Abstract
Background
The hospital setting provides an opportunity to re-engage people living with HIV (PLWH) in HIV care. We developed and implemented a protocol to identify PLWH in a hospital setting. The aim of the current study was to report on our strategy to recruit hospitalized HIV patients into an intervention study, and to report on lessons learned for future studies.
Methods
Our protocol was developed based on experience of our research staff in recruiting HIV patients as well as clinical input from providers and administrators on delivering care in hospitalized settings. We identified hospitalized PLWH between 2010 and 2013 who were potentially eligible for an intervention study. Patients were identified by review of electronic medical records and clinician referral, followed by in-person screening to confirm eligibility. We examined factors related to identifying and enrolling hospitalized patients, and documented lessons learned.
Results
Key strategies included systematic medical record review followed by in-person screening, collaboration with staff, and flexibility in recruitment logistics. We identified 1,801 PLWH hospitalized during the 3-year study period. 84% (n=1,514) met the met the inclusion criteria based on medical record review. Of these, 48% (n=733) were ineligible. Among eligible patients, 59% (n=460) were enrolled. Only 3% (n=23) of eligible patients declined; 84% (n=321) were not enrolled because they were discharged before enrollment. Lessons learned included 1) needing to identify patients and deliver the intervention before hospital discharge, 2) limiting the complexity of the intervention, and 3) having research staff available on weekends and after hours.
Conclusions
Targeted recruitment of hospitalized populations is a feasible and productive approach for finding and engaging PLWH who are newly diagnosed or out of routine care.
Keywords: HIV, retention in care, inpatient recruitment, intervention studies
INTRODUCTION
In the United States, an estimated 50,000 people are infected with HIV each year and approximately 1.2 million are living with HIV1. Expanded HIV testing strategies have been employed to increase the number of people who are aware of their HIV infection, especially people who use emergency care centers or other acute care facilities2. Linkage to and engagement in HIV care continues to be a challenge for newly diagnosed persons as well as those with known HIV infection who are out of routine care. Only 72% of newly diagnosed persons are linked to care within 4 months of diagnosis and 59% of those who enter care are retained3. Significant barriers to engagement in care include stigma4; 5, lack of social support6, financial barriers7 and difficulty navigating the healthcare system8. Interventions to retain people living with HIV (PLWH) in routine HIV care are needed.
Community based outreach strategies to find and re-engage PLWH who are out of routine care have been studied, and can be successful, but are labor intensive and relatively low yield5; 8–15. New strategies are needed for finding and re-engaging persons with known HIV infection who are out of routine care for both program and research purposes. Recruiting hospitalized patients who are out of routine care is a promising strategy that has been used in two recent clinical trials of approaches to improve retention in HIV care16, 17. Hospitalized PLWH are often out of routine care and have poor rates of outpatient follow-up18. The hospital setting may provide a unique opportunity to engage these patients in HIV care and test interventions to improve re-engagement in care. In addition to being more accessible, hospitalized patients have an acute illness, potentially leading to greater motivation and readiness to engage in outpatient care. However, there are reasonable concerns that hospitalized patients may be too ill to participate in a program or research study, and that lengths of stay often are too short to allow for recruitment and engagement in a program or research study prior to hospital discharge.
We developed and implemented a formalized strategy to identify and recruit hospitalized patients who were newly diagnosed or out-of-care and tested it in a randomized, controlled trial of a peer mentoring intervention for persons who are out of routine care. The Mentor Approach for Promoting Patients Self-Care (MAPPS) intervention study was a prospective, randomized, controlled trial to examine whether a peer mentoring program targeting hospitalized PLWH who are either out of routine care or recently diagnosed improves linkage and retention in HIV care. Results from this study were previously published17. The MAPPS intervention had no overall impact on engagement in care or viral suppression. Here, we report on the approach, implementation, and process-outcomes of identifying, screening, and enrolling hospitalized PLWH for this study. Results speak to the potential utility of this approach to identify whether out-of-care PLWH constitute a sizable proportion of hospitalized patients and to establish the feasibility of engaging this population in intervention programs. Findings inform both research and practice for administrators and clinicians seeking to find these patients and engage them in care.
