Abstract
In polygynous primates, a greater reproductive variance in males have been linked to their reduced life expectancy relative to females. The mortality patterns of monogamous pair-bonded primates, however, are less clear. We analyzed the sex differences in mortality within wild (NMales = 70, NFemales = 73) and captive (NMales = 25, NFemales = 29) populations of Azara’s owl monkeys (Aotus azarae), a socially and genetically monogamous primate exhibiting biparental care. We used Bayesian Survival Trajectory Analysis (BaSTA) to test age-dependent models of mortality. The wild and captive populations were best fit by the logistic and Gompertz models, respectively, implying greater heterogeneity in the wild environment likely due to harsher conditions. We found that age patterns of mortality were similar between the sexes in both populations. We calculated life expectancy and disparity, the latter a measure of the steepness of senescence, for both sexes in each population. Males and females had similar life expectancies in both populations; the wild population overall having a shorter life expectancy than the captive one. Furthermore, captive females had a reduced life disparity relative to captive males and to both sexes in the wild. We interpret this pattern in light of the hazards associated with reproduction. In captivity, where reproduction is intensely managed, the risks associated with gestation and birth are tempered so that there is a reduction in the likelihood of captive females dying prematurely, decreasing their overall life disparity.
Keywords: senescence, pair-bond, monogamy, biparental care, life expectancy, Keyfitz’ entropy
INTRODUCTION
A growing body of evidence indicates that many polygynous primate species conform to the characteristic mammalian trend of reduced lifespan in males relative to females [Bronikowski et al., 2011]. A smaller proportion of males reaching the oldest age classes have been documented in several primate clades, including the great apes [Hill et al., 2001; Müller & Wrangham, 2014; Wich et al., 2009], Old World monkeys[Drickamer, 1974; Fedigan & Zohar, 1997; Rajpurohit & Sommer, 1991], New World monkeys [Robinson, 1988], and strepsirhines [Kraus et al., 2008]. Since many of these taxa are characterized by greater intrasexual competition among males than females, this pattern is often interpreted as the result of sexual selection favoring male competitive ability in the context of greater age-independent mortality at every age class, with an associated cost to their longevity [Kirkwood, 1977; Williams, 1957]. Thus, reduced male longevity may be owed both to higher rates of age-independent mortality associated with male mating tactics [Alberts & Altmann, 1995; Hoffman et al., 2008; Kraus et al., 2008], and greater increases in mortality with age.
Relatively less research on mortality patterns has been conducted on monogamous pair-bonded primates, which offer the potential to evaluate the influence of sexual dimorphism and competition regimes on age and sex-specific mortality. Emerging evidence suggests that these features are associated with alike lifespans for males and females [Clutton-Brock & Isvaran, 2007]. For example, captive owl (Aotus sp.) and titi (Callicebus sp.) monkeys show similar lifespans for males and females [Allman et al., 1998]. In natural settings, male and female muriquis (Brachyteles hypoxanthus) [Bronikowski et al., 2011] and brown mouse lemurs (Microcebus rufus) [Zohdy et al., 2014], two sexually monomorphic species with commensurate levels of intrasexual competition, show no sex-based differences in survival. In some instances, slight survival advantages might be experienced by males relative to females: higher female mortality, from the onset of adulthood to the age of 18 years, has been reported for the Milne–Edwards’ sifaka (Propithecus edwardsi), a monomorphic species with a flexible social systemin which both males and females exhibit similar levels of intrasexual competition and dispersal [Tecot et al., 2013].
We examined sex differences in the age-specific mortality trajectories of Azara’s owl monkey (Aotus azarae). Owl monkeys are one of the few socially monogamous primates, living in small groups consisting typically of an adult heterosexual pair, an infant, and a few juveniles and/or sub adults [Huck et al., 2011]. This particular species is the only monogamous primate for which no extra-pair paternity has been reported, a trait it shares with a small number of monogamous mammals [Huck et al., 2014]. The genus is generally nocturnal, with only Azara’s owl monkey exhibiting cathemerality with regular periods of activity in the morning and evening hours [Fernandez-Duque, 2003; Fernandez-Duque et al., 2010; Wright, 1989]. Owl monkeys are sexually monomorphic with no apparent differences between the sexes in body mass or size, and only moderate enlargement of canines in males relative to females [Fernandez-Duque, 2011]. The sexes cannot even be easily differentiated by external genitalia [Fernandez-Duque & Rotundo, 2003]. Male and females have similar growth rates [Huck et al., 2011], both disperse from their natal groups [Fernandez-Duque, 2009], and both experience comparable levels of intrasexual competition from owl monkey “floaters,” recently-dispersed solitary individuals who challenge established pairs and sometimes replace resident individuals, often with no impact on the pair’s extant offspring [Fernandez-Duque & Huck, 2013; Huck & Fernandez-Duque, 2012]. Finally, as is typical, but not exhaustive of monogamous primates, owl monkeys display biparental care: a clear division of labor in the rearing of offspring where females are responsible for nursing, while males are responsible for transporting, playing, and grooming the young [Dixson & Fleming, 1981; Rotundo et al., 2005]. In both captivity and the wild, adult males transfer food to offspring at a greater rate than females [Wolovich et al., 2006; Wolovich et al., 2008].
We examined two populations of Azara’s owl monkeys, one in the wild and one in captivity. In our analyses we used the R package BaSTA [Colchero et al., 2012; Colchero & Clark, 2012], an algorithm for age-specific survival analysis when age information is missing. This method has been showed to recover unbiased estimates of mortality with small sample sizes. We used this algorithm to test for differences in age-specific mortality between sexes and between environmental contexts. In addition to estimating age-specific trajectories of mortality, we calculated two metrics that describe separate dimensions of aging [Baudisch, 2011]. The first, life expectancy, establishes the time scale over which changes in mortality happen. The second, life disparity, indicates the steepness of senescence irrespective of time scale. Disparity also indicates how much variability there is in the distribution of the age of death: the lower the disparity, the steeper the increase in mortality with age [Keyfitz & Caswell, 2005; Vaupel, 1986].
