Introduction
Allergic Sensitization, Asthma, and Sleep
Associations between allergic sensitization, decreased pulmonary function, and increased asthma morbidity in children have been demonstrated consistently across studies.1–3 Specific allergic sensitivity to dog, cat, alternaria, but especially to cockroach and rodents have been associated with increased bronchial responsiveness,4 increased risk of ED visits for asthma,5 and higher asthma morbidity.3,6–10
Sleep is an important outcome to evaluate when considering allergen sensitization in asthma, because it can be a vulnerable time for children with asthma for various physiologic reasons.11,12 To date, most studies of allergic asthma and sleep in children are based on self11 report assessments using diary data,13,14 which are subject to poor recall and bias. More objective assessments of sleep quality in asthma are needed.11
Urban minority children appear to be at greater risk. Children from African American and Latino, predominantly Puerto Rican, backgrounds have poor asthma outcomes.15,16 In particular, African Americans are two times more likely to report nocturnal asthma compared to non-Latino Whites (NLWs) in genetic studies.17 African Americans, as early as age 2 years, are also disproportionately affected by higher allergic sensitization compared to NLWs of the same age.18 Our previous work has shown that both urban African Americans and Latinos with asthma have higher total sleep problem scores compared to NLWs,19 and that well-controlled asthma and better lung function were more likely in children with “good” sleep health.20 We have yet to study the contribution of allergic sensitization to objective measures of sleep quality in urban children with asthma. Addressing this gap in the literature has important implications for environmental and behavioral interventions to promote good sleep health in urban minority groups.
Current Study
This study first investigated the association between increased allergic sensitization (number of positive allergy tests measured by skin prick test or specific IgE) and children’s sleep outcomes (measured objectively using actigraphy; sleep quality or efficiency, variability in sleep efficiency, mean number of awakenings and sleep duration) in a carefully evaluated urban sample of children with persistent asthma. We expected that higher allergic sensitization would be associated with poorer sleep quality and duration. Given higher levels of morbidity in minority groups,21 we expected this association would be more pronounced in children from African American and Latino backgrounds compared to NLW children. Second, we determined whether sleep outcomes differed across sensitization to specific indoor perennial allergens (dust mite, cat, dog, cockroach, mouse), potentially relevant to the bedroom environment. Based on prior literature in urban environments, we expected that children with cockroach and mouse sensitization would have poorer sleep outcomes. Finally, we sought to determine whether asthma status indicators (FEV1, FEV1 variability, and asthma control) moderated associations between allergic sensitization and sleep outcomes, and expected these associations to be more robust in the context of lower FEV1, greater FEV1 variability, and poor asthma control.
Methods
Data for this study were collected from a larger study, Project NAPS (Nocturnal Asthma and Performance in School, citation blinded for review), that examines the co-occurrence of asthma and allergic rhinitis symptoms, sleep quality, and academic function in urban children with persistent asthma (7–9 years of age) across one academic year. Data presented in this sub-study include the 196 participants who completed allergy testing at the clinic study visit that occurred during the fall/early winter monitoring period. The Institutional Review Board at Rhode Island Hospital approved the study protocol.
Participants were recruited from the four largest school districts in an urban Northeastern US city, from hospital and ambulatory pediatric clinics, and from a hospital-based asthma educational program by convenience sampling. Inclusion criteria specified child age 7–9 years, child’s legal guardian was willing to participate, caregiver race/ethnicity was self-identified as black/African American, Latino (Dominican or Puerto Rican), or NLW, and child attended public school in one of the targeted school districts in [blinded for review]. Children needed to meet criteria for persistent asthma either by prescription for asthma controller medication or recurrent asthma symptoms, e.g., daytime symptoms greater than 2 days a week, nocturnal symptoms greater than 2 times a month, short acting beta agonist use greater than 2 days a week, at least some limitation in normal activity, and/or 2 or more oral steroid bursts per year.22 Exclusion criteria were moderate to severe cognitive impairment as determined by school placement, use of stimulant medication for attention deficit/hyperactivity disorder, additional pulmonary disease or chronic health condition, or a diagnosed sleep disorder (e.g., restless leg syndrome) that would confound the primary hypotheses of the larger study. Children with sleep disordered breathing (SDB) were not excluded as this is highly comorbid among this population.
Data for the current study were collected during the fall and early winter period of each study year (August 15– December 31 from 2010 to 2014). At the initial study visit, demographic information and medication use were collected, followed by a second session at the hospital-based clinic at least 2 weeks later, when asthma severity and allergy status were evaluated. The clinic visit was followed by a one month home-monitoring period in which children wore an actigraph to assess sleep quality and used a handheld device to assess FEV1. Midway through the monitoring period, study staff conducted a home visit to download and review electronic sleep and pulmonary function data. At the end of the monitoring period, asthma control was assessed.