METHODS
Study population
Study participants include PLWH who were hospitalized at the Ben Taub General Hospital (BTGH) between August 2010 and August 2013. BTGH is the largest publically-funded hospital in Houston, TX, USA and has about 24,000 admissions each year, or between 50 and 75 admissions daily. It is part of the Harris Health System, which provides care regardless of ability to pay, and is affiliated with Baylor College of Medicine.
Participant identification and recruitment procedures
Our strategy to identify PLWH who were out of routine care involved several steps and was implemented by a research team member with access to electronic medical records. We used a two-stage screening process which consisted of a review of the electronic record (EMR) followed by an in-person screening interview for PLWH who appeared eligible and out of routine care based on medical record information. There were four methods of identifying hospitalized PLWH: 1) conducting a manual review of the census of each nursing unit for new admissions who had HIV infection or who had clinical conditions associated with HIV infection whose HIV testing was pending; 2) running an application in the EMR which searched for patients whose current location was noted as inpatient at BTGH and whose “problem list” included HIV or AIDS. The “problem list” contains current and pre-existing inpatient and outpatient diagnoses. However, the “problem list” requires manual updating by providers, so may not capture all patients with HIV infection, especially persons with new or recent diagnoses or persons who are new to the Harris Health System; 3) research staff routinely visited hospital nursing units to inquire about new admissions; and 4) research staff solicited referrals from attending and resident physicians and HIV testing personnel throughout the hospital. Medical records for PLWH identified by these methods were then manually reviewed by research staff to confirm HIV and assess out-of-care status. Coordinators attempted to tally PLWH identified by these methods who were discharged before their screening and/or enrollment could be completed.
PLWH who were not in HIV care and met the following study criteria based on medical record review were screened in person for inclusion in the study: 1) age≥18 years; 2) able to provide informed consent and complete a baseline survey in English or Spanish; 3) expected to be hospitalized at least one more night (to provide time for the intervention to be delivered); 4) no known plans for discharge to an institutional setting, including jail or prison; 5) intending to seek follow-up care at Thomas Street Health Clinic (TSHC); and 6) not already enrolled in a research study with prospective follow-up (because that might impact their retention in HIV care). “In HIV care” was defined as having at least 3 consecutive viral loads <400 copies/mL and having completed HIV primary care visits in at least 3 of the last 4 quarter-years in the 12-months prior to the hospitalization. All others were considered not in care, including all PLWH who were diagnosed in the last 12 months and persons wishing to transfer to TSHC, since by design they could not meet the definition of “in care.” Research staff conducted an in-person screening for all patients who appeared to meet the criteria for being out of HIV care. Patients who were clearly ineligible or were too sick to participate were not interviewed. During the in-person screening, out of routine care status was confirmed. Patients who were met all eligibility criteria were added to the list of eligible patients and subsequently recruited for the study.
Immediately following in-person screening, eligible patients were invited to participate in an intervention program (the MAPPS study). The research staff described the study as a randomized trial to help patients with HIV learn to manage their HIV and get into outpatient HIV care following hospital discharge. Patients were informed that they would be randomly assigned to meet with either a peer mentor or patient educator twice during their hospitalization if they agreed to participate. Patients were informed that study participation while hospitalized included completing a comprehensive baseline survey that took between 1 to 2 hours, and completing two sessions with an interventionist, each lasting between 15 and 45 minutes. The initial visit included obtaining informed consent, completing the baseline interview, and participating in the first of two sessions with the interventionist. The second in-person session with the interventionist occurred within the following 1–3 days and could be conducted by telephone if the patient had been discharged. Following hospital discharge, the patient would receive five telephone calls from the interventionist over the next 10 weeks to check-in and support the patient in navigating the healthcare system. For patients who were willing to participate, research staff immediately obtained informed consent and determined whether the patient was assigned to the peer mentor or patient educator based on the random number scheme previously generated by the study statistician. The peer mentor or patient educator assigned to the patient was contacted by the research coordinator and provided with the patient name and hospital room number. The peer mentor or patient educator would meet with the patient for their first session on the same day, or as soon as possible. The research staff was responsible for conducting the baseline survey prior to the patient’s first visit with the interventionist. Typically the baseline survey was administered immediately after the interventionist was contacted. The survey was administered via computer using the Audio-Computer Assisted Self Interview (ACASI) Software. Research staff was available to provide assistance as needed.