We compared these two measures between the sexes to evaluate the hypothesis that the intra-sexual competition regime has an impact on mortality patterns between the sexes. We predicted that there would be no differences in the model parameter estimates, life expectancies, and life disparities between the sexes within captive and wild populations of owl monkeys due to their similar levels of intrasexual competition. We also investigated differences in life expectancy and disparity across populations. Some research indicate that senescence is plastic in variable contexts within a single species [Austad, 1993; Jones, 2011; Lemaître et al., 2013], in concert with the prediction by Williams [1957] that senescence would be greater in the context of greater age-independent mortality [but see Caswell, 2007]. We predicted decreased life expectancy and disparity in the wild owing to greater sources of age-independent mortality.
METHODS
The research herein was approved by the corresponding provincial authorities in Argentina and various Institutional Animal Care and Use Committees in the United States, and is in accordance with the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates.
Study Populations and Monitoring Procedures
A wild population of Azara’s owl monkeys has been studied since 1996 as part of the Owl Monkey Project in the Argentinean Gran Chaco [Fernandez-Duque et al., 2001]. The study area occupies approximately 5,000 hectares of gallery forest that grow along the Pilagá and Guaycolec rivers in the 25,000 ha Guaycolec Ranch, Formosa Province, Argentina (58°11′W, 25° 58′S). This area of Formosa Province is an alluvial plain consisting of a mixture of grasslands, savanna, and xeric thorn and gallery forests. The population is monitored regularly to collect demographic data, including group size, age classes, presence and absence of infants, dispersal events, disappearances, and replacements of reproducing adults [Huck et al., 2014]. The number of groups monitored each year changes, but between eight and ten groups have been monitored regularly for 18 years [Wartmann et al., 2014]. The size of the groups varies between 2 and 6 individuals, with most groups having usually a pair of reproducing adults and two or three non-reproducing individuals. All observed animals are classified as adults, subadults, juveniles, or infants [Huck et al., 2011]. Sex can only be recorded from individuals who have been unequivocally identified given the very small sexual dimorphism characteristic of the species [Fernandez-Duque, 2011]; so, since 2001, 162 individuals have been marked and/or fitted with collars to facilitate identification [Fernandez-Duque & Rotundo, 2003]. A more detailed description of demographic data collection is presented elsewhere [Fernandez-Duque, 2009].
The procedure for capturing a subject is as follows: subjects are anesthetized with a small amount of ketamine hydrochloride (25–50 mg/kg; Vetanarcol, Konig, Argentina) delivered via disposable 0.5-cc darts from a blowpipe between 1999 and 2001 and from a CO2-powered rifle after that [Fernandez-Duque & Rotundo, 2003]. Once captured, the subject is examined, marked, and fitted with a collar. For the estimation of demographic parameters, we considered an animal to be alive in a year when it was either captured or sighted. We analyzed the birth, death, and sighting records of 143 (NMales = 70, NFemales = 73) individuals during a 13-year period (1997–2012). The year of birth was known for all subjects, but the year of death was only known for 27 subjects. Common causes for incomplete death records are the subject’s movement outside the project’s monitoring range or that it was still live at the conclusion of the sampling period.
The captive owl monkey colony is located at the Keeling Center for Comparative Medicine and Research in Bastrop, Texas, USA. We examined demographic records of 54 individuals (NMales = 25, NFemales = 29) collected between 1985 and 2013. Subjects are housed as family units, a social arrangement that reflect the most frequent social organization of wild groups: a monogamous breeding pair and three to five non-reproducing individuals. Reproduction is sometimes controlled through female oral contraceptives. We included in the analyses all subjects who died a natural death or who were euthanized because of their imminent death as reported in their health records. In captivity, there were also instances when timings of births and deaths were unknown, for example when animals were bought, sold, or traded, or if they were still alive. The year of birth was unknown for one individual; the age of death was unknown for 30 individuals, who were still alive at the conclusion of the sampling period.
Data Analyses
We compiled and analyzed the data using BaSTA (Bayesian Survival Trajectory Analysis) following the procedures detailed by Colchero et al. BaSTA employs a Markov Chain Monte Carlo (MCMC) algorithm that combines Metropolis sampling for the survival parameters and latent states (the unknown times of birth and death) and direct sampling for recapture and sighting probabilities [Colchero & Clark, 2012]. We tested four models in each population with identical simulation specifications. We used 150,000 MCMC iterations with a burn in of 15,001 iterations. We thinned the resulting MCMC chain to reduce serial autocorrelation between consecutive parameter estimates by taking every 150th estimate. We ran five simulations per model to assess model convergence. Each model included a categorical covariate “sex”, which we specified using a “fused” covariate structure. This structure allowed us to estimate the impact of levels of this covariate as a linear function of the survival parameters, in a similar way to how they are handled in generalized linear models (GLMs). We used the Kullback–Leibler discrepancy (KLc) to measure how differently the sex of the animal impacts survival. Values closer to 0.5 imply that there is a minimal difference in survival parameters between the sexes; values closer to 1 imply major differences [Kullback & Leibler, 1951; McCulloch, 1989]. Comparing this analytical approach to more traditional tests, when using a Welch’s t-test to compare means of variables with similar variances, a p-value of 0.05 is reached when KLc 0.65. Thus, one can interpret a KLc > 0.65 as indicative of a difference between the means that is unlikely to occur if the distributions of the two variables were the same. Model fit was assessed using the Deviance Information Criterion (DIC). Due to our resighting procedure in the wild being biased toward individuals that had been radio collared, we conditioned our analyses for both populations to survival to age 2. We do not usually capture and collar individuals younger than 1 year of age because of the risks associated with darting individuals of smaller size; these biases preclude us from accurately analyzing mortality during the first 2 years of life.