Standardized training procedures for device use were provided throughout to encourage protocol adherence and ensure appropriate use of the devices.20 Assessments were administered in English or Spanish according to participants’ preference, and standardized procedures were used for the translation of measures.23
Measures
Demographic and Descriptive Information
Primary caregivers provided demographic information (see Table 1). Poverty status was determined by dividing the family’s annual income by the Federal per capita poverty threshold for a family of that size.24
Table 1.
Sample characteristics (n= 196)
| Demographic variable | |
|---|---|
| Child age in years, M (SD) | 8.31 (.87) |
| Male (%) | 53 |
| Caregiver race/ethnicity (%) | |
| Black | 34 |
| Latino | 51 |
| Non-Latino White | 15 |
| At/below poverty threshold (%) | 70 |
| Number of people in household, M (SD) | 4.70 (1.76) |
| Asthma Severity (%) | |
| Mild persistent | 56 |
| Moderate persistent | 33 |
| Severe persistent | 11 |
| Asthma poorly controlled (%) | 44 |
| Asthma medications (%) | |
| Inhaled corticosteroid | 70 |
| Long-acting beta agonists | 5 |
| AR medications (%) | |
| Intranasal steroid | 11 |
| Antihistamine use (%) | |
| 1st generation antihistamine | 18 |
| 2nd generation antihistamine | 26 |
| FEV1% predicted, M (SD) | 83.9 (12.80) |
| Variability in FEV1% predicted, M (SD) | 14.97 (5.96) |
| Risk for sleep disordered breathing (%) | 49 |
| BMI percentile for age, M (SD) | 74.32 (25.22) |
| Sleep health indicators | |
| Sleep efficiency (%), M (SD) | 86.54 (3.48) |
| Variability in sleep efficiency, M (SD) | 4.17 (1.87) |
| Sleep duration (minutes), M (SD) | 554.49 (35.04) |
| Mean number of awakenings per night, M (SD) | 5.35 (2.42) |
Asthma Severity
The clinic study visit consisted of a medical history, physical examination, allergy testing, pulmonary function testing, and review of medications to confirm the diagnosis of asthma and classify asthma severity according to NHLBI EPR-3 guidelines.22
Allergy Testing
Allergy skin prick testing was performed (Multi Test II device, Lincoln Diagnostics; Decatur, IL) with allergen extracts in addition to positive (histamine 6mg/ml) and negative (50% glycerin) control after adequate medication washout. The following extracts were used from Greer laboratories: D. pteronyssinus (10,000 au/ml), D. farinae (10,000 au/ml), dog ep. (1:20 w/v), cat hair (10,000 BAU/ml), cockroach mix (German and American) (1: 20 w/v), mold mix 1 (1:20 w/v), Cladosporium herbarum (1:40 w/v), mouse (1:20 w/v), ragweed mix (1:20 w/v), Eastern tree mix (1:20 w/v), grass mix 7 (100,000 BAU/ml), Timothy (100,000 BAU/ml), plantain (1:20 w/v), oak (red) (1:20 w/v), birch (red) (1:20 w/v), and maple (sugar) pollen (1:20 w/v). The results of a test were considered to be positive if the maximum diameter of the wheal was at least 3mm after subtraction of the negative control.
Participants who could not washout of antihistamine (n=10), underwent specific IgE testing (sIgE) (Quest Diagnostics, Chantily, VA) to Northeast Environmental Panel 76 which contains the following allergens: Dermatophagoides pteronyssinus (d1), Dermatophagoides farinae (d2), cat dander (e1), dog dander (e5), Timothy grass (g6), cockroach (i6), Penicillium notatum (m1), Cladosporium herbarum (m2), Aspergillus fumigatus (m3), Alternaria alternata (m6), maple (Box Elder) (t1), birch (t5), oak (t7), elm (t8), common ragweed (short) (w1), giant ragweed (tall) (w3), English plantain (w9), and Lamb’s quarters (goose foot) (w10), with the addition of mouse (e88) and mouse epithelia (e76). A result was considered positive if it was greater than or equal to 0.35kU/L.
Evaluated allergens were subsequently grouped into a categorical variable (Positive/Negative) from either the skin test or sIgE for the following 10 allergen categories: mold, dust mite, dog, cat, cockroach, grass, ragweed, spring trees, plantain weed, and mouse. We used two variables to measure atopy: (1) Number of positive allergy tests was a continuous variable ranging from 0 to 10 positive allergy tests. This continuous variable included participants with zero positive results. (2) After reviewing the distribution of positive allergy tests, we created a second categorical variable, degree of atopy, for the participants with at least one positive test, to reflect increasing categories of sensitization: 1–2, 3–5, and 6–10 positive allergy tests.
