Abstract
Background:
Patients with DMD experience progressive restrictive respiratory disease and eventual respiratory failure. Standard of care guidelines command changes in disease management when forced vital capacity percent of predicted (FVC% p) falls below clinically relevant thresholds. The Phase 3 DELOS trial in patients with DMD demonstrated that idebenone reduces the loss of peak expiratory flow and FVC compared to placebo (Buyse GM, et al.; Lancet 2015; 385 : 1748-57).
Objective:
Post-hoc analyses were conducted to assess whether treatment with idebenone could reduce the risk of patients dropping below clinically meaningful thresholds of FVC% p.
Methods:
The DELOS trial enrolled DMD patients 10–18 years of age not using glucocorticoids to receive idebenone (N = 31) or placebo (N = 33) for 12 months. Change from baseline in FVC and FVC% p was assessed by hospital spirometry and analyzed by mixed model of repeated measures and slope analysis and proportions of patients falling below clinically meaningful thresholds of FVC% p were compared.
Results:
The change over 1 year in FVC and FVC% p showed a consistent pattern in favor of idebenone treatment across multiple analysis methods and fewer patients in the idebenone group declined by a margin of 10% or more in FVC and FVC% p compared to placebo. There were also fewer patients in the idebenone group (15%) with a decline below FVC% p of 50% compared to the placebo group (25%) and fewer patients in the idebenone group (28%) showed a decline below FVC% p of 50% or 40% or 30% compared to the placebo group (43%).
Conclusions:
These data added to the consistency and clinical meaningfulness of findings from the DELOS trial showing that idebenone can slow the loss of pulmonary function in patients with DMD.
Keywords: Pulmonary function, respiration, idebenone, Duchenne muscular dystrophy, forced vital capacity
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the most common and devastating type of muscular dystrophy [1]. With age, progressive respiratory muscle weakness affecting thoracic accessory muscles and the diaphragm causes restrictive respiratory disease, impaired clearance of airway secretions, recurrent pulmonary infections due to ineffective cough, and eventually respiratory failure [2–5].
Decline in forced vital capacity (FVC) or FVC as percent of predicted (FVC% p) is the best validated pulmonary function predictor of early morbidity and mortality in patients with DMD [6–8]. Decline in FVC% p starts at around the age of 7 years and reaches the threshold of 80%, typically defined as the lower limit or the normal range, at around the age of 10 years. Thereafter FVC% p declines almost linearly at an absolute rate of approximately 5–8% per year until the age of ∼20 years when FVC% p reaches a floor [9–11]. Data from the comprehensive CINRG Duchenne Natural History Study [12] further demonstrate that the decline in FVC% p is significantly correlated with increased rate of hospitalization [11].
Contemporaneous recommendations for the assessment of pulmonary function in DMD are available [6, 7, 13]. Moreover, standard of care guidelines recommend regular monitoring of pulmonary function as soon as DMD patients become non-ambulant, reach the age of 12 years or present with a FVC% p of below 80% [6, 14]. Recent assessments however demonstrate overall poor compliance with these care guidelines [15, 16] exposing patients to potential health risks associated with pulmonary function decline.
The risk to present with moderate pulmonary insufficiency is linked to FVC% p falling below 50%. Specifically, when FVC% p drops below 50% DMD patients may experience early clinical symptoms such as nocturnal hypoventilation, waking at night, morning headaches and problems with concentration. Furthermore, patients are considered at increased risk for post-procedural respiratory insufficiency when they undergo general anaesthesia or procedural sedation. These risks increase even further once the FVC% p declines below 30% [17]. Hence, the probability to present moderate pulmonary insufficiency is linked to FVC% p of <50% and severe pulmonary insufficiency in DMD is considered when patients have FVC% p <30% [14, 17, 18]. Preoperative training in and postoperative use of non-invasive ventilation is recommended for patients with FVC% p <50% and considered mandatory for patients with FVC% p <30% [19]. In addition, a drop in FVC% p below 40% is recognized as a threshold below which manual and mechanical assisted cough techniques are necessary to support effective airway clearance [14, 19]. Transition between these clinically relevant pulmonary function categories determined by thresholds for FVC% p commands a change in patient care and management as recommended by standard of care guidelines [14, 19] (Table 1).
Table 1.