Models of Aging
We tested four mortality models in each population (Table I). The first three of these models are age-dependent. The Gompertz model assumes an exponential increase in the probability of death with age [Gompertz, 1825; Pletcher, 1999]. Historically, the Gompertz function is, despite certain limitations, the most utilized [Promislow et al., 1999], particularly for non-human primates [Bronikowski et al., 2002; Bronikowski et al., 2011]. In this model, mortality increase, or actuarial senescence, is represented as an exponential function of age, with a scale parameter given by b0, and a rate parameter b1. The Gompertz model has been criticized for its inability to recover the mortality plateaus that are occasionally observed at older ages in humans [Horiuchi & Wilmoth, 1997; Kannisto et al., 1994] and flies [Carey et al., 1992; Fukui et al., 1993]. An alternative to the Gompertz model is the Weibull model [Pinder et al., 1978], which models mortality as a power function of age and, although it cannot reproduce late-life plateaus, it can model deceleration in late-life mortality. Finally, the logistic function is a three-parameter model that fits an S-shaped curve. This model has been shown to be useful in describing the slowing down of mortality in older ages. The logistic model results from a proportional hazards Gompertz mortality model where the proportional hazards term follows a γ distribution. This γ-distributed random variable reflects heterogeneity in frailty among individuals in a population [Vaupel et al., 1979; Vaupel & Yashin, 1985].
TABLE I.
Tested Mortality Functions
| Title | Formula | |
|---|---|---|
| Gompertz |
|
|
| Weibull |
|
|
| Logistic |
|
|
| Additional shape-defining parameters |
|
|
| Exponential |
|
The tested mortality (μ), functions given age (x), and parameters (b). The Gompertz, Weibull, and logistic functions were all extended by additional shape-defining parameters, the Makeham c term for age independent mortality, and an initial declining Gompertz function with parameters a1 and a2. The exponential function is age-independent and assumes constant mortality with age.
We extended these three mortality functions with three additional shape-defining parameters, a constant parameter, the Makeham c, which captures age-independent mortality [Pletcher, 1999], and a declining Gompertz function, which captures the decline in mortality early in life that is characteristic of primates and mammals generally [Gage, 1998; Siler, 1979]. We refer to these modifications as “bathtub” models (i.e., Gompertz-bathtub etc.); henceforth all mentions of the mortality function by name will imply the addition of these parameters. Although we have conditioned our study populations to reaching an age of 2 years, we have no a priori reason to think that a decline in mortality is not still in progress during the earliest years. The inclusion of an initial declining phase in the models will, therefore, help recover the baseline mortality levels at the onset of adulthood. Finally, in addition to these models, we also tested the exponential function [Cox & Oakes, 1984], which assumes that mortality is constant at all ages. While a substantial body of evidence has now emerged to disprove the notion that there is no actuarial senescence in wild vertebrate populations [Jones et al., 2008], we included it as a null model against which to test our age-specific mortality functions.
Analysis of the Pace and Shape of Senescence
We examined two different axes of senescence. First, we characterized the time-scale over which aging occurs using life expectancy at age 2, calculated as:
where is the survival function. Second, we examined the steepness of senescence, independent of the time-scale, by examining the degree to which there is a variation in the age at death or lifespan disparity. As our measure of disparity, we calculated Keyfitz’ entropy [Keyfitz & Caswell 2005; Vaupel 1986] from age 2 as:
A low disparity implies that mortality is concentrated around a specific age, whereas a high disparity implies that mortality is widely spread at different ages. Together, these axes of senescence have been referred to as the “pace” and “shape” of aging [Baudisch, 2011; Vaupel et al., 2004]. We calculated KLc to estimate the level of overlap between our estimates for each of these metrics.
RESULTS
Both populations showed pronounced mortality increases with age as indicated by the universally poor fit of the exponential model (Table II). We found that the two population were best described by different models of mortality: the wild population by the logistic model, which implies a deceleration of senescence in older ages and possibly higher heterogeneity in mortality (Fig. 1, bottom-right panel), and the captive population by the Gompertz model, which implies an exponential increase with age (Fig. 2, bottom-right panel).
TABLE II.
Ranked Model Performances
| Wild population
|
Captive population
|
||||||
|---|---|---|---|---|---|---|---|
| Model | Shape | DIC | Rank | Model | Shape | DIC | Rank |
| Logistic | Bathtub | 1047 | 1 | Gompertz | Bathtub | 417 | 1 |
| Gompertz | Bathtub | 1052 | 2 | Weibull | Bathtub | 435 | 2 |
| Weibull | Bathtub | 1053 | 3 | Logistic | Bathtub | 341 | 3 |
| Exponential | Simple | 1459 | 4 | Exponential | Simple | 504 | 4 |
The ranked performance of the Gompertz, Weibull, logistic, and exponential mortality functions fit to the wild and captive populations, as measured by their Deviation Information Criterion (DIC) scores.
Fig. 1.