Sleep Disordered Breathing
Primary caregivers completed the Sleep Related Breathing Disorders subscale of the Pediatric Sleep Questionnaire, a well-validated and reliable measure of SDB.25
Environmental Control Subscale
Caregivers reported child exposure to environmental triggers and family efforts to control these exposures during a well-validated semi-structured interview, the Family Asthma Management System Scale (FAMSS).26 Trained interviewers rated participants on an environmental control domain, using a subscale from 1 to 9 to assess child exposure to secondhand tobacco smoke, carpeting in the child’s room, family pets, problems with pests, as well as family knowledge and implementation of typical asthma trigger controls. Lower environmental control scores indicated higher levels of exposures to triggers, as well as fewer family efforts to control trigger exposure.26 Interviews were audio-recorded and coded according to a standardized coding system.26
Lung Function
During the monitoring period, children’s lung function was measured twice daily by a handheld computerized spirometer (Jaeger AM2; VIASYS Healthcare; Yorba Linda, CA). Participants were instructed to complete 3 “blows” prior to any asthma or allergy medications in the morning and evening. The highest of 3 FEV1 % predicted values per trial was retained. A series of data cleaning and reduction steps were used, which are available upon request.27 FEV1 variability, a measure of poor asthma control, was measured by coefficient of variation.28
Sleep Measures
Sleep methods have been described in detail previously.20 Participants wore an actigraph on their non-dominant wrist for the monitoring period (MiniMitter Company, Bend, OR, USA). Sleep outcome variables were computed as the aggregate across the monitoring period. Three sleep quality variables were included: (1) sleep efficiency was defined as the % time asleep divided by the total time in bed; (2) variability in sleep efficiency was the standard deviation across all monitored days, and (3) mean # of awakening per night of at least 3 minutes in duration. Our final sleep outcome variable included sleep duration, defined as the total time between evening sleep onset and morning waking.20
Asthma control
At the end of the monitoring period, parents and children completed the Childhood Asthma Control Test (cACT),29 a well-validated questionnaire of asthma control for this age group. Item scores are summed for a total score (0–27) with lower scores indicating poorer asthma control. A score 19 and below denotes poorly controlled asthma.
Statistical Analysis Plan
Analyses were conducted using IBM SPSS Statistics for Windows, V23.0 (IBM Corp., Armonk, NY, USA). For preliminary analyses, a chi-square was used to evaluate differences in race/ethnic group by poverty threshold. An analysis of variance (ANOVA) was used to determine differences in environmental control scores and number of positive allergy tests across race/ethnic group; post-hoc Least Significant Difference (LSD) analyses were used to pinpoint differences between groups. Child age, sex, poverty threshold, environmental control score, antihistamine use, and risk for SDB were evaluated as potential covariates in associations between number of positive allergy tests and sleep outcome variables using Pearson r correlations and t-test analyses. We controlled for covariates that were significantly associated with both predictor and outcome variables in subsequent analyses.
To determine associations between number of positive allergy tests (0–10 continuous variable) and sleep outcomes in addressing our first hypothesis, we used hierarchical regressions and controlled for significant covariates. An ANOVA with post-hoc LSD analyses determined differences in sleep outcomes across degree of atopy (1–2, 3–5, and 6–10 positive allergy tests) and race/ethnic groups. We also looked within each race/ethnic group and used (1) Pearson’s r correlations to determine associations between number of positive allergy tests and each sleep outcome, and (2) ANOVAs to determine differences in sleep outcomes across degree of atopy.
To address our second hypothesis, t-test analyses were conducted to determine differences in sleep outcome variables by sensitization to specific indoor allergens (yes/no sensitized to mouse, cockroach, dust mite, cat, or dog).
Finally, we tested FEV1, FEV1 variability, and asthma control as moderators in associations between number of positive allergy tests and sleep outcome variables for the entire group to address our third hypothesis. In hierarchical regressions predicting sleep variables, covariates were entered as the first step, number of positive allergy tests second, the moderator third, and the interaction term between number of positive allergy tests and the moderator as the final step. Prior to moderation analyses, predictor and moderator variables were centered and a product term was created from the centered predictor and moderator. In analyses with a significant interaction term, the moderator was dichotomized by median split and follow-up regression analyses were conducted examining the association between number of positive allergy tests and the sleep outcome at high and low levels of the moderating variable. Simple slopes were also plotted to aid in interpretation of moderation results.
Results
Descriptive Data
Characteristics of the 196 child participants who underwent allergy testing in Project NAPS can be found in Table 1. Child participants studied were 53% male, 85% minority and 70% living at or below poverty threshold, 44% moderate/severe persistent in asthma severity, and 70% on inhaled corticosteroid (ICS) therapy by self-report. Allergic sensitization and environmental control data are presented in Table 2. For seasonal pollen, 4.6% of participants were ragweed-sensitized and in-season. Other types of pollen were not applicable to the time-frame of this study.
Table 2.