Clinically relevant thresholds of FVC% p according to standard of care guidelines
| FVC% p category | Patient status | Medical recommendation/standard of care |
| <50% | Moderate pulmonary insufficiency | Special precautions during surgery (e.g. scoliosis surgery); recommended postoperative use of non-invasive ventilation |
| <40% | Signs and symptoms of hypoventilation | Volume recruitment/deep lung inflation techniques; Manual and mechanical assisted cough techniques |
| <30% | High risk of hypoventilation | Nocturnal ventilation; mandatory postoperative use of non-invasive ventilation |
The DELOS trial was a prospectively planned, multi-center, phase 3 clinical trial evaluating the efficacy of idebenone (Raxone® 150 mg tablets, Santhera Pharmaceuticals, Switzerland) compared to placebo in patients 10–18 years of age at baseline and not using concomitant glucocorticoids prior to and during the 12-month trial [20]. The DELOS trial reached its primary endpoint showing a significant difference in favor of idebenone treatment in peak expiratory flow expressed as percent of predicted (PEF% p). Supportive outcomes were seen for additional expiratory and inspiratory pulmonary function endpoints [20, 21]. Additional analyses of the DELOS trial showed that more patients in the placebo group compared to the idebenone group experienced bronchopulmonary adverse events (BAEs) including airway infections [22]. In addition, the number of serious adverse events leading to hospital admissions due to respiratory causes was higher in the placebo group as was the increased use of antibiotics typically prescribed to treat bronchopulmonary complications [22]. These findings indicate that the protective effect of idebenone on pulmonary function is clinically meaningful and therefore we hypothesized that in the DELOS trial more patients in the idebenone group would remain above the clinical thresholds of FVC% p of 50%, 40% and 30% than patients in the control group.
PATIENTS AND METHODS
Patients
A detailed description of the DELOS trial design and methodology has been published previously [20]. In short, pulmonary function data were obtained from patients participating in the DELOS trial conducted between July 2009 and December 2012 in study centers located in Belgium, Germany, the Netherlands, Switzerland, France, Sweden, Austria, Italy, Spain and the USA. Patients aged 10–18 years with a documented and confirmed diagnosis of DMD were eligible for enrolment. Study participants had to have stopped taking glucocorticoids (GC) at least 12 months prior to study entry and were not allowed to take GC during the 52-week study period. Furthermore, only patients who had reached the stage of pulmonary function decline with a baseline PEF% p <80% were included. There were no selection criteria for ambulatory status or for any dystrophin gene mutation type. Patients dependent on assisted ventilation at screening and/or baseline (defined as non-invasive nocturnal ventilation, daytime non-invasive ventilation or continuous invasive ventilation) were excluded from the study. The intent-to-treat (ITT) population consisted of 64 patients; 33 patients were randomized to the placebo group, 31 patients received idebenone 900 mg/day (3 times 2 tablets with meals).
The trial and any changes to the protocol were approved by relevant national authorities and the institutional review boards or independent ethics committees in the countries of the participating centers and conducted in accordance with good clinical practice and the principles of the Declaration of Helsinki. Prior to any study-related procedure, written informed consent was obtained from all patients and/or parents or guardian. This study is registered with ClinicalTrials.gov, number NCT01027884, and the overall outcome was reported previously [20–22].
Assessment of Forced Vital Capacity (FVC)
Forced vital capacity (FVC) was assessed in the sitting position using a Pneumotrac Spirometer 6800 (Vitalograph, UK) during hospital visits at screening, at baseline (within 6 weeks from screening) and at Weeks 13, 26, 39 and 52 with the aid of a qualified, trained and certified evaluator and in accordance with the American Thoracic Society/European Respiratory Society guidelines [23]. To account for maturational changes, FVC (measured in L) was normalized to percent of predicted (% p) using the equations from the third National Health and Nutrition Examination Survey (NHANES III) for interpreting spirometry results [24]. Height estimates were derived from ulnar length measures [25, 26].