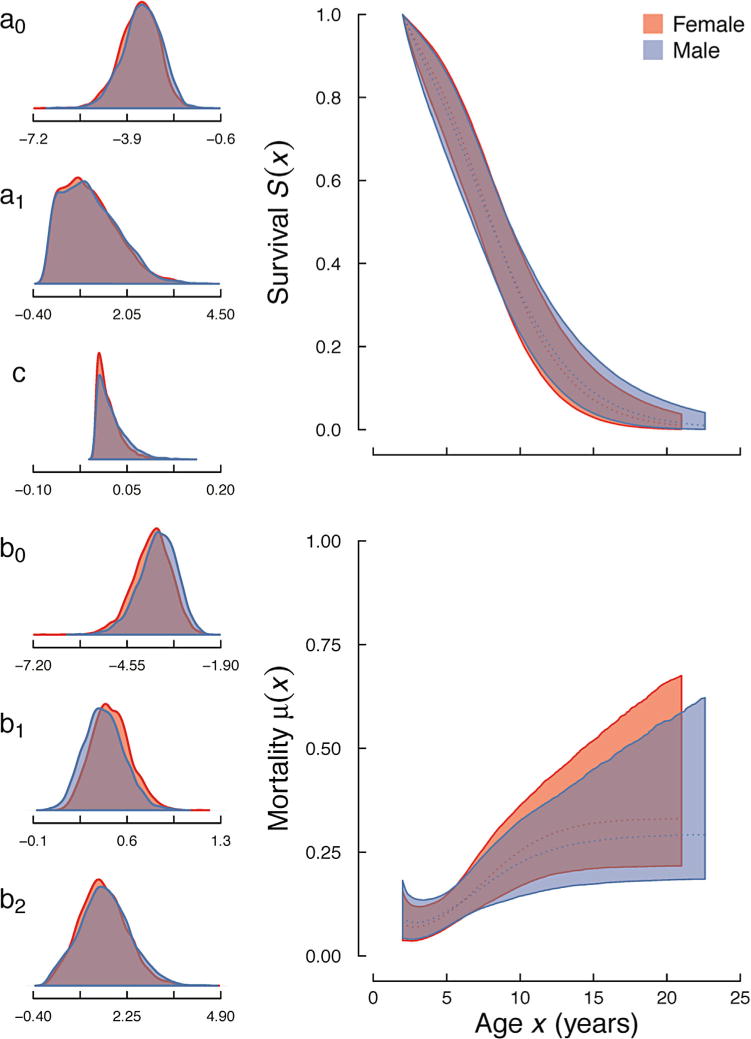
Age-specific mortality and survival trajectories for a wild population of Azara’s owl monkeys (Aotus azarae) in Formosa Province, Argentina. Female (red) and male (blue) estimated survival and mortality curves of the logistic model. Colored areas surrounding the curves represent the 95% confidence intervals. Density plots for the model parameters (see Table I) are depicted left of the mortality and survival profiles.
Fig. 2.
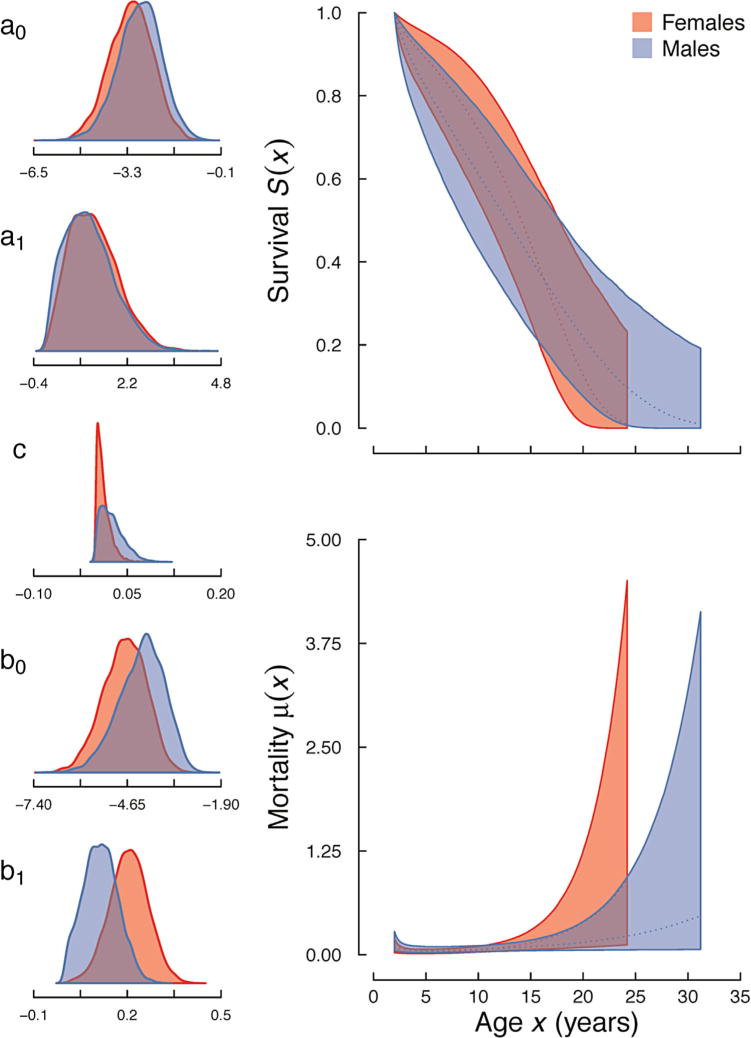
Age-specific mortality and survival trajectories for a captive population of owl monkeys (Aotus spp.) at the Keeling Center of Comparative Medicine. Female (red) and male (blue) estimated survival and mortality curves of the Gompertz model. Colored areas surrounding the curves represent the 95% confidence intervals. Density plots for the model parameters (see Table I) are depicted left of the mortality and survival profiles.