Measures
Demographic characteristics (age, biological sex, race/ethnicity), recent laboratory results (CD4 cell count, HIV viral load), and whether persons were in HIV care at Thomas Street Health Center (TSHC), the largest, publically funded HIV clinic in Houston, TX, was collected for all participants screened for this study. TSHC, serving approximately 5,000 patients annually, is part of the Harris Health System and shares a single EMR with BTGH and the rest of the health system. Whether a participant was currently in HIV care was determined based on evidence in the EMR as defined in the eligibility criteria section, or by self-report of being in care in an outside facility if applicable. A person was considered newly diagnosed with HIV infection if they were diagnosed during the current hospital stay, including testing done in the emergency department visit that led to the hospital admission.
Statistical analysis
To estimate the relative density of PLWH who are out of routine care among hospitalized patients, we calculated the proportion of PLWH who were out of routine care among all hospitalized PLWH identified during the study time period. To examine patient and clinical factors associated with being out of HIV care at the time of hospitalization compared to those hospitalized and currently engaged in HIV care, univariate analyses were conducted. To determine the willingness of hospitalized patients to engage in a research study, we compared the proportion of patients willing to participate in the study to those not willing to participate among eligible patients. To examine whether willingness to participate varied by demographic or clinical features, we compared eligible patients who were willing to participate to those who were not. Wald chi-square tests and accompanying p-values were used to assess statistical significance. All data were analyzed using SAS (SAS Version 9.2, SAS Institute, Cary, North Carolina, USA).
The study protocol, including a waiver for individual consent for medical record review prior to in-person screening, was approved by the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals.
RESULTS
Using our protocol, we identified and screened 1,801 PLWH hospitalized at BTGH. Among these patients, 287 persons (16%) had a viral load less than 400 copies/mL and were in care at TSHC and 84% (n=1,514) were identified as potentially out of routine care and viremic according to data available in the EMR (Figure 1). Among the 1,514 patients identified as out of routine care and preliminarily eligible based on medical record review, 44 (3%) were not in care at TSHC and had a VL<400 copies/mL, 145 (10%) were in care at TSHC but had a VL>400 copies/mL, and 1,325 (87%) were both not in care in TSHC and had a VL>400 copies/mL. PLWH who were younger (p<0.01), male (p=0.03), and Black or African American (p<0.01) were more likely to be preliminary eligible (Table 1).
Figure 1.
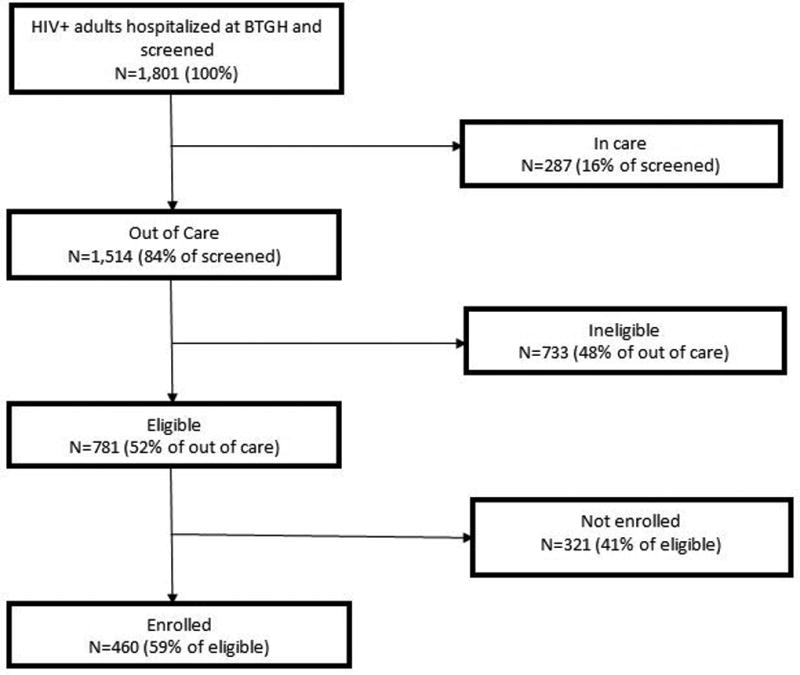
Flow diagram for study enrollment
Table 1.
