Abstract
Maternal obesity induces chronic inflammatory responses that impact the fetus/neonate during the perinatal period. Inflammation, iron regulation, and myelination are closely interconnected and disruptions in these processes may have deleterious effects on neurodevelopment. Hepcidin levels are increased in response to inflammation causing subsequent decreases in ferroportin and available iron needed for myelination. Our current studies were designed to test the hypotheses that: 1) maternal high fat diet (HFD) prior to and during pregnancy is sufficient to induce inflammation and alter iron regulation in the brain of the offspring, and 2) HFD exposure is associated with altered myelination and neurobehavioral deficits in the offspring. Our data revealed modest increases in inflammatory cytokines in the serum of dams fed HFD prior to pregnancy compared to dams fed a control diet (CD). Early increases in IL-5 and decreases in IL-10 were observed in serum at PN7 while IL-5 remained elevated at PN21 in the HFDexposed pups. At PN0, most cytokine levels in whole brain homogenates were higher in the pups born to HFD-fed dams but were not different or were lower than in pups born to CD-fed dams at PN21. Conversely, the inflammation mediated transcription factor Nurr77 remained elevated at PN21. At birth, brain hepcidin, ferroportin, and l-ferritin levels were elevated in pups born to HFD-fed dams compared to pups born to CD-fed dams. Hepcidin levels remained elevated at PN7 and PN21 while ferroportin and l-ferritin levels were lower at PN7 and were not different at PN21. Decreases in myelination in the medial cortex were observed in male but not in female pups born to maternal HFD-fed dams at PN21. These structural changes correlated with changes in behavior (novel object recognition) in at 4 months in males only. Our data indicate that maternal obesity (HFD) results in disruption of iron regulation in the brains of the offspring with structural and neurobehavioral deficits in males.
Keywords: Maternal obesity, High fat diet, Inflammation, Hepcidin, Neurodevelopment
1. Introduction
Pre-pregnancy maternal obesity is a worldwide problem affecting approximately one third of women of childbearing age in the United States (Ogden et al., 2013). Maternal obesity confers significant maternal and fetal/neonatal health risks during the perinatal period including preeclampsia/gestational hypertension, gestational diabetes mellitus (Scott-Pillai et al., 2013), preterm delivery (Cnattingius et al., 2013; Khatibi et al., 2012), stillbirth (Salihu, 2011; Yao et al., 2014), and delivery complications such as need for cesarean section or shoulder dystocia (Magann et al., 2013; Ruager-Martin et al., 2010; Scott-Pillai et al., 2013; Stothard et al., 2009; Turcksin et al., 2014). Infants born to obese mothers are also at an increased risk for long-term health problems including obesity and metabolic syndrome (Boney et al., 2005). More recent research indicates that children born to obese mothers are also at an increased risk for impaired neurodevelopment and learning disabilities (Hinkle et al., 2013; Reynolds et al., 2014), although the biological mechanisms involved are not well understood.
Obesity is a state of chronic inflammation and offspring of obese mothers are exposed to inflammatory mediators throughout gestation and lactation. Animal studies suggest that the inflammation induced in the offspring in response to maternal obesity is due to either high fat diet or the obesity itself. In rodents, increases in specific cytokines such as IL-1b or IL-6 in the brain are correlated to impaired cognition and memory suggesting that obesity-related inflammation directly influences neurological function (Bilbo and Tsang, 2010; Pistell et al., 2010; White et al., 2009; Yan et al., 2011). Diet-induced obesity (DIO) rodent models have thus far provided insights into the potential mechanisms underlying these associations including decreased DNA methylation in the brain (Carlin et al., 2013), alterations in neural networks that regulate food intake (Stachowiak et al., 2013), and changes in hippocampal development in the offspring (Niculescu and Lupu, 2009) but these pathways have not been extensively defined.
Inflammation, iron regulation, and myelination are closely interconnected and disruption in these processes may have deleterious effects on neurodevelopment (Lieblein-Boff et al., 2013; Urrutia et al., 2013; Wang et al., 2008). Importantly, hepcidin is a critical hormone that regulates iron homeostasis by negatively controlling ferroportin expression (Oates, 2007). Hepcidin levels typically decrease during normal pregnancy, likely due to a pregnancy-related iron deficiency (van Santen et al., 2013). Hepcidin signaling is increased in the setting of inflammation (Nemeth et al., 2004; Urrutia et al., 2013; Wang et al., 2008) and leptin levels (Chung et al., 2007) are also increased in obese pregnant women (Dao et al., 2013). Since hepcidin is primarily stored in oligodendrocytes and iron is an essential component for myelination, changes in hepcidin levels may negatively impact neurodevelopment by reducing myelination (Lieblein-Boff et al., 2013; Oates, 2007; Rossi, 2005). Urrutia et al. reported a change in iron content and a disruption in iron regulation in response to acute inflammation which resulted in iron accumulation in neurons and microglia (Urrutia et al., 2013). They also reported that disruption of iron homeostasis was due to changes in hepcidin and ferroportin expression. Wang et al. identified increases in hepcidin in the cortex and substantia nigra due to LPS administration (Wang et al., 2008). Leiblein-Boff et al. established relationships between inflammation, iron homeostasis and neurological behaviors in a development model induced by bacterial infection (Lieblein-Boff et al., 2013). These studies found that inflammation decreased iron availability, increased locomotor activity, decreased motor coordination and resulted in hypomyelination and decreased oligodendrocyte number in the white matter and motor cortex of the mice in adulthood. Collectively, these data strongly support a mechanistic link between obesity, inflammation, iron homeostasis, hypomyelination, and neurological function. Thus, the present studies tested the hypothesis that maternal obesity associated inflammation would result in inflammation in the offspring which would, in turn, be associated with altered iron availability, myelination, and neurocognitive behavior.
2. Materials and methods
2.1. Animals
All animal studies were carried out in accordance with a protocol approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children’s Hospital (Columbus, OH). Three-week-old female C57Bl/6J mice were purchased from Jackson Labs. Mice were housed in the facility for one week to allow habituation. At four weeks of age, mice were randomly assigned to either the high fat (Harlan Teklad TD.06414, 60% calories from fat, Madison, WI) or control diet (Harlan Teklad TD.08806, 10% calories from fat, Madison, WI). The mice were weighed weekly. Diets were stored at 4 °C and diet in cages was replaced weekly to prevent degradation. At the start of the ninth week on experimental diet, the female mice were paired for breeding continuously for two weeks. A subset of female mice was sacrificed prior to breeding for blood and tissue collection prior to pregnancy. Male mice were maintained on standard chow except for the two week breeding periods when they were exposed to the respective experimental diet. Visibly pregnant mice were individually housed prior to giving birth. Pups were weighed and euthanized at postnatal days (PN) 0, 7, and 21. A subset of male and female pups were weaned onto standard chow (Teklad 2020X) at day 21 and underwent behavioral testing at 12 weeks of age then returned to the home cage and were euthanized at 4 months (16 weeks) of age for tissue collection.