We found very small differences in the distributions of parameter estimates for the wild population (all KLc ≤ 0.54; Table III; Fig. 1, left-most panels). For the captive population, we found some differences between the sexes. The parameter estimates for the captive Makeham c and b1 parameters indicate slightly greater age-independent mortality for males (KLc = 0.74) and greater age-dependent increases in mortality for females (Klc = 0.81). Otherwise, all other parameter estimates indicate similar mortality (all other KLc values ≤ 0.59).
TABLE III.
Coefficient Estimates and Mean KLc Values
| Wild population, logistic | Captive population, Gompertz | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Parameter | Estimate
|
Standard error
|
KLc | Parameter | Estimate
|
Standard error
|
KLc | ||||
| M | F | M | F | M | F | M | F | ||||
| a0 | −3.37 | −3.46 | 0.68 | 0.66 | 0.51 | a0 | −2.88 | −3.14 | 0.77 | 0.76 | 0.53 |
| a1 | 1.09 | 1.07 | 0.72 | 0.71 | 0.5 | a1 | 1.16 | 1.24 | 0.68 | 0.67 | 0.5 |
| c | 0.03 | 0.02 | 0.02 | 0.02 | 0.54 | C | 0.03 | 0.01 | 0.02 | 0.01 | 0.74 |
| b0 | −3.68 | −3.84 | 0.53 | 0.54 | 0.52 | b0 | −4.15 | −4.56 | 0.67 | 0.64 | 0.59 |
| b1 | 0.42 | 0.48 | 0.15 | 0.14 | 0.53 | b1 | 0.12 | 0.2 | 0.06 | 0.06 | 0.81 |
| b2 | 1.62 | 1.57 | 0.71 | 0.66 | 0.5 | ||||||
Parameter estimates and their standard errors from the best-fitting mortality function for the males (M) and females (F) of each population. The Kullback–Leibler Discrepancy Calibration (KLc) measures differences between the sexes: values closer to 0.5 indicate no difference. Numbers in bold indicate a difference between the means that is unlikely to occur if the distributions of the two variables were the same.
Life expectancies were similar for both sexes in the captive (e2 Females = 11.7; e2 Males = 11.8; KLc = 0.57) and wild populations (e2 Females = 6.6, e2 Males = 6.7; Klc = 0.51; Table IV; Fig. 3A). Captive owl monkeys were expected to live an average of 5.1 more years after reaching age 2 than wild owl monkeys who reach that age. Wild males and females also had similar distributions in lifespan disparity (Wild: H2 Females = 0.63, H2 Males = 0.68, KLc = 0.62; Fig. 3B). Captive females had a lower lifespan disparity than captive males, wild males, and wild females (Captive: Hx Females = 0.45; all KLcs ≥ 0.9); suggesting that, in captivity, fewer females were dying prematurely. The lifespan disparity of captive males was more similar to that of wild males and females (Captive: Hx Males = 0.66; both KLcs ≤ 0.72).
TABLE IV.
Life Expectancies, Disparities, and Mean KLc Values
| Pairwise KLc values
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Metric estimates
|
Wild females
|
Captive males
|
Captive females
|
|||||
| e2 | H2 | e2 | H2 | e2 | H2 | e2 | H2` | |
| Wild males | 6.7 | 0.68 | 0.51 | 0.62 | 0.99 | 0.64 | 1 | 0.98 |
| Wild females | 6.6 | 0.63 | – | – | 0.99 | 0.72 | 1 | 0.95 |
| Captive males | 11.8 | 0.66 | – | – | – | – | 0.57 | 0.9 |
| Captive females | 11.7 | 0.45 | – | – | – | – | – | – |
The estimated life expectancies (e2) and disparities (H2) based on the parameters of the logistic and Gompertz models fit to the wild and captive samples, respectively. Pairwise The Kullback–Leibler Discrepancy Calibration (KLc) values closer to 1 indicate no overlap in parameter estimates; values closer to 0.5 indicate complete overlap. Numbers in bold indicate a difference between the means that is unlikely to occur if the distributions of the two variables were the same.
Fig. 3.
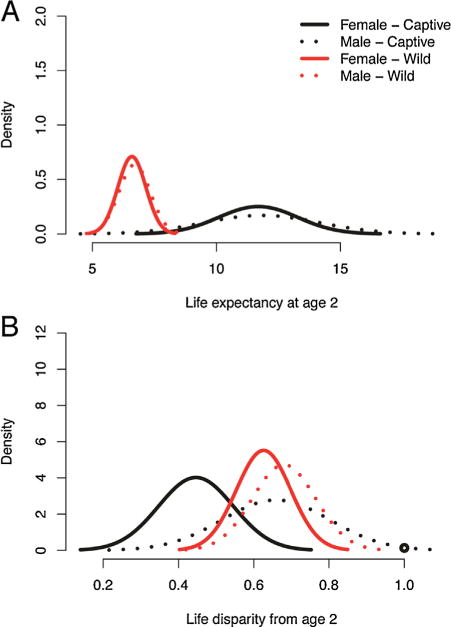
Female and male estimated life expectancy (A) and life disparity (B) in a wild population of Azara’s owl monkeys in Formosa Province, Argentina (red) and a captive population at the Keeling Center for Comparative Medicine (black).
DISCUSSION
We found some support for our primary hypothesis that age-specific mortality rates do not vary much between the sexes. We detected that there were no marked differences between parameter estimates, life expectancies, and life-disparities of males and females in the wild population of owl monkeys. Alternatively, we found similar life expectancies, but dissimilar lifespan disparities, in the captive population. This pattern of the expectation and distribution of the age at death in the captive population is consistent with a cross-over in mortality, whereby males experience greater mortality early in life, but lower levels later in life [Vaupel & Yashin, 1985]; female mortality eventually overtakes male mortality through steeper actuarial senescence (Fig. 2, bottom-right panel).