Comparison of patient and clinical characteristics of all screened patients by preliminary study criteria based on in care status and viral load. Preliminary study eligibility based on electronic medical record information. (n=1,801)
| Ineligible | Preliminary Eligible | ||||
|---|---|---|---|---|---|
|
| |||||
| In Care at TSHC and VL<400 copies/mL (n=287) % (N=232 |
Not in care at TSHC and VL<400 copies/mL (n=44) % |
In Care at TSHC and VL>400 copies/mL (n=145) % |
Not in care at TSHC and VL>400 copies/mL (n=1,325) % |
Overall p-value |
|
|
| |||||
| Age | <0.01 | ||||
| <30 | 4.2 | 9.0 | 12.4 | 11.5 | |
| 30–39 | 14.3 | 18.2 | 17.9 | 26.5 | |
| 40–49 | 35.5 | 43.2 | 37.9 | 36.2 | |
| 50–59 | 32.4 | 20.5 | 26.2 | 21.2 | |
| 60+ | 13.6 | 9.1 | 5.5 | 4.7 | |
| Sex | 0.03 | ||||
| Male | 64.8 | 64.8 | 75.0 | 72.4 | |
| Female | 35.2 | 35.2 | 25.0 | 27.6 | |
| Race/ethnicity | <0.01 | ||||
| Black (non-Hispanic) | 56.4 | 61.4 | 70.3 | 60.9 | |
| White (non-Hispanic) | 12.9 | 15.9 | 7.6 | 15.0 | |
| Hispanic | 29.3 | 15.9 | 20.7 | 22.7 | |
| Missing | 1.4 | 6.8 | 1.4 | 1.4 | |
Research staff conducted in-person screening among the 1,514 patients who were preliminary eligible based on information in the EMR. Less than 1% of preliminarily eligible patients declined to participate in the in-person screening. This additional screening reduced the number of patients who were eligible to 781 (51%) (Figure 1). The most common reasons for ineligibility were not intending to use TSHC after discharge (47%), unable to provide informed consent (15%), and having a planned discharged to an institution (10%).
Compared to eligible patients, ineligible patients were more likely to be female (p=0.02) and had a viral load less than 400 copies/mL (p<0.01) (Table 2). Significant differences by eligibility were also observed in race/ethnicity; White (non-Hispanic) PLWH were more likely to be ineligible compared to other racial/ethnic groups (p=0.02). No differences in CD4+ count were observed, and the median CD4 was very low in both groups (138 and 140 cells/uL).
Table 2.