Mice were anesthetized with intraperitoneal injections of ketamine/xylazine (150 mg/kg: 15 mg/kg, respectively) for tissue collection. Cardiac puncture was performed after anesthesia for blood collection. Brains were either collected and frozen in liquid nitrogen immediately or prepared for fixation. For brains collected on PN7, the intact skulls were submerged in 4% paraformaldehyde (PFA) for 24 h after which the skulls were removed and the brains were returned to 4% PFA for an additional 24 h of fixation. Brains were washed and stored in phosphate buffered saline (PBS) and embedded in paraffin. For brains collected at PN21 or later, whole body perfusions were performed with cold saline followed by 4% PFA. Brains were quickly removed and placed in 4% PFA for 24 h prior to washing with PBS and embedding in paraffin. Coronal sections were cut serially at 4 μm from paraffin blocks and collected on positively charged slides.
2.2. Real time quantitative polymerase chain reaction
RNA was isolated from brain tissues using TRIzol (Invitrogen) and RNeasy kit (Qiagen) following the manufacturer’s protocols. RNA (2 μg) was reverse transcribed per instructions using Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). PCR with Maxima SYBR green master mix (ThermoScientific) was performed on an Eppendorf Realplex Master Cycler using custom DNA primers (Integrated DNA Technologies). Primer sequences are provided in Table 1.
Table 1.
Primer sequences for RT-PCR.
| Primer | Forward Sequence (5′ → 3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Il-1beta | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| TNFa | AGAAGTTCCCAAATGGCCTC | TTGTCTTTGAGATCCATGC |
| TGFb | CCTGTCCAAACTAAGGCTCG | TGTTGTACAAAGCGAGCACC |
| Nur77 | CACTGGCGTTTGACATGGAAG | TGTGGGTGTGAACCATCTTTC |
| Nurr1 | GTGTTCAGGCGCAGTATGG | TGTATTCTCCCGAAGAGTGGTAA |
| Hepcidin | GCAGAAGAGAAGGAAGAGAGACACC | TGTAGAGAGGTCAGGATGTGGCTC |
| Ferroportin | ACCAAGGCAAGAGATCAAACC | AGACACTGCAAAGTGCCACAT |
| l-Ferritin | AGGGCGTAGGCCACTTCTT | CTGGGTTTTACCCCATTCATCTT |
2.3. ELISA
Dams were fasted for four hours prior to serum collection for leptin/insulin testing. After lethal anesthesia, blood was collected through cardiac puncture. Serum was separated and analyzed using the Meso Scale Discovery mouse metabolic kit (K15124C-1) and the proinflammatory panel kit V-PLEX™ (K15048D-1).
2.4. Western Blot
Western blots analyses were performed as previously described (Graf et al., 2014). Whole brain homogenates were separated by electrophoresis on 4–12% bis-tris gels and transferred to nitrocellulose membranes. Membranes were probed with antibodies to proteolipid protein (1:500, Abcam, ab28486). Membranes were then probed with a HRP-conjugated goat anti-rabbit secondary (1:12,000; Biorad, 170-6515) and developed using enhanced chemiluminescence (GE Healthcare). Relative densitometry was determined using Image Quant Software (Molecular Dynamics).
2.5. Immunostaining
Tissue sections were obtained were from the cerebral cortex at the level of the internal capsule and thalamus. Slides were deparaffinized at 60 °C for 40 min and then submerged in heated citrate buffer for antigen retrieval prior to immunostaining. Immunofluorescent staining was performed on PN7 tissue sections using an anti-Olig2 primary antibody (1:750; Millipore, AB9610) and a donkey anti-mouse fluorescent secondary (1–500; Invitrogen, A- 21206). Slides from tissues obtained at PN21 or 4 mon were evaluated by immunohistochemistry using anti-Myelin Basic Protein (MBP) primary antibody (1:200, Abcam, ab62631), detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody (1–600; Biorad, 170-6516) and visualized with NovaRed (Vector). The lateral cortex is defined as the area lateral to the caudoputamen in the perirhinal or ectorhinal cortical areas. The medial cortex is defined as the cortical region superior to the cingulum. Quantification was performed from 3 to 4 individual mice in two distinct regions conducted by two individuals who were blinded to the experimental conditions. Data was then averaged from the blinded individuals and statistically analyzed.
2.6. Behavioral testing
At 12 weeks of age, mice underwent Y maze and novel object recognition assessments. The mice were handled daily for one week prior to experiments in order to reduce stress during testing. Working memory was assessed with Y-maze testing as previously described (Mandillo et al., 2008). Testing was conducted in an opaque plastic Y-maze (starting arm 8″ × 1.75″, other arms 6″ × 1.75″). Mice were then placed at the end of the starting arm facing away from the center of maze and allowed to freely explore the maze for 5 min. Arm entry was only recorded if all four of the mouse’s limbs entered the arm. The order of arms entered, total number of arm entries, and latency to leave the first arm were recorded. Percent spontaneous alteration was calculated by dividing the number of actual alterations by the number of possible alterations (total arm entries/2).
Recognition memory was assessed through novel object recognition testing as described (Bevins and Besheer, 2006) in a rectangular Plexiglas chamber (16″ × 9″ × 7″). For three days prior to testing, each mouse was placed in the empty testing chamber for three minutes to allow habituation. On the fourth day, the mouse was brought to the room 30 min prior to testing. In the familiarization phase, the animal was placed in the arena equidistant from two identical objects and allowed to freely explore for 3 min, then returned to the home cage for 30 min. The mouse was then placed in the arena with a familiar object and a novel object and allowed to freely explore for 3 min with the time spent exploring each object recorded.
All observations were recorded by two experimenters blinded to the conditions and then data was averaged and statistically analyzed.
2.7. Statistical analysis
For each analysis, only 2 pups per given litter (1 male and 1 female) were included to eliminate dam/litter effects. Data are presented as mean ± SEM and were analyzed by Student’s t test (Fig. 1), 2-way ANOVA (for effects within a specific time), and Multivariate analysis using a General Linear Model for effects including diet, sex, and time followed by Tukey’s post-hoc analyses. Statistics were performed using GraphPad Prism or SPSS software (IBM version 21).
Fig. 1.