Statistical analysis
Changes from baseline in FVC and FVC% p for the ITT population were calculated as described [20] by a mixed model for repeated measurements (MMRM) with treatment group, visit, and interaction between treatment group and visit used as fixed factors in the model and baseline assessments used as a covariate. The MMRM was calculated using changes from baseline to all post-baseline visits (weeks 13, 26, 39 and 52) as response variables. This model was used to estimate the treatment difference at week 52 (pre-defined primary analysis), at the other visits and an overall difference over weeks 13–52. A sensitivity analysis was conducted following a recently described method to calculate the annual rate of decline of FVC using a random coefficient regression model with random slopes and intercepts [27]. For this, a random coefficient regression model with random slope (intercept for random effect of time of assessment expressed as years) and random intercept was fitted to the changes from baseline. All post-baseline values were included as response variables in the model. In addition to the random slopes and intercepts, the model included the fixed effect for treatment group (idebenone or placebo) and the baseline value as a covariate. The time to crossing a clinically relevant threshold of FVC% p (<50%, <40%, <30% or any of the three thresholds) and the time to clinically relevant decline in FVC or FVC% p (absolute or relative decline >10%) or the time to any decline in FVC were analysed with two different approaches. First, the time to first event (crossing a threshold or experiencing a decline) was analyzed using each of the criteria given above. Secondly, the time to first persistent event was analyzed. An event was classified as persistent if the FVC or FVC% p value stayed below the threshold at all subsequent assessments until the end of the study. The analyses of crossing the clinically relevant thresholds were conducted among the subset of subjects who were applicable for crossing the threshold (i.e. had a baseline above the threshold), while the analyses of decline by the 10% margin were conducted among all subjects. The event-based data were evaluated with hazard ratios calculated using the Cox proportional hazards model. The patients who did not have an event were censored at the time of the last FVC assessment. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Patient characteristics and general disease status
Patient characteristics at baseline for the ITT population (N = 64) and separated by treatment group (idebenone: N = 31, placebo: N = 33) have been described previously [20, 28]. Briefly, patients 10–18 years of age were enrolled resulting in an average age of the study population of 14.3 (SD 2.7) years [idebenone: 13.5 (SD: 2.7); placebo: 15.0 (SD: 2.5)]. Participating patients were already in an advanced disease stage as seen in the high proportion of non-ambulant patients (92.2%) and the overall high Brooke upper limb function score [29]. Only 7 of 64 patients (10.9%) had a Brooke score of 1 or 2, but 38 of 64 (59.4%) patients had already reached a Brooke score of 5 or 6 which implicates that patients could no longer raise their hands to their mouth [28]. At baseline, both treatment groups had comparable FVC [idebenone: 1.9 L (SD 0.5); placebo: 1.9 L (SD: 0.5)] and FVC% p [idebenone: 55.3% (SD 15.8); placebo: 50.4% (SD: 20.0)].
Change in FVC and FVC% p by treatment group over the 52-week study period
The change in FVC from baseline to each study visit (at week 13, 26, 39 and 52) and for the overall change to weeks 13–52 assessed by MMRM and the slope analysis assessed by the random coefficient regression model [27] are presented in Table 2. All analyses showed a non-significant change from baseline for the idebenone group but a statistically highly significant decline in the placebo group. Estimated differences between treatment groups for the change in FVC calculated with MMRM from baseline to week 52 reached a significance level of p = 0.050, while the changes to the other visits, the analyses taking into account all post-baseline visits or the analysis using the random coefficient regression model, all reached statistically significant differences in favor of the idebenone treatment group. The resulting overall difference across all post-baseline visits (weeks 13–52) in absolute FVC in favor of idebenone reached 122–136 mL depending on the analysis method applied. When FVC was normalized to percent of predicted factoring in patient age and using ulna length as a surrogate measure for height [24, 25, 26] resulted in a comparable outcome (Table 3). The difference between treatment groups for FVC% p using the MMRM at week 52 resulted in a non-significant trend (p = 0.08), while the MMRM analyses at other visits, MMRM analyses taking into account all post-baseline visits or analyses using the random coefficient regression model reached statistically significant differences in favor of the idebenone treatment group.
Table 2.
Change from Baseline in FVC [mL] by treatment group and between-treatment group difference by analysis method
| Analysis method | Idebenone (N = 31) | Placebo (N = 33) | Group Difference | |||
| Estimated | p-value | Estimated | p-value | Estimated | p-value | |
| Change | Change | Difference | ||||
| (95% CI) | (95% CI) | (95% CI) | ||||
| MMRM at week 13 | 13 (–51, 78) | 0.679 | –80 (–143, –17) | 0.014 | 93 (3, 183) | 0.043 |
| MMRM at week 26 | 10 (–62, 81) | 0.787 | –147 (–217, –77) | <0.001 | 157 (56, 257) | 0.003 |
| MMRM at week 39 | 14 (–68, 95) | 0.739 | –148 (–223, –72) | <0.001 | 161 (50, 272) | 0.005 |
| MMRM at week 52 | –41 (–139, 56) | 0.402 | –175 (–267, –83) | <0.001 | 134 (–0, 268) | 0.050 |
| MMRM for weeks 13–52 | –1 (–64, 61) | 0.972 | –137 (–197, –77) | <0.001 | 136 (50, 223) | 0.003 |
| Slope analysis for weeks 13–52 | –8 (–66, 49) | 0.770 | –131 (–187, –75) | <0.001 | 122 (48, 197) | 0.002 |
Estimated changes and differences are in mL. MMRM: mixed model for repeated measurements; slope analysis: random coefficient regression model.
Table 3.
