Abstract
Using fetal biomagnetometry, this study measured changes in fetal heart rate to assess discrimination of two rhythmically different languages (English, Japanese). Two-minute passages in English and Japanese were read by the same female bilingual speaker. Twenty-four mother-fetus pairs (mean gestational age=35.5 weeks) participated. Fetal magnetocardiography was recorded while the participants were presented first with Passage 1, a passage in English, and then, following an 18-minute interval, with Passage 2, either a different passage in English (English-English Condition: N=12) or in Japanese (English-Japanese Condition: N=12). The fetal magnetocardiogram was reconstructed following Independent Components Analysis decomposition. Mean inter-beat intervals were calculated for a 30-second baseline interval directly preceding each Passage, and for the first 30 seconds of each Passage. We then subtracted the mean inter-beat interval of the 30-second baseline interval from that of the first 30-second interval, yielding an inter-beat interval change value for each Passage. A significant interaction between Condition and Passage indicated that the English-Japanese Condition elicited a more robust inter-beat interval change for Passage 2 (Novelty Phase) than for Passage 1 (Familiarity Phase), reflecting a faster heart rate during Passage 2, while the English-English Condition did not. This effect demonstrates that fetuses are sensitive to the change in language from English to Japanese. These findings provide the first evidence for fetal language discrimination as assessed by fetal biomagnetometry, and support the hypothesis that rhythm constitutes a prenatally available building block in language acquisition.
Keywords: prenatal language ability, rhythm-based language discrimination, fetal magnetocardiography
Introduction
Research on early language development suggests that infants can discriminate among languages as early as a few days after birth; neonates can discriminate between their parents’ native language and another language [1], and between foreign languages [1,2]. The language pairs that infants can discriminate are argued to belong to different rhythm classes, leading researchers to propose the Rhythm-based Language Discrimination Hypothesis, positing that infants initially utilize rhythmic characteristics to discriminate languages [3].
Evidence for rhythm-based language discrimination ability in neonates raises the question of whether this ability is also present prenatally. If so, this may provide the child with one of the very first building blocks in acquiring their native language(s).
While some components of speech are attenuated in the womb, intonation can be transmitted to the fetal inner ear [4]. There is also some evidence for fetal sensitivity to acoustic characteristics of speech, including recognition of the mother’s voice [5], recognition of passages with rhyming speech [6], and discrimination of some speech sounds [7]. Postnatal infants can also distinguish languages based on rhythm when presented with speech sounds that were low-pass filtered [1] to resemble those available to the fetus in utero. While this suggests that rhythm-based language discrimination ability may feasibly be present pre-birth, it remains an open question whether fetuses indeed possess this ability in utero.
Fetal cognitive processing can be assessed via changes in heart rate. The brain influences heart rate through well-studied neural mechanisms, and there is considerable evidence suggesting heart rate change as an index of cognitive processing, including in fetuses [8]. Kisilevsky et al. (2009) presented fetuses with two speech recordings to test whether they are sensitive to a change in speech from English (a stress-timed language) to Chinese (often argued to be a syllable-timed language), as compared to when they were presented with two English recordings [9]. Fetal heart rate, measured by ultrasound, increased when the language switched from English to Chinese, as compared to when two English passages were presented in succession. These results were taken to suggest that fetuses can distinguish their native language from a foreign language based on their rhythmic properties.
However, the approach used by Kisilevsky et al. (2009) has some potential limitations. First, the English and Chinese stimuli were recorded by different speakers, raising the question of to what extent the fetal responses may have been due to speaker-related sound differences. Second, the rhythmic contrast between English and Chinese remains controversial, with some claiming Chinese is not syllable-timed, but stress-timed like English [10]. Kisilevsky et al. (2009) do not provide quantitative evidence that their English and Chinese stimuli differ rhythmically, making it difficult to determine to what extent their effects are due to rhythmic differences.
