Cortical Bone Mass in Female Mice. J Bone Miner Res. 2016 Mar;31(3):606–14. doi: 10.1002/jbmr.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, et al. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol. 2013 Apr;27(4):649–56. doi: 10.1210/me.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013 Jan;123(1):394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002 Jun;224(2):245–51. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 49.Bilic-Curcic I, Kronenberg M, Jiang X, Bellizzi J, Mina M, Marijanovic I, et al. Visualizing levels of osteoblast differentiation by a two-color promoter-GFP strategy: Type I collagen-GFPcyan and osteocalcin-GFPtpz. Genesis. 2005 Oct;43(2):87–98. doi: 10.1002/gene.20156. [DOI] [PubMed] [Google Scholar]
- 50.Ferron M, Lacombe J, Germain A, Oury F, Karsenty G. GGCX and VKORC1 inhibit osteocalcin endocrine functions. J Cell Biol. 2015 Mar 16;208(6):761–76. doi: 10.1083/jcb.201409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong ZA, Zahatnansky J, Snider J, Van Wieren E, Diegel CR, Williams BO. Wntless spatially regulates bone development through beta-catenin-dependent and independent mechanisms. Dev Dyn. 2015 Oct;244(10):1347–55. doi: 10.1002/dvdy.24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hetherington M, Doe B, Hay D. Mouse care and husbandry. In: Jackson IJ, Abbott CM, editors. Mouse Genetics and Transgenics: A Practical Approach. Oxford University Press; 2000. [Google Scholar]
- 53.Chiang C, Chiu M, Moore AJ, Anderson PH, Ghasem-Zadeh A, McManus JF, et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res. 2009 Apr;24(4):621–31. doi: 10.1359/jbmr.081217. [DOI] [PubMed] [Google Scholar]
- 54.Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, et al. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007 Mar;22(3):347–56. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 55.Windahl SH, Borjesson AE, Farman HH, Engdahl C, Moverare-Skrtic S, Sjogren K, et al. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vicent GP, Nacht AS, Zaurin R, Font-Mateu J, Soronellas D, Le Dily F, et al. Unliganded progesterone receptor-mediated targeting of an RNA-containing repressive complex silences a subset of hormone-inducible genes. Genes Dev. 2013 May 15;27(10):1179–97. doi: 10.1101/gad.215293.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maller JL. The elusive progesterone receptor in Xenopus oocytes. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammes SR. The further redefining of steroid-mediated signaling. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2168–70. doi: 10.1073/pnas.0530224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16794–9. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW, et al. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006 Dec;20(12):3146–64. doi: 10.1210/me.2006-0129. Epub 2006/09/09. [DOI] [PubMed] [Google Scholar]
- 61.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999 Nov;104(10):1363–74. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001 Jun;2(6):467–75. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 63.Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000 Oct 16;151(2):311–20. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015 Jul 16;523(7560):313–7. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Systemic bone turnover and cortical bone formation in the Prx1; PRcKO mice.
(A). Serum osteocalcin, and CTX-1 were analyzed in 2- and 6-month-old Prx1-Cre; PRcKO mice. (B). Mineralized surface was measured at the endocortical and periosteal bone surface of the middle femurs in intact WT and Prx1; PRcKO mice at 2 and 6 months of age.
Supplementary Figure 2. Characterization of Bglap; PRcKO mice.
(A). A representative image of Bglap-Cre; mT/mG distal femur shows that Bglap-Cre is active primarily in osteoblasts. (B). MicroCT analysis was performed on 2- and 6-month-old Bglap; PRcKO mice. Serum osteocalcin (C) and CTX-1 (D) were analyzed in 6-month-old Bglap; PRcKO mice.