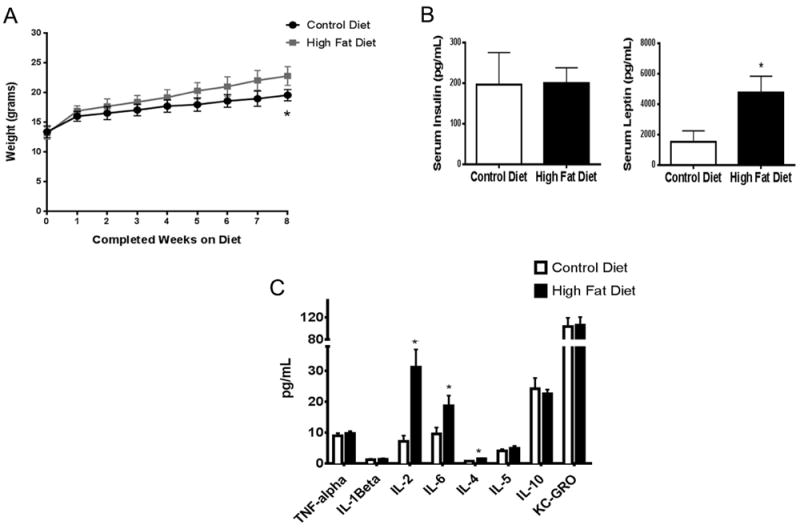
Effects of maternal HFD prior to pregnancy. Female mice were placed on HFD for 8 weeks. Total body weight (A) was assessed, and serum insulin, leptin levels (B), and cytokines levels (C) were measured using MSD multiplexed plates (see Section 2). Weight data was analyzed by Linear regression (p < 0.0001) and insulin, leptin, and cytokine data were analyzed by t-test, p < 0.05, n = 8–9. “*”denotes statistical significance.
3. Results
3.1. Weight gain, leptin levels, and inflammatory cytokines in prepregnant CD or HFD fed mice
Initial studies determined that the earliest significant difference in body weight between HFD and CD fed females was detected after eight weeks on experimental diets (Fig. 1A) (Linear regression, p < 0.0001, df = 218, F = 117.3). Based upon these data, females were maintained on their assigned diet for 8 weeks prior to mating. Serum analyses at 8 weeks revealed that HFD was not associated with changes in serum insulin levels (Fig. 1B) but was associated with increased serum leptin levels compared to CDfed females (Fig. 1B) (t-test, p = 0.034).
To assess the pre-pregnancy impact of HFD exposure on global inflammation, a multiplex assay for inflammatory cytokines/chemokines was performed on serum collected from females after 8 weeks of diet exposure. As shown in Fig. 1C, significant increases in IL-2, IL-4, and IL-6 levels were observed in HFD-fed females compared to CD females (t-test, p = 0.008, p = 0.013, p = 0.033, respectively) indicating that HFD elicited a pro-inflammatory state.
3.2. Weight gain and serum cytokine levels in offspring of dams fed HFD
Birth weights were not significantly different between pups born to HFD or CD fed dams. Univariate Analysis of Variance indicated an effect of diet (0.000), sex (0.038), and time (0.000) with interactions between sex and time (0.010), and diet and time (0.000) (df = 15, F = 153.012, adjusted R squared = 0.912) (Fig. 2A). Using Tukey’s post hoc analysis, by PN7 our data indicated a significantly greater increase in body weight in both male and female offspring of HFD-fed mothers compared to their CDfed counterparts. Similar increases in body weights in the offspring from HFD-fed mothers were also detected at PN21 but no differences were observed at 4 months after returning to control diet at weaning.
Fig. 2.
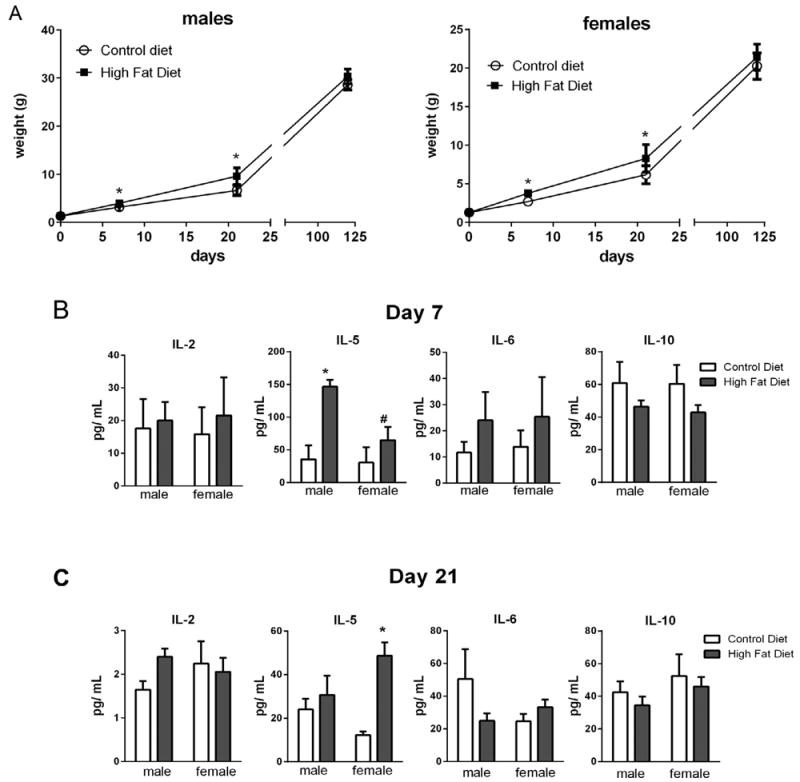
Effects of maternal HFD on pup weights and serum cytokine levels. Pups born to dams fed either CD or HFD were weighed at birth, PN day 7, and PN day 21 (A). Serum was collected at PN day 7 (B) and PN day 21 (C) and cytokines levels were measured using MSD multiplexed plates (see Section 2). Weight data (A) were analyzed by Univariate Analysis of Variance and indicated an effect of diet (0.000), sex (0.038), and time (0.000) with interactions between sex and time (0.010), and diet and time, n = 9– 21. Cytokine data were analyzed by multivariate regression using diet, sex, and time as variables; effects of diet (0.008) and time (0.000) were indicated, n = 4–5. Between subject effects were diet for IL-10 (0.040) and IL-5 (0.007) and time for IL-2 (0.000) and IL5 (0.009). Within group differences indicated by Tukey’s post hoc analyses were denoted as follows “*”statistically different than CD same sex; “#”statistically different than same diet different sex, p < 0.05.