In the current study, we investigate whether fetuses in the third trimester can use rhythmic characteristics to discriminate languages, building upon Kisilevsky et al. (2009) but making several modifications to address the above concerns, and introducing a precise measure of fetal heart rate, fetal magnetocardiography (MCG). Unlike the fetal electrocardiogram (ECG) that records electrical currents generated by the fetal heart from electrodes applied to the maternal abdomen, MCG measures the magnetic fields surrounding the electrical current. Unlike ECG, MCG is not distorted by the fetal vernix caseosa or the maternal body. This allows for precise detection of fetal R-waves without loss of signal, necessary for calculating fetal cardiac metrics. In addition, we test two languages whose rhythmic contrast is uncontroversial, English (a stress-timed language) and Japanese (a mora-timed language [11]). We also provide an acoustic analysis to confirm the rhythmic difference between our English and Japanese stimuli. Finally, our English and Japanese stimuli are spoken by the same bilingual speaker to ensure that differences are due to change in language rather than change in speaker.
Methods
Participants
Participants included 24 mother-fetus pairs recruited from the University of Kansas Medical center and broader Kansas City community. Gestational age ranged from 32–39 weeks (M=35.5, SD=1.76) and maternal age from 22–41 years old (M=29.4, SD=4.25); all mothers were native English speakers. Twelve fetuses were female, 11 male, and the gender of 1 fetus was unknown at the time of testing. All participants provided their written informed consent to participate and were compensated for their participation.
Stimuli
The stimuli included 3 2-minute audio-recorded passages from a children’s book: two different passages in English (Make Way for Ducklings by R. McClosky) and one in Japanese (Kamosan Otoori, the Japanese translation of Make Way for Ducklings, by S. Watanabe). They were read by a 28-year-old female bilingual speaker of English and Japanese and recorded in an anechoic chamber using a solid-state digital audio recorder (Marantz PMD671) and a cardioid microphone (Electrovoice-N/D-767).
To quantify the rhythmic differences between the two languages, we conducted an acoustic analysis of the recorded passages. We measured %V, the proportion of vocalic intervals (i.e., vowels) within a recorded sentence [12], for the first 30 seconds of each passage, and compared these values across the English and Japanese stimuli. A one-way ANOVA revealed a significant difference in %V between English (40.8) and Japanese (48.7) (F(1,14) = 10.323, p < 0.007), confirming that our English and Japanese stimuli are rhythmically different.
Procedure
Participants were tested at the Hoglund Brain Imaging Center, University of Kansas Medical Center. The procedure was approved by the Institutional Review Board at the University of Kansas Medical Center. Participants sat quietly in a reclined support chair in a magnetically shielded room, while the fetal MCG was recorded using an 83-channel fetal biomagnetometer (CTF Systems, Inc.). The stimuli were presented on a speaker outside of the shielded room, and delivered through tubing passed through a small opening into the room. The tubing was connected to a plastic cone, positioned approximately 1 cm above the maternal abdomen, over the position of the fetal head, determined by ultrasound. The stimuli were presented at an average of 95 dB; the mother wore a sound-attenuating headset during stimulus presentation.
Following Kisilevsky et al. (2009), mother-fetus pairs were exposed to two passages over the two phases of sound presentation. The Familiarization Phase began with a 2-minute silent interval (Pre-passage silence), followed by a 2-minute presentation of the first passage (Familiarization Passage) and a 16-minute silent interval (Post-passage silence). The Novelty Phase then began, starting with a 2-minute silent interval (Pre-passage silence), followed by a 2-minute presentation of the second passage (Novelty Passage), and concluded with a 2-minute Post-passage silent interval (Post-passage silence). Stimuli were presented using Presentation (Neurobehavioral Systems, Inc.).
Each maternal-fetal pair was randomly assigned to one of two Conditions. In the English-English Condition (N=12), the Familiarization Passage and the Novelty Passage were both in English. In the English-Japanese Condition (N=12), the Familiarization Passage was in English, and the Novelty Passage in Japanese. If the fetuses in the English-Japanese Condition show a greater heart rate change during the presentation of the Novelty Passage (when the language switches from English to Japanese), than those in the English-English Condition (when the passage switches from one English passage to another English passage), this would suggest fetal discrimination between the two languages. The speaker of the passages is kept constant across languages. Moreover, since the Familiarization and Novelty passages are different English passages in the English-English Condition, the content of the two passages changes in both Conditions. Thus, the effect of language change is disentangled from speaker and content of the passages.