Allergic Sensitization Data (n= 196)
| Variable | |
|---|---|
| Number of positive allergy tests (0–10), M (SD) | 3.18 (2.47) |
| Degree of atopy (%)* | |
| 1–2 positive allergy tests | 29 |
| 3–5 positive allergy tests | 36 |
| 6–10 positive allergy tests | 18 |
| Proportion sensitized to allergens (%) | |
| Mouse | 37 |
| Cockroach | 28 |
| Dust mite | 61 |
| Cat | 32 |
| Dog | 25 |
| Ragweed | 24 |
| Spring Trees | 47 |
| Grass | 28 |
| Plantain pollen | 10 |
| Mold | 28 |
| Mean environmental control score (1–9), M (SD) | 3.74 (2.56) |
| Pest exposure (%) | 40 |
| Carpet in sleeping room | 43 |
| Dog ownership | 24 |
| Cat ownership | 11 |
| Smoke exposure | 41 |
17% of the sample did not have a positive allergy test
Preliminary Analyses
Differences in variables by race/ethnic group and child sex are presented in Table 3. Child age was significantly correlated with sleep duration (r = −.28, p =.00), with younger children experiencing longer sleep duration. Children who were at or below poverty threshold had more variability in their sleep efficiency than children above poverty threshold (t(169) = −2.91, p = .004. Environmental control scores from the FAMSS, risk for SDB, and first generation antihistamine use were not associated with number of positive allergy tests, degree of atopy, or any sleep outcomes. Second generation antihistamine use was only associated with sleep duration (t(175) = −2.164, p =.032). Given that second generation antihistamine use was not associated with our predictor variables, it was not included as a covariate in analyses with sleep duration.
Table 3.
Preliminary analyses
| Differences by race/ethnicity | ||||||
|
| ||||||
| Blacka (n=66) | Latinob (n=100) | NLWc (n=30) | Test statistic | p | Post hoc comparisons | |
|
| ||||||
| At or below poverty threshold | 66% | 79% | 46% | X2(2)= 11.75 | .003** | -- |
| Mean environmental control score (1–9) | 3.80 (2.59) | 4.04 (2.61) | 2.60 (2.04) | F(2, 192) = 3.78 | .025* | a>c; b>c; a=b |
| # of positive allergy tests (0–10) | 3.92 (1.79) | 2.92 (2.48) | 2.43 (1.79) | F(2, 193) = 5.11 | .007** | a>b; a>c; b=c |
| Degree of atopy,† | X2(4)= 11.02 | .026** | -- | |||
| 1–2 | 21% | 30% | 43% | |||
| 3–5 | 39% | 33% | 37% | |||
| 6–10 | 29% | 16% | 3% | |||
|
| ||||||
| Differences by child sex | ||||||
|
| ||||||
| Male (n=104) | Female (n=92) | Test statistic | p | |||
|
| ||||||
| # of positive allergy tests (0–10) | 3.56 (2.53) | 2.76 (2.36) | t(194) = 2.27 | .024* | ||
| Sleep efficiency | 85.93 (3.84) | 87.26 (2.85) | t(177) = −2.61 | .01* | ||
| Variability in sleep efficiency | 4.23 (1.71) | 4.11 (2.04) | t(177) = .44 | .66 | ||
| # of night awakenings | 5.72 (2.81) | 4.91 (1.81) | t(180) = 2.27 | .024** | ||
| Sleep duration | 553.63 (36.0) | 555.51 (34.05) | t(177) = −.36 | .72 | ||
Note.
p <.05,
p< .01;
Test statistic with X2(df) refers to chi-square, F(df) refers to ANOVA, and t(df) refers to t-test.
11% of Black, 21% of Latino, and 17% of NLW children did not have a positive allergy test.
Associations between allergic sensitization and objective sleep outcomes
Controlling for child sex, number of positive allergy tests (0–10) predicted mean sleep efficiency (ΔF(1,176) = 5.60, β = −.18, p = .02) and mean number of night awakenings (ΔF(1,179) = 4.04, β = .15, p = .046). Children with a higher number of positive allergy tests experienced less efficient sleep and more night awakenings. Number of positive allergy tests was also significantly associated with variability in sleep efficiency (β =.22, p=.003), such that children with more positive allergy tests had more variability in their sleep efficiency. No significant association emerged between number of positive allergy tests and sleep duration.
For those with at least one positive test, variability in sleep efficiency also differed across degree of atopy (F(2, 147) = 6.36, p = .002). Children with 3–5 or 6–10 positive allergy tests experienced more variability in sleep efficiency (M = 4.36 vs. 4.92, respectively) than children with 1–2 positive allergy tests (M = 3.51). Mean sleep efficiency, night awakenings and sleep duration did not differ across degree of atopy.
Race/ethnic differences in sleep outcomes
African American children experienced less efficient sleep than both Latino and NLW children (F(2, 176) = 3.67, p = .027, η2 = .04). Within ethnic groups, no significant associations emerged between number of positive allergy tests/degree of atopy and sleep outcomes in African American and NLW children. However, within Latino children, number of positive allergy tests was significantly associated with variability in sleep efficiency (r =.30, p = .004). Latino children with more positive allergy tests experienced more variability in their sleep efficiency than Latinos with less positive allergy tests. Latino children with 6–10 positive allergy tests also experienced more variability in sleep efficiency than Latinos with 1–2 or 3–5 positive allergy tests (F(2, 70) = 6.43, p = .003). Similarly, mean sleep duration was lowest for Latinos in the 6–10 positive allergy test category.