To determine if weight gain correlated with changes in inflammatory cytokine levels, serum cytokine levels were measured at PN7 and PN21. Cytokine data were analyzed by Multivariate regression and effects of diet (0.008), time (0.000) were indicated (df = 7; F = 211.618). Between subject effects indicated an effect of diet for IL-10 (0.040) and IL-5 (0.007), an effect of time for IL-2 (0.000) and IL5 (0.009) (adjusted r squared = 0.151 (IL-10), 0.661 (IL-2), 0.402 (IL-5), and 0.040 (IL-6)). Tukey’s post hoc analyses indicated differences between individual points as indicated in Fig. 2B. No significant differences were observed in IL-6 levels at either time point. In contrast, IL-2 levels were substantially lower at PN21 than at PN7 in both HFD and CD groups. There was an effect of diet on IL-10 concentrations but no effect of time or sex. Interestingly, IL-5 levels were at least 2-fold higher in the HFD-exposed pups compared to CD-exposed pups at PN7 and IL-5 levels were also elevated in females at PN21 (Fig. 2B).
3.3. Neuroinflammation in the offspring
To assess neuroinflammation in the brains of offspring, mRNA was isolated from whole brain tissues at PN0, PN7, or PN21 and IL-1β, TGFβ, Nurr77, Nurr1, and TNFα levels were determined using qRT-PCR. Overall, exposure to HFD was associated with significant changes in the expression of IL-1β, TGFβ, Nurr77, Nurr1, or TNFα at most time points. Cytokine data were analyzed by Multivariate regression and effects of diet (0.045) and time (0.000) were indicated (df = 5; F = 12.283). Between subject effects indicated an effect of sex (0.044) and time (0.000) for IL-1β; an effect of diet (0.017) and time (0.011) for Nurr77; and an effect of time for Nurr1 (0.022) (adjusted r squared = 0.454 (IL-1β), 0.010 (TGFβ), 0.347 (Nurr77), 0.195 (Nurr1), and 0.061 for (TNFα)). No interactions were indicated. Tukey’s post hoc analyses indicated differences between individual points as denoted in Table 2. Greater levels of IL1β, TNFα, and Nurr1 were detected in the brains of pups exposed to maternal HFD at PN0 (Table 2). More striking was the increased expression of Nurr77 in the male but not in female pups born to HFD-fed dams. Nurr77 levels were 2.5 times greater in the male pups from HFD-fed dams at PN7 and were almost 4 times greater at PN21 than in male pups born to CD-fed dams. Nurr77 was increased in the brains of female pups born to HFD-fed dams at PN21 only. Overall, our data indicated significant effects of HFD on brain cytokine levels throughout the lactation period.
Table 2.
Cytokine mRNA expression levels in whole brain homogenates.
| Cyotkine | Day of life | CD
|
HFD
|
||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| IL-1β | 0 | 1.01 ± 0.10 | 1.14 ± 0.27 | 1.77 ± 0.21 | 1.85 ± 0.15 |
| 7 | 1.05 ± 0.13 | 1.02 ± 0.11 | 1.33 ± 0.21 | 0.73 ± 0.06# | |
| 21 | 1.05 ± 0.21 | 1.02 ± 0.15 | 0.37 ± 0.11* | 0.73 ± 0.02 | |
| TNFα | 0 | 1.01 ± 0.09 | 1.13 ± 0.33 | 1.93 ± 0.33 | 2.39 ± 0.33* |
| 7 | 1.04 ± 0.16 | 1.05 ± 0.25 | 0.54 ± 0.12 | 0.58 ± 0.07 | |
| 21 | 1.02 ± 0.15 | 0.55 ± 0.15 | 0.89 ± 0.12 | 1.10 ± 0.03 | |
| TGFβ | 0 | 1.07 ± 0.22 | 1.07 ± 0.20 | 0.91 ± 0.08 | 1.01 ± 0.08 |
| 7 | 1.03 ± 0.11 | 1.02 ± 0.09 | 1.65 ± 0.30 | 1.77 ± 0.26 | |
| 21 | 1.05 ± 0.21 | 1.00 ± 0.06 | 1.46 ± 0.35 | 1.18 ± 0.05 | |
| Nurr77 | 0 | 1.18 ± 0.37 | 1.01 ± 0.11 | 1.44 ± 0.13 | 1.16 ± 0.17 |
| 7 | 1.06 ± 0.15 | 1.07 ± 0.18 | 2.67 ± 0.45*# | 1.02 ± 0.19 | |
| 21 | 1.02 ± 0.14 | 1.03 ± 0.16 | 3.91 ± 0.16* | 2.70 ± 0.13*# | |
| Nurr1 | 0 | 1.00 ± 0.04 | 1.08 ± 0.15 | 1.64 ± 0.20* | 1.22 ± 0.16 |
| 7 | 1.04 ± 0.12 | 1.03 ± 0.13 | 0.89 ± 0.11 | 0.91 ± 0.20 | |
| 21 | 1.01 ± 0.10 | 1.02 ± 0.13 | 0.95 ± 0.03 | 0.81 ± 0.05 | |
Cytokines were measured in whole brain homogenates by RT-PCR. Data were analyzed by Multivariate regression and effects of diet (0.045) and time (0.000) were indicated. Between subject effects indicated an effect of sex (0.044) and time (0.000) for IL-1β; an effect of diet (0.017) and time (0.011) for Nurr77; and an effect of time for Nurr1 (0.022). Tukey’s post hoc analyses are indicated, p < 0.05, n = 3–4
denotes statistically different than CD same gender;
denotes statistically different than same diet different gender.
3.4. Alterations in iron metabolism-related gene expression in offspring in the first week of life
Expression of the iron regulatory proteins hepcidin, ferroportin, and l-ferritin were measured by qRT-PCR in whole brain homogenates from pups at PN0, PN7, or PN21 (Table 3). Iron protein data were analyzed by Multivariate regression and no overall effects were indicated (df = 3; F = 9.753). Between subject analyses indicated an effect of diet (0.018) for hepcidin; and time (0.0049) for l-ferritin; (adjusted r squared = 0.106 (hepcidin), 0.044 (ferroprotin), and 0.020 (l-ferritin)). No interactions were indicated. Individual two-way ANOVAs were performed to analyze differences between diets and sex within each time point with Tukey’s post hoc analyses and are designated on Table 3. At day 0, higher RNA transcript levels of all three proteins were detected in the pups from HFD-fed dams. Increases in hepcidin mRNA expression in the HFD-exposed pups persisted through PN7 but were not different than in pups born to CD-fed dams at PN21. Alternatively, expression of ferroportin and l-ferritin in pups born to HFD-fed dams were decreased at PN7 relative to CD pups and no differences were detected at PN21 (Table 3). Two-way ANOVA indicated an effect of HFD exposure on hepcidin, ferroportin, and l-ferritin mRNA expression when compared to CD at both PN0 and PN7 independently. While we observed differences in the RNA levels of these iron regulating proteins, we did not observed overt increases in free iron in brain tissue sections stained with Prussian blue stain (data not shown).