Change from Baseline in FVC% p by treatment group and between-treatment group difference by analysis method
| Analysis method | Idebenone (N = 31) | Placebo (N = 33) | Group Difference | |||
| Estimated | p-value | Estimated | p-value | Estimated | p-value | |
| Change | Change | Difference | ||||
| (95% CI) | (95% CI) | (95% CI) | ||||
| MMRM at week 13 | –0.48 (–2.44, 1.48) | 0.626 | –3.75 (–5.67, –1.83) | <0.001 | 3.27 (0.51, 6.03) | 0.021 |
| MMRM at week 26 | –1.69 (–3.77, 0.38) | 0.108 | –6.41 (–8,44, –4.38) | <0.001 | 4.72 (1.80, 7.63) | 0.002 |
| MMRM at week 39 | –2.86 (–5.86, 0.13) | 0.061 | –7.84 (–10.64, –5.03) | <0.001 | 4.97 (0.86, 9.09) | 0.019 |
| MMRM at week 52 | –5.67 (–8.36, –2.99) | <0.001 | –8.95 (–11.47, –6.42) | <0.001 | 3.27 (–0.43, 6.97) | 0.082 |
| MMRM for weeks 13–52 | –2.68 (–4.68, –0.68) | 0.009 | –6.74 (–8.65, –4.82) | <0.001 | 4.06 (1.28, 6.84) | 0.005 |
| Slope analysis for weeks 13–52 | –2.55 (–4.38, –0.72) | 0.007 | –6.69 (–8.47, –4.90) | <0.001 | 4.13 (1.73, 6.54) | <0.001 |
MMRM: mixed model for repeated measurements; slope analysis: random coefficient regression model.
Proportion of patients with decline in FVC and FVC% p
The proportion of patients with a decline in FVC and FVC% p by any margin is shown in Table 4. The analysis only considered events where the decline was persistent until the end of the study, not allowing for subsequent recovery. There was a higher proportion of patients in the placebo group with decline in FVC and FVC% p compared to the idebenone group with statistically significant hazard ratios, indicating that patients in the idebenone group had a reduced risk in declining in FVC.
Table 4.
Proportion of patients with persistent decline in FVC% p and FVC by any margin at any time point during the study
| Patients with persistent decline by any margin from Baseline to Week 52 | Idebenone (N = 31) | Placebo (N = 33) | HR (95% CI) p-value |
| FVC% p | 24 (77.4%) | 30 (90.9%) | 0.43 (0.24, 0.75) p = 0.003 |
| FVC [L] | 16 (51.6%) | 27 (81.8%) | 0.36 (0.19, 0.67) p = 0.002 |
Hazard ratio (95% CI) calculated by Cox model for the time to first persistent decline event.
In other pulmonary illnesses a decline in FVC or FVC% p of ≥10% is considered clinically relevant [30, 31]. Data from the DELOS trial were therefore analyzed to establish the proportions of patients in the idebenone and placebo groups with a decline by the margin of 10% or more calculated as absolute or relative decline. For all three analyses (Table 5) there were higher proportions of patients in the placebo group with a decline of 10% or more compared to the idebenone group. The hazard ratios (HR) for the analysis of patients with a relative decline of 10% in FVC% p and FVC were statistically significant, indicating a higher risk for patients in the placebo group falling by this margin, as illustrated also in the Kaplan-Meier plot for the time to 10% relative persistent decline in FVC% p (Fig. 1). The Kaplan-Meier plot for the time to 10% relative decline (first event analysis) is shown in Supplementary Material Figure 1.
Table 5.
Proportion of patients with decline in FVC% p and FVC by 10% or more at any time point during the study
| Patients with decline of 10% or more from Baseline to Week 52 | Event type | Idebenone (N = 31) | Placebo (N = 33) | HR (95% CI) p-value |
| FVC% p: 10% absolute | first event | 8 (25.8%) | 14 (42.4%) | 0.67 (0.28, 1.61) p = 0.367 |
| persistent | 5 (16.1%) | 12 (36.4%) | 0.50 (0.17, 1.44) p = 0.197 | |
| FVC% p: 10% relative | first event | 21 (67.7%) | 27 (81.8%) | 0.46 (0.26, 0.81) p = 0.008 |
| persistent | 18 (58.1%) | 25 (75.6%) | 0.54 (0.30, 1.00) p = 0.048 | |
| FVC [L]: 10% relative | first event | 9 (29.0%) | 20 (60.6%) | 0.35 (0.16, 0.77) p = 0.009 |
| persistent | 7 (22.6%) | 16 (48.5%) | 0.39 (0.16, 0.95) p = 0.039 |
Event type: “first event” analysis allows for subsequent improvement; “persistent” defined as a decline that stays below the threshold at all subsequent assessments until the end of the study. Hazard ratio (95% CI) calculated by Cox model.