Sensitization to specific indoor perennial allergens
We found associations in several mean sleep outcomes with cockroach and cat sensitization. Cockroach sensitization was associated with shorter sleep duration. Cat sensitization was associated with less efficient sleep and more variability in sleep efficiency (Table 4). Sleep outcomes did not differ across sensitization to other indoor allergens (e.g., mouse, dust mite, dog).
Table 4.
Sleep outcomes by cockroach and cat sensitization
| No sensitizationa | Sensitizationb | t | df | p | |
|---|---|---|---|---|---|
| Cockroach | (n=128) | (n=50) | |||
| Sleep efficiency | 86.69 (3.28) | 86.34 (3.74) | .62 | 176 | .54 |
| Variability in sleep efficiency | 4.08 (1.71) | 4.43 (2.25) | −1.12 | 176 | .26 |
| Night awakenings | 5.29 (2.32) | 5.34 (2.48) | −.12 | 176 | .91 |
| Sleep duration | 558.45 (34.46) | 554.78 (35.14) | 2.37 | 176 | .02* |
|
| |||||
| Cat | (n=122) | (n = 57) | |||
| Sleep efficiency | 86.92 (3.33) | 85.73 (3.67) | 2.62 | 177 | .03* |
| Variability in sleep efficiency | 3.97 (1.65) | 4.61(2.22) | 4.87 | 177 | .03* |
| Night awakenings | 5.18 (2.42) | 5.70 (2.40) | −1.37 | 180 | .17 |
| Sleep duration | 554.08 (34.82) | 555.36 (35.79) | −.23 | 177 | .82 |
Note..
p < .01;
p < .05
Moderation analyses in associations between number of positive allergy tests and sleep outcomes
We considered FEV1, FEV1 variability, and asthma control as moderators in associations between number of positive allergy tests and sleep efficiency, variability in sleep efficiency, and mean number of night awakenings. We controlled for child sex in the models including sleep efficiency and number of night awakenings.
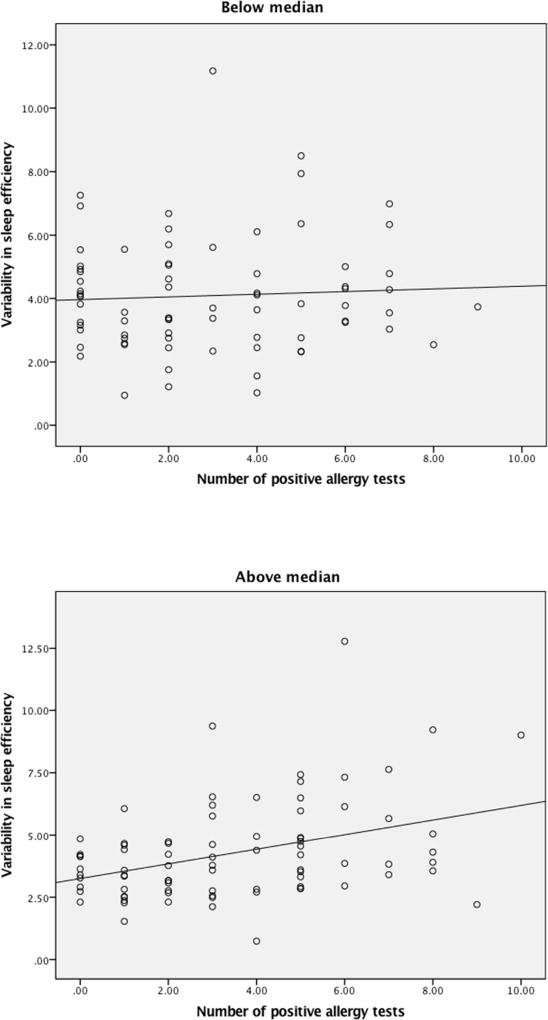
FEV1 variability was a significant moderator in the association between number of positive allergy tests and variability in sleep efficiency (β = .16, p =.04, R2 adjusted = .07; see Table 5). FEV1 variability for each participant was computed by dividing the standard deviation of FEV1 across the monitoring period by the mean FEV1 (across the monitoring period) and multiplying by 100. The median FEV1 variability score for the sample was calculated and dichotomized by median split. Scores above the median were labeled as “1” (high level) and scores below the median were labeled as “0” (low level). Follow-up regression analyses were conducted examining the association between number of positive allergy tests and variability in sleep efficiency (See Figure 1). For children above the median FEV1 value of variability, more allergy tests were associated with more variability in sleep efficiency (β = .38, p =.00, R2 adjusted = .13). On the other hand, for children below the median (less variability in FEV1), there was no significant association between number of positive allergy tests and variability in sleep efficiency (β = .04, p =.62, R2 adjusted = −.01). We did not find that FEV1 or asthma control were significant moderators in associations between number of positive allergy tests and sleep outcomes.