The difference in male and female lifespan disparity is an unanticipated result. One interpretation is that a greater portion of males are dying prematurely; however, we have difficulty identifying possible mechanisms through which this selection may operate since males and females exhibit an impressive monomorphism and are subject to similar environmental and social circumstances in captivity. One possible explanation relates to the costs of gestation and how reproduction is managed in the captive colony [Hoffman et al., 2008; Tardif et al., 2008]. In our captive sample, otherwise similar mortality patterns (as evidenced by the wild sample) are likely to be influenced by the use of contraceptives to manage the population. Birth control can reduce the risks normally associated with the first birth by delaying it until the mother is more fully developed, thus reducing the selective mortality experienced by females early in life and pushing female mortality below that of males. However, it is unclear why female mortality increases at a faster rate with age than males. It is possible that unnaturally large birth intervals imparted by contraception may impact maternal mortality; however, presently we cannot evaluate this relationship.
From an evolutionary perspective, the rate of mortality increase is expected to be influenced by the degree of external, age-independent mortality experienced by a population [Kirkwood, 1977; Williams, 1957]. We find support for this hypothesis in that Azara’s owl monkey has both similar levels of intra-sexual competition and age-specific mortalities. However, an alternative hypothesis was presented by Allman and colleagues [1998], who argued that natural selection favors longevity in the sex most responsible for rearing offspring. Since owl monkeys exhibit biparental care, similarities in mortality may be due to selection pressures increasing male longevity, rather than the lack of selection for male competitive ability that in turn shortens male lifespan. Unfortunately, we are unable to discriminate these two hypotheses, because the owl monkeys we examined in this study exhibit both similar levels of intrasexual competition and biparental care.
The affiliative nature of male–female relationship in owl monkeys represents a major adaptation, and it is possible that the existence of a bond between partners buffers against, and possibly reduces, differences in mortality between the sexes. In primates there is a documented role for an individual’s social bonds to influence their own and their offspring’s longevity [Nakamura et al., 2014; Silk et al., 2003; Silk et al., 2009; Silk et al., 2010]. Furthermore, emerging evidence across diverse taxa indicates an influence of partners within mating dyads on each other’s longevity [Monaghan et al., 2011; Šešlija et al., 2008]. Examining how each owl monkey pairmate influences the other’s age-specific mortality would be an interesting direction of research.
We also investigated the degree to which actuarial senescence in this species exhibited plasticity in various environments. It is notable that the mortality trajectories of the two populations were best-fit by different mortality functions. The S-shaped logistic mortality trajectory of the wild population can be indicative of an overall greater degree of heterogeneity, whereby frail individuals are more likely to be selected out due to harsher environmental conditions in the wild than in captivity. This is consistent with our results where we find that the captive population is best-fitted by the Gompertz model, which is a special case of the logistic model where heterogeneity is absent. Our prediction that life expectancy would be longer in captivity was supported by the analysis, an unsurprising result considering the greater mortality risk from external sources (i.e., predation) at all age classes in the wild. However, if our previous interpretation of life disparity is true (i.e., captive females are anomalous because of the use of contraceptives), then that would indicate that the steepness of senescence is not different between wild and captive populations; thus, we do not find support for our final prediction that greater external mortality in the wild leads to steeper senescence. It may be possible that the anomalous nature of our captive sample precludes our ability to accurately test this prediction. However, recent theoretical considerations may also cast doubt on the validity of the prediction itself [Caswell, 2007].
The forested habitat, partial nocturnality, arboreality, and relatively small size of the monogamous Azara’s owl monkey present frequent challenges to demographic data collection, and by extension to the examination of the influence of competition regime on age-specific mortality. For our analysis, we used BaSTA, a tool for fitting mortality models to data sets compromised by small sample sizes and incomplete census records. These are frequent limitations of primate field data, so we encourage future research to consider this method. Additionally, it would facilitate the testing of specific models of mortality, rather than simply examining survivorship, which conflates age-dependent and age-independent sources of mortality [Pletcher, 1999]. As long-term demographic data on primate populations become increasingly available, the examination of various mortality functions fit to the data, together with the exploration of different axes of aging, such as life-expectancy and life disparity will help tease apart the underlying mechanisms and improve our understanding of the ways in which primates age.
Acknowledgments
The authors would like to thank A. Scheuerlein for his helpful comments on earlier versions of the manuscript. EFD acknowledges the financial support during all these years from the Wenner-Gren Foundation, L.S.B. Leakey Foundation, National Geographic Society, National Science Foundation (BCS-640 0621020, BCS-837921, BCS-904867, BCS-924352), Trio Research Program (Boettner Center for Pensions and Retirement Security, National Institutes of Aging P30 AG012836-19) and the Eunice Shriver Kennedy National Institute of Child Health and Development Population Research Infrastructure Program (R24 HD-044964-11), the University of Pennsylvania Research Foundation, and the Zoological Society of San Diego. Special thanks to M. Rotundo, V. Dávalos, and C. Juárez for all these years of contributing their hard work to the demographic monitoring reported here. Thanks to all the students, volunteers, and assistants who helped in data collection and to Mr. F. Middleton, Manager of Estancia Guaycolec, and Ing. A. Casaretto (Bellamar Estancias) for the continued support for the Owl Monkey Project. The Owl Monkey Project has had continued approval for all research presented here by the Subsecretary of Ecology and Natural Resources and the Ministry of Production of Formosa Province in Argentina and by the IACUC committees of the Zoological Society of San Diego (2000–2005) and of the University of Pennsylvania (2006–2014).