Table 3.
Iron metabolizing protein mRNA levels in whole brain homogenates.
| Day of life | CD
|
HFD
|
Two-Way ANOVA | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Hepcidin | 0 | 1.02 ± 0.09 | 1.11 ± 0.21 | 1.56 ± 0.31 | 1.92 ± 0.43 | Effect of diet |
| 7 | 1.03 ± 0.11 | 1.08 ± 0.19 | 1.46 ± 0.25 | 1.27 ± 0.21 | Effect of diet | |
| 21 | 1.04 ± 0.20 | 1.02 ± 0.16 | 1.17 ± 0.07 | 0.93 ± 0.09 | ||
| Ferroportin | 0 | 1.01 ± 0.06 | 1.07 ± 0.15 | 1.30 ± 0.13 | 1.43 ± 0.16 | Effect of diet |
| 7 | 1.01 ± 0.05 | 1.01 ± 0.05 | 0.53 ± 0.04* | 0.75 ± 0.12 | Effect of diet | |
| 21 | 1.02 ± 0.15 | 1.05 ± 0.22 | 1.08 ± 0.09 | 1.16 ± 0.14 | ||
| l-ferritin | 0 | 1.02 ± 0.08 | 1.00 ± 0.04 | 1.49 ± 1.23 | 1.23 ± 0.11 | Effect of diet |
| 7 | 1.00 ± 0.04 | 1.02 ± 0.08 | 0.54 ± 0.06* | 0.68 ± 0.14 | Effect of diet | |
| 21 | 1.02 ± 0.15 | 1.00 ± 0.04 | 0.96 ± 0.06 | 1.11 ± 0.03 | ||
Iron regulatory proteins were measured in whole brain homogenates by RT-PCR. Data were analyzed by Multivariate regression using diet, gender, and time as co-variates and no overall effects were indicated. Data within day of life was analyzed by two-way ANOVA and Tukey’s post hoc, p < 0.05, n = 3–4. Multivariate analysis indicated significant differences in hepcidin. Effects across sex and diets within a time are noted on the table.
denotes statistically different than CD same gender;
denotes statistically different than same diet different gender.
3.5. Myelination is not substantially disrupted after maternal HFD exposure
Oligodendrocyte precursors were assessed using Oligo-2 immunofluorescence in tissue sections at PN7 (Fig. 3A, medial cortex). The area of Oligo-2 positivity (green fluorescence) was measured in the medial and lateral cortex regions (see Section 2 for details) relative to the total brain area per high power field (HPF) (Fig. 3B). The data were analyzed by two-way ANOVA with no significant differences indicated (lateral cortex, effect of gender p = 0.077, effect of diet p = 0.074; medial cortex, effect of gender = 0.059). Using multiple t-tests, the relative Oligo-2 fluorescence was lower in the lateral cortex in the HDF males compared to CD males (p = 0.04, df = 10).
Fig. 3.
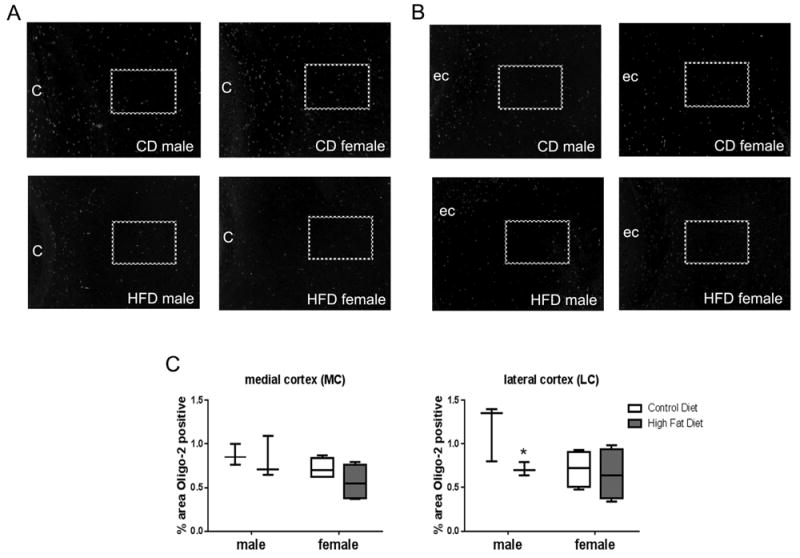
Brain tissue sections from PN 7 pups were stained for oligo-2 protein. Fixed brain sections were incubated with oligo-2 antibody followed by a fluorescent secondary antibody (AlexaFluor 488 nm). Medial cortex (A) and lateral cortex (B) fluorescence were quantified as percent positive staining to total area using threshold settings in Image J software. “C” denotes cingulum; “ec” denotes external capsule; and boxes denote areas quantified. Plot box indicates 25–75 percentile, line indicates median, and whiskers are minimum and maximum values. Data were analyzed by two-way ANOVA, no statistical effects were observed. Multiple t-test indicated a difference between CD and HFD in males in the lateral cortex, “*”statistically different than CD same sex, n = 3–4.
As an assessment for general myelination, tissues from PN21 mice were examined by Western blot for proteolipid protein (PLP) levels. No differences were observed between pups fed by dams eating CD verses HFD (Fig. 4A). Since deficits in myelination could occur in specific areas of the brain, we assessed myelination at PN21 and 4 months of life using immunohistochemistry for myelin basic protein. The area of MBP positivity (dark brown) was measured relative to the brain area per HPF (Fig. 4B and D). MBP levels were analyzed by Multivariate regression and an effect of diet (0.017) and interaction between sex and diet were indicated (0.022) (df = 7; F = 10.193). Between subject effects indicated an effect of sex (0.022) in the medial cortex; diet in the medial cortex (0.006) and an interaction between sex and diet (0.006) (adjusted r squared = 0.045 (lateral cortex), 0.340 (medial cortex). Individual differences were analyzed with Tukey’s post hoc test and are indicated in Fig. 4. MPB levels were lower in the medial cortex of male pups born to HFD-fed dams at PN21 (Fig. 4C). Although there was a trend toward lower MBP levels in the male pups from HFD-fed dams at 4 months, this did not reach statistical significance by the methods utilized for analyses (Fig. 4E). Post hoc analyses revealed significantly lower levels of MBP staining in the medial cortex in males at PN21 (Fig. 4B and C). At 4 months, an interaction between sex and diet were indicated in the lateral cortex (Fig. 4D and E).