Data Coding and Analysis
After Independent Components Analysis (ICA) decomposition of the biomagnetometer recording, the fetal MCG was reconstructed from the MCG-relevant ICA components. We calculated inter-beat intervals (IBI) for each fetus, and generated mean IBIs for the 30-second interval directly preceding each of the two Passages (Familiarization, Novelty), and for the first 30 seconds of each Passage, using QRSTool [13] available at www.psychofizz.org. We derived an IBI-Change variable, capturing fetal heart rate change from the pre-passage silence to the passage itself, by subtracting the mean IBI of the 30-second interval directly preceding the Passage from that of the first 30 seconds of the Passage.
Results
For the English-English Condition, mean IBI-Change was −4.1 for the Familiarization Phase and 1.4 for the Novelty Phase. For the English-Japanese Condition, mean IBI-Change was 8.1 for the Familiarization Phase and −16.1 for the Novelty Phase (see Figure 1). Note that negative numbers reflect reduced IBI, or faster heart rate, during the passage as compared to its pre-passage silent interval, and positive numbers reflect greater IBI, or reduced heart rate, during the passage as compared to its pre-passage silent interval.
Figure 1.
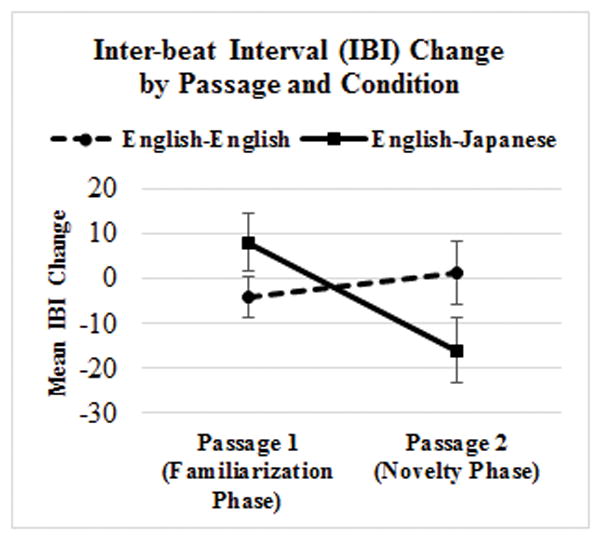
Mean IBI-Change across Passage and Condition
A Mixed ANOVA with Condition (English-English vs. English-Japanese) as a between-subjects factor and IBI-Change Differential (IBI-Change for Familiarization Passage vs. IBI-Change for Novelty Passage) as a within-subject factor yielded non-significant effects of Condition (F(1,22) = .156, p = .696) and IBI-Change Differential (F(1,22) = 2.335, p = .141). Crucially, there was a significant Condition x IBI-Change Differential interaction (F(1,22) = 5.837, p = .024). Post-hoc pairwise comparisons revealed that the IBI-Change Differential was significantly different between two Phases for the English-Japanese Condition (p = .011), but not for the English-English Condition (p = .537). The English-Japanese Condition elicited a more robust IBI Change for Passage 2 (Novelty Phase) than for Passage 1 (Familiarity Phase), reflecting reduced IBI, or faster heart rate, during Passage 2, while the English-English Condition did not.
Discussion
The current study utilized fetal biomagnetometry for the first time to investigate fetal language processing and demonstrated that the fetus is indeed sensitive to a change in language from English to Japanese, two languages with different rhythmic characteristics. This sensitivity is attributable to the rhythmic properties of the languages and is not simply due to a change in the acoustic content of the passages (Passage 1 and 2 were different in both Conditions), or due to a change in speaker (speaker was the same across Passage and Condition). Our findings converge with those of Kisilevsky et al. (2009), providing further support for the Rhythm-based Language Discrimination hypothesis and suggesting that cortical tuning to native-language properties begins in utero, as previously hypothesized largely based on findings from neonates [14]. This pre-birth sensitivity may subserve the development of memory traces for language in auditory cortex, supporting post-natal language learning [15].