Table 5.
Results testing moderators in associations between number of positive allergy tests and sleep outcomes (n = 156)
| Variability in Sleep Efficiency | ||||
|---|---|---|---|---|
|
| ||||
| β | B | 95% CI for B | R2 adjusted | |
| Number of positive allergy tests | .23** | .17 | .06 – .29 | |
| FEV1 variability | .001 | .00 | −.05 – .05 | |
| Allergy tests × FEV1 variability | .16* | .02 | .001 – .04 | .07 |
Note.
p < .05,
p < .01;
Results for models with nonsignificant interaction terms are not shown.
Figure 1.
Number of positive allergy tests and variability in sleep efficiency by variability in FEV1 (median split)
Discussion
To our knowledge, this is the first study to measure allergic sensitization in asthma and objective measures of sleep using actigraphy in children. In this sample of urban school-children with persistent asthma, the majority of whom reported using ICS therapy, we found that children who were sensitized to more aeroallergens had lower overall sleep efficiency, more variability in their sleep efficiency, and more night awakenings. These are important findings given that to date, most measures of sleep in pivotal studies of nocturnal asthma14,17 have used subjective diary data. Not only did we demonstrate a link between allergic sensitization and specific sleep outcomes assessed through objective methods, but we found the association to be moderated by FEV1 variability. For children with increased FEV1 variability (an objective measure of poor asthma control), an increased number of positive allergy tests was associated with more changes in children’s sleep efficiency (an objective measure of poor sleep). This variability in sleep efficiency can be an important marker of uncontrolled asthma or an indicator of frequent disruptions related to wakings due to asthma or other urban stressors.
Our study also found objective measures of sleep outcomes to be associated with cockroach sensitization, but not mouse sensitization. In the literature, there has been an ongoing debate regarding which of these two allergens is more significantly linked with morbidity in urban environments, sometimes revealing paradoxical results.30–34 We also found cat sensitization to be associated with sleep outcomes. Neither dust mite nor dog sensitization were associated with sleep outcomes in this urban group. Recent studies from the Inner City Asthma Consortium (ICAC) have found that multiple allergen sensitization (not just individual allergens) has effects on asthma severity and is related to difficult-to-control asthma.35,36 Likewise, seasonal data show evidence for the role of multiple allergic sensitization in increased asthma exacerbations during the fall season as inner-city children return to school.37 Our findings suggest an association between increased number of positive allergy tests and poor sleep outcomes during a similar time frame in urban children with asthma.
This sample was primarily minority in race/ethnicity. Our findings within the Latino subgroup suggest that Latino children with more positive allergy tests experienced more variability in sleep efficiency, and that the sub-group with 6–10 positive allergy tests were at higher risk for more variable sleep efficiency and less sleep duration than Latinos with 1–5 positive tests. This is interesting because African American children in our sample had a higher mean number of positive allergy tests and higher proportion in the 6–10 positive category over other groups, but did not follow the same patterns in increased sensitization and sleep as Latinos. To date, genetic studies do not indicate a clear and overwhelming role for genetic ancestry in the observed disparity in allergic sensitization for African American and Latinos, which may suggest a greater influence of local exposures or location of residence on atopy.38,39 We found no differences in environmental control scores, however, between our African American and Latino sub-groups. Further study is needed with more proportionate sampling of ethnic sub-groups.
Avenues for Intervention
Studies of nocturnal asthma cite potential therapies for nocturnal asthma which include chronopharmacologic dosing strategies during exacerbations (e.g., dose prednisone at 3pm), use of long acting beta-agonists (LABAs) in conjunction with ICS, and use of sustained release theophylline to achieve peak concentration when airflow limitation is greatest.40 Notably, although 70% of our sample reported ICS therapy, only 5% of our sample reported using LABAs. Use of combination therapy with LABAs presents a clear target for future intervention in similar samples.