Fig.1.
Kaplan-Meier analysis for the time to 10% relative decline in FVC% p (persistent event analysis).
Proportion of patients falling in FVC% p below clinically relevant thresholds
Twenty patients in the idebenone group of the DELOS trial presented with baseline FVC% p above 50%. From these, 4 patients (20%) fell below FVC% p of 50% during the study period. In the placebo group 16 patients presented with baseline FVC% p above 50% with 7 patients (44%) falling below 50% during the study (Table 6). Similarly, higher proportions of patients in the placebo group were also falling below the FVC% p of 40% and 30% thresholds respectively. Kaplan-Meier analysis for the time to first decline below FVC% p of 50% (in those with FVC% p above 50% at baseline) indicate that this clinically relevant pulmonary function threshold was reached later in patients in the idebenone group compared to the placebo group with a hazard ratio of 0.34 (95% CI: 0.10–1.18; p = 0.089) in favor of idebenone. The corresponding Kaplan-Meier plot for the time to decline below FVC% p of 50% (first event analysis) is shown in Supplementary Material Figure 2.
Table 6.
Proportion of patients in whom FVC% p fell below 50%, 40% or 30% at any time during the study by event type
| Patients falling | Event Type | Idebenone n (%) | Placebo n (%) | Hazard Ratio* (95% CI) |
| in FVC% p below | ||||
| 50% | first event | 4 of 20 (20%) | 7 of 16 (44%) | 0.34 (0.10, 1.18) |
| persistent | 3 of 20 (15%) | 4 of 16 (25%) | 0.48 (0.11, 2.16) | |
| 40% | first event | 4 of 24 (17%) | 4 of 20 (20%) | 0.58 (0.13, 2.60) |
| persistent | 4 of 24 (17%) | 4 of 20 (20%) | 0.61 (0.14, 2.74) | |
| 30% | first event | 4 of 29 (14%) | 7 of 28 (25%) | 0.59 (0.17, 2.10) |
| persistent | 3 of 29 (10%) | 6 of 28 (21%) | 0.51 (0.12, 2.15) | |
| any threshold** | first event | 10 of 29 (34%) | 16 of 28 (57%) | 0.51 (0.23, 1.14) |
| persistent | 8 of 29 (28%) | 12 of 28 (43%) | 0.60 (0.24, 1.49) |
Event type: “first event” analysis allows for subsequent improvement; “persistent” defined as a decline that stays below the threshold at all subsequent assessments until the end of the study. *Hazard Ratios from Cox model. **each patient is only counted once.
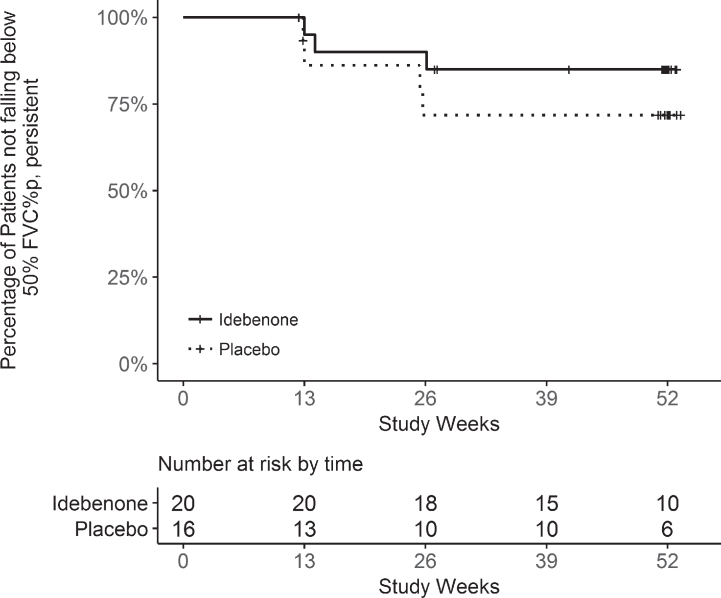
An analysis for patients with a decline below FVC% p of 50% which persisted until the end of the study, resulted in 3 of 20 patients (15%) in the idebenone group compared to 4 of 16 patients (25%) in the placebo group crossing this threshold with in a non-significant HR of 0.48 (95% CI: 0.11, 2.16). The corresponding Kaplan-Meier analysis is shown in Fig. 2. Similarly there were fewer patients in the idebenone group falling below the 40% or 30% thresholds for FVC% p (Table 6), of which the difference in patients falling below 30% is of particular clinical relevance as this threshold is associated with high risk of hypoventilation.