Comparison of patient and clinical characteristics by final eligibility status among patients who were preliminarily eligible. (n=1,514)
| Eligible (N=781) % |
Ineligible (n=733) % |
p-value | |
|---|---|---|---|
| Age | 0.49 | ||
| <30 | 11.1 | 11.9 | |
| 30–39 | 26.5 | 24.3 | |
| 40–49 | 37.3 | 35.7 | |
| 50–59 | 21.5 | 24.8 | |
| 60+ | 3.6 | 3.3 | |
| Sex | 0.02 | ||
| Male | 74.4 | 68.9 | |
| Female | 25.6 | 31.1 | |
| Race/ethnicity | 0.02 | ||
| Black (non-Hispanic) | 62.7 | 60.9 | |
| White (non-Hispanic) | 12.2 | 16.6 | |
| Hispanic | 24.1 | 20.5 | |
| Missing | 1.0 | 2.1 | |
| Most recent CD4+ count (cells/uL)* | 0.36 | ||
| <200 | 60.1 | 60.6 | |
| 200–500 | 23.5 | 25.5 | |
| ≥500 | 16.4 | 13.9 | |
| Most recent viral load (copies/mL)** | <0.01 | ||
| ≤400 | 22.8 | 29.5 | |
| >400 | 77.2 | 70.5 |
Missing CD4+ count: n=129
Missing viral load: n=257
Patients not in care at TSHC could potentially be in care elsewhere
Among the 781 patients who were eligible, 59% (n=460) were willing to enroll in the study (Figure 1). Among the 321 eligible patients who did not enroll, only 3% declined enrollment and 18% were too sick to complete enrollment. The vast majority (84%) of patients did not enroll because they were discharged before enrollment could be completed. Among those who enrolled, 10.7% (n=49) were newly diagnosed. The mean length of stay for enrolled patients was 8.6 days (standard deviation = 7.8). Information on length of stay was not collected for patients who were not enrolled. No differences in enrollment were observed by age or gender (Table 3). However, race/ethnicity differences were observed; Hispanic PLWH were less likely to be enrolled (p<0.01) compared to other racial/ethnic groups. Viral load was higher (p=0.03) and CD4+ cell count was lower (p=0.03) in PLWH who enrolled compared to those who did not enroll.
Table 3.
Comparison of patient and clinical characteristics by enrollment status among the 781 eligible patients.
| Enrolled (n=460) % |
Not enrolled (n=321) % |
p-value | |
|---|---|---|---|
| Age | 0.68 | ||
| <30 | 11.7 | 10.3 | |
| 30–39 | 27.6 | 24.9 | |
| 40–49 | 35.9 | 39.2 | |
| 50–59 | 20.7 | 22.4 | |
| 60+ | 4.1 | 3.2 | |
| Sex | 0.48 | ||
| Male | 73.3 | 75.8 | |
| Female | 26.7 | 24.2 | |
| Race/ethnicity | <0.01 | ||
| Black (non-Hispanic) | 66.7 | 57.0 | |
| White (non-Hispanic) | 13.9 | 9.7 | |
| Hispanic | 19.4 | 30.8 | |
| Missing | 0.0 | 2.5 | |
| Most recent CD4+ count (cells/uL) | 0.03 | ||
| <200 | 64.0 | 54.1 | |
| 200–500 | 21.1 | 27.2 | |
| ≥500 | 14.9 | 18.7 | |
| Most recent viral load (copies/mL) | 0.03 | ||
| <=400 | 20.2 | 27.3 | |
| >400 | 79.8 | 72.7 |
Missing CD4+ count: n=31
Missing viral load: n=67
DISCUSSION
In this study, we sought to determine the feasibility of implementing our protocol for recruiting hospitalized PLWH who were out of routine care, how commonly out-of-care PLWH are found in hospitalized populations, and the willingness of hospitalized PLWH to enroll in a research study focused on engagement in outpatient HIV care. We also examined demographic and clinical differences in eligibility and enrollment to inform future studies or programs targeting hospitalized PLWH. We found that it was feasible to implement our protocol to rapidly and efficiently identify HIV-positive patients who were out of routine care. Through the implementation of our protocol, we identified 1,801 hospitalized PLWH during a 3-year period. Further, a significant proportion of hospitalized PLWH were out of routine care with a detectable VL. Using our two stage approach, we found that 84% of hospitalized PLWH appeared to be out of routine care and/or had a VL >400 copies/mL and 781 were confirmed out of routine care and met the study eligibility criteria. Patients’ willingness to participate in a research study to re-engage in HIV care was relatively high; 59% were successfully enrolled and able to complete the study requirements. The vast majority of these enrolled PLWH were diagnosed with HIV before hospitalization; only 11% were newly diagnosed.