Fig. 4.
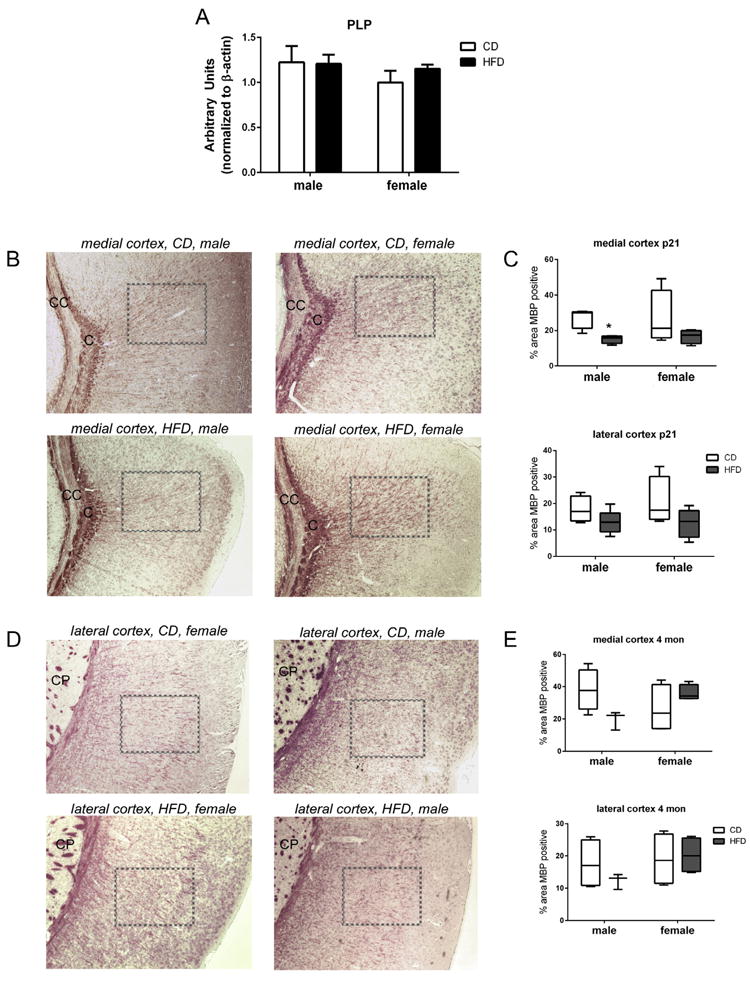
Assessments of myelin in brain tissues. (A) Western blot analysis was performed on whole brain homogenates at PN21. Digital densitometry analysis indicated no differences in PLP levels between diets or sex at this time point. Brain tissue sections from PN day 21 (B, medial cortex and D, lateral cortex) and 4 month pups were stained for MBP. “C” denotes the cingulum; “CC” denotes the corpus callosum; “CP” denotes the caudaputamen; boxes denote the area quantified. Fixed brain sections were incubated with MBP antibody and visualized with DAB. MBP was quantified as percent total positive staining using threshold settings in Image J software. Plot box indicated 25–75 percentile, line indicated median, and whiskers are minimum and maximum values. Data were analyzed by multivariate regression using diet, sex, and time as variables; an effect of diet (0.017) and interaction between sex and diet were indicated (0.022). Between subject effects indicated sex (0.022) in the medial cortex; diet in the medial cortex (0.006) and an interaction between sex and diet (0.006). Within group differences indicated by Tukey’s post hoc analyses were denoted as follows: “*”denotes statistically different than CD same sex, p < 0.05, n = 3–4.
3.6. Male HFD-exposed offspring display behavioral changes in novel object recognition testing
Weekly observation of pups from birth to 4 months did not indicate any obvious behavioral abnormalities. Of note, pups born to HFD mothers were excessively groomed by the dams starting at approximately 14 days of age, leading to some hair loss on both male and female pups. Once pups were weaned, their hair grew back in a normal pattern and no other differences in behavior were noted.
Behavioral testing was completed over a 2 day period at 4 months of age. Novel object assessments were performed including time exploring in the chamber and time exploring the novel object. Using two-way ANOVA, an effect of sex (p = 0.047) was indicated in exploring the novel object. Multiple t-tests indicated a significant decrease in the percentage of the total time spent exploring the novel object in male offspring of HFD-exposed dams when compared to male offspring of CD-exposed dams (p = 0.030, df = 19) (Fig. 5A). These differences were not detected in female offspring. Y-maze assessments included the numbers of times the arms entered the maze and the percent spontaneous alternations. No differences between groups were observed with either parameter (Fig. 5B).
Fig. 5.
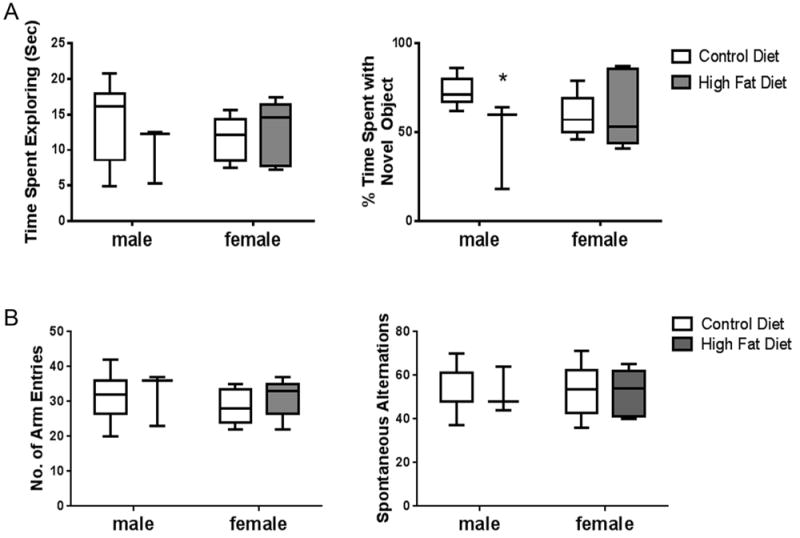
Novel object recognition and Y maze testing. Time spent exploring a novel object and percent of total time spent exploring a novel object were assessed as described in Section 2 (A). Number of arm entries and spontaneous alternations were measured for Y maze as described in Section 2 (B). Plot box indicates 25–75 percentile, line indicates median, and whiskers are minimum and maximum values. Data were analyzed by two-way ANOVA and no statistical differences were observed but multiple t-test indicated a difference between CD and HFD in males in % time spent with novel object, “*”statistically different than CD same sex, p < 0.05, n = 8–9.