Fig.2.
Kaplan-Meier analysis for the time to decline below 50% of FVC% p from baseline to week 52 (persistent event analysis).
The analysis for patients falling below any of the FVC% p thresholds of 50%, 40% or 30% resulted in 34% of patients in the idebenone group compared to 57% in the placebo group, again indicating a benefit for the idebenone group with a HR of 0.51(95% CI: 0.23–1.14; p = 0.099). The corresponding Kaplan-Meier plot for the time to decline below any of the FVC% p thresholds (first event analysis) is shown in Supplementary Material Figure 3. Assessing for persistent decline below any of the FVC% p thresholds also resulted in a higher proportion of patients in the placebo group (43%) compared to the idebenone group (28%) with a non-significant HR of 0.60 (95% CI: 0.24, 1.49). The corresponding Kaplan-Meier analysis is shown in Fig. 3.
Fig.3.
Kaplan-Meier analysis for time to decline below any of the 50%, 40% or 30% thresholds for FVC% p from baseline to week 52 (persistent event analysis).
DISCUSSION
This work provides additional data from the prospectively planned, placebo-controlled Phase 3 DELOS trial which enrolled 10–18 year old DMD patients who were in pulmonary function decline stage and who did not use concomitant glucocorticoids [20]. Participating patients had progressed to an advanced disease stage as seen in the high proportion of study participants who were already non-ambulant at baseline and the high proportion of patients unable to raise their hands to their mouth [28].
Decline in forced vital capacity is currently the best predictor for tracking pulmonary decline in patients with DMD [6–8]. Motivated by standard of care guidelines which define clinically relevant thresholds for FVC% p this work assessed the risk of patients participating in the DELOS trial in crossing any of these thresholds in the course of the 1-year study period. Although not planned prospectively, as these guidelines were published after the start of the DELOS trial, these analyses appear relevant in the context of patient support and care. The DELOS trial was not formally powered for the analyses presented here but the consistency in the outcomes observed clearly indicates that idebenone holds the potential in altering the rate of decline of pulmonary disease in patients with DMD as defined by crossing clinically relevant thresholds for FVC.
Interpretation of these findings is limited to adolescent patients who were not using GCs during the trial (either had stopped taking GC at least 12 months prior to enrolment or had never taken GCs). Recent data from the CINRG Duchenne Natural History Study have shown that the beneficial effect of GCs on pulmonary function is partial and limited in time [12, 32]. These data showed that onset of pulmonary function loss is delayed by ∼2-3 years in patients receiving GCs compared to patients not on GC therapy. Current dataset showed that once pulmonary function decline is established, the year-to-year drop in mean FVC% p by year of age on average is similar in all patients (i.e. those using GCs vs. GC-naïve or previously GC-treated patients) down to 20 years of age where the FVC% p approaches a floor [9–11]. Importantly, despite the use of GC, pulmonary function decline continues linearly and unabated from the age of approximately 7 years through to the early twenties.
The difference between treatment groups for the 1-year change in FVC and FVC% p in favour of idebenone reported here is comparable to treatment effects seen in other pulmonary diseases. Specifically, the treatment effect for FVC of 122–136 mL (depending on analysis method) described in Table 2 compares favorably with treatment effects observed for approved medications for the treatment of idiopathic pulmonary fibrosis (IPF), for which treatment effects of 110–120 mL were reported [20, 27, 33]. The treatment effect in absolute FVC of the DELOS trial is particularly relevant as it is observed in young patients, whilst data for IPF are from adult patients. Likewise, the treatment effect in favor of idebenone for FVC% p in the DMD DELOS trial are also comparable for the effect sizes reported for IPF medications [20, 27, 33].
One analysis presented here assesses the effect of idebenone in reducing the risk of falling in FVC% p by 10% or more. For IPF it is well documented that an absolute persistent decline of FVC% p by 10% represents an established marker of disease progression and mortality. Specifically, it was shown that the risk of death was nearly 5-fold higher for patients with decline in FVC% p of ≥10% (e.g. [30, 31] and literature cited therein). In addition, the minimal clinically important difference (MCID) in FVC% p for IPF is an absolute change between 2% and 6% [30]. Moreover, in IPF the relevance of a decline in FVC% p by 10% calculated as relative margin has been considered [34] and authors conclude that assessment of the relative change in FVC% p of 10% is relevant as more events are assessed compared to the calculation of absolute change. Both assessment methods correlated well with mortality outcomes.