Despite the organizational complexity of operating within a large, publically-funded hospital setting, it was feasible to identify and locate hospitalized PLWH. However, we found that multiple strategies were needed to quickly identify hospitalized PLWH who were out of routine care before hospital discharge. Use of the EMR was essential to rapidly identify a pool of potentially eligible patients. Research staff scanned medical records daily for new admissions, a process that was facilitated by the Harris Health System’s EMR that could be accessed from the research offices. Approximately 16% of all identified patients were deemed ineligible based on medical record review only. The EMR also included an application that listed all patients currently hospitalized who had HIV infection in their problem list, but this was less useful because it relies on a provider manually entering HIV infection into the problem list. To ensure that all potential patients were identified, including those missed by medical record review, research staff routinely visited hospital nursing units and sought referrals from infectious disease specialists and other practitioners on the clinical services providing care for hospitalized PLWH. Another source of identifying patients was the BTGH emergency center’s routine HIV testing program. Routine screening helped identify persons with HIV infection, including many diagnosed elsewhere but not known positive in our system19. These latter recruitment methods were most fruitful when persons were newly diagnosed with HIV infection during the course of their hospital stay, since the census review focused on what was known at the time of admission. We estimate that a research coordinator spent between 2 and 3 hours daily on these tasks.
We also found an in-person screening was necessary to confirm out of routine care status and that eligibility criteria were met. We estimate that a research coordinator spent between 2 and 3 hours daily on tracking preliminarily eligible persons and conducting in-person screening. Although this component increased the complexity of our protocol and required extra staff time to quickly locate patients and conduct the screening, we found that almost half of those preliminarily eligible were in care elsewhere or did not meet the study eligibility criteria.
Significant differences in patient eligibility were observed by gender and race/ethnicity. The majority of the eligible hospitalized PLWH were Black men. Previous studies have shown higher rates of poor retention in Black PLWH compared to other demographic groups20–22. Although African-American PLWH constituted the largest proportion of out-of-care patients identified for this study, a significant number of Hispanic PLWH were also identified. In the general population, more Hispanic persons (32.8%) and non-Hispanic African-American persons (20.9%) than non-Hispanic white persons (15.2%) lacked a usual source of care23. The racial/ethnic disparities observed in our study are therefore consistent with previous research.
Compared to all other groups, white men were least likely to meet our study eligibility criteria. The most common reason for study ineligibility among these patients was that they did not intend to use TSHC after discharge. This was an unexpected finding; we incorrectly assumed that patients who received inpatient care at the public, county hospital would intend to seek outpatient HIV care at the public, county clinic. We found that some patients preferred to receive their HIV care at a local Federally Qualified Health Centers (FHQC) and other clinics that receive Ryan White Treatment Modernization Act funding. Uninsured and underinsured PLWH from those clinics will often use the Harris Health System for emergency and inpatient care. In retrospect, we should have collected data to validate our assumption about healthcare preferences of our target study population. This is an important lesson learned from our study. Further, one of the local FQHC sites is more focused on men who have sex with men, which may partially explain our finding. TSHC is a dedicated HIV clinic, and anecdotal reports suggest that stigma associated with receiving care at TSHC was also a concern among some patients who chose to receive HIV care at another clinic.
Despite patients being hospitalized, their willingness to participate in a study focused on linkage to outpatient HIV care was relatively high. Almost 60% of all eligible patients enrolled. However, special consideration must be given to the logistics of recruitment and duration of the intervention during hospitalization. Among the 321 patients who were eligible but not enrolled, the vast majority of potentially eligible patients did not enroll because enrollment and/or the intervention could not be completed prior to hospital discharge. Many eligible patients were ready to be discharged shortly after they were identified for the study. Since our protocol required at least the baseline survey and one in-person visit with the interventionist to occur before discharge, our ability to enroll patients with a short length of hospital stay was limited. For those who remained hospitalized for at least one night, the uncertainty of when they would be discharged presented a challenge to delivering the intervention as designed. In addition, limited staff and mentor availability on the weekends reduced our capacity to enroll and deliver the intervention. Although we do not know how many patients would have enrolled, weekend staffing would have certainly increased enrollment among eligible patients. Future efforts to increase enrollment among hospitalized patients should consider additional staffing to maximize the number of patients who complete enrollment and receive the intervention before discharge.