4. Discussion
Obesity causes systemic inflammation that affects all organ systems including the brain (Pistell et al., 2010). This is also true in the pregnant female in which the offsprings are influenced by maternal chronic inflammation (Bilbo and Tsang, 2010). Consistent with other reports, we detected increases in body weight, increases in leptin, but no differences in insulin levels or evidence of insulin resistance in the dams. (Guo and Jen, 1995; Kang et al., 2014; Srinivasan et al., 2006a,b) (Fig. 1A and B). Increases in serum cytokine levels were observed in the dams fed HFD prior to pregnancy. The elevations in IL-6 are reported in both humans and animal models of obesity but the elevations in IL-2 and IL-4 were unexpected and may provide clues to obesity associated disruptions in T-cell-mediated immunity (Morin et al., 2016) (Fig. 1C).
At the time of birth, there were no differences in body weights between CD and HFD in the pups but at PN7 and PN21 the pups being nursed by dams fed HFD weighed more than pups nursed by dams fed CD (Fig. 2A). Serum cytokine analyses from these pups indicated that IL-5 and IL-10 levels were inversely affected by maternal diet with IL-5 levels elevated and IL-10 levels suppressed by HFD (Fig. 2B). While these changes in cytokine levels suggest that pups are experiencing a modest inflammatory response, it is unclear if these differences are driven by increases in pup weight (obesity in the pup) or are an effect of maternal systemic inflammation.
To assess the effects of maternal HFD on neuroinflammation in the pups, cytokine levels were measured in whole brain homogenates (Table 2). We detected greater levels of IL-1β, TNFα, and Nurr1 in HFD-exposed pups at birth but these elevations were no longer detected after PN7. These findings suggest that the maternal HFD induces an acute neuroinflammatory response in utero that resolves postnatally. Later effects were observed in expression levels of TGFβ and Nurr77 which suggests activation of alternative pathways in the offspring may occur with maturation. Nurr77 is a steroid/thyroid hormone receptor and is classified as an orphan nuclear receptor with no known ligands (Davis and Lau, 1994). Specific subcellular localization of Nurr77 directs alternative functions; nuclear Nurr77 is a transcription factor that enhances transcription of oncogenic genes while mitochondrial Nurr77 induces apoptosis by inhibiting BCL-2 function (Balasubramanian et al., 2012; Lin et al., 2004). In the brain, Nurr77 can be induced by nicotine exposure (Belluardo et al., 2005) or by hypoxia (Balasubramanian et al., 2012) suggesting that it may play a role in the deleterious effects associated with inflammatory responses.
Iron is tightly regulated in all organs but even more so in the brain. Dysregulation can cause oxidative stress and cell injury (Cnattingius et al., 2013). Iron is an essential component in the progression of myelination in developing neurons (Dao et al., 2013). Hepcidin is responsible for iron regulation (Oates, 2007), specifically during neurodevelopment. Hepcidin dysregulation is associated with inflammation and oxidative stress (Lieblein-Boff et al., 2013; Nemeth et al., 2004; Urrutia et al., 2013). In our studies, early markers of acute neuroinflammation, specifically at PN0, were associated with increases in iron regulatory proteins (Table 3). Levels of hepcidin, ferroportin, and l-ferritin were all increased at birth in pups born to HFD-fed dams. These data are consistent with the report of Wang et al. which demonstrated that LPS induces hepcidin and disrupts iron regulation in the brain (Wang et al., 2008). Increased levels of hepcidin persisted through PN7 with corresponding decreases in ferroportin and l-ferritin. By PN21, near the end of myelination, levels of these iron regulatory proteins were similar to the pups that were born to and nursed by CD fed dams. There are multiple ways in which to interpret our data. First, the inflammation and dysregulation of iron identified in the pups could be a consequence of maternal obesity. This interpretation would mean that maternal influence is greatest in utero and at birth then reduced at PN7 when maternal influence is limited to nursing, and virtually absent by PN21 when pups are weaned. Alternatively, birth through 21 days is a time of active brain growth, extension of neuronal processes, migrating oligodendrocytes, and myelination. Potentially, the dysregulation of iron correlates with a time during which normal physiological demands are greatest; during early brain development. This would imply that dysregulation becomes less obvious as the brain matures. Regardless of the cause for disruption in iron homeostasis, we speculate that observed alterations in iron metabolism are likely to negatively impact brain development.
As an outcome measure for disrupted iron metabolism, we investigated myelination in vivo. Oligo-2 immunostaining was performed as an indicator of oligodendrocyte survival. At PN7, we observed lower levels in males in the lateral cortex (Fig. 3). To assess myelin development and integrity at a later stage of development, we measured PLP and MBP levels. Myelin levels, as assessed by western blot using PLP concentrations in whole brain homogenates, were not different (Fig. 4A). Histological measurements of MBP staining demonstrated significantly less total staining in the medial cortex at PN21 and at 4 months in only the male offspring born to maternal HFD-fed dams (Fig. 4). The significance of the relative differences between medial and lateral cortex MBP measurement at PN7 and PN21 are not known but may relate to timing of maturation in these specific areas. Interestingly, trends toward decreased myelination in both the medial and lateral cortex in males are seen at 4 months suggesting that these differences may persist in a more subtle manner. As we have shown, this model of maternal HFD does not produce acute inflammation or overt responses and thus the observed changes in the offspring have been subtle. We speculate however, that these subtle changes could have long term detrimental effects. The histological differences observed in myelination correlated with a decrease in the percent total time spent on a novel object. We interpret these data to represent a difference in attention and cognitive function in male offspring (Fig. 5). A weakness to this interpretation is that anxiety, linked to obesity and subsequent behaviors could be responsible for the differences in behavior assessed in the pups (Peleg-Raibstein et al., 2012; Sasaki et al., 2014). Although the reasons for the observed sex differences are unclear, our findings could have significant clinical relevance and this is an important point of the present studies. In humans, male offspring experience a disproportionate incidence of neurobehavioral delays and disorders such as autism or ADHD (Rivera et al., 2015; Sullivan et al., 2012). Our data would suggest that males are more sensitive to maternal inflammation and that more robust responses in male verses female offspring account for observed and reported alterations in neurobehavior. Though the mechanisms responsible for these alterations were not the focus of the present studies, future investigations will focus on elucidation of potential mechanisms by which exposure to inflammation in utero and postnatally alter neurodevelopment.