References
- Alberts SC, Altmann J. Balancing costs and opportunities: dispersal in male baboons. The American Naturalist. 1995;145:279–306. [Google Scholar]
- Allman J, Rosin A, Kumar R, Hasenstaub A. Parenting and survival in anthropoid primates: caretakers live longer. Proceedings of the National Academy of Sciences. 1998;95:6866–6869. doi: 10.1073/pnas.95.12.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Retarded senescence in an insular population of Virginia opossums (Didelphis-virginiana) Journal of Zoology. 1993;229:695–708. [Google Scholar]
- Baudisch A. The pace and shape of ageing. Methods in Ecology and Evolution. 2011;2:375–382. [Google Scholar]
- Bronikowski AM, Alberts SC, Altmann J, et al. The aging baboon: comparative demography in a non-human primate. Proceedings of the National Academy of Sciences. 2002;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski AM, Altmann J, Brockman DK, et al. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science. 2011;331:1325–1328. doi: 10.1126/science.1201571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- Caswell H. Extrinsic mortality and the evolution of senescence. Trends in Ecology and Evolution. 2007;22:173–174. doi: 10.1016/j.tree.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society B: Biological Sciences. 2007;274:3097–3104. doi: 10.1098/rspb.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colchero F, Clark JS. Bayesian inference on age-specific survival for censored and truncated data. Journal of Animal Ecology. 2012;81:139–149. doi: 10.1111/j.1365-2656.2011.01898.x. [DOI] [PubMed] [Google Scholar]
- Colchero F, Jones OR, Rebke M. BaSTA: an R package for Bayesian estimation of age-specific survival from incomplete mark-recapture/recovery data with covariates. Methods in Ecology and Evolution. 2012;3:466–470. [Google Scholar]
- Cox DR, Oakes D. Analysis of survival data. London, UK: Chapman and Hall; 1984. p. 208. [Google Scholar]
- Dixson AF, Fleming D. Parental behavior and infant development in owl monkeys (Aotus trivirgatus griseimembra) Journal of Zoology. 1981;194:25–39. [Google Scholar]
- Drickamer LC. A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatologica. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Fedigan LM, Zohar S. Sex differences in mortality of Japanese macaques: twenty-one years of data from the Arashiyama West population. American Journal of Physical Anthropology. 1997;102:161–175. doi: 10.1002/(SICI)1096-8644(199702)102:2<161::AID-AJPA2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E. Influences of moonlight, ambient temperature and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai) Behavioral Ecology and Sociobiology. 2003;54:431–440. [Google Scholar]
- Fernandez-Duque E. Natal dispersal in monogamous owl monkeys (Aotus azarai) of the Argentinean Chaco. Behaviour. 2009;146:583–606. [Google Scholar]
- Fernandez-Duque E. Rensch’s rule, Bergman’s effect and adult sexual dimorphism in wild monogamous owl monkeys (Aotus azarai) of Argentina. American Journal of Physical Anthropology. 2011;146:38–48. doi: 10.1002/ajpa.21541. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Huck M. Till death (or an intruder) do us part: intrasexual-competition in a monogamous primate. PLoS One. 2013;8:e53724. doi: 10.1371/journal.pone.0053724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Rotundo M. Field methods for capturing and marking Azarai night monkeys. International Journal of Primatology. 2003;24:1113–1120. [Google Scholar]
- Fernandez-Duque E, Rotundo M, Sloan C. Density and population structure of owl monkeys (Aotus azarai) in the Argentinean Chaco. American Journal of Primatology. 2001;53:99–108. doi: 10.1002/1098-2345(200103)53:3<99::AID-AJP1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, De la Iglesia H, Erkert HG. Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS One. 2010;5:e12572. doi: 10.1371/journal.pone.0012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui HH, Xiu L, Curtsinger JW. Slowing of age-specific mortality rates in Drosophila melanogaster. Experimental Gerontology. 1993;28:585–599. doi: 10.1016/0531-5565(93)90048-i. [DOI] [PubMed] [Google Scholar]
- Gage TB. The comparative demography of primates: with some comments on the evolution of life histories. Annual Review of Anthropology. 1998;27:197–221. doi: 10.1146/annurev.anthro.27.1.197. [DOI] [PubMed] [Google Scholar]
- Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society of London. 1825;115:513–583. doi: 10.1098/rstb.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Boesch C, Goodall J, et al. Mortality rates among wild chimpanzees. Journal of Human Evolution. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, et al. Sex differences in survival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S, Wilmoth JR. Age patterns of the life table aging rate for major causes of death in Japan, 1951–1990. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1997;52:B67–B77. doi: 10.1093/gerona/52a.1.b67. [DOI] [PubMed] [Google Scholar]
- Huck MG, Fernandez-Duque E. Children of divorce: effects of adult replacements on previous offspring in Argentinean owl monkeys. Behavioral Ecology and Sociobiology. 2012;66:505–517. [Google Scholar]
- Huck MG, Rotundo M, Fernandez-Duque E. Growth and development in wild owl monkeys (Aotus azarai) of Argentina. International Journal of Primatology. 2011;32:1133–1152. [Google Scholar]
- Huck MG, Fernandez-Duque E, Babb P, Schurr T. Correlates of genetic monogamy in socially monogamous mammals: insights from Azara’s owl monkeys. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140195. doi: 10.1098/rspb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JH. Primates and the evolution of long-slow life histories. Current Biology. 2011;21:R708–R717. doi: 10.1016/j.cub.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Gaillard J-M, Tuljapurkar S, et al. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecology Letters. 2008;11:664–673. doi: 10.1111/j.1461-0248.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- Kannisto V, Lauritsen J, Thatcher AR, Vaupel JW. Reductions in mortality at advanced ages: several decades of evidence from 27 countries. Population and Development Review. 1994;20:793–810. [Google Scholar]