Only 3% of eligible patients declined enrollment in the study. Although we attempted to minimize patient burden and maximize patients’ ability to participate, approximately 20% of eligible patients were too sick to enroll. The initial visit with the patient was rather extensive and could take approximately 2 to 3 hours. This visit included obtaining informed consent, conducting the baseline survey, and delivering the first session of the intervention. Breaks were often needed to manage patient fatigue. Despite the demands of the first visit, completion rates for the baseline survey and the first intervention session were high, at 96% and 98% respectively. For future studies in hospitalized PLWH, it is important to consider that more intensive demands on participant’s time would limit the number of patients who would be well enough to participate. Several of our enrolled patients struggled to complete the first visit. However, we found that many patients also enjoyed having the research coordinators and interventionists visit them, especially as they felt better nearer to discharge (Source: Qualitative component of MAPPS study – unpublished data).
We observed significant differences in enrollment by race/ethnicity. Hispanic PLWH were the least likely to enroll compared to all other race/ethnic groups. Low study enrollment among Hispanic PLWH may be attributable to stigma, disclosure, or cultural differences associated with study participation22. This finding can also be partly explained by logistical issues. Spanish speaking participants were required to be enrolled and consented by a Spanish speaking research coordinator, and see a Spanish speaking mentor or control interventionist. Although we had Spanish speaking coordinators, mentors, and control interventionists, we had to defer enrollment and randomization of Spanish speaking patients if a Spanish speaking coordinator, mentor, and control interventionist were not available. Subsequent studies in areas with substantial non-English speaking populations should ensure adequate staffing in all relevant languages, as budgets allow.
We acknowledge the limitations of this study. First, our study cohort was limited to only patients who elected to receive care at TSHC. Second, this manuscript is intended to provide a general overview of our approach and lessons learned and we did not conduct a formal process evaluation to assess the feasibility of our recruitment procedure. Third, we did not conduct a cost-effectiveness analysis to quantify the cost per patient recruited with our strategy. Lastly, it would be difficult to replicate our methodology exactly at another site. However, while these efforts to identify hospitalized PLWH were quite labor intensive, over the course of the 3-year enrollment period, we estimate there were about 72,000 patients who needed EMR review, and we identified 1801 PLWH among them, yielding one PLWH per every 40 patients admitted to BTGH. Hospitals like BTGH that serve populations with a high prevalence of HIV infection are important venues to find PLWH, including PLWH who are out of routine care.
In conclusion, hospitalized PLWH who are out of routine care can be successfully identified and recruited for research or programs focused on engagement HIV in care. Many hospitalized patients were interested and willing to participate. Very few patients declined despite being hospitalized. Logistic barriers constituted the primary barrier to enrollment, but these issues are surmountable with sufficient resources. While not all hospitals care for large populations of PLWH who are out of routine care, identifying otherwise hard to reach PLWH who are out of routine care while hospitalized is a promising approach to achieving research and programmatic goals to improve engagement in HIV care.