Although the underlying pathologies leading to life-threatening restrictive pulmonary disease are different between IPF and DMD, it is nevertheless of interest to calculate the proportion of patients from the DELOS trial with a decline in FVC% p as absolute and relative at any time point during the 52 week study. As shown, there were 36% of patients in the placebo group with a persistent absolute 10% -decline in FVC% p compared to only 16% in the idebenone group. These findings are supported by analyses of persistent relative decline in FVC% p by 10% or more. This is clinically meaningful as in DMD a decline in FVC% p by 10% is associated with increased risk of hypoventilation. Specifically, as shown by Sawnani and colleagues [35], for every 10% decline in FVC% p the odds of hypoventilation increase by 20% (p = 0.001). In agreement with these results, there was also a 2-fold higher proportion of patients declining by 10% in FVC (in mL) in the placebo group compared to the idebenone group.
In summary, the analyses presented here further add to the understanding of the clinical relevance of idebenone’s treatment effect in adolescent patients with DMD not using concomitant GCs. In combination with data previously published from the DELOS trial [20–22] the consistency for pulmonary function outcomes reported indicate that idebenone can slow the loss of pulmonary function in adolescent patients who have reached the pulmonary function decline stage. This is of particular relevance for patients already in the non-ambulatory stage who are unable to take GCs and for whom there is currently no alternative treatment option.
CONFLICTS OF INTEREST
GMB and OHM are investigators for clinical trials in DMD sponsored by Santhera Pharmaceuticals and act as scientific consultants for Santhera Pharmaceuticals. GMB was investigator for clinical trials in Duchenne muscular dystrophy sponsored by Prosensa, and GlaxoSmithKline. OHM has additional consulting relationships with Bristol-Myers Squib, Capricor Therapeutics, Pfizer, Catabasis Pharmaceuticals, Sarepta Pharmaceuticals, Fibrogen, Biogen, AveXis, and Roche. TM and ML are regular employees of Santhera Pharmaceuticals. CR acts as clinical data scientist for Santhera Pharmaceuticals. GMB and TM are co-inventors of relevant patent applications.
DELOS Study Group (sorted by country): Austria: G. Bernert, F. Knipp (Vienna). Belgium: G.M. Buyse, N. Goemans, M. Van den Hauwe (Leuven). France: T. Voit, V. Doppler, T. Gidaro (Paris); J.-M. Cuisset, S. Coopman (Lille). Germany: U. Schara, S. Lutz (Essen); J. Kirschner, S. Borell, M. Will (Freiburg). Italy: M.G. D’Angelo, E. Brighina, S. Gandossini (Lecco); K. Gorni, E. Falcier (Milan); L. Politano, P. D’Ambrosio, A. Taglia (Naples). The Netherlands: J.J.G.M. Verschuuren, C.S.M. Straathof (Leiden). Spain: J.J. Vílchez Padilla, N. Muelas Gómez (Valencia). Sweden: T. Sejersen, M. Hovmöller (Stockholm). Switzerland: P.-Y. Jeannet, C. Bloetzer (Lausanne). USA: S. Iannaccone, D. Castro (Dallas); G. Tennekoon, R. Finkel, C. Bönnemann (Philadelphia); C. McDonald, E. Henricson, N. Joyce (Sacramento); S. Apkon, R.C. Richardson (Seattle).
Supplementary Material
ACKNOWLEDGMENTS
The study was sponsored by Santhera Pharmaceuticals. The DELOS Study Group is indebted to the participating patients and their parents. The authors thank Sofia Männikkö (4Pharma, Finland) for support in statistical analyses. GMB is Senior Clinical Investigator of the Research Foundation Flanders (FWO Vlaanderen, Belgium).
SUPPLEMENTARY MATERIAL
The supplementary figures are available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170245.