The overall goal of intervention is to develop an integrated and multifaceted environmental control strategy41 that focuses on allergic triggers in the bedroom. Additional goals include increasing access to allergy testing to identify allergic sensitization, improving medication adherence particularly if nocturnal asthma is present prior to bed, and developing interventions focusing on sleep behaviors as demonstrated by our prior work.19
Limitations
This study is limited by its small sample size, convenience sampling, and disproportionate number of families per ethnic group. The disproportionate number of Latino participants skews the results of the pooled data for the association between degree of atopy and sleep outcomes and limits its general applicability. We also note that our moderational analyses were conducted on 156 participants due to missing PFT and sleep outcome data, which warrants replication in larger samples. We acknowledge that our environmental exposure data are limited. Indoor environmental exposures were not measured objectively, as collecting allergen samples was beyond the scope of our study design. Self-report of exposure to pests did not specify whether it was mouse or cockroach. Pollen counts were obtained from a regional source; however, the bulk of the monitoring period occurred outside the ragweed pollen season and were not applicable for the following reasons: recruitment began with the start of the schoolyear (end August/September); continued on a rolling basis; and skin testing followed by the month-long monitoring period did not overlap often with elevated ragweed pollen counts, which typically ended by September 30 (pollen data available upon request). For indoor allergens, associations between individual allergens and sleep outcomes need further study with objective sampling and larger numbers. Allergic sensitization on testing does not always translate into clinically significant symptoms caused by individual allergens42. Race also was measured by self-report as genetic sampling was beyond the scope of this study. Finally, neither allergic rhinitis nor weight status were a focus of this report. Associations between allergic sensitization, allergic rhinitis control, weight status, degree of sleep disordered breathing, and objective markers of sleep are future areas of study. Likewise, future studies should assess medication adherence, specifically access to LABAs, availability of rescue therapy at night, and the role of the environment in Latino sub-groups.
Conclusion
Results from this important study of urban school-children with persistent asthma suggest that increased allergic sensitization was associated with poor sleep outcomes objectively measured during a one-month monitoring period in the fall/early winter. The association between allergic sensitization and sleep outcomes depended on variability in lung function, so that children with more variable FEV1 were more at risk for increased variability in sleep efficiency. These results have important implications on intervening through several avenues (e.g. behavioral, environmental, access to testing) to enhance the sleep quality of this group, as sleep can affect multiple areas of children’s functioning. The value of this study is its real-time monitoring of sleep and asthma outcomes in a sample of difficult-to-reach, atopic school-age children.
Acknowledgments
We thank the participants and their families for their participation, and acknowledge the tireless work of the research assistants on Project NAPS. The authors also acknowledge the dedicated skin testing of nurse Diane Andrade, RNC.
Funding Source: Supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD057220 to D. Koinis Mitchell, PI).
Abbreviations
- ANOVA
Analysis of Variance
- cACT
Childhood Asthma Control Test
- CAMP
Childhood Asthma Management Program
- EPR-3
Third Expert Panel Report
- FAMSS
Family Asthma Management System Scale
- FEV1
Forced Expiratory Volume in 1 second
- ICAC
Inner City Asthma Consortium
- ICS
Inhaled Corticosteroid
- LABA
Long-acting Beta Agonist
- LSD
Least Significant Difference
- NAPS
Nocturnal Asthma and School Performance
- NHANES
National Health and Nutrition Examination Survey
- NHLBI
National Heart Lung and Blood Institute
- NLW
Non-Latino White
- RI
Rhode Island
- SDB
Sleep Disordered Breathing
- sIgE
Specific IgE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Not applicable
Conflicts of Interest: none
References
- 1.Sarpong SB, Karrison T. Skin test reactivity to indoor allergens as a marker of asthma severity in children with asthma. Ann Allergy Asthma Immunol. 1998;80(4):303–308. doi: 10.1016/S1081-1206(10)62973-0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J, Weiss ST. Relationship of skin test reactivity to decrements in pulmonary function in children with asthma or frequent wheezing. Am J Resp Crit Care Med. 1995;152(6):2176–2180. doi: 10.1164/ajrccm.152.6.8520794. [DOI] [PubMed] [Google Scholar]
- 3.Rao DR, Sordillo JE, Kopel LS, et al. Association between allergic sensitization and exhaled nitric oxide in children in the School Inner-City Asthma Study. Ann Allergy Asthma Immunol. 2015;114(3):256–257. doi: 10.1016/j.anai.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson HS, Szefler SJ, Jacobs J, et al. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104(4):775–785. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 5.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: Results from the 2005–2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract. 2013;1(5):501–508. doi: 10.1016/j.jaip.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstreich D, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 7.Arruda LK, Vailes LD, Ferriani VP, Santos ABR, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107(3):419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 8.Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: Relationships amongst sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Pongracic JA, Visness CM, Gruchalla RS, Evans R, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 10.Fishbein AB, Lee TA, Cai M, et al. Sensitization to mouse and cockroach allergens and asthma morbidity in urban minority youth: Genes-environments and Admixture in Latino American (GALA-II) and Study of African-Americans, Asthma, Genes, and Environments (SAGE-II) Ann Allergy Asthma Immunol. 2016;117(1):43–49. doi: 10.1016/j.anai.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Koinis-Mitchell D, Craig T, Esteban CA, Klein RB. Sleep and allergic disease: A summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130(6):1275–1281. doi: 10.1016/j.jaci.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg H, Cohen RI. Nocturnal asthma. Curr Opin Pulm Med. 2012;18(1):57–62. doi: 10.1097/MCP.0b013e32834d098e. [DOI] [PubMed] [Google Scholar]