Assessments took place in the third trimester of gestation, after the emergence of specific behavioral patterns linked to fetal state. Around 30–32 weeks gestation, centrally-mediated heart rate patterns along with breathing, body and eye movements allow for the identification of behavioral state. The coupling and integration of central and autonomic processes represents an important developmental transition to behavioral states that are similar to newborns. These behavioral states are linked to heart rate patterns. Because direct measures of fetal brain function are limited, metrics of heart rate and variability serve as a proxies to central control. The heart does not “hear,” therefore, we attribute the differences in heart rate to central auditory processing of the language stimuli [16]. Our novel approach to probing fetal language acquisition via heart rate change may ultimately provide a new tool for studying developmental language impairments.
Acknowledgments
Funding: This study was supported in part by an NIH Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179), awarded to the University of Kansas Medical Center
Footnotes
Conflicts of Interest: None declared
References
- 1.Mehler J, Jusczyk PW, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 2.Nazzi T, Bertoncini J, Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. J Exp Psychol Hum Percept Perform. 1998;24:756–766. doi: 10.1037//0096-1523.24.3.756. [DOI] [PubMed] [Google Scholar]
- 3.Mehler J, Dupoux E, Nazzi T, Dehaene-Lambertz G. Coping with linguistic diversity: the infant’s viewpoint. In: Morgan JL, Demuth K, editors. Signal to syntax: bootstrapping from speech to grammar in early acquisition. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 101–116. [Google Scholar]
- 4.Querleu D, Renard X, Versyp F, Paris-Delrue L, Crèpin G. Fetal hearing. Eur J Obstet Gynecol Reprod Biol. 1988;28:191–212. doi: 10.1016/0028-2243(88)90030-5. [DOI] [PubMed] [Google Scholar]
- 5.Kisilevsky BS, Hains SMJ, Lee K, Xie X, Ye HH, Zhang K, Wang Z. Effects of experience on fetal voice recognition. Psychol Sci. 2003;14:220–224. doi: 10.1111/1467-9280.02435. [DOI] [PubMed] [Google Scholar]
- 6.DeCasper AJ, Lecanuet JP, Busnel MC, Granier-Deferre C, Maugeais R. Fetal reactions to recurrent maternal speech. Infant Behav Dev. 1994;17:159–164. [Google Scholar]
- 7.Shahidullah S, Hepper PG. Frequency discrimination by the fetus. Early Hum Dev. 1994;36:13–26. doi: 10.1016/0378-3782(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 8.Kisilevsky BS, Hains SMJ. Exploring the relationship between fetal heart rate and cognition. Inf Child Dev. 2010;19:60–75. [Google Scholar]
- 9.Kisilevsky BS, Hains SMJ, Brown CA, Lee CT, Cowperthwaite B, Stutzman SS, et al. Fetal sensitivity to properties of maternal speech and language. Infant Behav Dev. 2009;32:59–71. doi: 10.1016/j.infbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Rouas JL. Modeling long and short-term prosody for language identification. International Speech Communication Association. 9th European Conf. on Speech Communication and Technology (INTERSPEECH’2005 - EUROSPEECH); 2005; Lisboa, Portugal. [Google Scholar]
- 11.Warner N, Arai T. Japanese mora-timing: a review. Phonetica. 2001;58:1–25. doi: 10.1159/000028486. [DOI] [PubMed] [Google Scholar]
- 12.Ramus F, Nesper M, Mehler J. Correlates of linguistic rhythm in the speech signal. Cognition. 1999;73:265–292. doi: 10.1016/s0010-0277(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 13.Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011;2:222. doi: 10.3389/fpsyg.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pino O. Fetal memory: the effects of prenatal auditory experience on human development. BAOJ Med Nursing. 2016;2:3. [Google Scholar]
- 16.Gustafson KM, Popescu EA. Fetal assessment using biomagnetometry: characterization of movement behaviors and the development of autonomic control. In: Reissland N, Kisilevsky BS, editors. Fetal development: research on brain and behavior, environmental influences, and emerging technologies. New York, NY: Springer; 2016. pp. 453–480. [Google Scholar]