REFERENCES
- [1]. Mah JK, Korngut L, Dykeman J, et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24:482–91. [DOI] [PubMed] [Google Scholar]
- [2]. McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–92. [DOI] [PubMed] [Google Scholar]
- [3]. Gozal D. Pulmonary manifestations of neuromuscular disease with special reference to Duchenne muscular dystrophy and spinal muscular atrophy. Pediatr Pulmonol. 2000;29:141–50. [DOI] [PubMed] [Google Scholar]
- [4]. Simonds AK. Respiratory complications of the muscular dystrophies. Semin Respir Crit Care Med. 2002;23:231–8. [DOI] [PubMed] [Google Scholar]
- [5]. Bourke SC. Respiratory involvement in neuromuscular disease. Clin Med (Lond). 2014;14:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Finder JD, Birnkrant D, Carl J, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170:456–65. [DOI] [PubMed] [Google Scholar]
- [7]. Finder J, et al. Pulmonary endpoints in duchenne muscular dystrophy: A workshop summary. American Journal of Respiratory and Critical Care Medicine (epub ahead of print). 2017. [DOI] [PubMed] [Google Scholar]
- [8]. Phillips MF, Quinlivan RC, Edwards RH, Calverley PM. Changes in spirometry over time as a prognostic marker in patients with Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2001;164:2191–4. [DOI] [PubMed] [Google Scholar]
- [9]. McDonald CM, et al. Longitudinal pulmonary function test outcome measures in Duchenne muscular dystrophy: Long-term natural history with ad without glucocorticoids. (submitted). 2017. [DOI] [PubMed] [Google Scholar]
- [10]. Mayer OH, Finkel RS, Rummey C, et al. Characterization of pulmonary function in Duchenne muscular dystrophy. Pediatr Pulmonol. 2015;50:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Mayer OH, Henricson EK, McDonald CM, Buyse GM. Advances in pulmonary care in duchenne muscular dystrophy. US Neurology. 2017;13:35–41. [Google Scholar]
- [12]. McDonald CM, Henricson EK, Abresch RT, et al. The cooperative international neuromuscular research group Duchenne natural history study- a longitudinal investigation in the era of glucocorticoid therapy: Design of protocol and the methods used. Muscle Nerve. 2013;48:32–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. LoMauro A, D’Angelo MG, Aliverti A. Assessment and management of respiratory function in patients with Duchenne muscular dystrophy: Current and emerging options. Therapeutics and Clinical Risk Management. 2015;11:1475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part Implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. [DOI] [PubMed] [Google Scholar]
- [15]. Landfeldt E, Peter Lindgrenc P, Bell CF, et al. Compliance to care guidelines for Duchenne Muscular Dystrophy. Journal of Neuromuscular Diseases. 2015;2:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Rodger S, Katherine L, Woods KL, et al. Adult care for Duchenne muscular dystrophy in the UK. J Neurol. 2015;262:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Birnkrant DJ, Panitch HB, Benditt JO, et al. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest. 2007;132:1977–86. [DOI] [PubMed] [Google Scholar]
- [18]. Humbertclaude V, Hamroun D, Bezzou K, et al. Motor and respiratory heterogeneity in Duchenne patients: Implication for clinical trials. Eur J Paediatr Neurol. 2012;16:149–60. [DOI] [PubMed] [Google Scholar]
- [19]. Birnkrant DJ, Bushby KM, Amin RS, et al. The respiratory management of patients with duchenne muscular dystrophy: A DMD care considerations working group specialty article. Pediatr Pulmonol. 2010;45:739–4. [DOI] [PubMed] [Google Scholar]
- [20]. Buyse GM, Voit T, Schara U, et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet. 2015;385:1748–57. [DOI] [PubMed] [Google Scholar]
- [21]. Buyse GM, Voit T, Schara U, et al. Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy. Pediatr Pulmonol. 2017;52(4):508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. McDonald CM, Meier T, Voit T, et al. Idebenone reduces respiratory complications in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:473–80. [DOI] [PubMed] [Google Scholar]
- [23]. American Thoracic Society/European Respiratory S. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- [24]. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- [25]. Gauld LM, Kappers J, et al. Prediction of childhood pulmonary function using ulna length. Am J Respir Crit Care Med. 2003;168:804–9. [DOI] [PubMed] [Google Scholar]
- [26]. Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Dev Med Child Neurol. 2004;46:475–80. [DOI] [PubMed] [Google Scholar]
- [27]. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. [DOI] [PubMed] [Google Scholar]
- [28]. Meier T, Rummey C, Leinonen M, et al. Characterization of pulmonary function in 10–18 year old patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2017;27:307–14. [DOI] [PubMed] [Google Scholar]
- [29]. Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: Patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–81. [DOI] [PubMed] [Google Scholar]
- [30]. du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1382–9. [DOI] [PubMed] [Google Scholar]
- [31]. Richeldi L. Assessing the treatment effect from multiple trials in idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Henricson EK, Abresch RT, Cnaan A, et al. The cooperative international neuromuscular research group Duchenne natural history study: Glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve. 2013;48:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Noble PW, Albera C, Bradford WZ. Pirfenidone in patients with idiopathic pulmonary fibrosis. (CAPACITY): Two randomised trials. Lancet. 2011;377:1760–69. [DOI] [PubMed] [Google Scholar]
- [34]. Richeldi L, Ryerson CJ, Lee JS, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67:407–11. [DOI] [PubMed] [Google Scholar]
- [35]. Sawnani H, Thampratankul L, Szczesniak RD, et al. Sleep disordered breathing in young boys with Duchenne muscular dystrophy. J Pediatr. 2015;166:640–645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.