The present studies were designed to test the hypothesis that maternal obesity induced inflammation would result in inflammation in the offspring which would, in turn, be associated with altered iron availability, altered myelination, and neurocognitive behavioral alterations. Collectively, our data suggest that maternal HFD induces neuroinflammatory responses in utero that correlate with dysregulation of iron homeostasis and associated reductions in myelination in male offspring. Similar to previous studies, these data suggest that these behavioral changes are likely to be programmed early in life by maternal influences and have potential for long-term consequences. Mechanistically, we speculate that the alterations we observed could be driven by localized increased hepcidin levels and altered iron regulation and that these alterations may impact processes other than myelination that could be involved in later behavioral changes. Further elucidation of the specific mechanisms responsible for sex-specific alterations in offspring born to obese mothers is necessary in order to develop preventive and treatment strategies.
Acknowledgments
This work was supported by Nationwide Children’s Hospital (to AEG, MDT and AJT), the American Heart Association (15GRNT2579003 to AJT), and the National Institutes of Health (K99/R00HL116769 to AJT).
Abbreviations
- HFD
high fat diet
- CD
control purified diet
- DIO
diet induced obesity
- PFA
paraformaldehyde
- PBS
phosphate buffered saline
- SEM
standard error of the mean
Footnotes
Conflict of interest statement
There are no conflicts of interest, financial or otherwise, to disclose by the authors.
References
- Balasubramanian S, Jansen M, Valerius MT, Humphreys BD, Strom TB. Orphan nuclear receptor Nur77 promotes acute kidney injury and renal epithelial apoptosis. J Am Soc Nephrol JASN. 2012;23:674–686. doi: 10.1681/ASN.2011070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluardo N, Olsson PA, Mudo G, Sommer WH, Amato G, Fuxe K. Transcription factor gene expression profiling after acute intermittent nicotine treatment in the rat cerebral cortex. Neuroscience. 2005;133:787–796. doi: 10.1016/j.neuroscience.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching- to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Carlin J, George R, Reyes TM. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One. 2013;8:e63549. doi: 10.1371/journal.pone.0063549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137:2366–2370. doi: 10.1093/jn/137.11.2366. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, Granath F. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is Hepcidin the link? J Perinatol. 2013;33:177–181. doi: 10.1038/jp.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, Lau LF. Endocrine and neurogenic regulation of the orphan nuclear receptors Nur77 and Nurr-1 in the adrenal glands. Mol Cell Biol. 1994;14:3469–3483. doi: 10.1128/mcb.14.5.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf AE, Haines KM, Pierson CR, Bolon B, Houston RH, Velten M, Heyob KM, Rogers LK. Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci. 2014;942:164–171. doi: 10.1016/j.lfs.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Sharma AJ, Kim SY, Schieve LA. Maternal prepregnancy weight status and associations with children’s development and disabilities at kindergarten. Int J Obes. 2013;37:1344–1351. doi: 10.1038/ijo.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, Fryer JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J Neuroinflamm. 2014;11:156. doi: 10.1186/s12974-014-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi A, Brantsaeter AL, Sengpiel V, Kacerovsky M, Magnus P, Morken NH, Myhre R, Gunnes N, Jacobsson B. Prepregnancy maternal body mass index and preterm delivery. Am J Obstet Gynecol. 2012;207(212):e211–217. doi: 10.1016/j.ajog.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lieblein-Boff JC, McKim DB, Shea DT, Wei P, Deng Z, Sawicki C, Quan N, Bilbo SD, Bailey MT, McTigue DM, Godbout JP. Neonatal E. coli infection causes neuro-behavioral deficits associated with hypomyelination and neuronal sequestration of iron. J Neurosci. 2013;33:16334–16345. doi: 10.1523/JNEUROSCI.0708-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Magann EF, Doherty DA, Sandlin AT, Chauhan SP, Morrison JC. The effects of an increasing gradient of maternal obesity on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2013;53:250–257. doi: 10.1111/ajo.12047. [DOI] [PubMed] [Google Scholar]
- Mandillo S, Tucci V, Holter SM, Meziane H, Banchaabouchi MA, Kallnik M, Lad HV, Nolan PM, Ouagazzal AM, Coghill EL, Gale K, Golini E, Jacquot S, Krezel W, Parker A, Riet F, Schneider I, Marazziti D, Auwerx J, Brown SD, Chambon P, Rosenthal N, Tocchini-Valentini G, Wurst W. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 2008;34:243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin SO, Poggi M, Alessi MC, Landrier JF, Nunes JA. Modulation of T cell activation in obesity. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6746. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009;27:627–633. doi: 10.1016/j.ijdevneu.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates PS. The role of hepcidin and ferroportin in iron absorption. Histol Histopathol. 2007;22:791–804. doi: 10.14670/HH-22.791. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LC, Inder TE, Neil JJ, Pineda RG, Rogers CE. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J Perinatol. 2014;34:688–692. doi: 10.1038/jp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194. doi: 10.3389/fnins.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E. Hepcidin – the iron regulatory hormone. Clin Biochem Rev. 2005;26:47–49. [PMC free article] [PubMed] [Google Scholar]
- Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010;86:715–722. doi: 10.1016/j.earlhumdev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Salihu HM. Maternal obesity and stillbirth. Semin Perinatol. 2011;35:340–344. doi: 10.1053/j.semperi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan PO. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience. 2014;272:92–101. doi: 10.1016/j.neuroscience.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004–2011. BJOG. 2013;120:932–939. doi: 10.1111/1471-0528.12193. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya JD, Strutt B, Hill DJ, Patel MS. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab. 2006a;290:E129–E134. doi: 10.1152/ajpendo.00248.2005. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006b;291:E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Oommen S, Vasu VT, Srinivasan M, Stachowiak M, Gohil K, Patel MS. Maternal obesity affects gene expression and cellular development in fetal brains. Nutr Neurosci. 2013;16:96–103. doi: 10.1179/1476830512Y.0000000035. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and metaanalysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, Chamlou KA, Grove KL. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int J Obes Suppl. 2012;2:S7–S13. doi: 10.1038/ijosup.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr. 2014;10:166–183. doi: 10.1111/j.1740-8709.2012.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, Nunez MT. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126:541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med CCLM/FESCC. 2013;51:1395–1401. doi: 10.1515/cclm-2012-0576. [DOI] [PubMed] [Google Scholar]
- Wang Q, Du F, Qian ZM, Ge XH, Zhu L, Yung WH, Yang L, Ke Y. Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone hepcidin in the cortex and substantia nigra in rat brain. Endocrinology. 2008;149:3920–3925. doi: 10.1210/en.2007-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis. 2011;17:1513–1522. doi: 10.1002/ibd.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ananth CV, Park BY, Pereira L, Plante LA Perinatal Research, C. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:457, e451–459. doi: 10.1016/j.ajog.2014.01.044. [DOI] [PubMed] [Google Scholar]