- Keyfitz N, Caswell H. Applied Mathematical Demography. New York: Springer; 2005. p. 512. [Google Scholar]
- Kirkwood T. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kraus C, Eberle M, Kappeler PM. The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate. Proceedings of the Royal Society B: Biological Sciences. 2008;275:1635–1644. doi: 10.1098/rspb.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullback S, Leibler RA. On information and sufficiency. The Annals of Mathematical Statistics. 1951;22:79–86. [Google Scholar]
- Lemaître J-F, Gaillard J-M, Lackey LB, Clauss M, Müller DWH. Comparing free-ranging and captive populations reveals intra-specific variation in aging rates in large herbivores. Experimental Gerontology. 2013;48:162–167. doi: 10.1016/j.exger.2012.12.004. [DOI] [PubMed] [Google Scholar]
- McCulloch RE. Local model influence. Journal of the American Statistical Association. 1989;84:473–478. [Google Scholar]
- Monaghan P, Heidinger BJ, D’Alba L, Evans NP, Spencer KA. For better or worse: reduced adult lifespan following early-life stress is transmitted to breeding partners. Proceedings of the Royal Society B: Biological Sciences. 2011;279:709–714. doi: 10.1098/rspb.2011.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MN, Wrangham RW. Mortality rates among Kanyawara chimpanzees. Journal of Human Evolution. 2014;66:107–114. doi: 10.1016/j.jhevol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Hayaki H, Hosaka K, Itoh N, Zamma K. Brief communication: orphaned male chimpanzees die young even after weaning. American Journal of Physical Anthropology. 2014;153:139–143. doi: 10.1002/ajpa.22411. [DOI] [PubMed] [Google Scholar]
- Pinder JE, III, Wiener JE, Smith JG. The Weibull distribution: a new method of summarizing survivorship data. Ecology. 1978;59:175–179. [Google Scholar]
- Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. Journal of Evolutionary Biology. 1999;12:430–439. [Google Scholar]
- Promislow DEL, Tatar M, Pletcher SD, Carey JR. Below-threshold mortality: implications for studies in evolution, ecology, and demography. Journal of Evolutionary Biology. 1999;12:314–328. [Google Scholar]
- Rajpurohit LS, Sommer V. Sex differences in mortality among langurs (Presbytis entellus) of Jodhpur, Rajasthan. Folia Primatologica. 1991;56:17–27. [Google Scholar]
- Robinson JG. Demography and group structure in wedgecapped capuchin monkeys, Cebus olivaceus. Behaviour. 1988;104:202–232. [Google Scholar]
- Rotundo M, Fernandez-Duque E, Dixon AF. Infant development and parental care in free-ranging Aotus azarai azarai in Argentina. International Journal of Primatology. 2005;26:1459–1473. [Google Scholar]
- Šešlija D, Marečko I, Tucić N. Sexual selection and senescence: do seed beetle males (Acanthoscelides obtectus, Bruchidae, Coleoptera) shape the longevity of their mates? Journal of Zoological Systematics and Evolutionary Research. 2008;46:323–330. [Google Scholar]
- Siler W. A competing-risk model for animal mortality. Ecology. 1979;60:750–757. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TG, et al. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Tardif S, Araujo A, Arruda MF, et al. Reproduction and aging in marmosets and tamarins. In: Atsalis S, Margulis SW, Hop PR, editors. Primate reproductive aging: cross-taxon perspectives. Basel: Karger Pub; 2008. pp. 29–48. [Google Scholar]
- Tecot SR, Gerber BD, King SJ, Verdolin JL, Wright PC. Risky business: sex differences in mortality and dispersal in a polygynous, monomorphic lemur. Behavioral Ecology. 2013;24:987–996. [Google Scholar]
- Vaupel JW. How change in age-specific mortality affects life-expectancy. Population Studies. 1986;40:147–157. [Google Scholar]
- Vaupel JW, Yashin AI. Heterogeneity’s ruses: some surprising effects of selection on population dynamics. The American Statistician. 1985;39:176–185. [PubMed] [Google Scholar]
- Vaupel JW, Manton K, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Vaupel JW, Baudisch A, D€olling M, Roach DA, Gompe J. The case for negative senescence. Theoretical Population Biology. 2004;65:339–351. doi: 10.1016/j.tpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Wartmann FM, Juarez CP, Fernandez-Duque E. Size, site fidelity, and overlap of home ranges and core areas in the socially monogamous owl monkey (Aotus azarae) of northern Argentina. International Journal of Primatology. 2014;35:919–939. [Google Scholar]
- Wich SA, Shumaker RW, Perkins L, De Vries H. Captive and wild orangutan (Pongo sp.) survivorship: a comparison and the influence of management. American Journal of Primatology. 2009;71:680–686. doi: 10.1002/ajp.20704. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wolovich CK, Feged A, Evans S, Green SM. Social patterns of food sharing in monogamous owl monkeys. American Journal of Primatology. 2006;68:663–674. doi: 10.1002/ajp.20238. [DOI] [PubMed] [Google Scholar]
- Wolovich CK, Perea-Rodriguez JP, Fernandez-Duque E. Food transfers to young and mates in wild owl monkeys (Aotus azarai) American Journal of Primatology. 2008;70:211–221. doi: 10.1002/ajp.20477. [DOI] [PubMed] [Google Scholar]
- Wright PC. The nocturnal primate niche in the New World. Journal of Human Evolution. 1989;18:635–658. [Google Scholar]
- Zohdy S, Gerber BD, Tecot S, et al. Teeth, sex, and testosterone: aging in the world’s smallest primate. PLoS ONE. 2014;9:e109528. doi: 10.1371/journal.pone.0109528. [DOI] [PMC free article] [PubMed] [Google Scholar]