- 13.Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild to moderate asthma in the Childhood Asthma Management Program. J Allergy Clin Immunol Pract. 2002;110(3):395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 14.Horner CC, Mauger D, Strunk RC, et al. Most nocturnal asthma symptoms occur outside of exacerbations and associate with morbidity. J Allergy Clin Immunol. 2011;128(5):977–982. doi: 10.1016/j.jaci.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117(1):43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- 16.Moorman JE, Akinbami LJ, Bailey C, et al. National surveillance of asthma: United States, 2001–2010. Vital & health statistics Series 3, Analytical and epidemiological studies/[US Dept of Health and Human Services, Public Health Service, National Center for Health Statistics] 2012;Series 3(35):1–67. [PubMed] [Google Scholar]
- 17.Levin AM, Wang Y, Wells KE, et al. Nocturnal asthma and the importance of race/ethnicity and genetic ancestry. Am J Respir Crit Care Med. 2014;190(3):266–273. doi: 10.1164/rccm.201402-0204OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegienka G, Havstad S, Joseph CL, et al. Racial disparities in allergic outcomes in African Americans emerge as early as age 2 years. Clin Exp Allergy. 2012;42(6):909–917. doi: 10.1111/j.1365-2222.2011.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koinis-Mitchell D, Kopel SJ, Boergers J, et al. Asthma, allergic rhinitis, and sleep problems in urban children. J Clin Sleep Med. 2015;11(2):101–110. doi: 10.5664/jcsm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koinis-Mitchell D, Kopel SJ, Boergers J, et al. Good sleep health in urban children with asthma: A risk and resilience approach. J Pediatr Psychol. 2015;40(9):888–903. doi: 10.1093/jpepsy/jsv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94(1–8) [PubMed] [Google Scholar]
- 22.National Heart Lung and Blood Institute. Guidelnes for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 23.Canino G, Bravo M. The adaptation and testing of diagnostic and outcome measures for cross-cultural research. Int Rev Psychiatry. 1994;6(4):281–286. [Google Scholar]
- 24.U.S. Department of Health and Human Services. The 2005 HHS Poverty Guidelines. Washington, DC: U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 25.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 26.McQuaid EL, Walders N, Kopel SJ, Fritz GK, Klinnert MD. Pediatric asthma management in the family context: The Family Asthma Management System Scale. J Pediatr Psychol. 2005;30(6):492–502. doi: 10.1093/jpepsy/jsi074. [DOI] [PubMed] [Google Scholar]
- 27.Koinis-Mitchell DK, McQuaid EL, Seifer R, et al. Symptom perception in children with asthma: Cognitive and psychological factors. Health Psychol. 2009;28(2):226–237. doi: 10.1037/a0013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 29.Liu AH, Zeiger R, Sorkness CA, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 30.Ownby DR. Will the real inner-city allergen please stand up? J Allergy Clin Immunol. 2013;132(4):836. doi: 10.1016/j.jaci.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Phipatanakul W, Celedon J, Sredl D, Weiss S, Gold D. Mouse exposure and wheeze in the first year of life. J Allergy Clin Immunol. 2005;115(2):593–599. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forno E, Cloutier MM, Datta S, et al. Mouse allergen, lung function, and atopy in Puerto Rican children. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs TS, Forno E, Brehm JM, et al. Mouse allergen exposure and decreased risk of allergic rhinitis in school-aged children. Ann Allergy Asthma Immunol. 2014;113(6):614–618. e612. doi: 10.1016/j.anai.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. J Allergy Clin Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 35.Pongracic JA, Krouse RZ, Babineau DC, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138(4):1030–1041. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu AH, Babineau DC, Krouse RZ, et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J Allergy Clin Immunol. 2016;138(4):1042–1050. doi: 10.1016/j.jaci.2016.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teach SJ, Gergen PJ, Szefler SJ, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135(6):1465–1473. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JJ, Burchard EG, Choudhry S, et al. Differences in allergic sensitization by self-reported race and genetic ancestry. J Allergy Clin Immunol. 2008;122(4):820–827. doi: 10.1016/j.jaci.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar R, Nguyen EA, Roth LA, et al. Factors associated with degree of atopy in Latino children in a nationwide pediatric sample: the Genes-environments and Admixture in Latino Asthmatics (GALA II) study. J Allergy Clin Immunol. 2013;132(4):896–905. doi: 10.1016/j.jaci.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland ER. Nocturnal asthma. J Allergy Clin Immunol. 2005;116(6):1179–1186. doi: 10.1016/j.jaci.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Baxi SN, Phipatanakul W. The role of allergen exposure and avoidance in asthma. Adolesc Med State Art Rev. 2010;21(1):57. [PMC free article] [PubMed] [Google Scholar]
- 42.Zarei M, Remer CF, Kaplan MS, et al. Optimal skin prick wheal size for diagnosis of cat allergy. Annals of Allergy, Asthma & Immunology. 2004;92(6):604–610. doi: 10.1016/S1081-1206(10)61425-1. [DOI] [PubMed] [Google Scholar]