ABBREVIATIONS
- HIV
Human Immunodeficiency Virus
- TSHC
Thomas Street Health Center
Footnotes
CONFLICTS OF INTEREST: K. Amico has disclosed the following potential conflicts of interest: an educational grant from Gilead Sciences through the University of Michigan. All other authors reported no potential conflicts of interest. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.Centers for Disease Control and Prevention. Vital Signs: HIV Diagnosis, Care, and Treatment Among Persons Living with HIV - United States, 2011. Morbidity and Mortality Weekly Report. 2014 Nov 25;63 [PMC free article] [PubMed] [Google Scholar]
- 2.Hoxhaj S, Davila JA, Modi P, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58:S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 4.Logie C, Gadalla TM. Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care. 2009;21:742–753. doi: 10.1080/09540120802511877. [DOI] [PubMed] [Google Scholar]
- 5.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21(Suppl 1):S40–S48. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 6.Gardner LI, Marks G, Metsch LR, et al. Psychological and behavioral correlates of entering care for HIV infection: the Antiretroviral Treatment Access Study(ARTAS) AIDS Patient Care STDS. 2007;21:418–425. doi: 10.1089/apc.2006.0115. [DOI] [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, del RC, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford JB. The promise of outreach for engaging and retaining out-of-care persons in HIV medical care. AIDS Patient Care STDS. 2007;21(Suppl 1):S85–S91. doi: 10.1089/apc.2007.9983. [DOI] [PubMed] [Google Scholar]
- 9.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 10.Cabral HJ, Tobias C, Rajabiun S, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21(Suppl 1):S59–S67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 11.Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. AIDS Patient Care STDS. 2007;21(Suppl 1):S77–S84. doi: 10.1089/apc.2007.9984. [DOI] [PubMed] [Google Scholar]
- 12.Rajabiun S, Cabral H, Tobias C, Relf M. Program design and evaluation strategies for the Special Projects of National Significance Outreach Initiative. AIDS Patient Care STDS. 2007;21(Suppl 1):S9–19. doi: 10.1089/apc.2007.9991. [DOI] [PubMed] [Google Scholar]
- 13.Rumptz MH, Tobias C, Rajabiun S, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDS. 2007;21(Suppl 1):S30–S39. doi: 10.1089/apc.2007.9989. [DOI] [PubMed] [Google Scholar]
- 14.Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni ML, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-positive people. AIDS Patient Care STDS. 2007;21(Suppl 1):S68–S76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- 15.Tobias C, Cunningham WE, Cunningham CO, Pounds MB. Making the connection: the importance of engagement and retention in HIV medical care. AIDS Patient Care STDS. 2007;21(Suppl 1):S3–S8. doi: 10.1089/apc.2007.9992. [DOI] [PubMed] [Google Scholar]
- 16.Metsch LR, Feaster DJ, Gooden L, et al. Incentives on viral supression among hosptalized patients with HIV infection and substance use. JAMA. 2016;316(2):156–170. doi: 10.1001/jama.2016.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giordano TP, Cully J, Amico KR, Davila JA, Kallen MA, Hartman C, Wear J, Buscher A, Stanley M. A randomized trial to test a peer mentor intervention to improve outcomes in persons hospitalized with HIV infection. Clin Infect Dis. 2016 May 2; doi: 10.1093/cid/ciw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metsch LR, Bell C, Pereyra M, et al. Hospitalized HIV-positive patients in the era of highly active antiretroviral therapy. Am J Public Health. 2009;99:1045–1049. doi: 10.2105/AJPH.2008.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flash CA, Pasalar S, Hemmige V, Davila JA, Hallmark CJ, McNeese M, Miertschin N, Ruggerio MC, Giordano TP. Benefits of a Routine Opt-Out HIV testing and linkage to care program for previously diagnosed patients in publically funded emergency departments in Houston, TX. J Acquir Immune Defic Syndr. 2015;69(Suppl):S8–S15. doi: 10.1097/QAI.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert M. Racial and ethnic differences in health insurance coverage and usual source of health care, 2002. Rockville (MD): Agency for Healthcare Research and Quality; 2006. MEPS Chartbook No. 14. AHRQ Pub. No. 06-0004. [Google Scholar]
- 21.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-positive subjects in a large healthcare system in the United States. AIDS Patient Care STDS. 2013;27:442–449. doi: 10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-positive persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62:356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang BN, Giordano TP, Kim JH. Sociocultural and structural barriers to care among undocumented Latino immigrants with HIV infection. J Immigr Minor Health. 2012;14:124–131. doi: 10.1007/s10903-011-9542-x. [DOI] [PubMed] [Google Scholar]


